Abstract
Aims: Fasting and postprandial hypertriglyceridemia (PHTG) are caused by the accumulation of triglyceride (TG)-rich lipoproteins and their remnants, which have atherogenic effects. Fibrates can improve fasting and PHTG; however, reduction of remnants is clinically needed to improve health outcomes. In the current study, we investigated the effects of a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), K-877 (Pemafibrate), on PHTG and remnant metabolism.
Methods: Male C57BL/6J mice were fed a high-fat diet (HFD) only, or an HFD containing 0.0005% K-877 or 0.05% fenofibrate, from 8 to 12 weeks of age. After 4 weeks of feeding, we measured plasma levels of TG, free fatty acids (FFA), total cholesterol (TC), HDL-C, and apolipoprotein (apo) B-48/B-100 during fasting and after oral fat loading (OFL). Plasma lipoprotein profiles after OFL, which were assessed by high performance liquid chromatography (HPLC), and fasting lipoprotein lipase (LPL) activity were compared among the groups.
Results: Both K-877 and fenofibrate suppressed body weight gain and fasting and postprandial TG levels and enhanced LPL activity in mice fed an HFD. As determined by HPLC, K-877 and fenofibrate significantly decreased the abundance of TG-rich lipoproteins, including remnants, in postprandial plasma. Both K-877 and fenofibrate decreased intestinal mRNA expression of ApoB and Npc1l1; however, hepatic expression of Srebp1c and Mttp was increased by fenofibrate but not by K-877. Hepatic mRNA expression of apoC-3 was decreased by K-877 but not by fenofibrate.
Conclusion: K-877 may attenuate PHTG by suppressing the postprandial increase of chylomicrons and the accumulation of chylomicron remnants more effectively than fenofibrate.
Keywords: K-877 (Pemafibrate), SPPARMα, Postprandial hypertriglyceridemia, Fenofibrate, Chylomicron remnants
See editorial vol. 25: 126–127
1. Introduction
Fasting hypertriglyceridemia is an independent risk factor for coronary heart disease (CHD)1, 2), even when patients are treated with statins3). Patients with high fasting triglyceride (TG) level (≥ 150 mg/dL) have a high risk of CHD and are encouraged to receive secondary checkups and medical intervention. Similarly, postprandial hypertriglyceridemia (PHTG) reflects the CHD risk2, 4), as Zilversmit suggested over 30 years ago5). In PHTG, the TG-rich lipoproteins (TRLs) predominantly increase during the postprandial state, and their hydrolyzed products, remnant lipoproteins (or remnants), are increased during the fasting state6). The accumulation of intestine-derived chylomicron (CM) remnants has been shown to be highly atherogenic6, 7), as have liver-derived very low-density lipoprotein (VLDL) remnants and low-density lipoprotein (LDL) particles. Thus, treatments to ameliorate the accumulation of atherogenic CM remnants are needed.
Fibrates are recommended for the management of hypertriglyceridemia in the Japan Atherosclerosis Society Guidelines 20128). Fenofibrate improves cardiovascular outcomes in combination with a decrease in fasting TG levels in patients with a high risk status for CHD, such as those with diabetes9–11). Fenofibrate attenuates postprandial increases in TG and remnant lipoproteins in patients with hypertriglyceridemia12), partially through decreasing CM production in the small intestine13). Fibrates activate a class of intracellular receptors known as peroxisome proliferator-activated receptors (PPARs), especially PPARα. Because PPARs modulate carbohydrate and fat metabolism and adipose tissue differentiation, the activation of PPARα upregulates the transcription of multiple genes that facilitate lipid metabolism such as apolipoprotein (apo)A-5 and lipoprotein lipase (LPL), and downregulates that of apoC-3. These proteins modulate the amount of free fatty acid utilized for synthesis and secretion of apoB-containing lipoproteins14). However, the currently used fibrates are weak PPARα-agonists or pan-PPAR agonists; therefore, they have less selectivity for PPARα and have a high risk of causing unwanted side effects15). Therefore, a novel member of the selective PPARα modulator (SPPARM α) family designed to have higher PPARα agonistic activity and selectivity than fibrates, named K-877 (Pemafibrate), was produced16). In patients with hypertriglyceridemia, the administration of K-877 (0.025–0.4 mg, for 12 weeks) has been found to decrease fasting TG levels (−30.9% to −42.7% from the baseline) more potently than fenofibrate at 100 mg (−29.7%, p < 0.001)17). K-877 also decreased levels of VLDL-cholesterol, CM-cholesterol, remnant lipoprotein cholesterol, apoB, and apoC-3 without causing a significant increase in the incidence of adverse events. These results suggest that K-877 is also effective for ameliorating impaired metabolism of TRLs and remnant lipoproteins in patients with hypertriglyceridemia; however, its mechanism is unknown.
In the current study, we examined whether K-877 ameliorates PHTG by using a mouse model with diet-induced hypertriglyceridemia and investigated the consequent effects on TRL metabolism.
2. Materials and Methods
2.1. Animals and OFL Test
Male C57BL/6J mice were housed in a temperature- and humidity-controlled facility with a 12-h light/dark cycle and fed a normal chow diet. From eight to twelve weeks of age (for four weeks), mice were fed a high-fat diet (HFD, Oriental Bio Laboratories, Chiba, Japan) only, HFD containing 0.0005% K-877, or HFD containing 0.05% fenofibrate (n = 10/group) (Fig. 1A). This concentration was adopted from the former experiment using mouse for investigating lipid-lowering effect of K-87718). K-877 decreased TG levels and increased HDL-cholesterol levels significantly when it was used in this concentration and toxic responses were not observed in this former study18). The weight gain and dietary intake of all mice were recorded. When the mice were twelve weeks of age, oral fat loading (OFL) tests were performed through administration of olive oil by gavage (17 µL/g body weight) after an overnight fast. Blood (50 µL) was drawn from an orbital vein with a capillary micropipette (Ringcaps, Hirschmann Laborgerate, Eberstadt, Germany) while mice were under 2% isoflurane anesthesia. Mice used for the experiment were anesthetized by an intraperitoneal injection of medetomidine (0.3 mg/kg), midazolam (4 mg/kg), and butorphanol (5 mg/kg). Adequate anesthesia was maintained by monitoring the respiration rate and the lack of response to paw pinching. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Osaka University Graduate School of Medicine and was conducted in accordance with the Public Health Service (PHS) Policy on the Human Care and Use of Laboratory Animals, which was incorporated into the Institute for Laboratory Animal Research (ILAR) Guide for the Care and Use of Laboratory Animals.
Fig. 1.
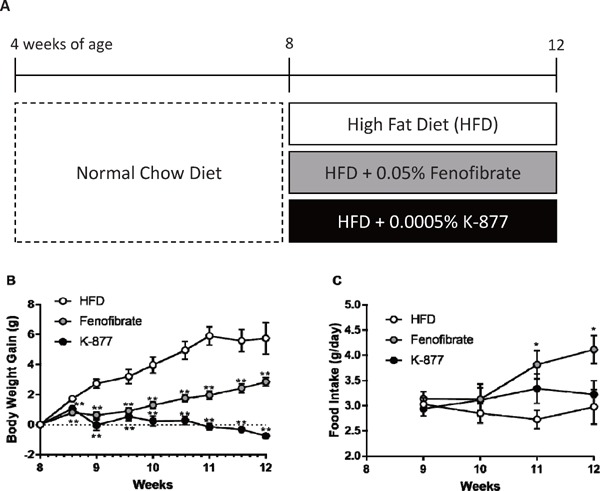
Body weight gain and food intake in mice fed after treatment with K-877 and fenofibrate.
Mice (n = 10, male, C57B6/J) were fed a normal chow diet up to 8 weeks of age, and they were then fed with a HFD only, an HFD containing 0.0005% K-877, or an HFD containing 0.05% fenofibrate for 4 weeks (A). Body weight gain from the baseline level (g) (B) and food intake (g/day) (C) were observed in these groups from 8 to 12 weeks of age. *: p < 0.05, **: p < 0.01 compared to HFD only.
2.2. Measurements
Plasma levels of TG, total cholesterol (TC) and FFA were measured by enzymatic methods (Wako Pure Chemical Industries, Tokyo, Japan), and these levels were compared at each time point. Areas under the curve (AUC) were calculated with the trapezoidal method. Lipoprotein profiles of plasma 4 hours after OFL were compared using high performance liquid chromatography (HPLC) (Liposearch, Skylight Biotech, Tokyo, Japan)13). LPL activities were compared among the 3 groups during the fasting state (LPL Activity Assay Kit, Roar Biomedical, Inc., New York, USA); we administered sodium heparin (1U/10gBW) via left carotid vein and blood samples were collected from right carotid vein 15 minutes after the injection.
2.3. Protein Analysis
Plasma samples were diluted in 1:100 in SDS sample buffer and incubated at 37°C overnight. Samples were applied to an SDS-PAGE gel, separated using SDS running buffer and subsequently transferred onto polyvinylidene difluoride membranes. The membranes were incubated for one hour at room temperature in 5% skim milk (Wako Pure Chemical Industries, Tokyo, Japan) in TBS with 0.1% Tween-20 (TBS-T) as blocking buffer. They were then probed with a rabbit polyclonal anti-apolipoprotein B (ApoB) antibody (1:1000, Meridian Life Science Inc., Ohio, USA) in TBS-T overnight at 4 °C for the detection of apoB-48 and apoB-100 proteins. Then, they were probed with an anti-rabbit secondary antibody conjugated to HRP (1:100,000, GE Healthcare, Uppsala, Sweden) in TBS-T for 30 minutes at room temperature. The reactions were developed with ChemilumiOne Ultra (Nacalai Tesque, Kyoto, Japan). Images were analyzed by ImageQuant LAS 4000 mini (GE Healthcare, Uppsala, Sweden).
2.4. Quantitative Real-Time PCR
Messenger RNA expressions in jejunum enterocytes and liver were measured with quantitative real-time PCR (qRT-PCR). The mouse intestines were divided into three segments and washed with PBS, and tissues containing enterocytes in the center segment were harvested from mice before and three hours after OFL. Briefly, total RNA was extracted from snap-frozen, homogenized enterocytes with an RNeasy Mini kit (Qiagen, Hilden, Germany). The RNA was reverse-transcribed using a SuperScript VILO cDNA Synthesis Kit (Thermo Fisher Scientific, Massachusetts, USA). Diluted cDNA was used as the template to quantify the relative concentrations of mRNA. The sequences of primers were designed with Primer3 (http://biotools.umassmed.edu/bioapps/primer3_www.cgi). The sequence and information for primers used in this study are shown in Table 1. qRT-PCR was performed using Fast SYBR Green master mix and a 7900 Sequence Detection System (Thermo Fisher Scientific). The relative gene expression values were normalized to those of GAPDH gene expression using the comparative Ct (threshold cycle) method according to the manufacturer's instructions.
Table 1. Primers used for mRNA quantification by real-time RT-PCR.
| Gene | Forward(‘5-’3) | Reverse(‘5-’3) |
|---|---|---|
| ApoB | TGGGATTCCATCTGCCATCTCGAG | GTAGAGATCCATCACAGGACAATG |
| Npc1l1 | GCTTCTTCCGCAAGATATACACTCCC | GAGGATGCAGCAATAGCCACATAAGAC |
| Srebp1c | CTGACAGGTGAAATCGGCG | GCATGTCTTCAAATGTGCAATCC |
| Mttp | CATGTCAGCCATCCTGTTTG | CTCGCGATACCACAGACTGA |
| Apoc3 | TCAGATCCCTGAAAGGCTAC | ATAGCTGGAGTTGGTTGGTC |
| Gapdh | ACTCCACTCACGGCAAATTC | TCTCCATGGTGGTGAAGACA |
2.5. Statistical Analysis
All data are presented as mean ± SEM. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). We used a modified version of R commander designed to add statistical functions frequently used in biostatistics18). Student's t-tests, one-way ANOVA, and Holm's multiple comparison tests were performed as appropriate. p < 0.05 was considered statistically significant.
3. Results
3.1. Body Weight and Dietary Intake
Body weight gain (approximately 6 gram per 4 weeks) was observed in mice fed an HFD, but this gain was suppressed by addition of fenofibrate or K-877 to the HFD (Fig. 1B). Notably, body weight was decreased when mice were fed an HFD containing K-877 from the baseline to twelve weeks of age (Fig. 1B). In all groups, other causes of weight loss such as diarrhea were not observed. Food intake was increased in mice fed an HFD containing fenofibrate, but there were no significant differences among mice fed an HFD only and those with HFD containing K-877 (Fig. 1C).
3.2. OFL Test and LPL Activity
OFL tests were performed after the four week feeding of HFD only, HFD containing fenofibrate or K-877. Fasting levels of plasma TG in mice fed an HFD containing fenofibrate or K-877 were significantly lower than those with HFD only. TG increases after OFL were also decreased (Fig. 2A). Thus, the AUC of plasma TG level (AUC-TG) was markedly decreased by fenofibrate or K-877 (Fig. 2B). FFA levels were also decreased by the addition of fenofibrate or K-877 in mice fed an HFD (Fig. 2C and 2D). However, fasting plasma TC levels were higher in mice with HFD containing fenofibrate or K-877 than those fed an HFD only, but there was no increase after OFL (Fig. 2E and 2F). There were no differences in plasma HDL-cholesterol (HDL-C) levels during the fasting state and after OFL (Fig. 2G). Fasting LPL activity was substantially increased in mice fed an HFD containing fenofibrate or K-877 (Fig. 2H).
Fig. 2.
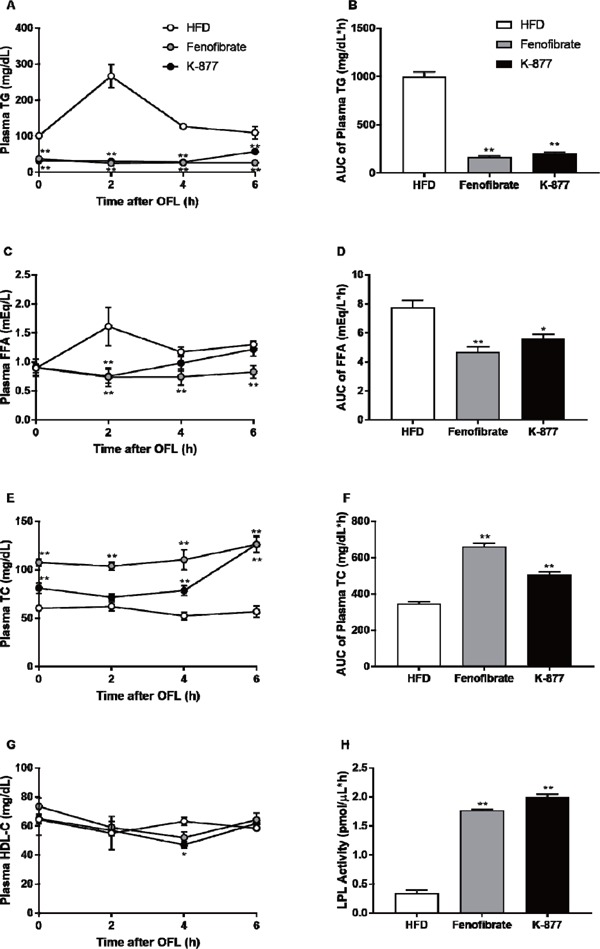
Oral fat loading test after treatment with K-877 and fenofibrate.
At twelve weeks of age, the OFL test was performed using olive oil by gavage (17 µL/g body weight) after an overnight fast. Blood (50 µL) was drawn from an orbital vein, and plasma levels of TGs (A, B), FFA (C, D), TC (E,F) and HDL-cholesterol (HDL-C) (G) were measured, and these levels were compared on the basis of incremental AUC. Lipoprotein lipase (LPL) activities were compared among the three groups during the fasting state. *: p < 0.05, **: p < 0.01 compared with HFD only.
3.3. Lipoprotein Profile and ApoB-48/ApoB-100 Mass
Lipoprotein profiles three hours after OFL were analyzed by the HPLC method13). The addition of fenofibrate or K-877 strikingly decreased TG levels of lipoproteins in CM, VLDL and LDL (Fig. 3A). Cholesterol levels of lipoproteins were increased in LDL and decreased in HDL by the addition of fenofibrate or K-877 (Fig. 3B). During fasting, both apoB-48 and apoB-100 levels were higher in mice fed an HFD containing fenofibrate or K-877 than in mice fed an HFD only (Fig. 3C). ApoB-48 levels were increased after OFL in mice fed an HFD only but not in mice fed an HFD containing fenofibrate or K-877 (Fig. 3C). Moreover, the increase in apoB-100 levels after OFL was ameliorated in mice fed an HFD containing K-877 (Fig. 3D).
Fig. 3.
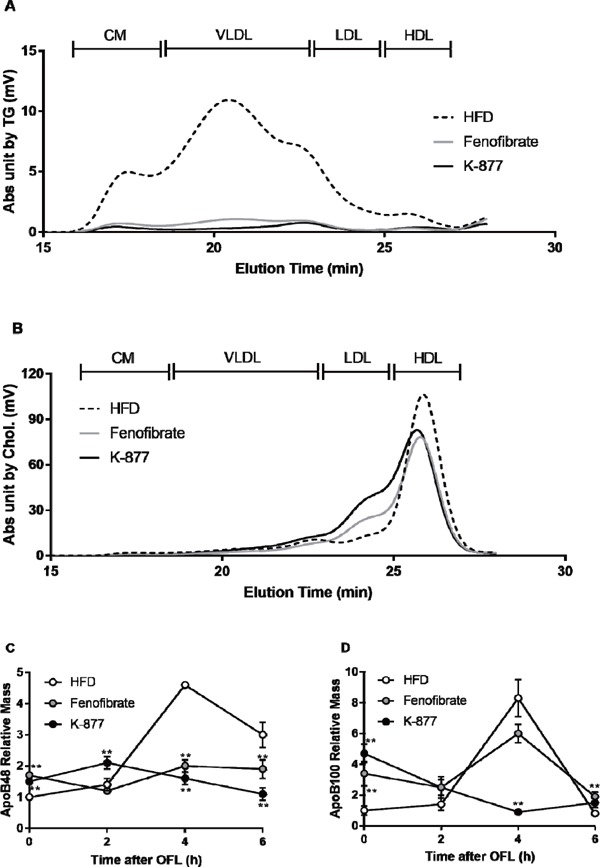
Lipoprotein profile and apolipoprotein B-48/B-100 relative mass of plasma after the oral fat load
Lipoprotein profiles were analyzed and compared among the 3 groups by measurement of TG and cholesterol concentrations of every sub-fractionated sample 4 hours after OFL by using HPLC. Increases in apoB-48 and apoB-100 concentrations after OFL were compared by western blot using a rabbit polyclonal anti-apoB antibody. *: p < 0.05, **: p < 0.01 compared with HFD only.
3.4. Quantitative Analyses of mRNA Expression
Messenger RNA expression of genes related to lipoprotein metabolism were analyzed in intestinal epithelial cells and hepatocytes during fasting and three hours after OFL (Fig. 4). In intestines, mRNA expression of Apob (Fig. 4A) and Npc1l1 (Fig. 4B) was substantially inhibited by fenofibrate and K-877 during fasting and after OFL. However, mRNA expression of Mttp (Fig. 4C) and Srebp1c (Fig. 4D) was significantly enhanced in mice fed an HFD containing fenofibrate during fasting and after OFL compared with HFD only. In contrast, this increase was not observed in mice fed an HFD only or HFD containing K-877 (Fig. 4C, 4D). In hepatocytes, there were no differences in ApoB mRNA expression among the three groups (Fig. 4E). K-877 suppressed ApoC3 mRNA expression during fasting and after OFL but fenofibrate did not (Fig. 4F).
Fig. 4.
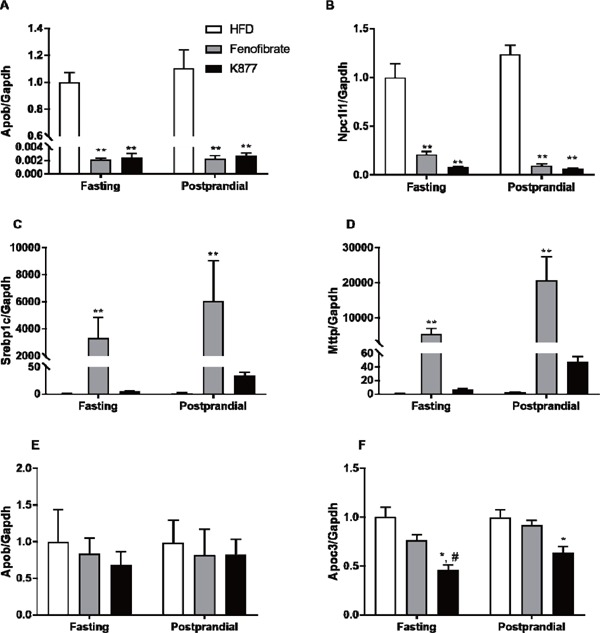
Fasting and postprandial messenger RNA expressions of genes related to lipoprotein metabolism after treatment with K-877 and fenofibrate.
Gene expression in the intestinal epithelial cells and hepatocytes was measured with qRT-PCR. Intestinal mRNA expressions of Apob(A), Npc1l1(B), Srebp1c(C) and Mttp(D) were compared among the 3 groups, and hepatic mRNA expressions of ApoB(E) and ApoC3(F) were compared in the same manner. The relative gene expression values were normalized to those of GAPDH gene expression. *: p < 0.05, **: p < 0.01 compared with HFD only, #: p < 0.05, ##: p < 0.01 compared with HFD containing fenofibrate.
4. Discussion
4.1. K-877 and PHTG
In the current study, we provide the first evidence that K-877, a novel selective PPARα modulator (SPPARMα), attenuates PHTG in mice fed an HFD. K-877 decreased fasting levels and postprandial increases in plasma TG and apoB-48 and ameliorated the postprandial accumulation of TRLs, including CM remnants (Fig. 2 and 3). Similarly to K-877, we reported that fenofibrate also decreased postprandial TG and apoB-48 levels and ameliorated the postprandial accumulation of TRLs, including CM remnants, in an animal model of PHTG, CD36 knockout (CD36-KO) mice13, 19). TG contents of lipoproteins in the size from small CM particle to large VLDL particle (elution time; 18–22 min.) were relatively lower in mice fed an HFD containing K-877 (Fig. 3A) than those containing fenofibrate; it is supposed that K-877 is more effective for decreasing TG and CM remnants during the postprandial state than fenofibrate. Moreover, the dose of K-877 was one-hundredth of that of fenofibrate; it is supposed that K-877 is more efficient for decreasing TG and CM remnants than fenofibrate. In the current study, we investigated the mechanisms underlying PHTG amelioration by both fenofibrate and K-877. In our former study, we have found that the addition of fenofibrate markedly decreases the intestinal production of CM into intestinal lymph and also decreases mRNA expression of apoB in the intestinal epithelium without affecting any other genes related to CM assembly and production13). In the current study, both fenofibrate and K-877 decreased the intestinal mRNA expression of ApoB and Npc1l1 (Fig. 4A and 4B) and enhanced LPL activity (Fig. 2H); these effects are related to the attenuation of the postprandial increase in small CM remnants20). The decreased mRNA expressions of ApoB and Npc1l1 in mice fed an HFD containing K-877 or fenofibrate may be related to decreased numbers of CM particle; thus postprandial apoB-48 levels were decreased in these mice (Fig. 3C). There was no significant difference in hepatic mRNA expressions of apoB and Npc1l1 (data not shown). Fenofibrate substantially increased the intestinal mRNA expression of Srebp1c and Mttp, whereas K-877 did not increase their expression (Fig. 4C and 4D). Because Srebp-1c is a regulator of genes required for de novo lipogenesis, and Mttp has a role in the intestinal assembly of CM particles with TG, their increased expression may consequently enhance the production of CM in the intestine. TG contents in CM and CM remnants are smaller in mice fed an HFD containing K-877 than those containing fenofibrate (Fig. 3A); the postprandial clearance of CM remnants may be faster in K-877 than fenofibrate. Therefore, K-877 may suppress the intestinal CM production more efficiently than fenofibrate without affecting the expression of Srebp1c and Mttp. Both fenofibrate and K-877 enhanced LPL activity (Fig. 2H), that attenuates the postprandial increase in CM remnants. On the other hand, there was no significant difference in hepatic mRNA expressions of Mttp and Srebp1c (data not shown). Additionally, K-877 not only increased LPL activity but also decreased the hepatic expression of apoC-3, an inhibitor of LPL activity, more effectively than fenofibrate, which may cause an enhanced hydrolysis of CM and CM remnants. Although we did not analyze mRNA expressions of LPL or apoA-5 that are related to a PPARα stimulation, we confirmed that K-877 significantly downregulates the mRNA expression of apoC-3 that mainly inhibits LPL activity (Fig. 4F). These changes might be related to the upregulation of LPL activity and the decrease in CM and CM remnants. These results strongly suggested that K-877 suppresses the postprandial increase in CM remnants more effectively and strongly compared with fenofibrate.
4.2. Comparison of K-877 and Fibrates
Fibrates are a PPARα-agonist clinically used for improving hypertriglyceridemia and hypo-HDL-cholesterolemia in patients with dyslipidemia; however, their selectivity is so weak that fibrate use is limited because of dose-related adverse effects, such as nausea, renal and liver dysfunction, or myopathy. Fibrates are not only PPARα-agonists but also PPARγ-agonists, and the adverse effects caused by stimulating PPARγ occur when fibrates are used at high doses21). In contrast, K-877, pemafibrate, activates PPARα selectively in very low doses in vitro and in vivo, decreases CM production from the intestine, stimulates reverse cholesterol transport by promoting macrophage cholesterol efflux, and exert anti-inflammatory activities22). In the current study K-877 attenuated PHTG at considerably lower doses than fenofibrate (one-hundredth dose of fenofibrate). The use of low doses may be safer and more effective for avoiding the side effects observed in fenofibrate. A PPARα stimulation causes weight loss by the decreased CM production from the intestine22). To the contrary, a PPARγ agonist, thiazolidinedione (TZD) causes body weight gain partially by increasing citrate synthase activity and fatty acid synthase activity in adipose tissue, and enhances the food consumption23). K-877 that is a selective PPARα modulator did not cause enhanced food intake; however, fenofibrate that stimulates both PPARα and PPARγ did (Fig. 1C). It was supposed that the higher levels of body weight gain in mice fed HFD containing K-877 than in mice fed HFD containing fenofibrate may be due to the increased food intake by an additional PPARγ stimulation. In Fig. 3C and 3C, postprandial increase in apoB-48 was suppressed by K-877 and fenofibrate; however, that in apoB-100 was suppressed by K-877 only. A PPARα stimulation by K-877 causes weight loss by the decreased CM production from the intestine, as postprandial increases of apoB-48 level and apoB-100 level are decreased. On the other hand, a PPARγ stimulation combined with a PPARα stimulation by fenofibrate may cause body weight gain and enhance the food consumption, it may relate to postprandial increase of apoB-100 in mice fed an HFD containing fenofibrate.G iven the selectivity of K-877 in vitro, K-877 is more suitable and flexible for improving atherogenic properties in patients with hypertriglyceridemia than fenofibrate.
4.3. Anti-Atherogenic Effects of K-877
Fibrates are used as a representative drug for hypertriglyceridemia and are also recognized as a useful treatment for preventing coronary events. Fenofibrate decreases serum TG levels and atherogenic remnant lipoproteins and also improves postprandial free fatty acid oxidation and inflammatory responses by decreasing postprandial levels of oxidized fatty acids, soluble VCAM-1, and soluble ICAM-124). The efficacy of fibrates in preventing coronary risk is critically different among the subjects investigated, partially because of the diversity of their metabolic profiles. Bezafibrate decreased mortality in patients with hypertriglyceridemia (BIP trial25)), and fenofibrate did not decrease CHD events in diabetic patients (FIELD trial26)). Fenofibrate has been found not to decrease CHD events in diabetic patients previously treated with simvastatin; however, this combination therapy significantly decreases CHD events in diabetic patients with both hypertriglyceridemia and hypo-HDL-cholesterolemia (ACCORD Lipid trial27)). These results suggest that fibrates may prevent CHD events in patients with accumulated remnants but are not necessarily effective in all cases. The atherogenicity in patients with fasting hypertriglyceridemia and PHTG has been reported to be due to the accumulation of TRLs and their remnant lipoproteins by many studies28, 29), and postprandial increases in CM remnants are more atherogenic than those in apoB-100-containing lipoproteins30). The accumulation of CM remnants can be evaluated by measuring serum apoB-48 levels with anti-apoB-48 antibodies31–33), and high fasting apoB-48 levels are related to the PHTG, intima-media thickening of the carotid artery, and the prevalence of coronary artery stenosis34–36). In the present study, we have shown that K-877 attenuates PHTG and decreases postprandial increases in CM remnants, thus suggesting that K-877 may effectively decrease ASCVD risk and the morbidity of cardiovascular events by ameliorating accumulation of CM remnants.
4.4. Limitations of this Study
There are several limitations in this study. First, hepatic mRNA expression of PPARα is very low, at 5%–10% of the levels found in mice37). Second, the plasma lipoprotein profile in mice is very different from that in humans, especially in regard to LDL and HDL, because mice lack expression of CETP38). The result that cholesterol levels of lipoproteins were increased in LDL and decreased in HDL by the addition of fenofibrate or K-877 might be specific in mouse. Thus, the effect of K-877 must be assessed in human clinical investigations.
5. Conclusions
The current study revealed that a new selective SPPARMα, K-877, effectively ameliorates postprandial accumulation of CM remnants in mice fed an HFD, when it is administered at a lower dose than fenofibrate. Therefore, K-877 therapy may be useful for preventing cardiovascular events, especially in patients with hypertriglyceridemia and accumulated CM remnants.
Acknowledgements
We thank Masumi Asaji, Ayami Saga, and Kyoko Ozawa for their excellent administrative and technical assistance. MS performed experiments in collaboration with TK, and MS and TK wrote the manuscript, which was reviewed by DM. Other authors provided discussion on experimental results. This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 15K01713, Grant-in-Aid for Scientific Research (C).
Conflict of Interest
SY received an advisory fee from Kowa Company, Ltd.. SY and DM received research funds as a joint research with Kowa Company, Ltd.. K-877 powder was provided under a material transfer agreement from Kowa Company, Ltd.
References
- 1). Hokanson JE, Austin MA: Plasma Triglyceride Level is a Risk Factor for Cardiovascular Disease Independent of High-Density Lipoprotein Cholesterol Level: A Metaanalysis of Population-Based Prospective Studies. Eur J Cardiovasc Prev Rehabil, 1996; 3: 213-219 [PubMed] [Google Scholar]
- 2). Iso H, Naito Y, Sato S, Kitamura A, Okamura T, Sankai T, Shimamoto T, Iida M, Komachi Y: Serum triglycerides and risk of coronary heart disease among Japanese men and women. Am J Epidemiol, 2001; 153: 490-499 [DOI] [PubMed] [Google Scholar]
- 3). Schwartz GG, Abt M, Bao W, DeMicco D, Kallend D, Miller M, Mundl H, Olsson AG: Fasting Triglycerides Predict Recurrent Ischemic Events in Patients With Acute Coronary Syndrome Treated With Statins. J Am Coll Cardiol, 2015; 65: 2267-2275 [DOI] [PubMed] [Google Scholar]
- 4). Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A: Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA, 2007; 298: 299-308 [DOI] [PubMed] [Google Scholar]
- 5). Zilversmit DB: Atherogenesis: a postprandial phenomenon. Circulation, 1979; 60: 473-485 [DOI] [PubMed] [Google Scholar]
- 6). Masuda D, Yamashita S: Postprandial Hyperlipidemia and Remnant Lipoproteins. J Atheroscler Thromb, 2017; 24: 95-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Fujioka Y, Ishikawa Y: Remnant lipoproteins as strong key particles to atherogenesis. J Atheroscler Thromb, 2009; 16: 145-154 [DOI] [PubMed] [Google Scholar]
- 8). Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K, Japan Atherosclerosis Society : Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan -2012 version. J Atheroscler Thromb, 2013; 20: 517-523 [DOI] [PubMed] [Google Scholar]
- 9). Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesäniemi YA, Sullivan D, Hunt D, Colman P, d'Emden M, Whiting M, Ehnholm C, Laakso M, FIELD study investigators : Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet, 2005; 366: 1849-1861 [DOI] [PubMed] [Google Scholar]
- 10). Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, Wang L, Kirkesseli S, Rocklin R, Bock B, Hamilton J, Ming JE, Radin A, Stahl N, Yancopoulos GD, Graham N, Pirozzi G: Effects of Combination Lipid Therapy in Type 2 Diabetes Mellitus. N Engl J Med, 2010; 362: 1563-1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Jun M, Foote C, Lv J, Neal B, Patel A, Nicholls SJ, Grobbee DE, Cass A, Chalmers J, Perkovic V: Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet, 2010; 375: 1875-1884 [DOI] [PubMed] [Google Scholar]
- 12). Rosenson RS, Wolff DA, Huskin AL, Helenowski IB, Rademaker AW: Fenofibrate therapy ameliorates fasting and postprandial lipoproteinemia, oxidative stress, and the inflammatory response in subjects with hypertriglyceridemia and the metabolic syndrome. Diabetes Care, 2007; 30: 1945-1951 [DOI] [PubMed] [Google Scholar]
- 13). Sandoval JC, Nakagawa-Toyama Y, Masuda D, Tochino Y, Nakaoka H, Kawase R, Yuasa-Kawase M, Nakatani K, Inagaki M, Tsubakio-Yamamoto K, Ohama T, Nishida M, Ishigami M, Komuro I, Yamashita S: Fenofibrate reduces postprandial hypertriglyceridemia in CD36 knockout mice. J Atheroscler Thromb, 2010; 17: 610-618 [DOI] [PubMed] [Google Scholar]
- 14). Fruchart JC: Peroxisome proliferator-activated receptoralpha (PPARα): At the crossroads of obesity, diabetes and cardiovascular disease. Atherosclerosis, 2009; 205: 1-8 [DOI] [PubMed] [Google Scholar]
- 15). Fruchart JC: Selective peroxisome proliferator-activated receptor α modulators (SPPARMα): The next generation of peroxisome proliferator-activated receptor α-agonists. Cardiovasc Diabetol, 2013; 12: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Yamazaki Y, Abe K, Toma T, Nishikawa M, Ozawa H, Okuda A, Araki T, Oda S, Inoue K, Shibuya K, Staels B, Fruchart JC: Design and synthesis of highly potent and selective human peroxisome proliferator-activated receptor α agonists. Bioorg Med Chem Lett, 2007; 17: 4689-4693 [DOI] [PubMed] [Google Scholar]
- 17). Ishibashi S, Yamashita S, Arai H, Araki E, Yokote K, Suganami H, Fruchart JC, Kodama T for the K-877-04 Study Group : Effects of K-877, a novel selective PPARα modulator (SPPARMα), in dyslipidaemic patients: A randomized, double blind, active- and placebo-controlled, phase 2 trial. Atherosclerosis, 2016; 249: 36-43 [DOI] [PubMed] [Google Scholar]
- 18). Hennuyer N, Duplan I, Paquet C, Vanhoutte J, Woitrain E, Touche V, Colin S, Vallez E, Lestavel S, Lefebvre P, Staels B: The novel selective PPARα modulator (SPPARMα) pemafibrate improves dyslipidemia, enhances reverse cholesterol transport and decreases inflammation and atherosclerosis. Atherosclerosis, 2016; 249: 200-208 [DOI] [PubMed] [Google Scholar]
- 19). Kanda Y: Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant, 2013; 48: 452-458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Masuda D, Hirano K, Oku H, Sandoval JC, Kawase R, Yuasa-Kawase M, Yamashita Y, Takada M, Tsubakio-Yamamoto K, Tochino Y, Koseki M, Matsuura F, Nishida M, Kawamoto T, Ishigami M, Hori M, Shimomura I, Yamashita S: Chylomicron remnants are increased in the postprandial state in CD36 deficiency. J Lipid Res, 2009; 50: 999-1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Sabine W, Lilli W, Katrin G, Jutta D, Claus L: Chylomicron remnants of various sizes are lowered more effectively by fenofibrate than by atorvastatin in patients with combined hyperlipidemia. Atherosclerosis, 2003; 171: 369-377 [DOI] [PubMed] [Google Scholar]
- 22). Raza-Iqbal S, Tanaka T, Anai M, Inagaki T, Matsumura Y, Ikeda K, Taguchi A, Gonzalez FJ, Sakai J, Kodama T: Transcriptome Analysis of K-877 (a Novel Selective PPARα Modulator (SPPARMα))-Regulated Genes in Primary Human Hepatocytes and the Mouse Liver. J Atheroscler Thromb, 2015; 22: 754-772. Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ: Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet, 2009; 373: 2125–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Larsen TM, Toubro S, Astrup A: PPARgamma agonists in the treatment of type II diabetes: is increased fatness commensurate with long-term efficacy? Int J Obesity, 2003; 27: 147-161 [DOI] [PubMed] [Google Scholar]
- 24). Rosenson RS, Wolff DA, Huskin AL, Helenowski IB, Rademaker AW: Fenofibrate therapy ameliorates fasting and postprandial lipoproteinemia, oxidative stress, and the inflammatory response in subjects with hypertriglyceridemia and the metabolic syndrome. Diabetes Care, 2007; 30: 1945-1951 [DOI] [PubMed] [Google Scholar]
- 25). Arbel Y, Klempfner R, Erez A, Goldenberg I, Benzekry S, Shlomo N, Fisman EZ, Tenenbaum A, BIP Study Group : Bezafibrate for the treatment of dyslipidemia in patients with coronary artery disease: 20-year mortality follow-up of the BIP randomized control trial. Cardiovasc Diabetol, 2016; 15: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesäniemi YA, Sullivan D, Hunt D, Colman P, d'Emden M, Whiting M, Ehnholm C, Laakso M, FIELD study investigators : Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet, 2005; 366: 1849-1861 [DOI] [PubMed] [Google Scholar]
- 27). ACCORD Study Group. Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC, Jr, Cushman WC, Simons-Morton DG, Byington RP: Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med, 2010; 362: 1563-1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Karpe F: Postprandial lipoprotein metabolism and atherosclerosis. J Intern Med, 1999; 246: 341-355 [DOI] [PubMed] [Google Scholar]
- 29). Toth PP: Triglyceride-rich lipoproteins as a causal factor for cardiovascular disease. Vasc Health Risk Manag, 2016; 12: 171-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Proctor SD: Intimal Retention of Cholesterol Derived From Apolipoprotein B100- and Apolipoprotein B48-Containing Lipoproteins in Carotid Arteries of Watanabe Heritable Hyperlipidemic Rabbits. Arterioscler Thromb Vasc Biol, 2003; 23: 1595-1600 [DOI] [PubMed] [Google Scholar]
- 31). Uchida Y, Kurano Y, Ito S: Establishment of monoclonal antibody against human Apo B-48 and measurement of Apo B-48 in serum by ELISA method. J Clin Lab Anal, 1998; 12: 289-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Sakai N, Uchida Y, Ohashi K, Hibuse T, Saika Y, Tomari Y, Kihara S, Hiraoka H, Nakamura T, Ito S, Yamashita S, Matsuzawa Y: Measurement of fasting serum apoB-48 levels in normolipidemic and hyperlipidemic subjects by ELISA. J Lipid Res, 2003; 44: 1256-1262 [DOI] [PubMed] [Google Scholar]
- 33). Hanada H, Mugii S, Okubo M, Maeda I, Kuwayama K, Hidaka Y, Kitazume-Taneike R, Yamashita T, Kawase R, Nakaoka H, Inagaki M, Yuasa-Kawase M, Nakatani K, Tsubakio-Yamamoto K, Masuda D, Ohama T, Matsuyama A, Ishigami M, Nishida M, Komuro I, Yamashita S: Establishment of chemiluminescence enzyme immunoassay for apolipoprotein B-48 and its clinical applications for evaluation of impaired chylomicron remnant metabolism. Clin Chim Acta, 2012; 413: 160-165 [DOI] [PubMed] [Google Scholar]
- 34). Meyer E, Westerveld HT, de Ruyter-Meijstek FC, van Greevenbroek MM, Rienks R, van Rijn HJ, Erkelens DW, de Bruin TW: Abnormal postprandial apolipoprotein B-48 and triglyceride responses in normolipidemic women with greater than 70% stenotic coronary artery disease: a case-control study. Atherosclerosis, 1996; 124: 221-235 [DOI] [PubMed] [Google Scholar]
- 35). Tanimura K, Nakajima Y, Nagao M, Ishizaki A, Kano T, Harada T, Okajima F, Sudo M, Tamura H, Ishii S, Sugihara H, Yamashita S, Asai A, Oikawa S: Association of serum apolipoprotein B48 level with the presence of carotid plaque in type 2 diabetes mellitus,” Diabetes Res Clin Pract, 2008; 81: 338-344 [DOI] [PubMed] [Google Scholar]
- 36). Masuda D, Sugimoto T, Tsujii K-I, Inagaki M, Nakatani K, Yuasa-Kawase M, Tsubakio-Yamamoto K, Ohama T, Nishida M, Ishigami M, Kawamoto T, Matsuyama A, Sakai N, Komuro I, Yamashita S: Correlation of fasting serum apolipoprotein B-48 with coronary artery disease prevalence. Eur J Clin Invest, 2012; 42: 992-999 [DOI] [PubMed] [Google Scholar]
- 37). Holden PR, Tugwood JD: Peroxisome proliferator-activated receptor alpha: role in rodent liver cancer and species differences. J Mol Endocrinol, 1999; 22: 1-8 [DOI] [PubMed] [Google Scholar]
- 38). Agellon LB, Walsh A, Hayek T, Moulin P, Jiang XC, Shelanski SA, Breslow JL, Tall AR: Reduced high density lipoprotein cholesterol in human cholesteryl ester transfer protein transgenic mice. J Biol Chem, 1991; 266: 10796-10801 [PubMed] [Google Scholar]


