Abstract
We report the case of a 69-year-old female with Fuchs endothelial dystrophy and posterior chamber in-the-bag intraocular lens, whom we treated with DMEK surgical technique. We encountered difficulties both during obtaining the endothelium from the young donor and during the intraocular unrolling and its application on the stroma. We evaluated both preoperative and postoperative the following parameters: visual acuity, slit lamp, and optical coherence tomography appearance of the cornea, corneal thickness, intraocular pressure, endothelial cell density. In all postoperative assessments, the endothelium was attached to the host's stroma, with small peripheral, inferior folding area. Short-term results were favorable with a clear cornea and significant improvement in visual acuity obtained in the first 3 months of postoperative follow-up.
Abbreviation:
BCVA = best-corrected visual acuity, RE = right eye, LE = left eye, OCT = optical coherence tomography, DMEK = Descemet membrane endothelial keratoplasty
Keywords: corneal graft, DMEK, Fuch’s endothelial dystrophy
Introduction
Great progress has been achieved lately in corneal surgery, especially in corneal grafting for endothelial failure. The once gold standard penetrating keratoplasty has been replaced by more advanced and superior techniques such as DSAEK (Descemet’s stripping automated endothelial keratoplasty), UT-DSAEK (ultra-thin DSAEK) and, most recently, DMEK [1]. DMEK is a partial-thickness corneal graft surgery in which only Descemet’s membrane and endothelium are replaced, unlike DSAEK and UT-DSAEK, in which the donor tissue includes a fine lamella of corneal stroma.
Today, numerous articles and studies show the superiority of DMEK versus DSAEK and UT-DSAEK in terms of BCVA, graft rejection [2,3], corneal topography [4,5], choice of patient [6]. Although still very used (for their more rapid learning curve), DSAEK and UT-DSAEK have lost a great part of their indications in the advantage of DMEK [7].
In the majority of cases, endothelial keratoplasty by DMEK is a method of choice for corneal pathologies with endothelial dysfunction [8] but also a challenge, because of both donor tissue preparation and its manipulation for positioning on the recipient's stroma.
Material and methods – Case report
A 65-year-old woman presented in our clinic with gradual decreased vision for the past two years, more significant in the RE. The patient was diagnosed with Fuchs’ dystrophy 3 years prior to presentation and she had cataract surgery of the RE two years earlier. She was also complaining of foreign body sensation and, sometimes, eye pain. Past medical history of the patient was insignificant with no family history of eye diseases. General review of systems was negative.
At presentation, BCVA was: RE = 0.1, LE = 0.7; manifest refraction was RE: +1,5 dcyl 160, LE: +3 dsf +1,25 dcyl 176; intraocular pressure on non contact tonometry was RE: 26 mmHg, LE: 23 mmHg; pachymetry was RE: 675 µm, LE: 631 µm. Slit-lamp examination of the RE revealed corneal beaten metal aspect, confluent guttae, descemets folds, significant stromal edema, epithelial edema with small defect areas, posterior chamber intraocular lens implanted in the bag. LE presented endothelial beaten metal appearance, confluent guttae, stromal edema, cortical opacities, and mild nuclear sclerosis of the lens. Dilated fundus examination was impossible to perform at RE, retinal angiosclerosis being shown at LE.
Specular microscopy of the corneal endothelium of the LE revealed decreased cell density (1100/ mm²) with abnormal cell morphology (polymegathism and pleomorphism) and the presence of endothelial guttae. Specular microscopy was not possible to perform at the RE. The anterior segment OCT showed increased central corneal thickness in both eyes, posterior corneal surface irregular and highly reflective, prominent formations in anterior chamber, small, hyporeflective intrastromal spaces.
The patient’s symptoms and examination were consistent with the diagnosis of Fuchs’ endothelial dystrophy in both eyes, complicated with bullous keratopathy in RE, cataract in LE, posterior chamber pseudophakia in RE.
Treatment options including medical therapy and surgical intervention were discussed with the patient and she elected surgery by Descemet’s membrane endothelial keratoplasty.
For the preparation of the donor tissue, we used the SCUBA (Submerged Cornea Using Backgrounds Away) technique (Fig. 1 A-D). We have centered the donor cornea with the endothelial side up on the punch block, we scored the peripheral cornea at the Schlemm’s canal level with a blunt instrument, we have gently stripped the endothelial tissue leaving a small adhesion in center, we have punched the donor with 8,5 mm trephine, lifted it from the stromal adhesion carefully using the “one touch” technique and stained it with trypan blue. The donor tissue, double rolled, was loaded into a glass injector by aspiration. The difficulty of the preparation was the younger age of the donor: the endothelial tissue was thin, prone to tears, and adherent to the corneal stroma.
Fig. 1 A.

Scoring the donor’s endothelium
Fig. 1 D.

Double rolled donor tissue in BSS
Fig. 1 B.

Partially stripping the endothelium after the coloration with trypan blue
Fig. 1 C.

Finalizing the stripping of the endothelium
The surgical technique we used for this patient was Descemet Membrane Endothelial Keratoplasty, with the following main steps (Fig. 2 A-E): performing retrobulbar anesthesia, desepitheliation for better visualization, marking the peripheral cornea at 9 mm diameter, creating 3 paracentesis, filling the anterior chamber with air, scoring and stripping Descemet’s membrane with reverse Sinskey hook following the peripheral markings, completing a descemetorhexis, keratotomy and removal of the Descemet membrane through the main wound, injecting acetylcholine intraocular solution into the anterior chamber to constrict the pupil, creating an inferior peripheral iridotomy, removing air and injecting BSS leaving a shallow anterior chamber, injecting the endothelium very carefully into the anterior chamber with double-roll up, suturing the wound with 10-0 nylon. Unscrolling and orientating the DMEK-graft using different “no-touch” techniques were very difficult due to the young donor’s age. The graft was properly positioned and centered on the recipient’s stroma but the persistence of peripheral folds could not be avoided. The graft position was secured by filling the anterior chamber with air and positioning the patient supine as much as possible until complete air resorption.
Fig. 2 A.

Cornea at the moment of surgery
Fig. 2 E.
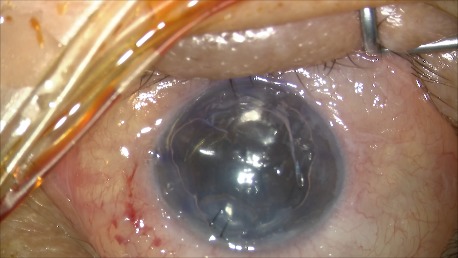
The graft is attached to the stroma, using air tamponade
Fig. 2 B.

Descemetorhexis after marking the cornea
Fig. 2 C.

Double rolled graft in the stable anterior chamber, well oriented
Fig. 2 D.

Unrolling and positioning the graft with the aid of an air bubble and tapping the cornea
Postoperatively, the patient received a bandage contact lens to protect the cornea until epithelial healing and topical treatment with antibiotic, steroids, lubricating eye drops, hypertonic solution. We used ciprofloxacin drops per 1 week and tobramycin/ dexamethasone combination four times a day, for 1 month. After the first month, dexamethasone drops were used three times a day for 2 months and were tapered by one drop at every 2 months, after that, dexamethasone was substituted with fluorometholone drops three times a day with progressive decreasing doses.
On postoperative week 1, the graft was attached but the corneal edema was significant and the visual acuity was decreased. Anterior segment OCT showed the attached endothelium graft with inferior peripheral folds (Fig. 3 A,B).
Fig. 3 A.

Anterior segment OCT at 1 week postoperatively shows irregular posterior corneal surface, increased central corneal thickness, attached endothelium graft
Fig. 3 B.

Anterior segment OCT at 1 week postoperatively shows inferior peripheral folds
On postoperative week 2, the corneal edema decreased and BCVA was 0.4, dilated fundus exam showed retinal angiosclerosis, and a good number of endothelial cells was found at cellular microscopy (Fig. 4 A-C).
Fig. 4 A.

Anterior segment examination at 2 weeks shows clear cornea
Fig. 4 C.

Anterior segment OCT at 2 weeks shows the attached endothelium
Fig. 4 B.

Endothelial cell density at 2 weeks is 1905 cells/ mm²
1 month postoperatively, BCVA was 0,6 and increased at 0,7 at 3 months after the surgical procedure. Good endothelial cells number and improved OCT aspects were observed: attached graft, decreasing central corneal thickness, stable peripheral folds (Fig. 5 A-D).
Fig. 5 A.
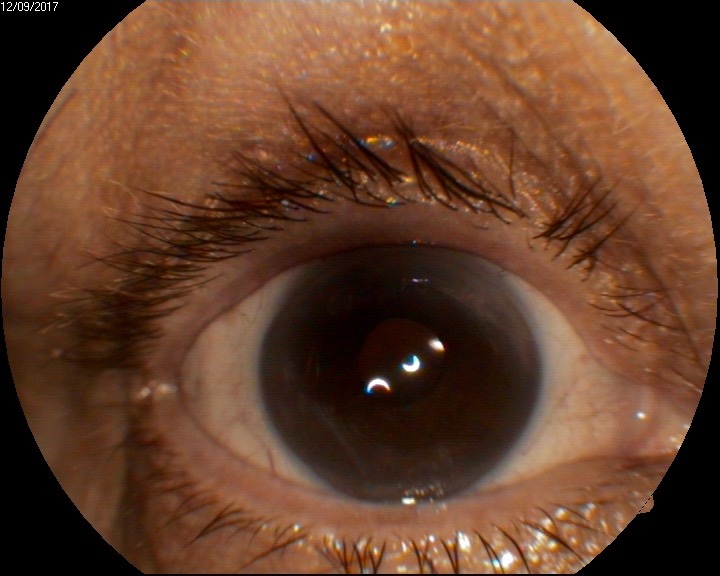
Anterior segment examination shows clear cornea
Fig. 5 D.

Anterior segment OCT shows stable inferior folds
Fig. 5 B.

Endothelial cell density at 3 months is 1705 cells/ mm²
Fig. 5 C.

Anterior segment OCT shows the attached endothelium
Conclusions
DMEK is an efficient alternative to penetrating keratoplasty, presenting many advantages in selected cases. The good BCVA obtained and the lower rejection risk makes DMEK a preferable technique for more and more surgeons. DMEK is a delicate, highly complex surgery so that it requires a longer learning curve to master standard reproducible techniques for corneal endothelium manipulation [1] but the learning curve does not correlate with the clinical outcome [9]. During a learning curve, an ideal DMEK candidate for endothelial failure due to Fuchs dystrophy should be pseudophakic, with no prior cataract surgery complications, no prior vitrectomy, nor glaucoma filtrating surgery [7] and have a good intraoperative visibility with or without desepitheliation.
The good BCVA obtained in our patient postoperatively (0.1 preoperatively to 0.7 at 3 months after surgery) is encouraging for resolving similar cases with this technique. There are some disadvantages though: the poor availability of donors and the necessity of selecting the donor for an optimal result.
References
- 1.Corneal surgeons overcoming challenges, maximizing outcomes with DMEK. Ocular Surgery News U.S. Edition. 2015 Apr 10; https://www.healio.com/ophthalmology/cornea-external-disease/news/print/ocular-surgery-news. [Google Scholar]
- 2.Bachmann B, Schaub F, Cursiefen C. Treatment of corneal endothelial disorders by DMEK and UT-DSAEK. Indications, complications, results and follow-up. Ophthalmology. 2016 Mar;113(3):196–203. doi: 10.1007/s00347-016-0221-0. [DOI] [PubMed] [Google Scholar]
- 3.Maier AK, Gundlach E, Gonnermann J, Klamann MK, Bertelmann E, Rieck PW, Joussen AM, Torun N. Retrospective contralateral study comparing Descemet membrane endothelial keratoplasty with Descemet stripping automated endothelial keratoplasty. Eye. 2015 Mar;29(3):327–332. doi: 10.1038/eye.2014.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi T, Hayashi T, Yamaguchi T, Yuda K, Kato N, Satake Y, Shimazaki J. Topographic characteristics after Descemet’s membrane endothelial keratoplasty and Descemet’s stripping automated endothelial keratoplasty. Plos One. 2017 Nov 30;12(11) doi: 10.1371/journal.pone.0188832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Dijk K, Droutsas K, Hou J, Sangsari S, Liarakos VS, Melles GR. Corneal higher-order aberrations after Descemet’s membrane endothelial keratoplasty. Ophthalmology. 2012;119(3):528–535. doi: 10.1016/j.ophtha.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Pavlovic I, Shajari M, Hermann E, Schmack I, Lencova A, Kohnen T. Meta-Analysis of Postoperative Outcome Parameters Comparing Descemet Membrane Endothelial Keratoplasty Versus Descemet Stripping Automated Endothelial Keratoplasty. Cornea. 2017 Dec;36(12):1445–1451. doi: 10.1097/ICO.0000000000001384. [DOI] [PubMed] [Google Scholar]
- 7. https://www.aao.org/eyenet/article/is-dmek-on-rise-2?december-2013.
- 8.Melles GR, Ong TS, Ververs B, van der Wees J. Descemet membrane endothelial keratoplasty (DMEK) Cornea. 2006 ;25(8):987–990. doi: 10.1097/01.ico.0000248385.16896.34. [DOI] [PubMed] [Google Scholar]
- 9.Dapena I, Ham L, Droustas K, van Dijk K, Moutsouris K, Melles GR. Learning Curve in Descemet's Membrane Endothelial Keratoplasty: First Series of 135 Consecutive Cases. Ophtalmology. 2011 Nov;118(11):2147–2154. doi: 10.1016/j.ophtha.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 10.Denniston AK, Barry RJ. Controversies in the Pharmacological Treatment of Uveitis. Curr Pharm Des. 2015 ;21(32):4682–4687. doi: 10.2174/1381612821666150909094907. [DOI] [PubMed] [Google Scholar]


