Abstract
Aims: We evaluated whether exercised-based cardiac rehabilitation (CR) can ameliorate the HDL function, i.e., cholesterol efflux capacity (CEC) and paraoxonase-1 activity in patients with acute coronary syndrome (ACS).
Methods: This study is a retrospective analysis of stored serum from patients with ACS following successful percutaneous coronary intervention. The CEC, measured by a cell-based ex vivo assay using apolipoprotein B-depleted serum and 3H-cholesterol labeled macrophages and arylesterase activity (AREA) at the onset or early phase of ACS, and the follow-up periods were compared between 69 patients who completed the five-month outpatient CR program (CR group) and 15 patients who did not participate and/or dropped out from CR program (non-CR group).
Results: Apolipoprotein A-I (apoA-I) and CEC significantly increased by 4.0% and 9.4%, respectively, in the CR group, whereas HDL-cholesterol and AREA were not changed during the follow-up periods in both groups. Among CR patients, the CEC significantly increased, irrespective of the different statin treatment, while HDL-cholesterol and apoA-I significantly increased in patients treated with rosuvastatin or pitavastatin. Although CEC and AREA were significantly correlated each other, there is a discordance between CEC and AREA for their correlations with other biomarkers. Both CEC and AREA were significantly correlated with apoA-I rather than HDL-cholesterol. Changes in CEC and those in AREA were significantly correlated with those in apoA-I (rho = 0.328, p = 0.002, and rho = 0.428, p < 0.0001, respectively) greater than those in HDL-cholesterol (rho = 0.312, p = 0.0042, and rho = 0.343, p = 0.003, respectively).
Conclusions: CR can improve HDL function, and it is beneficial for secondary prevention.
Keywords: High-density lipoprotein, Cholesterol efflux capacity, Apolipoprotein A1, Cardiac rehabilitation, Paraoxonase-1 activity
See editorial vol. 25: 128–130
Introduction
High-density lipoprotein (HDL)-mediated cholesterol efflux is the first step of reverse cholesterol transport (RCT), and cholesterol efflux capacity (CEC) is a key anti-atherogenic function of HDL1, 2). Recent large scale case-control studies and population-based prospective studies showed that HDL-mediated CEC from macrophage, measured by a cell-based ex vivo assay was significantly and inversely associated with the incidence of coronary artery disease (CAD), independent of established cardiovascular risk factors, including HDL cholesterol (HDL-C) or apolipoprotein A1 (apoA-I), and was a stronger predictor than HDL-C or apoA-I3–5). Even among patients with heterozygous familial hypercholesterolemia, low CEC was reported to be independently associated with the presence of atherosclerotic cardiovascular disease6). This suggests that altering CEC could be a novel therapeutic approach for the prevention of atherosclerotic cardiovascular disease7).
Besides CEC, HDL has several other anti-atherogenic properties including antioxidant capacity, antiinflammatory properties, and nitric oxide-promoting activity1). Paraoxonase-1 (PON1) is a plasma enzyme that is synthesized in the liver and resides on HDL particles, and has been shown to possess antioxidant properties resulting in the inhibition of the oxidation of low-density lipoprotein (LDL) particles8). PON1 hydrolyzes different kinds of substrates and act as paraoxonase to hydrolyze oxons like paraoxon and as arylesterase to hydrolyze aromatic esters like phenylacetate8). It has been reported that low PON1 activities in either paraoxonase activity (PONA) or arylesterase activity (AREA) were significantly associated with elevated risk for CAD9). Berrougui et al. showed that PON1 regulates the CEC by enhancing the interaction between apoA-I and ATP-binding cassette A1 (ABC A1)10).
Comprehensive cardiac rehabilitation (CR) has been shown to be beneficial in improving exercise capacity, quality of life, and/or prolonging survival in patients with CAD11). Recent our preliminary study was the first to show that a six-month CR program significantly increased CEC as well as HDL-C and apoA-I in 57 patients with acute coronary syndrome (ACS), whereas those changes were not seen in 11 patients who dropped out from the outpatient CR program; increases in CEC were significantly correlated to increases in HDL-C and apoA-I12). It is conceivable that CR may improve PON1 activity as well as CEC. To date, only one observational study reported that a 12-week CR program increased AREA in 37 patients with CAD13). In the present study, we investigated the effects of CR on CEC and AREA in patients with ACS.
Methods
Study Patients
The present study enrolled patients with ACS that followed successful percutaneous coronary interventions (PCI) between August 2005 and May 2015, and all of the studied patients were distinct from the patients in our previous study12). The diagnoses of ACS were based on electrocardiographic changes and coronary arteriograms. Serum samples were collected immediately before the emergency coronary angiography on admission of ACS and/or in a fasting state at the beginning of the CR program, and unused samples were stored for long-term storage at −80°C. The exclusion criteria included severe hepatic disease, end-stage renal disease such as hemodialysis, current treatment for malignancy, any other serious conditions, patients who took drugs for thyroid dysfunction, and patients with missed blood samples. The follow-up serum samples were collected in a fasting state at 6–18 months after the baseline sampling, and unused samples were stored for long-term storage at −80°C.
After applying these criteria, 70 male and 14 female patients were included in the present analysis. The patients were divided into two groups based on whether they completed the five-month outpatient CR program. The institutional review board of Showa University approved this protocol. The investigation conformed to the principles of the Declaration of Helsinki. The written informed consent was obtained from each patient included in the study.
Cardiac Rehabilitation Program and Definitions at Baseline
Comprehensive CR programs include exercise training, counseling on lifestyle modification, including diet therapy, smoking cessation, and physical activity, and encouragement to adhere to the medical treatment of physicians, nurses, physical therapists, and dieticians according to the Japanese Circulation Society guidelines11). Educational sessions were performed every month. The CR program began in the early phase and continued after hospital discharge a few times a week for five months. The exercise training consisted with supervised exercise sessions of gymnastics and a 30–min supervised aerobic exercise using a bicycle ergometer. All patients attended the CR program once and were encouraged to participate in the comprehensive CR program. Trained patients were encouraged to exercise at home, consisting mainly of brisk walking for 30–60 min, three to five times a week. The prescribed intensity was determined individually at 40%–60% of heart rate (HR) reserve (Karvonen's equation, k = 0.4–0.6), at an anaerobic threshold level obtained by cardiopulmonary exercise test (CPX), or at level 12–13 of the Borg scale for ratings of perceived exertion. Exercise tolerance was measured by CPX using a ramp protocol at the beginning and end of the CR program. After a 3-min rest on the bicycle ergometer in the upright position, the patients started pedaling at an intensity of 10 W for 4 min. (warm-up), followed by an incremental exercise at 10 or 20 W/min until exhaustion. A 12-lead electrocardiogram was continuously monitored, and blood pressure (BP) was measured once a minute with a sphygmomanometer. Respiratory flow was measured by breath-by-breath method using a gas analyzer (MINATO AE-300s; Minato Medical Science Co. Ltd., Japan). AT was determined by the V-slope method14). Oxygen consumption at peak exercise was regarded as peak VO2 and as an indices of exercise capacity.
The BP and HR were measured using an oscillometric device (Form/ABI, Colin Company, Ltd., Komaki, Japan) at the beginning of the CR program and at the follow-up period of blood sampling. Four pneumatic pressure cuffs, two electrocardiogram electrodes, and one microphone for detecting heart sounds were attached on both arms and ankles, wrists, and the left edge of the sternum, respectively, to record the volume waveform for the brachial and ankle arteries. The subjects were kept at rest in supine position for at least 5–10 min. The examination room was maintained at a standardized temperature. The Ankle Brachial Index (ABI) of less than 0.90 was diagnosed with peripheral artery disease (PAD). The diagnosis of hypertension was based on a history of hypertension or BP above 140 mmHg (systolic) or 90 mmHg (diastolic)15). Diabetes mellitus (DM) was defined as a fasting serum glucose value greater than 126 mg/dL, HbA1c greater than 6.5%, and/or current use of medication for DM15). Dyslipidemia was defined as the current use of lipid-lowering medication and/or meeting the criteria of the Japan Atherosclerosis Society for fasting serum lipid levels as follows: LDL-C ≥ 140 mg/dL, HDL-C < 40 mg/dL, or triglyceride ≥ 150 mg/dL15). A serum creatinine–based estimate of glomerular filtration rate (eGFR) was calculated as follows: eGFR = 194 × Cr−1.094 × Age−0.287 (× 0.739 for women)16). Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. Patients with a reported smoking habit of at least one cigarette per day on admission were classified as current smokers. Former smokers were defined as having previously smoked but ceased before the onset of ACS.
Targets for the controls of risk factors are smoking cessation, systolic BP < 140 mmHg17), diastolic BP < 90 mmHg17), LDL-C < 100 mg/dl15), non-HDL-C < 130 mg/dl 15), HDL-C ≥ 40 mg/dl 15), triglyceride < 150 mg/dl15), and HbA1c < 7.0%15).
Laboratory Measurement
Total-cholesterol, triglycerides, HDL-C, and glycated hemoglobin (HbA1c) were measured using standard laboratory procedures. HbA1c values that were measured as HbA1c (JDS) till March 31, 2012, were estimated by (= 1.019 × HbA1c [JDS] + 0.3)18). The non-HDL cholesterol (non-HDL-C) level was estimated by subtracting the HDL-C concentration from the total-cholesterol concentration. The LDL cholesterol (LDL-C) levels were measured with a direct homogenous assay (Sekisui Medical Co. Ltd. Tokyo, Japan), that was reported to be as accurate for post-prandial samples as for fasting samples19). Serum apolipoprotein levels were determined by an immunoturbidimetric assay (Daiichi Chemicals Co. Ltd. Tokyo, Japan). Malondialdehyde-modified LDL (MDA-LDL) was measured using an enzyme-linked immunosorbent assay20). Remnant lipoproteins (RLPs) were isolated from the serum to an immunoaffinity mixed gel containing anti-apoA-I and anti-apolipoprotein B 100 (apoB) monoclonal antibodies (Japan Immunoresearch Laboratories, Takasaki, Japan), and the cholesterol concentrations of the unbound fraction were measured as RLP cholesterol (RLP-C)21). The high-sensitivity C-reactive protein (hsCRP) level was measured by the Dade Behring BN assay22). Plasma brain natriuretic peptide (BNP) was measured by chemiluminescence-enzyme immunoassay.
Measurement of Cholesterol Efflux Capacity (CEC) and Arylesterase Activity (AREA)
Serum samples were kept frozen at −80°C until the assay for CEC and AEA. CEC was performed according to the methods used by Khera et al.3–5, 12). The murine macrophage cell lines J774.1 were purchased from RIKEN (Tsukuba, Japan), cultured in RPMI 1640 medium (Sigma-Aldrich, MO, USA), containing 10% fetal bovine serum, and kept under constant conditions of 5% carbon dioxide and a temperature of 37°C. J774.1 cells were plated in 24-well plates, grown to 80–90% confluence, and radiolabeled with 2 µCi/mL of 3H-cholesterol. ApoB-depleted serum was prepared by incubation with a 13% polyethylene glycol 6000 solution (Wako Pure Chemicals). Subsequently, an efflux medium containing 2.8% apoB-depleted serum was added and incubated for four hours. All procedures were performed in the presence of the acyl-coenzyme A: cholesterol acyltransferase inhibitor Sandoz 58–035 (2 µg/mL; Sigma-Aldrich, MO, USA) and 8-bromoadenosine 3′, 5′-cyclic monophosphate (0.3 mmol/L; Sigma-Aldrich, MO, USA). A liquid scintillation counter was used to quantify the efflux of radioactive cholesterol from the supernatants of each well, and the extraction of cell lysates through the equal mixture of hexane and isopropanol in control wells that was not exposed to the serum. CEC was measured by determining the percentage of radiolabeled cholesterol released (% cholesterol efflux) using the following formula: Percent efflux was calculated using the following formula: (cpm of 3H-cholesterol in media containing 2.8% apoB-depleted serum–cpm of 3H-cholesterol in serum-free mediums)/(cpm of 3H-cholesterol in cells extracted before the efflux step) × 100. All assays were performed in duplicate. The CEC of patients' sera were expressed as the relative values to the pooled serum for standardization.
The AREA was measured by using a commercially available assay kit (Mega Tip San ve Tic Ltd, Turkey). PON1 hydrolyzes phenyl acetate to phenol and acetic acid. The phenol was colorimetrically measured via oxidative coupling with 4-aminoantipyrine and potassium ferricyanide. Non-enzymatic hydrolysis of phenyl acetate was subtracted from total rate of hydrolysis. The molar absorptivity of the colored complex is 4000M−1cm−1 and one unit of AREA is equal to 1 mmol of phenyl acetate hydrolyzed per liter per min at 37°C. The interassay variation coefficient was less than 7%.
Statistics
All statistical analyses were performed using the IBM SPSS Statistics version 23 software package (IBM Institute). Baseline characteristics were compared between CR and non-CR using unpaired t-test for parametric variables and Mann-Whitney U test for non-parametric variables. Categorical variables were compared with chi-square tests. Changes in blood biomarkers between the baseline and the follow-up in two groups were tested with two-way repeated measures analysis of variance (ANOVA). For within-group comparisons, changes in the biomarkers between the baseline and the follow-up were analyzed using paired t-test for parametric variables and Wilcoxon signed rank test for non-parametric variables. Correlation coefficients between two biomarkers were determined by Spearman's rank analyses. All the statistical analyses were two-tailed. P < 0.05 was considered statistically significant.
Results
Clinical Characteristics and Laboratory Findings at Baseline and Follow-up
The clinical characteristics of the subjects are summarized in Table 1. Fifty-seven men and twelve women completed the five-month outpatient CR program (CR group), and thirteen men and two women unfortunately decided that they did not participate and/or dropped out from CR program (non-CR group). The mean number of CR sessions in CR group was 33.6, whereas that in non-CR group was 2.7. The percentages of patients with hypertension were significantly higher in the CR group, whereas those with dyslipidemia and DM were similar between the two groups. No patients took an alfa-glucosidase inhibitor or pioglitazone during the study period. The HR significantly decreased and BP, both systolic and diastolic, significantly increased during the follow-up period in the CR group, while those did not change in the non-CR group. Twenty percent of patients in the non-CR group and twenty-nine percent of patients in the CR group took statins at the baseline blood sampling. Only one patient in the CR group discontinued the statin before follow-up blood sampling because of mild elevation of serum transaminase and was treated with ezetimibe. Table 2 compares the laboratory findings at baseline and at follow-up. The baseline blood samples were collected at the beginning of CR in six male and one female patient in the CR group. The LDL-C, non-HDL-C, and ApoB significantly decreased at follow-up in both groups, whereas MDA-LDL, RLP-C, hsCRP, and BNP significantly decreased in the CR group alone. The LDL-C, non-HDL-C, triglyceride, ApoB, and RLP-C at follow-up were significantly lower in the CR group than those in the non-CR group. The HDL-C somewhat decreased in the non-CR group, and AREA non-significantly increased in both the non-CR and CR groups. The CEC and apoA-I significantly increased in the CR group alone (Fig. 1).
Table 1. Clinical characteristics at baseline and follow-up period.
| Non-CR (N = 15) | CR (N = 69) | |||
|---|---|---|---|---|
| Men/women (male %) | 13/2 (87%) | 57/12 (83%) | ||
| No of CR sessions | 2.7 ± 3.6 | 33.6 ± 13.4 | ||
| At baseline | ||||
| Age, years | 60.9 ± 14.7 | 66.7 ± 11.3 | ||
| Prior MI, n (%) | 0 (0.0) | 3 (4.3) | ||
| Prior PCI/CABG, n (%) | 1 (6.6) | 8 (11.6) | ||
| Prior stroke, n (%) | 1 (6.7) | 3 (4.2) | ||
| Prior PAD, n (%) | 0 (0.0) | 1 (1.4) | ||
| Prior malignancy, n (%) | 2 (13.3) | 3 (4.3) | ||
| Diagnosis of ACS | ||||
| UAP | 3 | 9 | ||
| anterior MI | 8 | 33 | ||
| lateral MI | 3 | 6 | ||
| inferior MI | 1 | 21 | ||
| LVEF, % | 52.1 ± 7.3 | 53.1 ± 9.8 | ||
| Risk factor | ||||
| Hypertension, n (%) | 6 (40.0%) | 49 (71.0%)* | ||
| Diabetes, n (%) | 6 (40.0%) | 21 (30.4%) | ||
| Dyslipidemia, n (%) | 10 (66.7%) | 60 (87.0%) | ||
| Prior lipid-lowering drug, n (%) | 3 (20%) | 20 (29%) | ||
| Physical characteristics | Baseline | Follow-up | Baseline | Follow-up |
| BMI, kg/m2 | 23.9 ± 3.4 | 24.2 ± 3.5 | 24.5 ± 4.1 | 24.1 ± 3.8 |
| HR, bpm | 71.7 ± 8.4 | 66.9 ± 10.4 | 69.8 ± 11.2 | 64.8 ± 10.5§§§ |
| SBP, mmHg | 118.1 ± 18.3 | 125.7 ± 14.5 | 115.4 ± 14.4 | 124.4 ± 16.2§§§ |
| DBP, mmHg | 72.3 ± 11.5 | 76.2 ± 12.4 | 69.2 ± 9.0 | 74.3 ± 9.8§§§ |
| Peak VO2, ml/min/kg | 17.8 ± 3.0 (6)† | N/A | 16.8 ± 3.8 | 18.5 ± 4.5§§§ |
| Smoking status | ||||
| Current/Former | 10/2 | 5/7 | 25/22 | 4/43* |
| Prior blood sampling | Follow-up | Prior blood sampling | Follow-up | |
| Cardiovascular medication | ||||
| Calcium channel blocker | 2 | 1 | 15 | 13 |
| ACE-I | 0 | 0 | 2 | 17* |
| ARB (Telmisartan) | 3 (1) | 10 (1) | 20 (7) | 37 (12) |
| Beta blocker | 1 | 13 | 10 | 56 |
| Diuretic | 0 | 3 | 4 | 8 |
| Aldosterone blocker | 0 | 1 | 1 | 10 |
| Antiplatelet drug | 2 | 15 | 14 | 69 |
| Anticoagulant | 0 | 3 | 2 | 9 |
| Glucose-lowering treatment | ||||
| Insulin | 0 | 1 | 0 | 1 |
| Sulfonyl urea or glinide | 2 | 1 | 6 | 5 |
| DPP-4 inhibitor | 2 | 3 | 7 | 13 |
| Metformin | 0 | 0 | 3 | 3 |
| Lipid-lowering treatment, statin n (dose, mg) | ||||
| None | 14 | 0 | 52 | 1 |
| Pravastatin | 0 | 2 (7.5 ± 3.5) | 1 (5.0) | 0 |
| Simvastatin | 0 | 0 | 1 (5.0) | 0 |
| Fluvastatin | 0 | 0 | 0 | 1 (20) |
| Prior blood sampling | Follow-up | Prior blood sampling | Follow-up | |
| Lipid-lowering treatment, statin n (dose, mg) | ||||
| Atorvastatin | 1 (10) | 5 (16.0 ± 5.5) | 9 (11.1 ± 5.5) | 35 (13.7 ± 4.9) |
| Rosuvastati | 0 | 7 (5.7 ± 2.8) | 2 (3.8 ± 1.8) | 24 (6.9 ± 4.9) |
| Pitavastatin | 0 | 1 (2.0) | 4 (1.5 ± 0.6) | 8 (1.8 ± 1.0) |
| Lipid-lowering treatment, non-statin | ||||
| Fibrate | 1 | 0 | 1 | 1 |
| n-3 PUFA | 2 | 0 | 2 | 8 |
| Ezetimibe | 0 | 0 | 0 | 4 |
Data are expressed as mean ± SD or number (%). The number in parenthesis† indicates actual number of analyzed cases. Risk factors and medication including prior lipid-lowering at baseline were evaluated at the blood sampling. *p < 0.05 compared with non-CR group by unpaired t-test or chi-square test. §p < 0.05, §§p < 0.01, §§p < 0.0001 compared with the baseline by paired t-test. N/A indicates not available. ACE-I = angiotensin-converting enzyme inhibitor; ACS = acute coronary syndrome; ARB = angiotensin II type 1 receptor blocker; CABG= coronary artery bypass graft surgery; CR = cardiac rehabilitation; DPP4 = dipeptidyl peptidase-4 inhibitor; LV EF = left ventricular ejection fraction by ultrasound Simpson method; MI = myocardial infarction; n-3 PUFA = n-3 polyunsaturated fatty acid; PAD = peripheral artery disease; PCI = percutaneous coronary intervention; UAP = unstable angina pectoris.
Table 2. Comparison of laboratory findings at baseline and follow-up period between non-CR and CR groups.
| Biomarkers (unit) | Whole patients |
Male patients |
||
|---|---|---|---|---|
| Non-CR (n = 15) | CR (n = 69) | Non-CR (n = 13) | CR (n = 57) | |
| LDL-C (mg/dL) | ||||
| baseline | 131.7 ± 42.9 | 127.5 ± 35.9 | 134.2 ± 40.0 | 126.6 ± 35.5 |
| follow-up | 94.8 ± 36.2§§ | 82.0 ± 19.2§§§ | 98.8 ± 36.5§§ | 82.6 ± 17.7*§§§ |
| Non-HDL-C (mg/dL) | ||||
| baseline | 150.8 ± 44.0 | 150.7 ± 38.8 | 153.4 ± 41.2 | 146.9 ± 36.9 |
| follow-up | 122.1 ± 44.1§ | 103.6 ± 21.0*§§§ | 126.7 ± 44.9§ | 103.4 ± 19.4*§§§ |
| TG (mg/dL) | ||||
| baseline | 120.2 ± 74.3 | 132.9 ± 85.5 | 118.6 ± 74.7 | 126.0 ± 66.3 |
| follow-up | 172.1 ± 101.1 | 115.6 ± 44.7* | 176.8 ± 103.7 | 115.2 ± 44.8* |
| MDA-LDL (U/L) | ||||
| baseline | 161.5 ± 60.9 | 157.2 ± 61.2 (66) | 163.6 ± 58.8 | 153.7 ± 53.3 (55) |
| follow-up | 126.4 ± 44.9 (14) | 107.4 ± 32.2§§§ | 130.8 ± 46.3 (12) | 108.4 ± 31.9*§§§ |
| RLP-C (mg/dL) | ||||
| baseline | 5.3 ± 2.9 | 6.4 ± 5.1 | 5.5 ± 2.9 | 5.6 ± 2.8 |
| follow-up | 6.3 ± 4.9 | 3.7 ± 1.6*§§§ | 6.7 ± 5.1 | 3.8 ± 1.7*§§§ |
| ApoB (mg/dL) | ||||
| baseline | 101.8 ± 28.9 | 103.9 ± 25.0 | 103.2 ± 27.7 | 102.8 ± 24.3 |
| follow-up | 86.3 ± 29.7§ | 74.6 ± 14.9*§§§ | 89.5 ± 30.2 | 74.4 ± 14.0*§§§ |
| HDL-C (mg/dL) | ||||
| baseline | 46.0 ± 10.5 | 44.9 ± 10.2 | 46.8 ± 11.1 | 44.1 ± 9.8 |
| follow-up | 42.7 ± 7.9 | 46.2 ± 9.4 | 42.1 ± 6.9 | 44.8 ± 9.0 |
| ApoA-I (mg/dL) | ||||
| baseline | 123.9 ± 18.2 | 121.1 ± 20.7 | 125.1 ± 19.4 | 119.3 ± 20.1 |
| follow-up | 123.0 ± 18.2 | 125.9 ± 18.6§ | 122.6 ± 16.4 | 123.0 ± 17.7 |
| CEC (relative value) | ||||
| baseline | 0.88 ± 0.14 | 0.87 ± 0.15 | 0.89 ± 0.13 | 0.87 ± 0.15 |
| follow-up | 0.89 ± 0.13 | 0.96 ± 0.18§§ | 0.91 ± 0.13 | 0.94 ± 0.16§§ |
| AREA (U/L) | ||||
| baseline | 685.3 ± 139.3 (12) | 768.0 ± 169.7 (60) | 675.8 ± 142.0 (11) | 755.8 ± 166.3 (50) |
| follow-up | 750.8 ± 291.3 (12) | 783.6 ± 167.5 (60) | 739.5 ± 302.8 (11) | 773.1 ± 160.2 (50) |
| HbA1c (%) | ||||
| baseline | 6.34 ± 1.48 | 6.28 ± 1.41 | 6.35 ± 1.59 | 6.25 ± 1.40 |
| follow-up | 6.17 ± 0.79 | 6.09 ± 0.62 | 6.16 ± 0.85 | 6.08 ± 0.61 |
| hsCRP (mg/dL) | ||||
| baseline | 0.97 ± 2.35 | 0.90 ± 2.15 | 1.10 ± 2.50 | 1.07 ± 2.33 |
| follow-up | 0.13 ± 0.28 | 0.12 ± 0.27§§ | 0.15 ± 0.30 | 0.13 ± 0.30§§ |
| BNP (pg/mL) | ||||
| baseline | 120.5 ± 134.0 | 101.8 ± 133.3 | 101.5 ± 113.6 | 100.7 ± 122.0 |
| follow-up | 58.2 ± 69.7 (14) | 64.5 ± 58.4§ (64) | 57.8 ± 73.5 (12) | 61.9 ± 57.9§ (52) |
| eGFR (ml/min/1.73 m2) | ||||
| baseline | 86.3 ± 19.7 | 71.8 ± 23.0* | 88.2 ± 20.5 | 72.6 ± 20.4* |
| follow-up | 74.8 ± 15.8§§ | 64.6 ± 18.2*§§§ | 77.2 ± 15.8§ | 65.6 ± 16.6*§§§ |
Data are expressed as mean ± SD. The number in parenthesis indicates actual number of analyzed cases. Seven samples at baseline in CR group were collected in fasting-state at the beginning of CR. The others were collected immediate before the emergency coronary angiography on admission. *p < 0.05 compared with non-CR group by student's t-test. §p< 0.05, §§ p < 0.01, §§§ p < 0.0001 compared with the baseline levels by paired t-test. Abbreviations as in text.
Fig. 1.
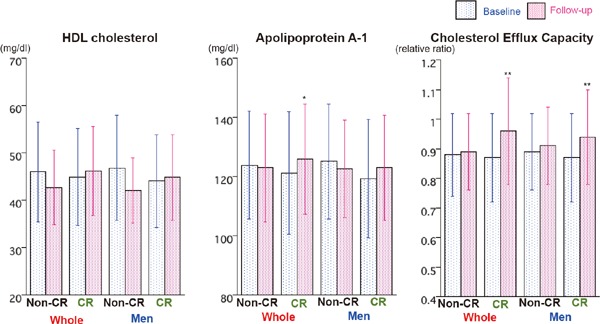
Comparisons of HDL-C, apoA-1, and CEC at baseline and follow-up period between CR and non-CR group. HDL-C, apoA-1, and CEC at baseline and follow-up period were compared between CR and non-CR groups in whole patients and male patients. Data are expressed as mean ± SD. Error bars indicate SD. *p < 0.05, ** p < 0.01 vs baseline by paired t-test. Abbreviations as in text.
Effects of Different Statins on Lipid Levels and CEC in Statin-naïve Patients
It has been reported that HDL-C-elevating effect, CEC, and anti-oxidative action of HDL differ among the types and doses of statins, and that pitavastatin and rosuvastatin have greater HDL-C- and apoA-I-elevating effects than atorvastatin23). Table 3 compares the laboratory findings at baseline and at follow-up in patients treated with atorvastatin and rosuvastatin or pitavastatin among patients without prior lipid-lowering treatment at baseline. A marked reduction of LDL-C, non-HDL-C, ApoB, and MDA-LDL was seen in the CR group as opposed to the non-CR group, irrespective of the different statin treatment. The HDL-C and ApoA-I significantly increased in only patients treated with rosuvastatin or pitavastatin in the CR group, while CEC significantly increased in the CR group, irrespective of the different statin treatment (Fig. 2).
Table 3. Effects of Atorvastatin and Rosuvastatin or Pitavastatin therapy on lipid levels, CEC and AREA between Non-CR and CR groups among patients without lipid-lowering treatment at baseline.
| Atorvastatin |
Rosuvastatin or Pitavastatin |
|||
|---|---|---|---|---|
| Non-CR (n = 4) | CR (n = 23) | Non-CR (n = 6) | CR (n = 24) | |
| men/women | 4/0 | 20/3 | 5/1 | 19/5 |
| dose of statin (mg/day) | 15.0 ± 3.5 | 13.9 ± 5.0 | R: 6.0 ± 2.9 (5) | R: 7.0 ± 5.3 (19) |
| P: 2 (1) | P: 2.0 ± 1.2 (5) | |||
| Non-statin drugs (number) | ||||
| Ezetimibe | 0 | 0 | 0 | 3 |
| n-3PUFA | 0 | 2 | 0 | 3 |
| Fibrate | 0 | 1 | 0 | 0 |
| BMI (kg/m2) | ||||
| baseline | 23.0 ± 2.0 | 24.0 ± 3.4 | 25.1 ± 4.9 | 24.8 ± 5.2 |
| follow-up | 23.2 ± 2.7 | 23.6 ± 2.9 | 25.6 ± 4.6 | 24.9 ± 5.3 |
| LDL-C (mg/dL) | ||||
| baseline | 153.5 ± 30.4 | 138.7 ± 28.4 | 133.2 ± 42.8 | 140.4 ± 35.2 |
| follow-up | 83.3 ± 11.6* | 77.0 ± 16.9*** | 93.2 ± 32.2* | 87.3 ± 19.6*** |
| Non-HDL-C (mg/dL) | ||||
| baseline | 178.8 ± 39.7 | 158.9 ± 31.4 | 150.7 ± 38.9 | 164.5 ± 37.5 |
| follow-up | 107.5 ± 17.6* | 97.0 ± 17.0*** | 121.2 ± 33.5 | 108.5 ± 21.6*** |
| ApoB (mg/dL) | ||||
| baseline | 117.3 ± 29.6 | 110.6 ± 18.9 | 101.7 ± 26.6 | 112.4 ± 26.2 |
| follow-up | 76.8 ± 13.4* | 71.0 ± 13.0*** | 87.5 ± 21.7 | 78.1 ± 15.3*** |
| MDA-LDL (U/L) | ||||
| baseline | 189.8 ± 77.2 | 178.9 ± 61.7 | 140.2 ± 49.5 | 169.7 ± 61.7 |
| follow-up | 119.5 ± 20.1 | 110.7 ± 34.9*** | 122.0 ± 32.1 | 115.2 ± 35.6*** |
| HDL-C (mg/dL) | ||||
| baseline | 50.3 ± 9.5 | 43.7 ± 9.6 | 47.5 ± 12.8 | 43.5 ± 10.0 |
| follow-up | 45.5 ± 7.0 | 44.1 ± 9.9 | 41.2 ± 5.8 | 48.0 ± 10.0* |
| ApoA-I (mg/dL) | ||||
| baseline | 133.3 ± 11.6 | 118.2 ± 18.4 | 123.2 ± 23.6 | 119.1 ± 19.8 |
| follow-up | 128.5 ± 7.7 | 123.4 ± 19.6 | 123.2 ± 22.2 | 128.6 ± 19.7* |
| CEC (relative value) | ||||
| baseline | 0.95 ± 0.14 | 0.88 ± 0.14 | 0.90 ± 0.09 | 0.86 ± 0.17 |
| follow-up | 0.89 ± 0.13 | 0.92 ± 0.16** | 0.93 ± 0.13 | 1.00 ± 0.19** |
| AREA (U/L) | ||||
| baseline | 605.2 ± 152.6 (3) | 749.3 ± 172.4 | 738.2 ± 163.5 (5) | 776.1 ± 155.7 (19) |
| follow-up | 667.9 ± 242.9 (3) | 729.5 ± 145.2 | 831.1 ± 417.6 (5) | 829.9 ± 200.3 (19) |
Data are expressed as mean ± SD or number. The number in parenthesis indicates actual number of analyzed cases. Seven samples at baseline were collected in fasting-state at the beginning of CR. The others were collected immediate before the emergency coronary angiography on admission. *p < 0.05, **p < 0.01, ***p < 0.0001 compared with the baseline by paired t-test. P = pitavastatin; R = rosuvastatin, other abbreviations as in text and Table 2.
Fig. 2.
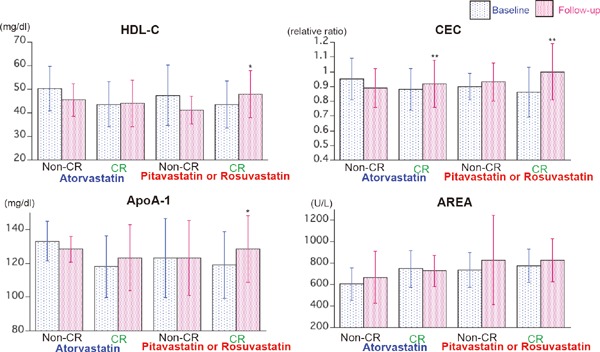
Comparison of HDL-C, ApoA-1, CEC, and AREA at baseline and follow-up period in patients treated with atorvastatin and those treated with rosuvastatin or pitavastatin in CR group who did not take any lipid-lowering drugs at baseline. *p < 0.05, **p < 0.01 compared with the baseline levels by paired t-test. Abbreviations as in text.
Effects of Medical Treatment and Smoking Cessation on CEC and AREA
There are marked differences in the smoking state and medical treatment at the follow-up periods between the non-CR and CR groups. Significantly more patients could stop smoking in the CR group. Fourteen patients took angiotensin-converting enzyme inhibitor (ACE-I), four patients took ezetimibe, and eight patients took an n-3 polyunsaturated fatty acid (n-3 PUFA) during the follow-up periods in the CR-group, whereas no patients took these drugs in the non-CR group. The CEC and AREA at baseline and at follow-up were compared between the non-CR and CR groups in patients who did not take any lipid-lowering drugs at baseline, patients who did not smoke at follow-up, and patients who did not take ACE-I, ezetimibe, and/or n-3 PUFA (Supplementary Fig. 1). Although AREA did not change in either the non-CR or CR group, the CEC significantly increased in the CR group alone in these subpopulations.
Supplementry Fig. 1.
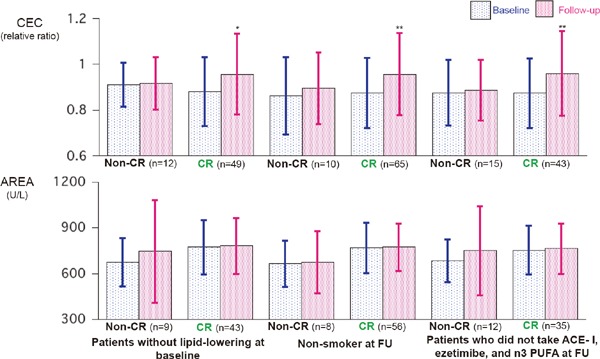
Comparison of CEC and AREA at baseline and follow-up period in patients who did not take any lipid-lowering drugs at baseline (left), patients who did not smoke at follow-up (FU) (center), and patients who did not take angiotensin-converting enzyme inhibitor (ACE-I), ezetimibe, and n-3 polyunsaturated fatty acid (n-3 PUFA) at FU *p = 0.001, **p < 0.0001 compared with the baseline levels by paired t-test.
Effects of Risk Factor Control on CEC in Patients with CR
Among CR patients, four patients continued smoking, and 83%, 97%, and 54% of patients achieved the targets for BP, HbA1c, and all lipid targets, respectively. The peak VO2 at the follow-up CPX in 69 patients was 18.5 ± 4.5 mL/min/kg. Lower cardiopulmonary fitness was defined as less than 19 mL/min/kg, and this level was as same as our previous report12). Significant increases in CEC were seen irrespective of the exercise capacity, and were seen in the patients who achieved risk factor control except all lipid targets (Fig. 3).
Fig. 3.
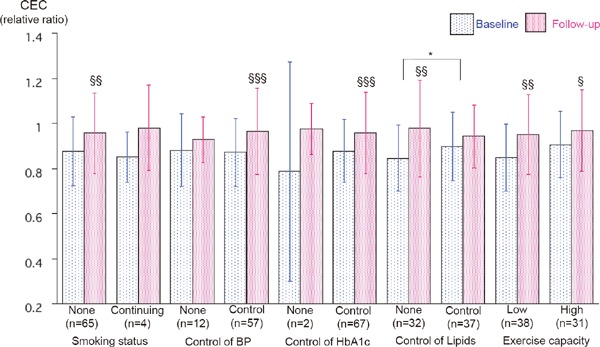
Effects of risk factor control and exercise capacity at follow-up period on CEC among patients in CR group. Data are expressed as mean ± SD. The number in parenthesis indicates actual number of analyzed cases. Seven samples at baseline were collected in fasting-state at the beginning of CR. The others were collected immediate before the emergency coronary angiography on admission. Target controlled blood pressure are defined as < 140 systolic and < 90 diastolic. Target controlled HbA1c levels are defined as < 7.0%. Target controlled lipid levels of LDL-C, non-HDL-C, HDL-C, and triglyceride are defined as < 100, < 130, ≥ 40, and < 150 mg/dl, respectively. *p < 0.05 for significant group difference by two way repeated AVOVA. §p < 0.05, §§p < 0.01, §§§p < 0.0001 compared with the baseline levels by paired t-test. Abbreviations as in text.
Correlation Coefficients between CEC or AREA and Other Variables
Table 4 shows Spearman correlation coefficients between CEC or AREA at baseline or at follow-up, and each of the counterparts of biomarkers, and those net changes in whole patients, patients who did not take any lipid-lowering drugs at baseline, and patients completed the CR program. Although CEC and AREA were significantly correlated each other (ρ = 0.364, P = 0.002, n = 84 at baseline and ρ = 0.274, P = 0.020, n = 72 at follow-up), correlation coefficients between CEC or AREA and other variables were different. The AREA at baseline and follow-up were negatively correlated with age and positively correlated with exercise capacity, while the CEC at baseline and follow-up were significantly positively correlated with HDL-C. Both CEC and AREA were positively associated with apoA-I. AREA at baseline was negatively correlated to hsCRP. Neither CEC nor AREA correlated to LDL-C, non-HDL-C, apoB, Lp(a), and HbA1c. Both changes in CEC and AREA were significantly associated with those in HDL and apoA-I. Fig. 4 shows the correlation coefficients between percent changes in CEC, AREA, HDL-C, and apoA1 in whole patients. The changes in these biomarkers were significantly correlated with each other. On the other hand, lack of association between percent changes in CEC or AREA and those in Peak VO2, triglyceride, MDA-LDL, and hsCRP were observed.
Table 4. Correlation coefficients between CEC or AREA and various parameters at baseline and follow-up.
| Cholesterol efflux capacity |
Arylesterase acitivity |
|||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | Net change | Baseline | Follow-up | Net change | |
| Age | −0.122 (84) | −0.110 (84) | NA | −0.268* (72) | −0.310** (72) | NA |
| −0.071 (61) | −0.136 (61) | −0.301* (52) | 0.413 (52) | |||
| −0.116 (69) | −0.150 (69) | −0.354** (60) | −0.390** (60) | |||
| Peak VO2 | 0.111 (75) | 0.122 (84) | 0.049 (69) | 0.339** (65) | 0.380** (60) | −0.012 (60) |
| 0.261 (54) | 0.197 (49) | 0.171 (49) | 0.357* (47) | 0.428** (43) | 0.044 (43) | |
| 0.085 (69) | 0.122 (69) | 0.049 (69) | 0.331* (60) | 0.380** (60) | −0.012 (60) | |
| HDL-C | 0.346** (84) | 0.231* (84) | 0.305** (84) | 0.194 (72) | 0.198 (72) | 0.339** (72) |
| 0.383** (61) | 0.363** (61) | 0.261* (61) | 0.170 (52) | 0.162 (52) | 0.345* (52) | |
| 0.334** (69) | 0.228 (69) | 0.247* (69) | 0.249 (60) | 0.193 (60) | 0.312* (60) | |
| Apo A1 | 0.357** (84) | 0.227* (84) | 0.331** (84) | 0.376** (72) | 0.281* (72) | 0.412*** (72) |
| 0.390** (61) | 0.354** (61) | 0.344** (61) | 0.329* (52) | 0.224 (52) | 0.330* (52) | |
| 0.312** (69) | 0.262* (69) | 0.326** (69 | 0.441*** (60) | 0.248 (60) | 0.379** (60) | |
| TG | 0.152 (84) | 0.171 (84) | 0.020 (84) | 0.179 (72) | 0.331** (72) | 0.113 (72) |
| 0.155 (61) | 0.145 (61) | 0.041 (61) | 0.142 (52) | 0.434** (52) | 0.031 (52) | |
| 0.128 (69) | 0.340** (69) | 0.112 (69) | 0.202 (60) | 0.413** (60) | 0.154 (60) | |
| MDA-LDL | 0.168 (84) | 0.126 (84) | 0.004 (80) | 0.003 (70) | 0.065 (72) | 0.141 (70) |
| 0.254* (61) | 0.142 (60) | 0.113 (60) | 0.166 (52) | 0.121 (52) | 0.214 (52) | |
| 0.110 (66) | 0.229 (69) | −0.086 (66) | 0.117 (58) | 0.133 (60) | 0.210 (58) | |
| BNP | 0.113 (84) | −0.137 (78) | −0.144 (78) | −0.046 (72) | −0.099 (66) | −0.206 (66) |
| 0.096 (61) | −0.137 (58) | −0.215 (58) | −0.022 (52) | −0.217 (49) | −0.090 (49) | |
| 0.135 (69) | −0.250* (64) | −0.207 (64) | −0.040 (60) | −0.195 (55) | −0.297* (55) | |
| eGFR | 0.235* (84) | 0.159 (84) | 0.103 (84) | 0.106 (72) | 0.153 (72) | −0.070 (72) |
| 0.190 (61) | 0.180 (61) | 0.025 (61) | 0.035 (52) | 0.185 (52) | −0.124 (52) | |
| 0.258 (69) | 0.215 (69) | 0.021 (69) | 0.178 (60) | 0.184 (60) | −0.144 (60) | |
| hsCRP | −0.033 (84) | −0.036 (84) | −0.085 (84) | −0.256* (72) | −0.062 (72) | −0.150 (72) |
| −0.134 (61) | −0.107 (61) | −0.024 (61) | −0.292* (52) | −0.075 (52) | −0.040 (52) | |
| −0.035 (69) | −0.061 (69) | −0.173 (69) | −0.236 (60) | −0.074 (60) | −0.239 (60) | |
Data are expressed as Spearman's Rho between the levels at baseline or follow-up and those counterparts, and between the values of net change and those counterparts. The upper, middle, and lower figures show whole patients, patients who did not take lipid-lowering drugs at baseline, and patients that completed the CR program, respectively. The number in parenthesis indicates actual number of analyzed cases. Seven samples at baseline were collected in a fasting-state at the beginning of the CR. The others were collected immediately before the emergency coronary angiography on admission. *p < 0.05, **p < 0.01, ***p < 0.001. N/A= not available. Abbreviations as in text.
Fig. 4.
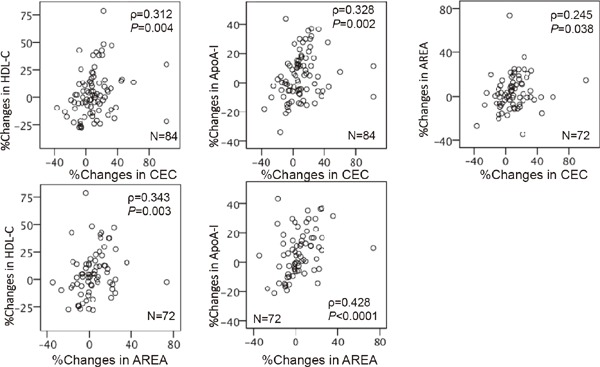
Correlation coefficients between changes in HDL-C, apoA-1, CEC, and AREA. The correlations were determined by Spearman's rank analyses.
Discussion
The present study confirmed that CR could increase the CEC irrespective of the concomitant of different statins, while the CEC was not changed under non-CR usual medical therapy in patients with ACS. These results are good agreement with our previous report of separate ACS patients12). In contrast to our previous report12), HDL-C did not increase in CR patients and this was affected by different statin treatment. There are three novel findings in this report. First, increases in CEC were significantly correlated to increases in AREA. Second, both increases in CEC and those in AREA were correlated with those in apoA1 rather than those in HDL-C. Third, there is a discordance between CEC and AREA for their correlations with other biomarkers. To the best of our knowledge, there was no study to investigate effects of CR on both CEC and AREA compared with non-exercise usual therapy in CAD patients. The results provide the effects of CR on functional properties of HDL and further support to CR for the secondary prevention.
Table 5 shows limited studies that investigated the effects of exercise training on quantity of HDL and HDL function (CEC and/or PON1)13, 24–33). These inconsistent results may be partially due to differences in studied population, HDL-C levels at baseline, exercise status, and concomitant therapy such as diet and medication. Among them, four studies compared both CEC and PON1 activity, and all these studies failed to show increases in HDL-C and apoA-1. Casella-Filho et al. reported that a 3-month aerobic exercise training increased both PONA and CEC without changes in BMI, in sedentary subjects with metabolic syndrome24). Sang et al. reported that exercise training increased PONA alone in patients with metabolic syndrome compared with non-exercise counterparts26). Aicher et al. reported that weight loss program significantly reduced both HDL-C and CEC, without changes in AREA in overweight or obese women27). In contrast, Králová Lesná et al. reported that a significant weight reduction was significantly associated with increases in CEC in young healthy obese women28). Cross-sectional studies reported that both HDL-C and CEC were significantly higher in athletes compared with non-athlete controls34, 35), and that both HDL-C and apoA-1 were significantly associated with CEC and Peak VO234). With regard to PON1 activity and physical activity, both positive correlation35–37) and lack of correlation38) were reported. The former studies support the positive association between AREA and peak VO2 in the present study. Tomas et al. reported an opposite effect of exercise training on PONA according to PON1-192 polymorphism39). A recent report with Turkish national judoists showed that an anaerobic judo training increased significantly PONA, AREA, and HDL-C, and that interaction of PON1-192 polymorphism was seen in not AREA but PONA33). In addition to genetic factors, regulation of PON1 activity is affected by environmental factors such as anxiety40), oxidative stress41), and inflammation42). In contrast to the previous report41), the present study failed to show an association between AREA and MDA-LDL as surrogate marker for oxidative stress, while AREA at baseline was significantly inversely associated with hsCRP. Furthermore, Kameyama et al. reported that postprandial increases in AREA and postprandial decreases in PON-1 lactonase activities in healthy men43). The baseline blood samples were collected at non-fasting state on admission in 92% of patients, and AREAs at baseline in these patients were slightly higher compared with the AREA in fasting state at the beginning of the CR (756.2 ± 170.2 vs 727.4 ± 129.0, respectively). These various factors may attenuate the changes in AREA induced by CR in our patients. Tang et al. reported that not PONA value but lower AREA predicted long-term cardiovascular risk in both primary and secondary prevention subjects although genetic determinants of PONA and AREA were not associated with cardiovascular risk. They showed that some single nucleotide polymorphisms were contrast association with PONA (positive) and AREA (negative), and that the relationship between AREA and its genetic determinants was lower than those with PONA44). These suggest that AREA differ from PONA, and we measured only AREA. Future studies are required to measure both AREA and PONA, and evaluate effects of various factors on CEC, AREA, and PONA in a large population.
Table 5. Effects of exercise-based lifestyle modification on HDL cholesterol, apolipoprotein A-1, cholesterol efflux capacity and PON-1 activities.
| Study population, age, number [reference] | Exercise mode, intensity, frequency, duration | HDL-C ApoA-1 | CEC PON-1 |
|---|---|---|---|
| Sedentary subjects with MS (50 ± 10y, 10M, 10F) Sedentary subjects without MS (45 ± 7y, 6M, 4F) as cont. [24] |
moderate intensity with bicycle ergometer, 45 min, 3/w, 3 mo | HDL-C → ApoA-1 → |
CEC↑ PONA↑ |
| DM (59 ± 7y 3M, 8F), Healthy control (50 ± 9y 1M, 10F) Sedentary DM (51 ± 10y 4M, 6F) as cont. [25] |
moderate intensity with bicycle ergometer, 40 min, 3/w, 4 mo | HDL-C → | CEC → PONA → |
| MS (59 ± 1y 9M, 18F) MS (61 ± 1y 4M, 8F) as cont. [26] |
walk/run training program, 30–60 min, 5/w, 10 w | HDL-C → ApoA-1 → |
CEC → PONA↑ |
| Overweight or obese women (46 ± 11y, 12DM, 88nonDM), No cont. [27] | walking steps (+5000 steps/day), +Reduced fat and total energy diet (−500 Cal.), | HDL-C↓ ApoA-1 → |
CEC↓ PONA → |
| Obese women (< 40y, 15F), No cont. [28] | increased physical activity, 60 min, 5/w, 9 w+Healthy diet | HDL-C → ApoA-1↓ |
CEC →* |
| CAD (66 ± 7y, 23M, 14F), No cont. [13] | moderate intensity, 30 min, 3/w, 12 w | HDL-C → | AREA↑ |
| Obese non-DM with MS (43 ± 11y, 40M), 22men completed the program Healthy volunteers (39 ± 10y, 26M) as cont. [29] | weight loss program by diet (1200 Cal) and exercise, 60 min, 3–5/w, 3 mo | HDL-C↑ (3 & 12mo) |
PONA ↓ (3mo) PONA ↑ (12mo) |
| Overweight or obese men (63y, 46–76y, 22M) with CVD risk, No cont. [30] | moderate intensity, 45–60 min, 21 d | HDL-C↓ ApoA-1 → |
PONA → |
| Sedentary healthy elders (69 ± 5y, 18M, 25F), No cont. [31] | mild to moderate intensity, ≥30 min, 2/w, 6 mo | HDL-C → | AREA → |
| DM (55 ± 8y, 7M, 7F), Healthy volunteers (48 ± 8y, 5M, 7F), No cont. [32] | mild to moderate intensity, 40 min, 3/w, 18 w | HDL-C → | AREA → |
| National judoists (18 ± 1y 18F), No cont. [33] | anaerobic exercise, 2 hr/day, 6/w, 5 mo | HDL-C↑ | PONA↑ AREA↑ |
ApoA-1 = apolipoprotein A-1; AREA = arylesterase activity; CAD = coronary artery disease; CEC = cholesterol efflux capacity; cont. = non-exercise control group; CVD = cardiovascular disease; d = day; DM = diabetes mellitus; erg = ergometer; F = female; hr = hour; HDL-C = HDL cholesterol; M = male; min = minute; mo = month; MS = metabolic syndrome; nonDM = non diabetes mellitus; PONA = paraoxonase activity; w = week; y = year; ↑ = increase; ↓ = decrease; → = no change.
*CEC was significantly increased when two females with lowest weight change were excluded.
The ninety-nine percent of patients took statins during the follow-up period. A meta-analysis of 25 clinical trials including one study in Japan45) showed that statin therapy was associated with a significant elevation of PONA and AREA46). However, this metaanalysis described that elevation of AREA following statin therapy varied in the type of statin. Significant effects were observed in trials with simvastatin and were not found in trials with atorvastatin and rosuvastatin47). Only one study from Miyamoto-Sasaki et al showed that 4-week treatment with 2 mg of pitavastatin significantly increased HDL-C by 9%, CEC by 8.6%, and AREA by 11% in 30 patients with dyslipidemia45). In the present study, no patient took simvastatin, and only nine patients took pitavastatin at follow-up. Nicholls et al. have reported that a 12-week statin monotherapy with 20 mg of atorvastatin, 10 mg of rosuvastatin or 40 mg of simvastatin significantly decreased CEC and preβ1 HDL compared with placebo in patients with dyslipidemia, and CEC at baseline was significantly correlated with HDL-C, apoA-I, and preβ1 HDL47). The present study showed that significant increases in HDL-C and apoA-I were seen in only CR patients treated with pitavastatin or rosuvastatin, while significant increases in CEC were observed in CR patients, irrespective of the types of statins. The reasons for the increases in CEC by CR have not been elucidated clearly. As the majority of the patients in CR group achieved targets of BP and HbA1c, it is difficult to investigate whether risk factor control affect the present results. Tanaka et al. reported that a 4-week administration of high dose eicosapentaenoic acids increased CEC although HDL-C and serum AREA did not change in 21 patients with dyslipidemia48). The increases in CEC were observed even when we excluded the patients who took n-3 PUFA, ezetimibe, and ACE-I. In non-CR group, the patients did not want to participate supervised CR program, and other unmeasured factors may have affected the results. Anyway, whatever the mechanism, our results confirmed that CR could improve HDL function. Future prospective studies are required to investigate the mechanisms of effects of CR and usual therapy for ACS on CEC and AREA.
A cross-sectional study with 205 ACS patients and 100 healthy subjects showed that the significant correlation between CEC and PONA was positive in healthy subjects and inverted in ACS patients, and that no correlation between CEC and HDL-C in both groups, although both CEC and PONA were significantly lower in ACS patients than in healthy subjects49). The present study showed positive correlation between CEC and AREA, and between changes in CEC and those in AREA. Both CEC and AREA were significantly associated with apoA-I rather than HDL-C. It has been reported that apoA-I is important for the stability and activity of PON1 on HDL50). A report of homozygous and heterozygous apoA-I deficiency showed that apoA-I is the major HDL component underlying both CEC and anti-oxidative activity51). HDL particle contains 2 to 5 molecules of apoA-I52) and inflammation replaces apoA-I in HDL particle with serum amyloid A53). In contrast to apoB, serum apoA-I could not be reflected as HDL particle number. Thus, it is difficult to evaluate CEC per HDL particle. The present study suggests that CR improve individual CEC, and that apoA-I may serve as surrogate biomarkers of HDL function.
The major limitation of the present study is a single center retrospective analysis with small sample size particularly in female patients with ACS and patients in non-CR group. Therefore, our results should be confirmed by prospective studies with more sample size in future. This study is associated with several other limitations. First, heterogeneity of HDL and composition of proteins and lipids of HDL were not examined. Second, lecithin cholesterol acyltransferase, cholesterol ester transfer protein, hepatic lipase and endothelial lipase were not measured. These factors play important roles in HDL metabolism. Third, genetic polymorphisms of PON1 and/or anxiety state on AREA were not evaluated. Fourth, regular physical activity was not measured in all patients. Fifth, we did not measure PONA and PON1 protein concentration. These factors should be investigated in future studies.
Conclusion
The present study of ACS patients demonstrated three findings. First, five-month outpatient CR program significantly improved CEC despite of no changes in HDL-C. Second, increases in CEC were significantly correlated to increases in AREA, however. there is a discordance between CEC and AREA for their correlations with other biomarkers. Third, both CEC and AREA were significantly correlated with apoA-I rather than HDL-C. These results suggest that one of the beneficial effects of the comprehensive CR can increase functional HDL particles. Again, future prospective studies should be conducted to evaluate these issues in larger number of sample size.
Acknowledgments
We are grateful for the technical guide of Drs. Katsunori Ikewaki, Makoto Ayaori, and Harumi Uto-Kondo from the Division of Anti-aging and Vascular Medicine, Department of Internal Medicine, National Defense Medical College, Tokorozawa, Japan. We would also like to thank the nursing stuff of the catheterization laboratory and all of the cardiologists at the Department of Cardiology of Showa University Hospital, for their valuable help with this study.
Disclosures
The authors have no conflicts to declare.
References
- 1). Brewer HB, Jr: The evolving role of HDL in the treatment of high-risk patients with cardiovascular disease. J Clin Endocrinol Metab, 2011; 96: 1246-1257 [DOI] [PubMed] [Google Scholar]
- 2). Rosenson RS, Brewer HB, Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L: Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation, 2012; 125: 1905-1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ: Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med, 2011; 364: 127-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW: HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med, 2014; 371: 2383-2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, Kastelein JJ, Boekholdt SM, Khaw KT, Wareham N, Rader DJ: Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol, 2015; 3: 507-513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Ogura M, Hori M, Harada-Shiba M: Association Between Cholesterol Efflux Capacity and Atherosclerotic Cardiovascular Disease in Patients With Familial Hypercholesterolemia. Arterioscler Thromb Vasc Biol, 2016; 36: 181-188 [DOI] [PubMed] [Google Scholar]
- 7). Brownell N, Rohatgi A: Modulating cholesterol efflux capacity to improve cardiovascular disease. Curr Opin Lipidol, 2016; 27: 398-407 [DOI] [PubMed] [Google Scholar]
- 8). Rajkovic MG, Rumora L, Barisic K: The paraoxonase 1, 2 and 3 in humans. Biochem Med (Zagreb), 2011; 21: 122-130 [DOI] [PubMed] [Google Scholar]
- 9). Zhao Y, Ma Y, Fang Y, Liu L, Wu S, Fua D, Wang X: Association between PON1 activity and coronary heart disease risk: A meta-analysis based on 43 studies. Mol Genet Metab, 2012; 105: 141-148 [DOI] [PubMed] [Google Scholar]
- 10). Berrougui H, Loued S, Khalil A: Purified human paraoxonase-1 interacts with plasma membrane lipid rafts and mediates cholesterol efflux from macrophages. Free Radic Biol Med, 2012; 52: 1372-1381 [DOI] [PubMed] [Google Scholar]
- 11). The Japanese Circulation Society Joint Working Group : Guidelines for Rehabilitation in Patients with Cardiovascular Disease (JCS 2012)–Digest Version–. Circ J, 2014; 78: 2022-2093 [DOI] [PubMed] [Google Scholar]
- 12). Koba S, Ayaori M, Uto-Kondo H, Furuyama1 F, Yokota Y, Tsunoda F, Shoji M, Ikewaki K, Kobayashi Y: Beneficial effects of exercise-based cardiac rehabilitation on high-density lipoprotein-mediated cholesterol efflux capacity in patients with acute coronary syndrome. J Atheroscler Thromb, 2016; 23: 865-877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Goldhammer E, Ben-Sira D, Zaid G, Biniamini Y, Maor I, Lanir A, Sagiv M: Paraoxonase activity following exercise-based cardiac rehabilitation program. J Cardiopulmonal Rehab Prev, 2007; 27: 151-154 [DOI] [PubMed] [Google Scholar]
- 14). Beaver WL, Wasserman K, Whipp BJ: A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol, 1986; 60: 2020-2027 [DOI] [PubMed] [Google Scholar]
- 15). Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K: Executive Summary of the Japan Atherosclerosis Society (JAS) Guidelines for the Diagnosis and Prevention of Atherosclerotic Cardiovascular Diseases in Japan -2012 Version. J Atheroscler Thromb, 2013; 20: 517-523 [DOI] [PubMed] [Google Scholar]
- 16). Imai E, Horio M, Watanabe T, Iseki K, Yamagata K, Hara S, Ura N, Kiyohara Y, Moriyama T, Ando Y, Fujimoto S, Konta T, Yokoyama H, Makino H, Hishida A, Matsuo S: Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol, 2009; 13: 621-630 [DOI] [PubMed] [Google Scholar]
- 17).The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res, 2014; 37: 253-392 [DOI] [PubMed] [Google Scholar]
- 18). Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T, Sanke T, Namba M, Hashiramoto M, Sasahara T, Nishio Y, Kuwa K, Ueki K, Takei I, Umemoto M, Murakami M, Yamakado M, Yatomi Y, Ohashi H, Committee on the Standardization of Diabetes Mellitus-Related Laboratory Testing of Japan Diabetes Society : International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. Diabetol Int, 2012; 3: 8-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Miida T, Nishimura K, Hirayama S, Miyamoto Y, Nakamura M, Masuda D, Yamashita S, Ushiyama M, Komori T, Fujita N, Yokoyama S, Teramoto T: Homogeneous Assays for LDL-C and HDL-C are Reliable in Both the Postprandial and Fasting State. J Atheroscler Thromb, 2017; 24: 583-599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Kotani K, Maekawa M, Kanno T, Kondo A, Toda N, Manabe M: Distribution of immunoreactive malondialdehyde-modified low-density lipoprotein in human serum. Biochim Biophys Acta, 1994; 1215: 121-125 [DOI] [PubMed] [Google Scholar]
- 21). Nakajima K, Saito K, Tamura A, Suzuki M, Nakano T, Adachi M, Tanaka A, Tada N, Nakamura H, Campos E, Havel RL: Cholesterol in remnant-like lipoproteins in human serum using monoclonal anti apoB-100 and anti apoA-1 immunoaffinity mixed gels. Clin Chim Acta, 1993; 223: 53-71 [DOI] [PubMed] [Google Scholar]
- 22). Roberts WL, Moulton L, Law TC, Farrow G, Cooper-Anderson M, Savory J, Rifai N: Evaluation of nine automated high-sensitivity C-reactive protein methods: Implications for clinical and epidemiological applications. Part 2. Clin Chem, 2001; 47: 418-425 [PubMed] [Google Scholar]
- 23). Yamashita S, Tsubakio-Yamamoto K, Ohama T, Nakagawa-Toyama Y, Nishida M: Molecular mechanisms of HDL-cholesterol elevation by statins and its effects on HDL functions. J Atheroscler Thromb, 2010; 17: 436-451 [DOI] [PubMed] [Google Scholar]
- 24). Casella-Filho A, Chagas AC, Maranhão RC, Trombetta IC, Cesena FH, Silva VM, Tanus-Santos JE, Negrão CE, da Luz PL: Effect of exercise training on plasma levels and functional properties of high-density lipoprotein cholesterol in the metabolic syndrome. Am J Cardiol, 2011; 107: 1168-1172 [DOI] [PubMed] [Google Scholar]
- 25). Ribeiro IC, Iborra RT, Neves MQ, Lottenberg SA, Charf AM, Nunes VS, Negrão CE, Nakandakare ER, Quintão EC, Passarelli M: HDL atheroprotection by aerobic exercise training in type 2 diabetes mellitus. Med Sci Sports Exerc, 2008; 40: 779-786 [DOI] [PubMed] [Google Scholar]
- 26). Sang H, Yao S, Zhang L, Li X, Yang N, Zhao J, Zhao L, Si Y, Zhang Y, Lv X, Xue Y, Qin S: Walk-run training improves the anti-inflammation properties of high-density lipoprotein in patients with metabolic syndrome. J Clin Endocrinol Metab, 2015; 100: 870-879 [DOI] [PubMed] [Google Scholar]
- 27). Aicher BO, Haser EK, Freeman LA, Carnie AV, Stonik JA, Wang X, Remaley AT, Kato GJ, Cannon RO, 3rd: Diet-induced weight loss in overweight or obese women and changes in high-density lipoprotein levels and function. Obesity (Silver Spring), 2012; 20: 2057-2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Králová Lesná I, Suchánek P, Kovár J, Poledne R: Life style change and reverse cholesterol transport in obese women. Physiol Res, 2009; 58 (Suppl 1): S33-S38 [DOI] [PubMed] [Google Scholar]
- 29). Liang KW, Lee WJ, Lee IT, Lee WL, Lin SY, Hsu SL, Wan CJ, Yu CY, Tsai IC, Fu CP, Ting CT, Sheu WH: Persistent elevation of paraoxonase-1 specific enzyme activity after weight reduction in obese non-diabetic men with metabolic syndrome. Clin Chim Acta, 2011; 412: 1835-1841 [DOI] [PubMed] [Google Scholar]
- 30). Roberts CK, Ng C, Hama S, Eliseo AJ, R. Barnard RJ: Effect of a short-term diet and exercise intervention on inflammatory/anti-inflammatory properties of HDL in overweight/obese men with cardiovascular risk factors. J Appl Physiol, 2006; 101: 1727-1732 [DOI] [PubMed] [Google Scholar]
- 31). Kotani K, Caccavello R, Mutou T, Yamada T, Taniguchi N, Gugliucci A: Association between reactive oxygen metabolites and paraoxonase 1 activity during a physical activity increase intervention with older Japanese people. Australas J Ageing, 2012; 31: 222-226 [DOI] [PubMed] [Google Scholar]
- 32). Iborra RT, Ribeiro IC, Neves MQ, Charf AM, Lottenberg SA, Negrão CE, Nakandakare ER, Passarelli M: Aerobic exercise training improves the role of high-density lipoprotein antioxidant and reduces plasma lipid peroxidation in type 2 diabetes mellitus. Scand J Med Sci Sports, 2008; 18: 742-750 [DOI] [PubMed] [Google Scholar]
- 33). Turgay F, Şişman AR, Aksu AÇ: Effects of anaerobic training on paraoxonase-1 enzyme (PON1) activities of high density lipoprotein subgroups and its relationship with PON1-Q192R phenotype. J Atheroscler Thromb, 2015; 22: 313-326 [DOI] [PubMed] [Google Scholar]
- 34). Olchawa B, Kingwell BA, Hoang A, Schneider L, Miyazaki O, Nestel P, Sviridov D: Physical Fitness and Reverse Cholesterol Transport. Arterioscler Thromb Vasc Biol, 2004; 24: 1087-1091 [DOI] [PubMed] [Google Scholar]
- 35). Brites F, Verona J, De Geitere C, Fruchart JC, Castro G, Wikinski R: Enhanced Cholesterol Efflux Promotion in Well-Trained Soccer Players. Metabolism, 2004; 53: 1262-1267 [DOI] [PubMed] [Google Scholar]
- 36). Senti M, Tomás M, Anglada R, Elosua R, Marrugat J, Covas MI, Fitó M: Interrelationship of smoking, paraox onase activity, and leisure time physical activity: a population-based study. Eur J Intern Med, 2003; 14: 178-184 [DOI] [PubMed] [Google Scholar]
- 37). Cakmak A, Zeyrek D, Atas A, Erel O: Paraoxonase activity in athletic adolescents. Pediatr Exerc Sci, 2010; 22: 93-104 [DOI] [PubMed] [Google Scholar]
- 38). Otocka-Kmiecik A, Lewandowski M, Szkudlarek U, Nowak D, Orlowska-Majdak M: Aerobic training modulates the effects of exercise-induced oxidative stress on PON1 activity: a preliminary study. Scientific World Journal, 2014; 2014: 230271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Tomás M, Elosua R, Sentí M, Molina L, Vila J, Anglada R, Fitó M, Covas MI, Marrugat J: Paraoxonase1-192 polymorphism modulates the effects of regular and acute exercise on paraoxonase1 activity. J Lipid Res, 2002; 43: 713-720 [PubMed] [Google Scholar]
- 40). Sklan EH, Lowenthal A, Korner M, Ritov Y, Landers DM, Rankinen T, Bouchard C, Leon AS, Rice T, Rao DC, Wilmore JH, Skinner JS, Soreq H: Acetylcholinesterase/paraoxonase genotype and expression predict anxiety scores in Health, Risk Factors, Exercise Training, and Genetics study. Proc Natl Acad Sci USA, 2004; 101: 5512-5517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Uzun H, Karter Y, Aydin S, Curgunlu A, Simşek G, Yücel R, Vehiyd S, Ertürk N, Kutlu A, Benian A, Yaldiran A, Ozturk E, Erdine S: Oxidative stress in white coat hypertension; role of paraoxonase. J Hum Hypertens, 2004; 18: 523-528 [DOI] [PubMed] [Google Scholar]
- 42). Kontush A. Chapman: Functionally defective high-density lipoprotein: A new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev, 2006; 58: 342-374 [DOI] [PubMed] [Google Scholar]
- 43). Kameyama N, Maruyama C, Kotani K, Caccavello R, Gugliucci A, Matsui S, Araki R, Maruyama T: Postprandial Paraoxonase 1 activity following consumption of recommended amounts of mixed meals in healthy males. J Atheroscler Thromb, 2016; 23: 225-232 [DOI] [PubMed] [Google Scholar]
- 44). Tang WHW, Hartiala J, Fan Y, Wu Y, Stewart AFR, Erdmann J, kathiresan S, The CARDIoGRAM Consortium. Roberts R, McPherson Allayee H, Hazen SL: Clinical and genetic association of serum paraoxonase and arylesterase activities with cardiovascular risk. Arterioscler Thromb Vasc Biol, 2012; 32: 2803-2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Miyamoto-Sasaki M, Yasuda T, Monguchi T, Nakajima H, Mori K, Toh R, Ishida T, Hirata K: Pitavastatin increases HDL particles functionally preserved with cholesterol efflux capacity and antioxidative actions in dyslipidemic patients. J Atheroscle Thromb, 2013; 20: 708-716 [DOI] [PubMed] [Google Scholar]
- 46). Ferretti G, Bacchetti T, Sahebkar A: effect of statin therapy on paraoxonase-1 status: A systematic review and meta-analysis of 25 clinical trials. Prog Lipid Res, 2015; 60: 50-73 [DOI] [PubMed] [Google Scholar]
- 47). Nicholls SJ, Ruotolo G, Brewer HB, Kane JP, Wang, Krueger KA, Adelman SJ, Nissen SE, Rader DJ: Cholesterol efflux capacity and pre-beta-1 HDL concentrations are increased in dyslipidemic patients treated with evacetrapib. J Am Coll Cardiol, 2015; 66: 2201-2210 [DOI] [PubMed] [Google Scholar]
- 48). Tanaka N, Ishida T, Nagao M, Mori T, Monguchi T, Sasaki M, Mori K, Kondo K, Nakajima H, Honjo T, Irino Y, Toh R, Shinohara M, Hirata K: Administration of high dose eicosapentaenoic acid enhances anti-inflammatory properties of high-density lipoprotein in Japanese patients with dyslipidemia. Atherosclerosis, 2014; 237: 577-583 [DOI] [PubMed] [Google Scholar]
- 49). Bounafaa A, Berrougui H, Ikhlef S, Essamadi A, Nasser B, Bennis A, Yamoul N, Ghalim N, Khalil A: Alteration of HDL functionality and PON1 activities in acute coronary syndromepatients. Clin Biochem, 2014; 47: 318-325 [DOI] [PubMed] [Google Scholar]
- 50). Deakin SP, James RW: Genetic and environmental factors modulating serum concentrations and activities of the antioxidant enzyme paraoxonase–1. Clin Sci, 2004; 107: 435-447 [DOI] [PubMed] [Google Scholar]
- 51). Rached F, Santos RD, Camont L, Miname MH, Lhomme M, Dauteuille C, Lecocq S, Serrano CV, Jr, Chapman MJ, Kontush A: Defective functionality of HDL particles in familial apoA-I deficiency: relevance of alterations in HDL lipidome and proteome. J Lipid Res, 2014; 55: 2509-2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52). Luscher TF, Landmesser U, von Eckardstein A, Fogelman AM: High-density lipoprotein Vascular protective effects, dysfunction, and potential as therapeutic target. Circ Res, 2014; 114: 171-182 [DOI] [PubMed] [Google Scholar]
- 53). Ansell BJ, Watson KE, Fogelman AM, Navab M, Fonarow GC: High-density lipoprotein function Recent advances. J Am Coll Cardiol, 2005; 46: 1792-1798 [DOI] [PubMed] [Google Scholar]


