Abstract
Introduction
We describe a case of metallic, angiographic coil migration, following radiological exclusion of a gastroduodenal artery pseudoaneurysm secondary to chronic pancreatitis.
Patients and Methods
A 55-year-old man presented to the out-patient clinic with chronic, intermittent, post-prandial, abdominal pain, associated with nausea, vomiting and weight loss. He was known to have chronic pancreatitis and liver disease secondary to alcohol abuse and previously underwent angiographic exclusion of a gastroduodenal artery pseudoaneurysm. During subsequent radiological and endoscopic investigation, an endovascular coil was discovered in the gastric pylorus, associated with ulceration and cavitation. This patient was managed conservatively and enterally fed via naso-jejunal catheter endoscopically placed past the site of the migrated coil. This patient is currently awaiting biliary bypass surgery for chronic pancreatitis, and definitive coil removal will occur concurrently.
Conclusions
Literature review reveals that this report is only the eighth to describe coil migration following embolisation of a visceral artery pseudoaneurysm or aneurysm. Endovascular embolisation of pseudoaneurysms and aneurysms is generally safe and effective. More common complications of visceral artery embolisation include rebleeding, pseudoaneurysm reformation and pancreatitis.
Keywords: Coil migration, Pseudoaneurysm, Visceral artery
Introduction
Visceral artery pseudoaneurysms or aneurysms (VAPA) of the splenic,1 gastroduodenal,2, 3 hepatic,2 gastroepiploic,4 superior mesenteric5 or inferior mesenteric6 (or any visceral branch of the coeliac axis, inferior mesenteric, superior mesenteric or renal arteries) arteries can form secondary to a variety of congenital, traumatic and inflammatory pathologies. Visceral artery aneurysms are found in 0.01–0.2% of routine autopsies but are being increasingly found incidentally in our ageing population;7 whereas visceral artery pseudoaneurysm formation is a rare but potentially fatal complication occurring in less than 2% of cases of chronic pancreatitis (CP),8 and even less frequently following acute pancreatitis and hepatopancreaticobiliary (HPB) surgery, 9 vasculitis or other inflammatory processes.10 Massive haemorrhage into the gastrointestinal (GI) tract or peritoneal cavity from VAPAs can result in death in 20–40% of cases.9, 11, 12 Whereas spontaneous thrombosis has been described rarely,4 VAPAs usually require treatment in the form of surgical,13 endovascular,14 or combined approaches.5
We describe a case, and review all similar reports, of coil migration following endovascular exclusion of a visceral artery pseudoaneurysm.
Case report
A 55-year-old man with known diagnoses of alcohol-related liver disease (Child-Pugh Grade B) and CP was routinely reviewed in the hepatopancreaticobiliary (HPB) outpatient clinic. During the consultation he complained of chronic abdominal pain that was significantly exacerbated post-prandially and associated with nausea, vomiting and weight loss. He was known to have multiple liver disease and CP-related complications, including biliary obstruction and previous placement of a common bile duct (CBD) stent; splenic and abdominal varices; splenic and portal vein occlusion; a dilated pancreatic duct; duodenal obstruction secondary to a large cyst in the head of the pancreas; a pseudocyst (Fig 1); and a large gastroduodenal artery (GDA) pseudoaneurysm (Fig 1).
Figure 1.

Computed tomography images reveal a large pancreatic pseudocyst (horizontal arrow) and associated gastroduodenal artery pseudoaneurysm (vertical arrow).
The GDA pseudoaneurysm had been discovered on routine computed tomography (CT) follow-up for CP 10 months prior to his current presentation. The pseudoaneurysm had been managed by endovascular insertion of 20 x 3mm metallic coils and thrombin injection, via a small, discrete neck. Angiography one day post initial endovascular exclusion revealed a residual 20% sac requiring further endovascular management with 12 x 3mm metallic coils, following which complete exclusion of the pseudoaneurysm was confirmed (Fig 2). His other past medical history included diabetes and steatorrhoea but he had now been abstinent from alcohol for over a year.
Figure 2.

Angiographic image, following endovascular coiling, reveals multiple coils (arrow) in a successfully excluded gastroduodenal artery pseudoaneurysm.
Examination in the outpatient clinic revealed spider naevi, epigastric and right upper quadrant tenderness, a palpable liver edge and evidence of significant weight loss. He was directly admitted for further investigation of his symptoms.
Blood tests revealed no gross abnormalities. However, a Gastrograffin follow-through revealed significant pyloric outlet and duodenal obstruction (Fig 3) with duodenal dilatation, which was confirmed on endoscopic retrograde cholangio-pancreatography (ERCP). Also revealed on ERCP was a metal coil in the pyloric region, associated with ulceration and cavitation, that could not be removed endoscopically (Fig 4). A sphincterotomy was performed, a new CBD stent inserted and a naso-jejunal (NJ) catheter passed. No fistula or direct pathway for migration between the GDA pseudoaneurysm and the pylorus was convincingly demonstrated on ERCP or follow-up CT.
Figure 3.

Gastrograffin follow-through images reveal the migrated endovascular coil (arrow) and contrast delay at the gastric outlet.
Figure 4.
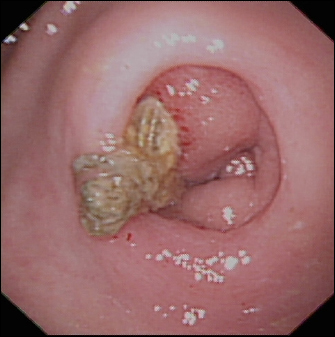
Oesophagogastroduodenoscopic images reveal an endovascular coil extending through the pyloric region of the stomach.
A duodenal stent was subsequently endoscopically inserted and the patient’s symptoms gradually resolved. He was discharged home well after 17 days as an in-patient, with NJ feeding and dietician care. However, in the longer term, conservative management with duodenal and biliary stenting failed to successfully relieve the patient’s symptoms, particularly following NJ-catheter removal and commencement of normal food intake. Therefore, despite the high risk associated with surgery, definitive management in the form of simultaneous biliary and gastric bypass is being considered following satisfactory pre-operative assessment (possibly including cardiopulmonary exercise testing) and, once his nutritional condition improves, surgical removal of the coil will take place concurrently.
Review methods
A computerised literature search of PubMed was made for all reports of coil migration following endovascular management of arterial pseudoaneurysms and aneurysms, with particular emphasis upon visceral arteries, and utilising the key-words: pseudoaneurysm, aneurysm, visceral artery, coil, and migration in various combinations. All reports written in English, or with an English abstract that contained pertinent information, were included.
This approach led to the review of seven reports of coil migration following endovascular treatment of visceral artery pseudoaneurysms or aneurysms (Table 1).
Table 1.
A summary of reports documenting the migration of endovascular coils from visceral arteries
| Author | Age (yrs) | Sex | Diagnosis | PMH | Site of vascular abnormality | Site of coil migration | Time from coil insertion | Management | Outcome |
| Pseudoaneurysm | |||||||||
| Skipworth et al (2009) | 55 | M | CP | Liver disease | Gastroduodenal artery | Gastric pylorus | 10 months | NJ-nutrition and future surgery | Well |
| Reed et al (2007) [20] | 50 | F | PCNL | Renal calculus | Renal artery branch AV fistula | Left uretero-vesical junction | 1 year | None – coil passed | Well |
| Shah et al (2007) [16] | 65 | F | CP | AP; DM | Splenic artery | Passage per rectum | 3 weeks | None-coil passed | Well |
| Turaga et al (2006) [17] | 65 | M | Cholecystectomy | Chole | Hepatic artery | CBD | 1 year | Open CBD exploration | Well |
| Ozkan et al (2002) [18] | 58 | M | CP | Chole | Hepatic artery | CBD | 2 years | Open CBD exploration | Well |
| Takahashi et al (2001) [15] | 59 | M | CP | AP | Splenic artery | Gastric body | 3 weeks | Open surgery (concurrent gastric carcinoma) | Well |
| Aneurysm | |||||||||
| Dinter et al (2007) [19] | 82 | F | Upper GI Bleed | Scl; GU | Coeliac trunk | Cardia/lesser gastric curve | 10 years | None | Fatal haemate mesis (aortogastric fistula) |
| Abad et al (1990) [21] | 18 | M | Unknown Aetiology | Unknown | Pulmonary artery | Right basal bronchus of inferior lobe | 6 weeks | Open surgery (right inferior lobectomy) | Well |
CBD – Common bile duct
Scl – Scleroderma
GI – Gastrointestinal
GU – Gastric ulcer
AP– Acute pancreatitis
Chole – Acute cholecystitis
CP – Chronic pancreatitis
PCNL – Percutaneous nephrolithotomy
DM – Diabetes
NJ-Nasojejunal
Review results
Two reports describe coil migration following endovascular exclusion of pancreatitis-related pseudoaneurysms. Takahashi et al15 describe the incidental intra-operative finding of a coil in the stomach, which migrated via an endoscopically confirmed gastropseudocystic fistula, three weeks after endovascular coiling of a large splenic artery pseudoaneurysm secondary to acute pancreatitis. Shah et al16 document the passage of two steel-wire coils from an embolised splenic artery pseudoaneurysm secondary to alcohol-related pancreatitis, via the GI tract, to be discovered in the patient’s stool three weeks following embolisation. Similarly to our case, no clear communication or fistula was definitively demonstrated by Shah et al.
Two further reports describe the sequelae of coil migration in patients who underwent embolisation of hepatic artery pseudoaneurysms – the first occurring following a difficult open cholecystectomy in a patient who later presented with ascending cholangitis resulting from coil migration into the CBD;17 and a further report describing the development of pancreatitis following coil erosion into the CBD in a patient who had undergone a difficult surgical dissection during cholecystectomy for acute acalculous cholecystitis.18 Other reports describe coil migration from a coeliac trunk aneurysm, via an aortogastric fistula and leading to fatal upper GI haemorrhage;19 from a renal artery aneurysm, through the renal collecting system to be passed via the urinary tract;20 and from a pulmonary artery aneurysm into the bronchus. 21
Further identified reports describe endovascular coil migration following the embolisation of a pseudoaneurysm, aneurysm or vascular abnormality from various nonvisceral arterial sites including through the middle ear in a patient who had undergone embolisation of an internal carotid pseudoaneurysm;22 from a superior gluteal vessel following embolisation of a haematoma;23 and multiple reports of migration from intracranial aneurysms to alternate sites within the intra-cerebral circulation.24–29
Discussion
Pseudoaneurysm formation of the GDA is a well-recognised complication of CP30 and a therapeutic endovascular approach is one of the major modalities employed during pseudoaneurysm management.31 We report the case of a patient in whom a coil used to embolise a GDA pseudoaneurysm migrated into the gastric pylorus, resulting in gastric ulceration and possibly contributing to outlet obstruction secondary to CP and pancreatic pseudocyst. This unique case represents only the eighth documented report of coil migration following endovascular exclusion of a visceral artery pseudoaneurysm or aneurysm (Table 1). Of note is the fact that the majority of authors were unable to effectively demonstrate a clear route of migration between the sites of coil insertion and resultant migration.
Endovascular methods of VAPA exclusion depend upon lesion size, location and flow rates. The most commonly employed endovascular techniques include embolisation, stent insertion and thrombin injection, all of which aim to exclude the VAPA from the circulation, while simultaneously preserving distal blood flow. A combination of materials, including metallic coils, gelfoam, hydrogel particles, acrylic glue or a combination of these, can now be used for embolisation.1
Afferent arterial embolisation can be employed in instances of pseudoaneurysm or aneurysm formation from visceral arteries with no significant collateral supply.3 However, VAPAs with a well-established collateral supply3 and high flow14 usually require embolisation of proximal and distal branches to enable successful exclusion and prevent backflow from the collateral circulation and thus may be capable of preventing the subsequent migration of coils.15 Narrow-necked pseudoaneurysms or aneurysms are best treated via direct delivery of coils into the sac;3 whereas wide-necked and large diameter vessels can be treated by stent insertion. 3 Low-flow VAPAs can often be treated with percutaneous thrombin injection alone.14
Literature review reveals that percutaneous endovascular embolisation techniques are generally safe and effective,1, 14 and are capable of achieving definitive haemostasis in 80–95% of emergency cases,8, 9, 11, 24 particularly when early recognition and treatment takes place.12 Some studies have therefore described an association of radiological embolisation strategies with a decreased incidence of blood transfusion and length of hospital stay, as well as lower re-bleed and mortality rates, as compared to surgery, when utilised in the emergency setting.32
More common complications following endovascular exclusion include bleeding or re-bleeding, often requiring repeat angiography16 and surgical intervention for ligation or repair of bleeding vessels8 and pseudoaneurysm recurrence, 14 both of which mandate careful follow-up. Postembolisation syndrome and infarction can occur in up to 30% of splenic artery pseudoaneurysm or aneurysm exclusions,33 although clinically significant infarction of the spleen, or other organs, is rare. Other complications of endovascular coiling include catheterisation failure,34 arterial dissection occasionally requiring angioplasty to prevent propagation and maintain vessel patency35 and initiation of acute episodes of pancreatitis.36
However, most studies to date have investigated the use of angioembolisation in pseudoaneurysms of various aetiologies and the specific applicability of these studies to pseudoaneurysms in CP remains unknown. Udd et al8 attempted to address this specific issue and identified a post-embolisation complication rate of 17%4, 23 in patients undergoing angioembolisation for bleeding pseudoaneurysms secondary to CP. The complications consisted of one coil being pushed into the main pancreatic duct and requiring endoscopic removal; one dissection of the bleeding artery (which led to bleeding cessation); one coil being pushed into the iliac artery and requiring operative intervention; and one pseudoaneurysm at the angiography inguinal puncture site.
Acknowledgements
James Skipworth, who is supported as the Jason Boas Fellow by the No Surrender Charitable Trust, drafted the manuscript. All authors have significantly contributed to, read and approved the final manuscript. All authors declare that they have no competing interests, that the submitted work is their own and that copyright has not been breached in seeking its publication.
References
- 1.Venkatesh SK, Kumar S, Baijal SS et al. Endovascular management of pseudoaneurysms of the splenic artery: experience with six patients. Australas Radiol 2005; 49: 283–288. [DOI] [PubMed] [Google Scholar]
- 2.Basile A, Ragazzi S, Piazza D et al. Hepatic artery pseudoaneurysm treated using stent-graft implantation and retrograde gastroduodenal artery coil embolization. Eur Radiol 2008; 18: 2579–2581. [DOI] [PubMed] [Google Scholar]
- 3.Chong WW, Tan SG, Htoo MM. Endovascular treatment of gastroduodenal artery aneurysm. Asian Cardiovasc Thorac Ann 2008; 16: 68–72. [DOI] [PubMed] [Google Scholar]
- 4.Vanlangenhove P, Defreyne L, Kunnen M. Spontaneous thrombosis of a pseudoaneurysm complicating pancreatitis. Abdom Imaging 1999; 24: 491–493. [DOI] [PubMed] [Google Scholar]
- 5.Saito T, Tsuchiya T, Kenjo A et al. Successful treatment of pseudoaneurysms of celiac and superior mesenteric arteries by combined endovascular and surgical approach. J Hepatobiliary Pancreat Surg 2008; 15: 444–448. [DOI] [PubMed] [Google Scholar]
- 6.Tulsyan N, Kashyap VS, Greenberg RK et al. The endovascular management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg 2007; 276–283; discussion 283. [DOI] [PubMed]
- 7.Kanazawa S, Inada H, Murakami T et al. The diagnosis and management of splanchnic artery aneurysms. Scand J Gastroenterol 1996; 31: 737–743. [DOI] [PubMed] [Google Scholar]
- 8.Udd M, Leppäniemi AK, Bidel S et al. Treatment of bleeding pseudoaneurysms in patients with chronic pancreatitis. World J Surg 2007; 31: 504–510. [DOI] [PubMed] [Google Scholar]
- 9.Balachandra S, Siriwardena AK. Systematic appraisal of the management of the major vascular complications of pancreatitis. Am J Surg 2005; 3: 489–495. [DOI] [PubMed] [Google Scholar]
- 10.Stanley JC, Wakefield TW, Graham LM et al. Clinical importance and management of splanchnic artery aneurysms. J Vasc Surg 1986; 3: 836–840. [PubMed] [Google Scholar]
- 11.Hyare H, Desigan S, Brookes JA et al. Endovascular management of major arterial hemorrhage as a complication of inflammatory pancreatic disease. J Vasc Interv Radiol 2007; 18: 591–596. [DOI] [PubMed] [Google Scholar]
- 12.Zyromski NJ, Vieira C, Stecker M et al. Improved outcomes in postoperative and pancreatitis-related visceral pseudoaneurysms. J Gastrointest Surg 2007; 11: 50–55. [DOI] [PubMed] [Google Scholar]
- 13.Upadhyaya PK, Chava S, Bin-Sangheer S et al. Delayed rupture of a splenic artery pseudoaneurysm after biliopancreatic diversion. Obes Surg 2008; 18: 890–892. [DOI] [PubMed] [Google Scholar]
- 14.Nicholson AA, Patel J, McPherson S et al. Endovascular treatment of visceral aneurysms associated with pancreatitis and a suggested classification with therapeutic implications. J Vasc Interv Radiol 2006; 17: 1279–1285. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi T, Shimada K, Kobayashi N et al. Migration of steel-wire coils into the stomach after transcatheter arterial embolization for a bleeding splenic artery pseudoaneurysm: report of a case. Surg Today 2001; 31: 458–462. [DOI] [PubMed] [Google Scholar]
- 16.Shah NA, Akingboye A, Haldipur N et al. Embolization coils migrating and being passed per rectum after embolization of a splenic artery pseudoaneurysm, ‘the migrating coil’: a case report. Cardiovasc Intervent Radiol 2007; 30: 1259–1262. [DOI] [PubMed] [Google Scholar]
- 17.Turaga KK, Amirlak B, Davis RE et al. Cholangitis after coil embolization of an iatrogenic hepatic artery pseudoaneurysm: an unusual case report. Surg Laparosc Endosc Percutan Tech 2006; 16: 36–38. [DOI] [PubMed] [Google Scholar]
- 18.Ozkan OS, Walser EM, Akinci D et al. Guglielmi detachable coil erosion into the common bile duct after embolization of iatrogenic hepatic artery pseudoaneurysm. J Vasc Interv Radiol 2002; 13: 935–938. [DOI] [PubMed] [Google Scholar]
- 19.Dinter DJ, Rexin M, Kaehler G et al. Fatal coil migration into the stomach 10 years after endovascular celiac aneurysm repair. J Vasc Interv Radiol 2007; 18: 117–120. [DOI] [PubMed] [Google Scholar]
- 20.Reed A, Suri R, Marcovich R. Passage of embolization coil through urinary collecting system one year after embolization. Urology 2007; 70: 1222 e17–18. [DOI] [PubMed] [Google Scholar]
- 21.Abad J, Villar R, Parga G et al. Bronchial migration of pulmonary arterial coil. Cardiovasc Intervent Radiol 1990; 13: 345–346. [DOI] [PubMed] [Google Scholar]
- 22.Chow MW, Chan DT, Boet R et al. Extrusion of a coil from the internal carotid artery through the middle ear. Hong Kong Med J 2004; 10: 215–216. [PubMed] [Google Scholar]
- 23.Yuan KC, Hsu YP, Fang JF et al. Delayed Hemorrhage Caused by Coil Migration After Transcatheter Arterial Embolization in Patient With Unstable Pelvic Fracture: A Case Report. J Trauma 2008; 66: 267–270. [DOI] [PubMed] [Google Scholar]
- 24.Beattie GC, Hardman JG, Redhead D et al. Evidence for a central role for selective mesenteric angiography in the management of the major vascular complications of pancreatitis. Am J Surg 2003; 185: 96–102. [DOI] [PubMed] [Google Scholar]
- 25.Phatouros CC, McConachie NS, Jaspan T. Post-procedure migration of Guglielmi detachable coils and Mechanical detachable spirals. Neuroradiology 1999; 41: 324–327. [DOI] [PubMed] [Google Scholar]
- 26.Kiyosue H, Okahara M, Tanoue S et al. Dispersion of coils after parent-artery occlusion of radiation-induced internal carotid artery pseudoaneurysm. AJNR Am J Neuroradiol 2004; 25: 1080–1082. [PMC free article] [PubMed] [Google Scholar]
- 27.Collignon FP, Friedman JA, Piepgras DG et al. Transcutaneous coil, stent, and balloon migration following endovascular treatment of a cervical carotid artery aneurysm. Case illustration. J Neurosurg 2003; 98: 1135. [DOI] [PubMed] [Google Scholar]
- 28.Iguchi H, Takayama M, Kusuki M et al. Transmucosal coil migration after endovascular management for carotid artery pseudoaneurysm: a late complication. Acta Otolaryngol 2007; 127: 447–448. [DOI] [PubMed] [Google Scholar]
- 29.Dagain A, Nataf F, Page P et al. Endovascular coil transfixing a cranial nerve five years after embolisation. Acta Neurochir (Wien) 2008; 150: 705–707; discussion 707. [DOI] [PubMed] [Google Scholar]
- 30.de Perrot M, Berney T, Bühler L et al. Management of bleeding pseudoaneurysms in patients with pancreatitis. Br J Surg 1999; 86: 29–32. [DOI] [PubMed] [Google Scholar]
- 31.Brountzos EN, Vagenas K, Apostolopoulou SC et al. Pancreatitis-associated splenic artery pseudoaneurysm: endovascular treatment with self-expandable stent-grafts. Cardiovasc Intervent Radiol 2003; 26: 88–91. [DOI] [PubMed] [Google Scholar]
- 32.Bergert H, Hinterseher I, Kersting S et al. Management and outcome of hemorrhage due to arterial pseudoaneurysms in pancreatitis. Surgery 2005; 137: 323–328. [DOI] [PubMed] [Google Scholar]
- 33.Piffaretti G, Tozzi M, Lomazzi C et al. Splenic artery aneurysms: postembolization syndrome and surgical complications. Am J Surg 2007; 193: 166–170. [DOI] [PubMed] [Google Scholar]
- 34.Mansueto G, Cenzi D, D’Onofrio M et al. Endovascular treatment of arterial bleeding in patients with pancreatitis. Pancreatology 2007; 7: 360–369. [DOI] [PubMed] [Google Scholar]
- 35.Mammen T, Joseph P, Sitaram V et al. Acute parent artery dissection as a complication of mesenteric endovascular coil embolisation for pancreatic pseudoaneurysm. Br J Radiol 2008; 81: e7–e10. [DOI] [PubMed] [Google Scholar]
- 36.Saltzberg SS, Maldonado TS, Lamparello PJ et al. Is endovascular therapy the preferred treatment for all visceral artery aneurysms? Ann Vasc Surg 2005; 19: 507–515. [DOI] [PubMed] [Google Scholar]


