Abstract
The advent of tyrosine kinase inhibitor (TKI) therapy markedly improved the outcome of patients with chronic-phase chronic myeloid leukemia (CML). However, the poor prognosis of patients with advanced-phase CML and the lifelong dependency on TKIs are remaining challenges; therefore, an effective therapeutic has been sought. The BCR–ABL p210 fusion protein’s junction region represents a leukemia-specific neoantigen and is thus an attractive target for antigen-specific T-cell immunotherapy. BCR–ABL p210 fusion-region-specific CD4+ T-helper (Th) cells possess antileukemic potential, but their function remains unclear. In this study, we established a BCR–ABL p210 b3a2 fusion-region-specific CD4+ Th-cell clone (b3a2-specific Th clone) and examined its dendritic cell (DC)-mediated antileukemic potential. The b3a2-specific Th clone recognized the b3a2 peptide in the context of HLA-DRB1*09:01 and exhibited a Th1 profile. Activation of this clone through T-cell antigen receptor stimulation triggered DC maturation, as indicated by upregulated production of CD86 and IL-12p70 by DCs, which depended on CD40 ligation by CD40L expressed on b3a2-specific Th cells. Moreover, in the presence of HLA-A*24:02-restricted Wilms tumor 1 (WT1)235–243 peptide, DCs conditioned by b3a2-specific Th cells efficiently stimulated the primary expansion of WTI-specific cytotoxic T lymphocytes (CTLs). The expanded CTLs were cytotoxic toward WT1235–243-peptide-loaded HLA-A*24:02-positive cell lines and exerted a potent antileukemic effect in vivo. However, the b3a2-specific Th-clone-mediated antileukemic CTL responses were strongly inhibited by both TKIs and interferon-α. Our findings indicate a crucial role of b3a2-specific Th cells in leukemia antigen-specific CTL-mediated immunity and provide an experimental basis for establishing novel CML immunotherapies.
Keywords: BCR-ABL, cancer immunotherapy, CML, dendritic cell, T helper cell
Introduction
Chronic myeloid leukemia (CML) is a clonal disease of hematopoietic stem cells that is characterized by the Philadelphia chromosome, which results from the t(9;22)(q34;q11) translocation and encodes one of two chimeric forms of the 210-kDa protein BCR–ABL p210, comprising the products of either the b2a2 or b3a2 exon junction. BCR–ABL p210 exhibits an aberrant tyrosine kinase activity that is central to CML pathogenesis.1 Currently, the use of tyrosine kinase inhibitors (TKIs) that block BCR–ABL p210 kinase activity is a well-established standard CML treatment and is particularly effective in improving the prognosis of chronic-phase CML patients.2 A recent study reported that after discontinuation of TKI treatment, sustained remission was increased because of the synergistic action of interferon (IFN)-α with TKIs.3 However, no effective treatment has been established for blastic crisis or disease relapse.4 Moreover, the lifelong dependence on TKIs owing to the presence of minimal residual disease is another remaining challenge in CML management.5
For the treatment of refractory CML, the only potentially curative strategy available currently is allogeneic hematopoietic stem cell transplantation (allo-HSCT) that elicits graft-versus-leukemia (GVL) reaction.6 However, because allo-HSCT is frequently associated with lethal transplant-related complications, including severe graft-versus-host disease (GVHD), a more specific leukemia immunotherapy, which avoids causing GVHD but maintains GVL reaction, has been sought.
In a recent study, tumors from patients who had received CTLA-4 inhibition treatment were analyzed, and the results showed that a high mutation load was associated with favorable clinical responses; this indicated that T cells specific for ‘neoantigens’ derived from tumor mutations play a pivotal role in antitumor immunity.7 The importance of tumor-specific antigens that are distinct from self-antigens is further supported by the successful results obtained using cancer vaccines that target neoantigens.8 As compared with tumor-associated self-antigens, tumor-specific neoantigens are considered to more effectively induce high-avidity T cells, because neoantigens would be recognized as foreign antigens and would therefore avoid inducing intrathymic tolerance. Because the junction region of BCR–ABL p210 represents a tumor neoantigen that is specifically expressed in leukemic cells, it is a promising target for T-cell-mediated immunotherapy, and the potential relevance of this region as a therapeutic target is underscored by its specific expression in CML cells, its essential role in leukemogenesis and its expression in stem cells.9 Notably, previous studies have shown that a synthetic b3a2 peptide derived from BCR–ABL p210’s junction region between exon 3 of BCR and exon 2 of ABL induces HLA class I-restricted CD8+ T lymphocytes and HLA class II-restricted CD4+ T lymphocytes.10, 11, 12, 13, 14
In antitumor immune responses, CD8+ cytotoxic T lymphocytes (CTLs) act as dominant effector cells by mediating direct tumor cell killing. By contrast, CD4+ T-helper (Th) cells play a crucial role in the efficient induction of CD8+ CTL-mediated antitumor immunity and the long-lasting functional memory CD8+ CTL responses.15 CD4+ Th cells also facilitate the entry of CD8+ CTLs into tumor sites.16 Notably, dendritic cells (DCs) play a critical role in the regulation of the tumor-specific immune responses that are mediated by CD4+ Th cells and CD8+ CTLs.17
Previously, b3a2-specific CD4+ Th cells were shown to proliferate in response to not only target cells loaded with the b3a2 peptide but also target cells that presented a b3a2 peptide that was endogenously processed.12, 13 Moreover, b3a2-specific CD4+ Th cells were reported to exhibit cytotoxicity against b3a2-peptide-loaded target cells.11 However, no study has clarified the role played by b3a2-specific CD4+ Th cells in eliciting downstream activation of antileukemic effector cells.
In this study, we established a b3a2-specific CD4+ Th clone (designated here as ‘SK’) and examined its cellular adjuvant properties for DCs. We found that the b3a2-specific CD4+ Th clone induced the maturation of b3a2-peptide-pulsed DCs, and that the licensed DCs efficiently stimulated the primary expansion of Wilms tumor 1 (WT1)-specific CTLs. The primed WT1-specific CTLs killed WT1-peptide-pulsed target cells. Moreover, treatment with therapeutic concentrations of TKIs hampered the leukemia antigen-specific CTL responses elicited by b3a2-specific CD4+ T cells, and the TKI dasatinib, in particular, strongly inhibited both DC and T-cell responses. By contrast, IFN-α enhanced DC maturation, but suppressed T-cell proliferation; consequently, SK–DC interaction-mediated CTL expansion was impaired.
Our findings demonstrate the antileukemic properties of b3a2-specific CD4+ T cells and indicate that these cells can potentially be used in adoptive immunotherapies against CML. To prevent the attenuation of the therapeutic action of b3a2-specific CD4+ T cells, attention must be paid to the effect of TKIs or IFN-α on antileukemic CTL responses mediated through DC maturation.
MATERIALS AND METHODS
Peptide, cytokines and chemicals
HLA-DR9 (DRB1*09:01)-restricted BCR–ABL b3a2 junctional peptide (ATGFKQSSKALQRPVAS) and HLA-A24 (A*24:02)-restricted modified WT1235–243 epitope peptide (CYTWNQMNL) were commercially synthesized and supplied at >90% purity (Toray Research Center, Kamakura, Japan). The modified WT1235–243 peptide contained a Y instead of the M present at amino-acid position 2 of the natural WT1235–243 peptide (CMTWNQMNL). The following reagents were from commercial sources: recombinant human interleukin-4 (rhIL-4) and recombinant human granulocyte–macrophage colony-stimulating factor (rhGM-CSF), Primmune (Osaka, Japan); rhIL-2 and rhIL-12, R&D Systems (Minneapolis, MN, USA); rhIL-15, PeproTech (Rocky Hill, NJ, USA); OK432, Chugai Pharmaceutical Co. (Tokyo, Japan); rhIFN-α-2a, HumanZyme (Chicago, IL, USA); imatinib (Ima), Focus Biomolecules (Plymouth Meeting, PA, USA); dasatinib (Dasa), Cellagen Technology (San Diego, CA, USA); and nilotinib (Nilo), Adipogen (San Diego, CA, USA).
Cells
We isolated peripheral blood mononuclear cells (PBMCs) from healthy donors as previously described.18 The human lung cancer cell line PC9 was cultured in RPMI-1640 medium (Sigma-Aldrich, St Louis, MO, USA) supplemented with heat-inactivated 10% fetal bovine serum. Mouse L-fibroblasts transfected with HLA class II genes were used as described previously.19 The use of cells isolated from the healthy adults was approved by the Ethics Committee of Aichi Cancer Center, and informed consent was obtained from all donors in accordance with the Declaration of Helsinki.
Transfectants
The complementary DNA (cDNA) encoding HLA-DR9 (DRB1*09:01) was kindly provided by Dr Hiroya Kobayashi (Asahikawa Medical College, Asahikawa, Japan). The cDNA encoding BCR–ABL p210 was purchased from Addgene (Cambridge, MA, USA).20 The cDNA encoding BCR–ABL p210, HLA-A24 (A*24:02), HLA-DRA or HLA-DR9 (DRB1*09:01), or the minigene encoding the HLA-A24-restricted modified WT1235–243 epitope was inserted into the lentiviral vector CSII-EF-MCS (RIKEN BioResource Center, Tsukuba, Japan); lentivirus transduction was performed as previously described.21
Antibodies used for functional assays
The following antibodies (Abs) used for functional assays were purchased from Abcam (Cambridge, UK), Beckman Coulter (Marseille, France), BioLegend (San Diego, CA, USA), eBioscience (San Diego, CA, USA), BD Biosciences (San Joe, CA, USA) or R&D Systems: anti-HLA-DP (B7/21, mouse IgG3), anti-HLA-DQ (SPVL3, mouse IgG2a), anti-HLA-DR (L243, mouse IgG2a), anti-HLA-ABC (W6/32, mouse IgG2a), anti-IL-4 (34019, mouse IgG2b), anti-IL-12 (C17.8, rat IgG2a), anti-CD3 (HIT3a, mouse IgG2a), anti-CD154 (CD40L; 40804, mouse IgG2b) and mouse IgG (eBM2a, mouse IgG2a).
Flow cytometry
The following monoclonal antibodies (mAbs) conjugated with fluorescein isothiocyanate, phycoerythrin or allophycocyanin were purchased from BD Biosciences, Beckman Coulter, BioLegend, eBioscience, Miltenyi Biotec (Auburn, CA, USA), R&D Systems or Caltag Laboratories (Hamburg, Germany): anti-CD4 (RPA-T4, mouse IgG1), anti-CD8α (B9.11, mouse IgG1), anti-CD40 (HB14, mouse IgG1), anti-CD45RA (HI30, mouse IgG1), anti-CD45RO (UCHL1, mouse IgG2a), anti-CD80 (2D10, mouse IgG1), anti-CD83 (HB15e, mouse IgG1), anti-CD86 (IT2.2, mouse IgG2b), anti-CD154 (CD40L; TRAP-1, mouse IgG1), anti-CD62L (DREG-56, mouse IgG1), anti-CCR4 (1G1, mouse IgG1), anti-CCR6 (53103, mouse IgG2b), anti-CCR7 (3D12, rat IgG2a), anti-CCR8 (191704, rat IgG2b), anti-CRTh2 (BM16, rat IgG2a), anti-CXCR3 (1C6, mouse IgG1), anti-HLA-DR (L243, mouse IgG2a), anti-TRBV22S1 (IMMU546, mouse IgG1), anti-TCR-αβ (IP26, mouse IgG1), anti-TCR-γδ (5A6.E9, mouse IgG1) and anti-CD279 (PD-1; EH12.2H7, mouse IgG1). The stained cell samples were analyzed using a FACSCalibur, Accuri C6 or FACSAria II flow cytometer (BD Biosciences), and the data were processed using the FlowJo software program (Tree Star, Ashland, OR, USA). Relative fluorescence intensity was calculated as the ratio of the mean fluorescence intensity (MFI) of specific markers to the MFI of isotype controls.
Generation of b3a2-peptide-specific HLA-DR*09:01-restricted CD4+ T cells
A b3a2-peptide-specific T-cell line was established from PBMCs obtained from HLA-DR*09:01-positive healthy donors through restimulation with 10 μM BCR–ABL b3a2 peptide, as described previously.11 The b3a2-peptide-specific T-cell clone was established using the limiting dilution procedure, and the established clone (‘SK’) was maintained through restimulation with 5 μM b3a2-peptide-pulsed autologous PBMCs.
Cell proliferation
Cell proliferation was quantified using the standard [3H]-thymidine incorporation assay, as described previously.21
51Cr release assay
Cytotoxic activity was measured using the standard 51Cr release assay, as described.21
Measurement of cytokines
Cytokine levels in culture supernatants were measured using enzyme-linked immunosorbent assays (hIFN-γ, hIL-4, hIL-10, hIL-17 and hIL-12p70; eBioscience).
Analysis of T-cell antigen receptor gene rearrangement in the T-cell clone
The V, D and J segments of the rearranged T-cell antigen receptor (TCR)-α and TCR-β chains of the b3a2-specific Th clone were identified as previously described.22 The gene segment nomenclature here follows the ImMunoGeneTics (IMGT) usage; the V, D and J segment usages were identified by comparing the resulting sequences against the IMGT database (http://www.imgt.org/), using an online tool (IMGT/V-QUEST).
Real-time quantitative reverse transcription PCR
To quantify transcripts, we performed real-time quantitative PCR on an ABI PRISM 7500 sequence detector (Applied Biosystems, Foster City, CA, USA) and used TaqMan Gene-Expression Assays (Life Technologies, Applied Biosystems, Carlsbad, CA, USA). The following probes were used: GATA3, Hs00231122_m1, and T-bet (TBX21), Hs00203436_m1 (Life Technologies, Applied Biosystems). All expression levels were normalized relative to GAPDH expression using the ddCt method.
Preparation of human monocyte-derived DCs
Human monocyte-derived DCs were induced as described.23 Briefly, CD14+ monocytes from PBMCs were cultured in medium containing rhGM-CSF and rhIL-4, and on day 6, nonadherent DCs were collected and used as immature DCs.
Induction of antigen-specific cytotoxic T-cell expansion
CD8+ T cells and DCs were obtained from the same donor from whom the b3a2-specific T-cell clone (SK) was established, and these were used for inducing antigen-specific CTLs in order to avoid alloreactive responses. CD8+ T cells were isolated from PBMCs through negative magnetic cell sorting using a CD8+ T-cell isolation kit (Miltenyi Biotec). DCs were cultured for 3 h in 96-well round plates in the presence or absence of the b3a2 peptide, and then the T-cell clone was added to the culture and incubated for 5 h. After 30-Gy irradiation, CD8+ T cells were added together with the WT1235–243 peptide. On day 7, we added 1 μCi of [3H]-thymidine to cultures, and after 16-h incubation, assessed the primary proliferative response of the CD8+ T cells using the [3H]-thymidine incorporation assay. In another experiment performed in the same manner on day 10, the frequencies of WT1-peptide-specific CTLs were determined by staining with the HLA-A*2402/WT1235–243 tetramer. We restimulated the resultant CD8+ T cells with the WT1235–243 peptide in the presence of 35-Gy-irradiated autologous PBMCs, and then used the cells in cytotoxicity assays.
In vivo experiments
All in vivo animal studies were approved by the animal experiment committee of the National Cancer Center. Six-week-old BALB/c Rag-2−/−Jak3−/− mice (RJ mice)24 were inoculated subcutaneously (s.c.), in the right-shaved shank, with the mixtures of K562 cells (5.0 × 105) transduced with HLA-A24 and the minigene encoding HLA-A24-restricted modified-WT1235–243 epitope (K562-A24-WT1 minigene cells) and either saline or WT1-specific CTLs (2.0 × 106). Where indicated, the mixtures of b3a2-peptide-loaded DCs (5.0 × 105) and SK cells (2.0 × 106) were intraperitoneally (i.p.) injected into mice at day −1. The mice were monitored for tumor growth and survival, and tumor size was measured at 7-day intervals until the mice died or were killed when the tumors exceeded 25 mm in diameter.
Statistical analysis
STATA version 13.0 (StataCorp LP, College Station, TX, USA) was used for all statistical analyses. In comparisons of multiple experimental groups, significance was assessed using a one-way analysis of variance with the Bonferroni post hoc test. In the statistical analysis of Kaplan–Meier survival curves, P-values were calculated using a log-rank (Mantel–Cox) test. P<0.05 was considered statistically significant, and significant differences are indicated in figures by asterisks.
Results
b3a2-specific Th cells recognize naturally processed BCR–ABL p210 in the HLA-DR9 context
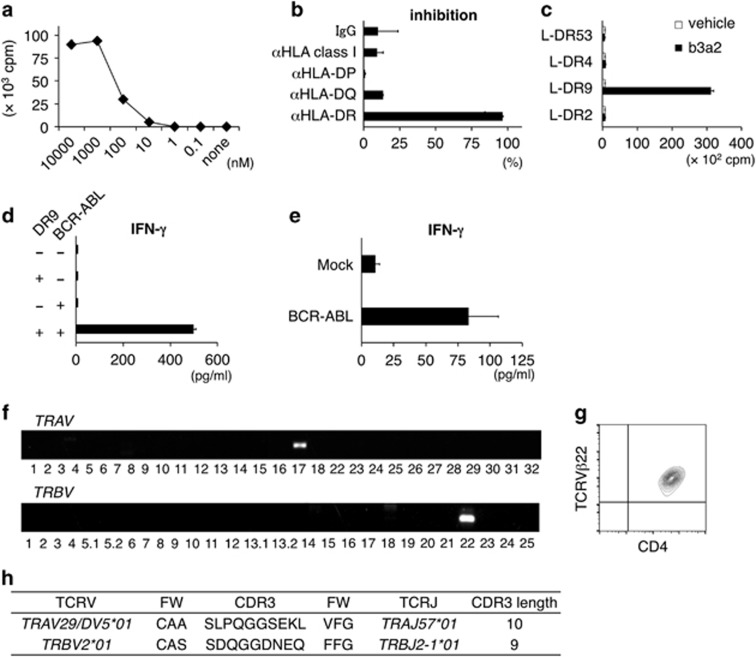
A previous report described the establishment of a b3a2-peptide-specific HLA-DRB1*09:01-restricted CD4+ Th-cell clone.11 According to this report, PBMCs were isolated from an HLA-DRB1*09:01-positive donor and stimulated with the b3a2 peptide. The obtained CD4+ T-cell clone, SK, proliferated when stimulated with the b3a2 peptide in the presence of autologous PBMCs. SK exhibited a dose-dependent proliferative response to stimulation with the b3a2 peptide (Figure 1a). This proliferative response to the peptide was inhibited when we added an anti-HLA-DR mAb, but not an anti-HLA-DQ or anti-HLA-DP mAb, which suggested that the recognition of the b3a2 peptide by SK TCR was restricted by HLA-DR (Figure 1b). To further examine the restricting molecule expressed by this clone, we used, as antigen-presenting cells (APCs), murine L-cells that were transfected with HLA-DRA*01:01 and HLA-DRB1*09:01 (L-DR9), DRB1*04:05 (L-DR4), DRB1*01:03 (L-DR53) or DRB1*15:02 (L-DR2). SK proliferated in response to b3a2-peptide-loaded L-DR9 cells, but not b3a2-peptide-loaded L-DR4, L-DR53 or L-DR2 cells (Figure 1c). These findings suggest that the proliferative response of SK is restricted by HLA-DR9.
Figure 1.
A b3a2-specific and HLA-DRB1*09:01-restricted CD4+ T-cell clone (SK). (a) Proliferative response of SK to graded concentrations of soluble b3a2 peptide. Autologous PBMCs were used as APCs. Proliferation was measured using the [3H]-thymidine incorporation assay. The presented values are the mean c.p.m. of duplicate cultures. (b) Inhibition of the proliferative response by anti-HLA antibodies. SK cells (5 × 104) were cocultured with irradiated PBMCs (5 × 105) in the presence of b3a2 peptide (0.1 μM) and indicated mAbs (10 μg/ml). (c) Proliferative response of SK cells to L-cells expressing the HLA-DR gene. SK cells (5 × 104) were cocultured with irradiated L-cell transfectants (4 × 104) prepulsed with the b3a2 peptide (10 μM). (d) IFN-γ secretion from SK cells (1 × 105) cocultured for 24 h with THP-1 cells (5 × 104) transduced with HLA-DR9 and/or BCR–ABL p210. (e) IFN-γ secretion from SK cells (5 × 104) cocultured for 24 h with autologous DCs (2.5 × 104) that were prepulsed with 150-Gy-irradiated THP-1 or BCR–ABL p210-transduced THP-1 cells (1 × 104). (b–e) Data shown are means±s.d. of triplicate cultures and are representative of three independent triplicate experiments. (f) Expression of the genes TRAV and TRBV. (g) Representative flow cytometry profiles of TCR-Vβ22 and CD4 surface expression on SK cells. (h) TCR gene usage and V–(D)–J junction region sequences of SK. APCs, antigen-presenting cells; DCs, dendritic cells; IFN-γ, interferon-γ mAbs, monoclonal antibodies; PBMCs, peripheral blood mononuclear cells; TCR, T-cell receptor.
SK produced IFN-γ in response to THP-1-DR9 cells expressing BCR–ABL p210 (Figure 1d), and also in response to autologous HLA-DR9-positive DCs fed with 150-Gy-irradiated THP-1 cells that expressed BCR–ABL p210. However, SK did not produce IFN-γ in response to DCs fed with THP-1 cells not expressing BCR–ABL p210 (Figure 1e). These data indicate that BCR–ABL p210—both exogenous and endogenous—can be naturally processed and presented to SK cells in the HLA-DR9 context.
TCR gene usage of the α- and β-chains expressed in b3a2-specific Th cells
The TCRVα and TCRVβ genes used in SK were TRAV29/DV5*01 and TRBV2*01, respectively (according to IMGT nomenclature; corresponding Arden names: TRAV21S1-ADV21S1 and TRBV22S1, respectively; Figure 1f).25 They also expressed TCRVβ22 at the protein level (Figure 1g). The CDR3 of TCRVα and TCRVβ was 10 and 9 amino-acid long, respectively (Figure 1h).
b3a2-specific Th cells exhibit a Th1 profile
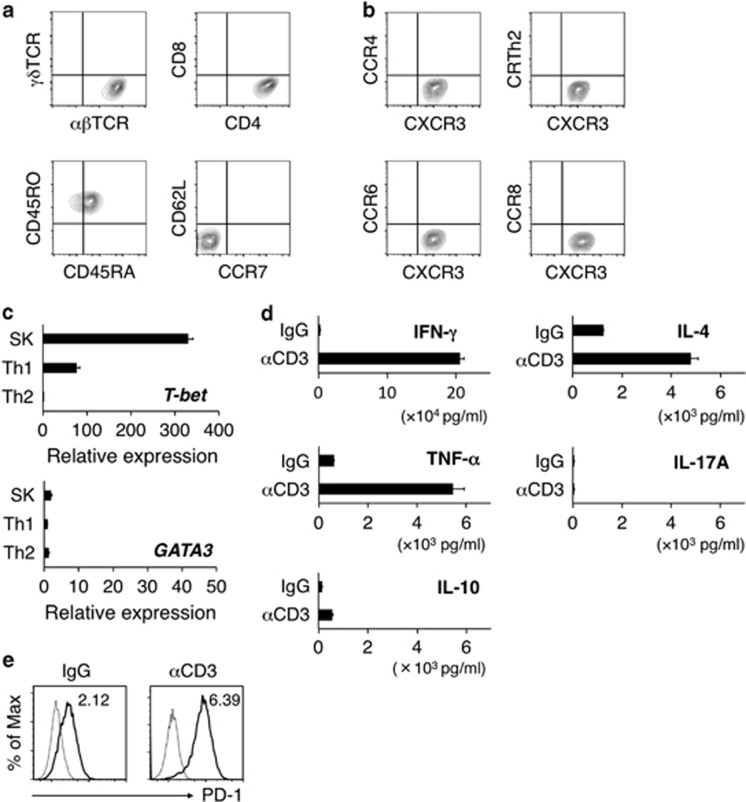
SK expressed TCR-αβ, CD4 and CD45RO, but not TCR-γδ or CD8α moreover, the cells did not express the major lymph node homing receptors CD62L and CCR7, which indicated the effector memory Th-cell phenotype (Figure 2a). SK expressed the Th1-associated chemokine receptor CXCR3, but not the Th2-associated chemokine receptors CRTh2 and CCR4, the Th17-associated chemokine receptor CCR6 or the regulatory T-cell-associated chemokine receptor CCR8 (Figure 2b). SK expressed T-bet and GATA3 at high and low levels, respectively, and produced very high levels of IFN-γ and TNF-α, and comparatively low levels of IL-4 and IL-10 (Figures 2c and d), but produced no IL-17 (Figure 2d). These data collectively suggest that SK presents a Th1 profile. When plate-bound anti-CD3 mAbs were used to stimulate SK, PD-1 surface expression was upregulated in the cells, which indicated the exhaustion of SK (Figure 2e).
Figure 2.
Th1 phenotype of the b3a2-specific CD4+ T-cell clone. (a) Representative flow cytometry profiles of surface αβTCR, γδTCR, CD4, CD8, CD45RO, CD45RA, CD62L and CCR7 on SK cells. (b) Surface expression of chemokine receptors (CCR4, CXCR3, CRTh2, CCR6 and CCR8). (c) Expression of transcription factors involved in Th1 and Th2 differentiation. After SK cells were stimulated for 24 h with plate-bound anti-CD3 mAbs (10 μg/ml), T-bet and GATA3 mRNA levels were quantified. To induce the Th1 and Th2 cells used as controls, PBMCs were stimulated with plate-bound anti-CD3 mAbs (10 μg/ml) under Th1 conditions (IL-12 plus anti-IL-4 Ab) and Th2 conditions (IL-4 plus anti-IL-12 Ab), respectively. The mRNA amounts (shown in a.u.) are normalized relative to the GAPDH mRNA amount. (d) Cytokine production by SK cells after 48-h stimulation with plate-bound control IgG or anti-CD3 mAbs (10 μg/ml). (c, d) Data shown are means±s.d. of triplicate cultures and are representative of three independent triplicate experiments. (e) PD-1 expression on SK. SK cells were stimulated with plate-bound control IgG or anti-CD3 mAbs (10 μg/ml) for 24 h. Staining histograms of PD-1 (solid line) and isotype-matched controls (dotted line) are shown. RFI is shown in the upper part of each panel. IL, interleukin; IgG, immunoglobulin G; IFN, interferon; mAbs, monoclonal antibodies; mRNA, messenger RNA; PBMCs, peripheral blood mononuclear cells; RFI, relative fluorescence intensity; Th, T helper; TCR, T-cell receptor.
b3a2-specific Th cells induce DC maturation and IL-12p70 production
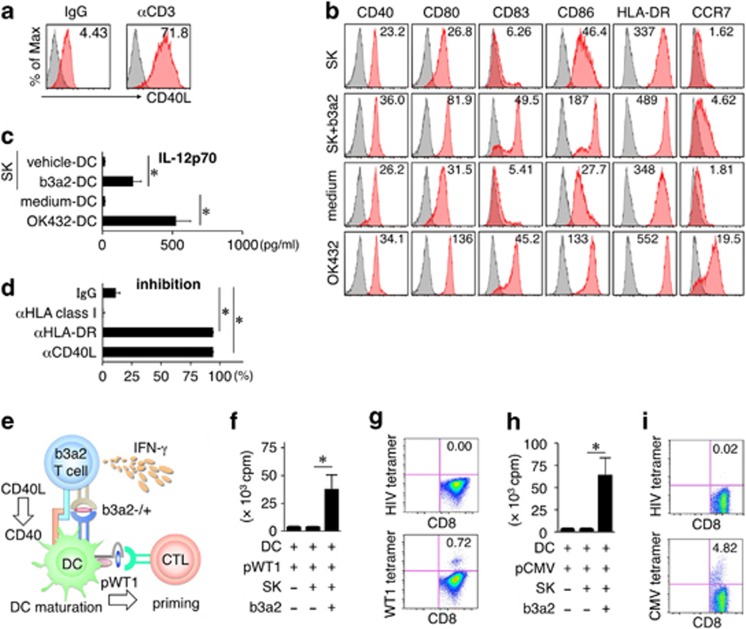
CD40L expressed on activated CD4+ Th cells can induce DC maturation.26 Stimulation of SK with plate-bound anti-CD3 mAbs resulted in the upregulation of CD40L surface expression in the cells (Figure 3a). Thus, we tested whether SK can induce DC maturation by culturing immature DCs with SK cells. In the presence of the b3a2 peptide, DCs increased their expression of CD40, CD80, CD83, CD86, HLA-DR and CCR7, and produced IL-12p70 in substantial amounts (Figures 3b and c). Next, we conducted a blocking experiment to examine how CD40 ligation affects IL-12p70 production, and found that treatment with a blocking Ab against HLA-DR or CD40L inhibited the production of IL-12p70 (Figure 3d). These results indicate that antigen-stimulated SK induces DC maturation and IL-12p70 production by DCs, and that this depends on CD40 ligation.
Figure 3.
Enhanced expansion of leukemia antigen-specific CTLs. (a) Representative flow cytometry profiles of surface CD40L on SK. SK cells were stimulated for 24 h with plate-bound control IgG or anti-CD3 mAbs (10 μg/ml). (b) Surface phenotype of DCs. Vehicle or b3a2 peptide DCs were cultured for 24 h with SK cells at a DC/SK ratio of 10:1. The expression of CD40, CD80, CD83, CD86, HLA-DR and CCR7 is shown. OK432 (10 μg/ml)-matured DCs and medium-control DCs served as controls. (a, b) Staining histograms of the indicated surface molecules (red) and isotype-matched controls (gray) are shown. RFI is shown in the upper part of each panel. (c) IL-12p70 production by DCs cultured with SK cells in the presence or absence of the soluble b3a2 peptide (1 μM). The controls for all other culture conditions were OK432-matured DCs and medium-control DCs. (d) HLA-DR- and CD40L-dependent production of IL-12p70 by DCs. b3a2 peptide DCs were cultured with SK cells for 24 h in the presence of the indicated blocking mAbs (10 μg/ml). (e) Schematic representation of the WT1-specific CTL priming assay. SK cells (5 × 103) and DCs (1 × 104)±b3a2 peptide (5 μM) were initially cocultured for 5 h to mature the DCs, after which the DCs and SK cells were irradiated and cultured with autologous CD8+ T cells (5 × 104) in the presence of the WT1 peptide (5 μM). (f) Proliferation of CD8+ T cells at day 7 in the WT1-specific CTL priming assay was measured as [3H]-thymidine incorporation. (g) Frequency of WT1/HLA-A24-tetramer-positive CD8+ T cells at day 10 in the WT1-specific CTL priming assay. (h) Proliferation of CD8+ T cells at day 7 in the CMV-specific CTL priming assay was measured as [3H]-thymidine incorporation. (i) Frequency of CMV/HLA-A24-tetramer-positive CD8+ T cells at day 10 in the CMV-specific CTL priming assay. (c, d, f, h) Data are representative of three independent triplicate experiments. Error bars, s.d.; *P<0.05, one-way ANOVA. (g, i) Representative flow cytometry profiles of three independent experiments. HIV-env/HLA-A24 tetramer was used as a control. ANOVA, analysis of variance; b3a2, b3a2 peptide; CTL, cytotoxic T lymphocyte; CMV, cytomegalovirus; DC, dendritic cell; IL, interleukin; Ig, immunoglobulin G; IFN-γ, interferon-γ pWT1, WT1 peptide; RFI, relative fluorescence intensity; WT1, Wilms tumor 1.
b3a2-specific Th cells efficiently induce the primary expansion of leukemia antigen-specific CTLs
CD4+ Th cells have been shown facilitate DC-mediated priming of major histocompatibility complex class I-restricted CD8+ CTLs.27, 28, 29 To determine whether SK can induce tumor antigen-specific CTL responses, vehicle- or b3a2-peptide-loaded DCs were cocultured for 5 h with SK cells, and these differentially conditioned DCs were loaded with the WT1 peptide, irradiated and subsequently cultured with CD8+ T cells (Figure 3e). We cultured the cells for 7 days and then evaluated the proliferation of CD8+ T cells. CD8+ T-cell proliferation stimulated by the WT1 peptide was markedly enhanced when SK/b3a2-peptide-conditioned DCs were used as APCs, but not when SK/vehicle-conditioned DCs were used (Figure 3f). The CTLs stimulated by SK/b3a2-peptide-conditioned DCs included WT1-tetramer-positive T cells (Figure 3g). When we used a cytomegalovirus (CMV) peptide instead of the WT1 peptide, we again observed enhanced proliferation of CD8+ T cells and CMV-tetramer-positive T cells upon using SK/b3a2-peptide-conditioned DCs (Figures 3h and i). These data indicate that SK activation by the b3a2 peptide produces cellular adjuvant properties that can boost diverse leukemia antigen-specific CTL responses through DC activation.
TKIs inhibit the leukemia antigen-specific CTL responses induced by b3a2-specific Th cells
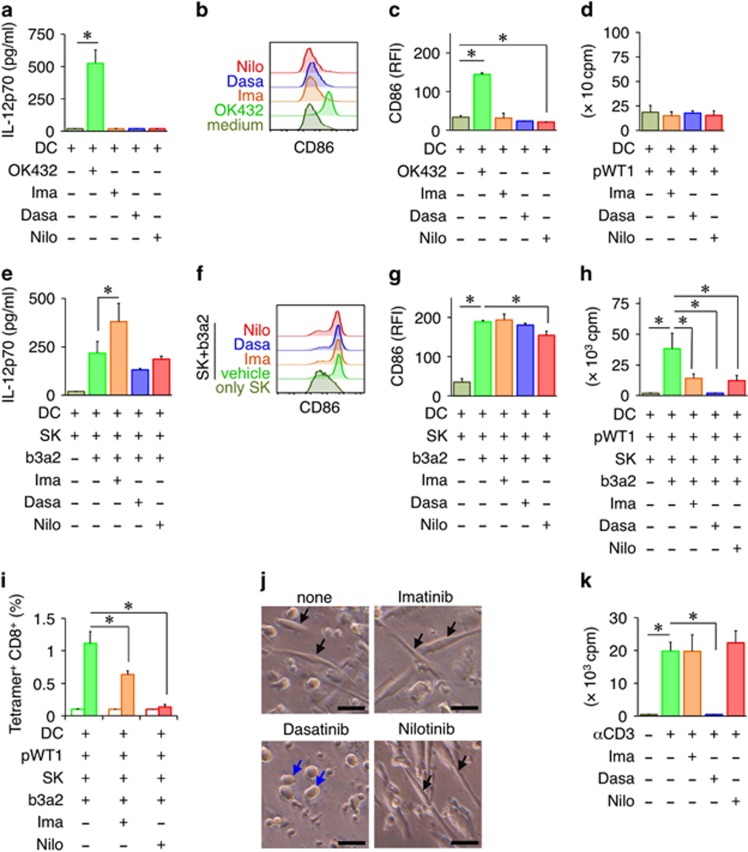
TKI therapy is the current gold standard in CML treatment, particularly in the chronic phase of the disease; however, TKIs are reported to modulate immune responses by inhibiting numerous kinases.30 Thus, we next examined how TKIs used at therapeutic concentrations affect the antileukemic CTL responses induced by the SK–DC interaction. In the absence of SK, TKIs alone did not exert any effect on DC maturation or the subsequent expansion of CD8+ T cells (Figures 4a–d). However, in the presence of SK/b3a2 peptide, IL-12p70 production from DCs was enhanced by imatinib, but was not affected by the other two TKIs tested (Figure 4e). Conversely, SK/b3a2-peptide-induced CD86 upregulation on DCs was inhibited by nilotinib (Figures 4f and g). Furthermore, all three TKIs strongly suppressed the expansion of WT1-specific CD8+ T cells induced by SK/b3a2-peptide-matured DCs (Figures 4h and i). Because dasatinib almost eliminated the expansion of WT1-specific CD8+ T cells, we could not determine the WT1 tetramer frequency in CD8+ T cells (Figure 4h). Intriguingly, among the three TKIs, only dasatinib completely abolished dendrite formation in DCs (Figure 4j), which might result in diminished T-cell stimulatory activity. Last, to examine the direct effect of TKIs on T-cell proliferation, CD8+ T cells cultured alone were stimulated with plate-bound anti-CD3 mAbs in the presence or absence of TKIs, and then cell proliferation was measured. Here dasatinib treatment completely suppressed the proliferation of CD8+ T cells, which indicated that this TKI strongly inhibits not only DC maturation but also T-cell proliferation (Figure 4k). Collectively, these results suggest that although the three TKIs exert distinct effects on DCs, they all inhibit the leukemia antigen-specific CTL responses induced by the interaction between SK and DCs.
Figure 4.
Suppression of leukemia antigen-specific CTL responses by TKIs. (a) Effect of TKIs on IL-12p70 production by DCs. b3a2 peptide DCs were cultured in the presence of the indicated TKIs for 24 h. (b) Representative flow cytometry profiles of CD86 on DCs. (c) RFI values indicating CD86 expression. (a–c) OK432-matured DCs and medium-control DCs served as controls. (d) Proliferation of CD8+ T cells at day 7 in the WT1-specific CTL priming assay in the absence of SK. Irradiated DCs pulsed with the WT1 peptide were cultured with autologous CD8+ T cells, with the indicated TKIs added to the cultures. After 7 days, T-cell proliferation was measured using the [3H]-thymidine incorporation assay. (e) Effect of TKIs on IL-12p70 production by SK-conditioned DCs. b3a2 peptide DCs were cultured with SK cells in the presence of the indicated TKIs for 24 h. (f) Representative flow cytometry profiles of CD86 on SK-conditioned DCs. Vehicle or b3a2 peptide DCs were cultured for 24 h with SK cells at a DC/SK ratio of 10:1 in the presence of the indicated TKIs. (g) RFI values indicating CD86 expression on SK-conditioned DCs. (h) Proliferation of CD8+ T cells at day 7 in the WT1-specific CTL priming assay in the presence of the indicated TKIs was measured as [3H]-thymidine incorporation. (i) Frequency of WT1/HLA-A24-tetramer-positive CD8+ T cells (filled box) at day 10 in the WT1-specific CTL priming assay in the presence of the indicated TKIs. The HIV tetramer (open box) served as the control. (j) Representative optical microscopy images of SK/b3a2-matured DCs in the presence of the indicated TKIs. The arrows indicate DCs. Scale bar, 20 μm. DCs treated with dasatinib appear spherical (blue arrows), whereas DCs treated with the other TKIs present a spindle-shaped morphology (black arrows). (k) Proliferation of CD8+ T cells stimulated with plate-bound anti-CD3 mAbs in the presence of the indicated TKIs. (a, c, d, e, g–i, k) Data are representative of three independent triplicate experiments. Error bars, s.d.; *P<0.05, one-way ANOVA. ANOVA, analysis of variance; b3a2, b3a2 peptide; CTL, cytotoxic T lymphocyte; Dasa, dasatinib; DC, dendritic cell; Ima, imatinib; Nilo, nilotinib; pWT1, WT1 peptide; RFI, relative fluorescence intensity; TKI, tyrosine kinase inhibitor; WT1, Wilms tumor 1.
IFN-α inhibits the leukemia antigen-specific CTL responses induced by b3a2-specific Th cells
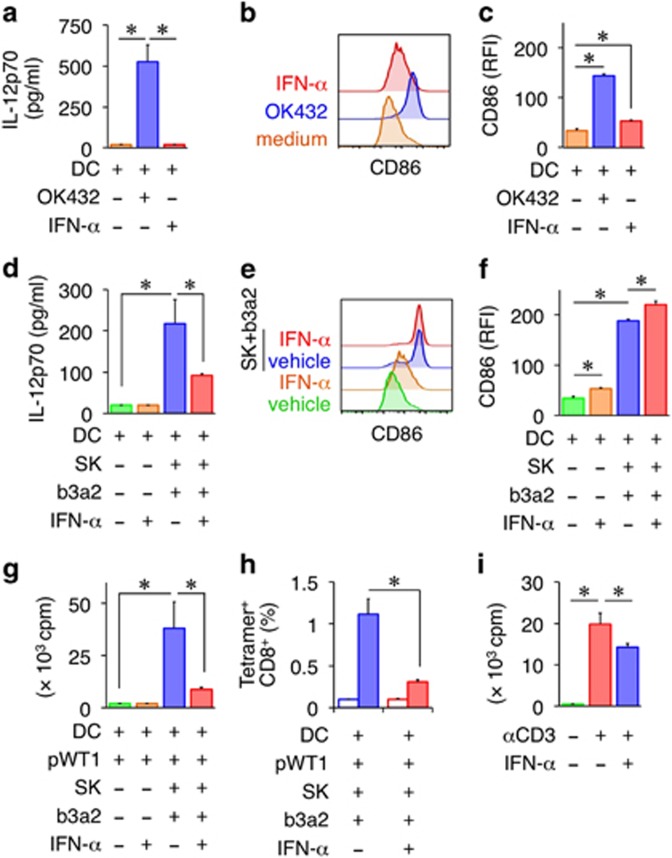
IFN-α has been reported to induce a CML-specific CTL response in CML patients.31 Moreover, the rate of TKI-free remission has recently been reported to increase in response to IFN-α maintenance therapy.3 Thus, we examined the effect of IFN-α on the antileukemic CTL responses induced by the SK–DC interaction. IFN-α treatment on its own did not stimulate IL-12p70 production by DCs, but slightly enhanced the expression of CD86 (Figures 5a–c). IFN-α also enhanced the upregulation of CD86 on SK-matured DCs, but suppressed IL-12p70 production (Figures 5d and e). Furthermore, the expansion of WT1-specific CD8+ T cells induced by SK-matured DCs was inhibited in the presence of IFN-α (Figures 5g and h). Next, to examine the direct effect of IFN-α on CD8+ T-cell proliferation, CD8+ T cells cultured alone were stimulated with plate-bound anti-CD3 mAbs in the presence or absence of IFN-α, and then the proliferative response was measured. CD8+ T-cell proliferation was potently inhibited by IFN-α (Figure 5i), which might be responsible for the suppressed expansion of WT1-specific CD8+ T cells. Collectively, these results suggest that IFN-α exerts inhibitory effects on both DCs and CD8+ T cells.
Figure 5.
Suppression of leukemia antigen-specific CTL responses by IFN-α. (a) Effect of IFN-α on IL-12p70 production by DCs. (b) Representative flow cytometry profiles of CD86 on DCs. DCs were cultured for 24 h in the presence or absence of IFN-α (1 ng/ml). (c) RFI values indicating CD86 expression. (a–c) OK432-matured DCs and medium-control DCs served as controls. (d) Effect of IFN-α on IL-12p70 production by SK-conditioned DCs. b3a2-peptide-loaded DCs were cultured with SK cells in the presence of IFN-α. (e) Representative flow cytometry profiles of CD86 on SK-conditioned DCs. Vehicle or b3a2 peptide DCs were cultured for 24 h with or without SK cells at a DC/SK ratio of 10:1 in the presence or absence of IFN-α. (f) RFI values indicating CD86 expression. (g) Proliferation of CD8+ T cells at day 7 in the WT1-specific CTL priming assay in the presence or absence of IFN-α was measured as [3H]-thymidine incorporation. (h) Frequency of WT1/HLA-A24-tetramer-positive CD8+ T cells (filled box) at day 10 in the WT1-specific CTL priming assay in the presence or absence of IFN-α. The HIV tetramer (open box) served as the control. (i) Proliferation of CD8+ T cells stimulated with plate-bound anti-CD3 mAbs in the presence of IFN-α. (a, c, d, f–i) Data are representative of three independent triplicate experiments. Error bars, s.d.; *P<0.05, one-way ANOVA. ANOVA, analysis of variance; b3a2, b3a2 peptide; CTL, cytotoxic T lymphocyte; DC, dendritic cell; IFN, interferon; mAbs, monoclonal antibodies; WT1, WT1 peptide; RFI, relative fluorescence intensity; WT1, Wilms tumor 1.
WT1-specific CTLs primed by the Th–DC interaction exert antileukemic effects
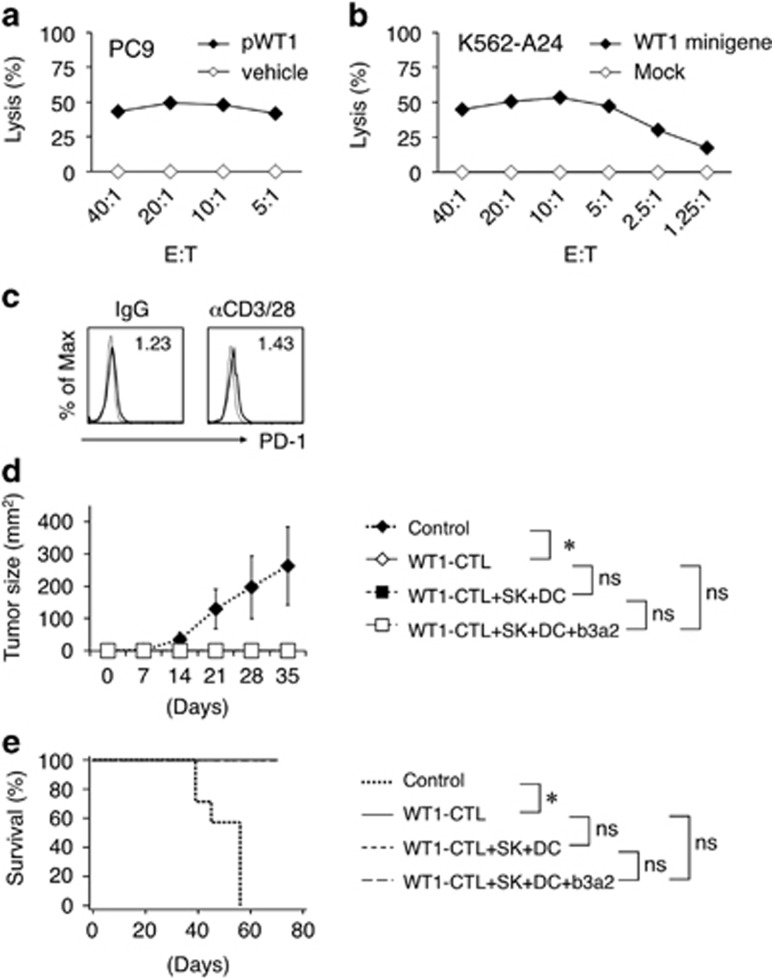
The WT1-peptide-specific CTLs primed by SK/b3a2-peptide-conditioned DCs were further expanded by repeatedly stimulating them with the WT1 peptide. These expanded WT1-peptide-specific CTLs exhibited WT1-specific cytotoxicity against WT1-peptide-loaded and HLA-A24-positive PC9 lung cancer cells, as well as against WT1-minigene-transduced and HLA-A24-expressing K562 cells (K562-A24-WT1 minigene cells) (Figures 6a and b). The WT1-peptide-specific CTLs expressed PD-1 at extremely low levels following stimulation with anti-CD3/28 mAbs (Figure 6c). To determine the in vivo antileukemic effects, the K562-A24-WT1 minigene cells were injected (s.c.) into RJ mice together with or without WT1-peptide-specific CTLs; tumor growth was completely inhibited in the presence of WT1-peptide-specific CTLs, which also yielded a survival advantage to the group (Figures 6d and e). However, the antileukemic effect was not further enhanced following the additional injection (i.p.) of b3a2-peptide-loaded DCs and SK cells, which suggested that the SK–DC interaction critically functions at the stage of WT1-peptide-specific CTL priming, but not thereafter. These data indicate that leukemia antigen-specific CTL priming by the SK–DC interaction can exert potent antileukemic effects.
Figure 6.
Antileukemia activity of CTLs primed by SK-conditioned DCs. (a) Cytotoxic activities of expanded WT1-specific CD8+ T cells against an HLA-A24-positive cell line (PC9) loaded with vehicle or WT1 peptide. (b) Cytotoxic activities against K562-A24 cells that were mock transduced or transduced with a minigene encoding the HLA-A24-restricted WT1 epitope. (a, b) Cytotoxicity was measured at 4 h in 51Cr release assays at the indicated effector/target (E:T) ratios. Data are representative of three independent duplicate experiments. (c) Representative flow cytometry profiles of PD-1 on expanded WT1-specific CD8+ T cells. The CD8+ T cells were stimulated with soluble control IgG or anti-CD3 mAbs (1 μg/ml) plus anti-CD28 mAbs (1 μg/ml) for 24 h. Staining histograms of PD-1 (solid line) and isotype-matched controls (dotted line) are shown. RFI is shown in the upper part of each panel. (d) In vivo inhibition of leukemia. RJ mice were s.c. coinjected with mixtures of K562-A24-WT1 minigene cells and either saline or WT1-specific CTLs. Where indicated, mixtures of SK cells and DCs±b3a2 peptide were i.p. injected into mice at day −1. The average tumor size for each group from day 0 to day 35 is shown. Error bars, s.d.; *P<0.05, one-way ANOVA; NS (e) Kaplan–Meier survival curves plotted for treated and control mice. *P<0.05, log-rank (Mantel–Cox) test; NS. (WT1-CTL, n=8; WT1-CTL+SK+DC, n=8; WT1-CTL+SK+DC+b3a2, n=8; no treatment, n=7). ANOVA, analysis of variance; CTL, cytotoxic T lymphocyte; DC, dendritic cell; i.p., intraperitoneally; NS, not significant; s.c., subcutaneously; RFI, relative fluorescence intensity; WT1, Wilms tumor 1.
Discussion
The treatment outcome of patients with chronic-phase CML has improved considerably since the advent of TKI therapy. However, several challenges remain, including the poor treatment outcome of patients with advanced-phase CML and the lifelong dependence on TKIs because of the presence of minimal residual disease. The b3a2 junction region of BCR–ABL p210 is a leukemia-specific neoantigen that is expressed only in CML cells, and thus represents an optimal target for T-cell-mediated immunotherapy. Here we have demonstrated for the first time that b3a2-specific CD4+ Th cells hold considerable potential for use in CML therapy because they can elicit a CTL-mediated antileukemic immune response, and further that TKIs and IFN-α inhibit this antileukemic immune response.
In our analysis performed using a BCR–ABL p210-transduced THP-1 model, we found that the b3a2 epitope can be naturally processed from endogenous and exogenous BCR–ABL p210 and presented to b3a2-specific Th cells (Figures 1d and e). These findings agree with previous results showing that b3a2-specific Th cells recognize the BCR–ABL fusion peptide that is naturally processed and presented by CML cells or APCs.11, 12, 13
Previously, a WT1-specific CD4+ T-cell clone was reported to play a crucial role in the expansion of WT1-specific CTLs,32 but the precise mechanisms through which the CD4+ T-cell clone produced additive effects on CTL priming were not identified. Our results here have demonstrated that the priming of antigen-specific CTLs was facilitated by the interaction of b3a2-specific CD4+ T cells with DCs. The costimulatory signals that licensed DCs provided are crucial for effectively stimulating and increasing the survival of antigen-specific CD8+ T cells.33 By contrast, cross-presentation by ‘unlicensed’ or immature DCs triggers a CD8+ T-cell response that is abortive and results in deletion or anergy and not in effector–CTL induction.34 Our results indicate that CD4+ T lymphocytes specific for leukemia antigens can harness DC licensing and lead to a subsequent antileukemic CTL response (Figure 3). In the leukemia environment in vivo, b3a2-specific Th cells are considered to condition the DCs that uptake leukemia cells and cross-present diverse leukemia-derived antigens, which results in the activation of diverse CTLs that are specific for distinct leukemia antigens. As compared with the adoptive transfer of single antigen-specific CTLs, this strategy might lower the risk of tumor evasion through immunoediting.35
We successfully primed CTLs specific for a mutant WT1 epitope that was used as a model (Figure 3g). By contrast, we failed to prime CTLs specific for the wild-type WT1 epitope (data not shown), which could be because of the comparatively lower frequencies in peripheral blood of CD8+ T cells expressing high-affinity TCR. A previous study provided evidence suggesting that immunogenic CTL epitopes can be generated from diverse mutations in the kinase domain of BCR–ABL that arise owing resistance to TKIs during CML treatment.36 The antigenicity of these mutation-derived CML neoantigens might be comparable to that of the mutant WT1 epitope used in this study, and these neoantigens might function as potent epitopes and induce multiple high-avidity CTLs.
Previously, b3a2-specific Th cells were demonstrated to promote CML colony growth.11 Moreover, in a murine CML model, IFN-γ secreted from leukemia-specific CD8+ CTLs was shown to induce CML stem cell proliferation.37 These reports suggest the possible exacerbation of CML by b3a2-specific Th cells. By contrast, we have demonstrated that b3a2-specific Th cells produce a beneficial effect in terms of the anti-CML immune response (Figure 3). However, we used an in vitro CTL priming model here to demonstrate the antileukemic effect, and thus additional studies are required to ascertain whether these antileukemic effects of b3a2-specific Th cells are reproduced in vivo.
In b3a2-specific Th cells, PD-1 was highly expressed following TCR stimulation, presumably owing to the ex vivo expansion process required for establishing antigen-specific T-cell clones (Figure 2e). In CML patients, the PD-1/PD-L1 interaction is involved in CTL activity inhibition and disease progression.38 Thus, concomitant checkpoint blockade could potentially enhance the antileukemic efficacy of b3a2-specific Th cells.
TKIs inhibit not only ABL but also numerous other kinases, some of which are recognized to be critical for immune system functions.30 Although TKIs are reported to inhibit various T-cell immune responses, the effect of TKIs on DC function remains poorly understood.39 Intriguingly, our results showed that dasatinib strongly suppressed the function of both CTLs and DCs; dasatinib treatment almost completely inhibited the expansion of antigen-specific CTLs (Figure 4h) and, notably, eliminated dendrite formation in mature DCs (Figure 4j). This agrees with a previous report showing that dasatinib-dependent inhibition of the phosphorylation of cytoskeletal proteins induced marked changes in carcinoma cell morphology (the cells shrank and contracted, and became less stretched).40 The impaired cytoskeletal rearrangements of mature DCs might hamper the establishment of efficient DC–T-cell interaction and the proliferation of antigen-specific CTLs.41 Thus, at least in vitro, TKIs, particularly dasatinib, counteract the antileukemic CTL responses induced through DC maturation. Conversely, dasatinib might be useful for controlling DC-mediated adverse responses such as acute GVHD.
Recently, IFN-α maintenance therapy was reported to increase the rate of TKI discontinuation in CML patients.3 IFN-α treatment has been shown to be associated with the emergence of proteinase-1-specific CTLs.31 However, we unexpectedly found that IFN-α attenuated the leukemia antigen-specific CTL responses elicited by b3a2-specific CD4+ T cells (Figures 5g and h). Type I IFN was reported to inhibit the production of proinflammatory cytokines, including IL-12p70, from Mycobacterium tuberculosis-infected macrophages in an IL-10-mediated manner.42 In this study, IFN-α markedly reduced IL-12p70 production from DCs, which, coupled with the inhibition of T-cell proliferation, explains the compromised antigen-specific CTL response following IFN-α treatment (Figures 5d and i). Although these findings are based on in vitro data, the inhibitory effect of IFN-α must be taken into account when considering therapeutic use of b3a2-specific Th cells.
For refractory CML, a promising treatment is adoptive T-cell therapy performed using a large amount of ex vivo-expanded b3a2-specific Th cells. Here b3a2 peptide stimulation led to 20–50-fold expansion of b3a2-specific Th cells relative to the initial cell number (data not shown). However, repeated stimulation resulted in exhaustion and an anergy state of the T-cell clone (Figure 2e). Together with the complex procedures required to isolate CD4+ T cells specific for relevant antigens, these technological issues hamper the general application of b3a2-specific Th cells in adoptive T-cell therapy.
One helpful strategy here could be to use induced pluripotent stem cells (iPSCs) derived from T cells. In our recent study, iPSCs were established from mature antigen-specific T cells and then redifferentiated into T cells that exhibited identical antigen specificity as the original T cells.43 Passage through pluripotency might provide an unlimited source of antigen-specific CD4+ T cells. As an alternative strategy, T cells could be genetically engineered to express tumor antigen-specific TCR.44 These approaches should yield large amounts of leukemia antigen-specific CD4+ T cells from a single elite CD4+ T-cell clone possessing antileukemic potential.
In conclusion, we have demonstrated that b3a2-specific Th cells exhibit effective antileukemic properties by inducing DC maturation and downstream activation of leukemia antigen-specific CTLs. Thus, these Th cells can be used to establish novel immunotherapies for refractory CML. Moreover, we found that TKIs and IFN-α inhibit the CTL responses, and thus we suggest that b3a2-specific Th cells should be used after reducing the leukemia burden using these anti-CML agents and then terminating their use. Last, the use of our CD4+ T-cell clone in combination with other recently developed technologies might represent a promising approach for establishing effective immunotherapies for refractory CML in the future.
Acknowledgments
CSII-EF, pCMV-VSV-G-RSV-Rev and pCAG-HIVgp were kindly provided by Dr H Miyoshi (RIKEN BioResource Center, Tsukuba, Japan). The cDNA encoding HLA-DRB1*09:01 (DR9) was kindly provided by Dr H Kobayashi (Asahikawa Medical College, Asahikawa, Japan). This study was performed as a research program of the Project for Development of Innovative Research on Cancer Therapeutics (P-DIRECT), Ministry of Education, Culture, Sports, Science and Technology of Japan. This study was supported by grants from Nagono Medical Foundation, the National Cancer Center Research and Development Fund (25-A-7) and the Takeda Science Foundation.
Footnotes
Shin Kaneko is a founder, shareholder and scientific adviser at AsTlym Co., Ltd. Hitoshi Kiyoi received research funding from Bristol-Myers Squibb, Chugai Pharmaceutical Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Zenyaku Kogyo and Fujifilm Corporation. The remaining authors declare no conflict of interest.
References
- Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood 2000; 96: 3343–3356. [PubMed] [Google Scholar]
- Hochhaus A, O'Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia 2009; 23: 1054–1061. [DOI] [PubMed] [Google Scholar]
- Burchert A, Saussele S, Eigendorff E, Muller MC, Sohlbach K, Inselmann S et al. Interferon alpha 2 maintenance therapy may enable high rates of treatment discontinuation in chronic myeloid leukemia. Leukemia 2015; 29: 1331–1335. [DOI] [PubMed] [Google Scholar]
- Hehlmann R, Saussele S. Treatment of chronic myeloid leukemia in blast crisis. Haematologica 2008; 93: 1765–1769. [DOI] [PubMed] [Google Scholar]
- Experts in Chronic Myeloid Leukemia. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood 2013; 121: 4439–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood 2008; 112: 4371–4383. [DOI] [PubMed] [Google Scholar]
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014; 371: 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacohen N, Fritsch EF, Carter TA, Lander ES, Wu CJ. Getting personal with neoantigen-based therapeutic cancer vaccines. Cancer Immunol Res 2013; 1: 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 2009; 15: 5323–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchia M, Korontsvit T, Xu Q, Mackinnon S, Yang SY, Sette A et al. Specific human cellular immunity to bcr-abl oncogene-derived peptides. Blood 1996; 87: 3587–3592. [PubMed] [Google Scholar]
- Yasukawa M, Ohminami H, Kaneko S, Yakushijin Y, Nishimura Y, Inokuchi K et al. CD4(+) cytotoxic T-cell clones specific for bcr-abl b3a2 fusion peptide augment colony formation by chronic myelogenous leukemia cells in a b3a2-specific and HLA-DR-restricted manner. Blood 1998; 92: 3355–3361. [PubMed] [Google Scholar]
- Mannering SI, McKenzie JL, Fearnley DB, Hart DN. HLA-DR1-restricted bcr-abl (b3a2)-specific CD4+ T lymphocytes respond to dendritic cells pulsed with b3a2 peptide and antigen-presenting cells exposed to b3a2 containing cell lysates. Blood 1997; 90: 290–297. [PubMed] [Google Scholar]
- Bosch GJ, Joosten AM, Kessler JH, Melief CJ, Leeksma OC. Recognition of BCR-ABL positive leukemic blasts by human CD4+ T cells elicited by primary in vitro immunization with a BCR-ABL breakpoint peptide. Blood 1996; 88: 3522–3527. [PubMed] [Google Scholar]
- Pawelec G, Max H, Halder T, Bruserud O, Merl A, da Silva P et al. BCR/ABL leukemia oncogene fusion peptides selectively bind to certain HLA-DR alleles and can be recognized by T cells found at low frequency in the repertoire of normal donors. Blood 1996; 88: 2118–2124. [PubMed] [Google Scholar]
- Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol 2004; 4: 595–602. [DOI] [PubMed] [Google Scholar]
- Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res 2010; 70: 8368–8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature 2007; 449: 419–426. [DOI] [PubMed] [Google Scholar]
- Liu TY, Uemura Y, Suzuki M, Narita Y, Hirata S, Ohyama H et al. Distinct subsets of human invariant NKT cells differentially regulate T helper responses via dendritic cells. Eur J Immunol 2008; 38: 1012–1023. [DOI] [PubMed] [Google Scholar]
- Tabata H, Kanai T, Yoshizumi H, Nishiyama S, Fujimoto S, Matsuda I et al. Characterization of self-glutamic acid decarboxylase 65-reactive CD4+ T-cell clones established from Japanese patients with insulin-dependent diabetes mellitus. Hum Immunol 1998; 59: 549–560. [DOI] [PubMed] [Google Scholar]
- He Y, Wertheim JA, Xu L, Miller JP, Karnell FG, Choi JK et al. The coiled-coil domain and Tyr177 of bcr are required to induce a murine chronic myelogenous leukemia-like disease by BCR/ABL. Blood 2002; 99: 2957–2968. [DOI] [PubMed] [Google Scholar]
- Zhang R, Liu T, Senju S, Haruta M, Hirosawa N, Suzuki M et al. Generation of mouse pluripotent stem cell-derived proliferating myeloid cells as an unlimited source of functional antigen-presenting cells. Cancer Immunol Res 2015; 3: 668–677. [DOI] [PubMed] [Google Scholar]
- Uemura Y, Senju S, Maenaka K, Iwai LK, Fujii S, Tabata H et al. Systematic analysis of the combinatorial nature of epitopes recognized by TCR leads to identification of mimicry epitopes for glutamic acid decarboxylase 65-specific TCRs. J Immunol 2003; 170: 947–960. [DOI] [PubMed] [Google Scholar]
- Uemura Y, Liu TY, Narita Y, Suzuki M, Nakatsuka R, Araki T et al. Cytokine-dependent modification of IL-12p70 and IL-23 balance in dendritic cells by ligand activation of Valpha24 invariant NKT cells. J Immunol 2009; 183: 201–208. [DOI] [PubMed] [Google Scholar]
- Ono A, Hattori S, Kariya R, Iwanaga S, Taura M, Harada H et al. Comparative study of human hematopoietic cell engraftment into BALB/c and C57BL/6 strain of rag-2/jak3 double-deficient mice. J Biomed Biotechnol 2011; 2011: 539748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden B, Clark SP, Kabelitz D, Mak TW. Human T-cell receptor variable gene segment families. Immunogenetics 1995; 42: 455–500. [DOI] [PubMed] [Google Scholar]
- Wiesel M, Oxenius A. From crucial to negligible: functional CD8(+) T-cell responses and their dependence on CD4(+) T-cell help. Eur J Immunol 2012; 42: 1080–1088. [DOI] [PubMed] [Google Scholar]
- Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med 1997; 186: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 1998; 393: 474–478. [DOI] [PubMed] [Google Scholar]
- Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 1998; 393: 480–483. [DOI] [PubMed] [Google Scholar]
- Hantschel O, Rix U, Superti-Furga G. Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and dasatinib. Leuk Lymphoma 2008; 49: 615–619. [DOI] [PubMed] [Google Scholar]
- Burchert A, Wolfl S, Schmidt M, Brendel C, Denecke B, Cai D et al. Interferon-alpha, but not the ABL-kinase inhibitor imatinib (STI571), induces expression of myeloblastin and a specific T-cell response in chronic myeloid leukemia. Blood 2003; 101: 259–264. [DOI] [PubMed] [Google Scholar]
- Fujiki F, Oka Y, Tsuboi A, Kawakami M, Kawakatsu M, Nakajima H et al. Identification and characterization of a WT1 (Wilms Tumor Gene) protein-derived HLA-DRB1*0405-restricted 16-mer helper peptide that promotes the induction and activation of WT1-specific cytotoxic T lymphocytes. J Immunother 2007; 30: 282–293. [DOI] [PubMed] [Google Scholar]
- Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity 1995; 3: 87–98. [DOI] [PubMed] [Google Scholar]
- Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity 2005; 22: 275–284. [DOI] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331: 1565–1570. [DOI] [PubMed] [Google Scholar]
- Cai A, Keskin DB, DeLuca DS, Alonso A, Zhang W, Zhang GL et al. Mutated BCR-ABL generates immunogenic T-cell epitopes in CML patients. Clin Cancer Res 2012; 18: 5761–5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurch C, Riether C, Amrein MA, Ochsenbein AF. Cytotoxic T cells induce proliferation of chronic myeloid leukemia stem cells by secreting interferon-gamma. J Exp Med 2013; 210: 605–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumprecht S, Schurch C, Schwaller J, Solenthaler M, Ochsenbein AF. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood 2009; 114: 1528–1536. [DOI] [PubMed] [Google Scholar]
- Wehner R, Wendisch M, Schakel K, Bornhauser M, Platzbecker U, Mohr B et al. Imatinib mesylate does not impair the immunogenicity of human myeloid blood dendritic cells. Leukemia 2006; 20: 1629–1632. [DOI] [PubMed] [Google Scholar]
- Caccia D, Micciche F, Cassinelli G, Mondellini P, Casalini P, Bongarzone I. Dasatinib reduces FAK phosphorylation increasing the effects of RPI-1 inhibition in a RET/PTC1-expressing cell line. Mol Cancer 2010; 9: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Alwan MM, Rowden G, Lee TD, West KA. The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J Immunol 2001; 166: 1452–1456. [DOI] [PubMed] [Google Scholar]
- McNab FW, Ewbank J, Howes A, Moreira-Teixeira L, Martirosyan A, Ghilardi N et al. Type I IFN induces IL-10 production in an IL-27-independent manner and blocks responsiveness to IFN-gamma for production of IL-12 and bacterial killing in Mycobacterium tuberculosis-infected macrophages. J Immunol 2014; 193: 3600–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Kaneko S, Kawana-Tachikawa A, Tajima Y, Goto H, Zhu D et al. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell 2013; 12: 114–126. [DOI] [PubMed] [Google Scholar]
- Qasim W, Thrasher AJ. Progress and prospects for engineered T cell therapies. Br J Haematol 2014; 166: 818–829. [DOI] [PMC free article] [PubMed] [Google Scholar]