Abstract
npr-9 encodes a homologue of the gastrin-releasing peptide receptor (GRPR) and is expressed in AIB interneurons. In this study, we investigated the role of NPR-9 in the neuronal control of innate immunity using the model system Caenorhabditis elegans. After exposure to Pseudomonas aeruginosa PA14, npr-9(tm1652) mutants showed resistance to infection, decreased PA14 colonization and increased expression of immunity-related genes. Nematodes overexpressing NPR-9 exhibited increased susceptibility to infection, increased PA14 colonization and reduced expression of immunity-related genes. In nematodes, ChR2-mediated AIB interneuron activation strengthened the innate immune response and decreased PA14 colonization. Overexpression of NPR-9 suppressed the innate immune response and increased PA14 colonization in nematodes with the activation of AIB interneurons mediated by ChR2 or by expressing pkc-1(gf) in AIB interneurons. We, therefore, hypothesize that NPR-9 regulates the innate immune response by antagonizing the activity of AIB interneurons. Furthermore, expression of GRPR, the human homologue of NPR-9, could largely mimic NPR-9 function by regulating innate immunity in nematodes. Our results provide insight into the pivotal role of interneurons in controlling innate immunity and the complex biological functions of GRPRs.
Keywords: AIB interneuron, Caenorhabditis elegans, innate immunity, neuronal basis, NPR-9
INTRODUCTION
Caenorhabditis elegans can be used to identify both virulence-related microbial genes and immune-based host genes.1, 2 In C. elegans, both genetic and functional genomic approaches have defined several molecular determinants of pathogen resistance. Among these, some conserved signal transduction pathways are found to be required for pathogen resistance. The conserved signal transduction pathways, at minimum, include p38 MAP kinase, Toll-like, insulin, transforming growth factor-β (TGF-β), Wnt/Hox and unfolded protein response signalling pathways.3, 4, 5, 6, 7, 8, 9, 10, 11 Studies in C. elegans can provide useful evolutionary and mechanistic insights into signal transduction and the physiology of innate immunity.12
Much is already known about the immune defence mechanisms in the intestinal epithelium of C. elegans, an essential line of defence against ingested pathogens.13 Recent studies have identified several neuronal signalling pathways capable of regulating the innate immune response, including the neuroendocrine network, the TGF-β pathway and specific neurotransmitters.5, 6, 14, 15, 16, 17 For example, exocytosis of neuropeptides from dense core vesicles suppressed innate immunity,14 and serotonin released from ADF sensory neurons may target the DAF-16/FOXO-signalling pathway to modulate innate immunity.6, 17 Moreover, it has been shown that sensory neurons of AQR, PQR and URX are required for the control of innate immunity,18 implying that specific neuronal circuits may control the innate immune response in nematodes.15
In C. elegans, there is evidence that G protein-coupled receptors (GPCRs) have an important role in the control of innate immunity.18, 19 For example, NPR-1, expressed in AQR PQR, and URX sensory neurons, OCTR-1, expressed in ASH and ASI sensory neurons, and FSHR-1 have been shown to regulate the innate immune response.19, 20, 21 In mammals, gastrin-releasing peptide receptor (GRPR) modulates numerous biological processes involved in metabolism, stress response, energy homeostasis, neurotransmission and behaviour.22 In C. elegans, the GRPR homologue, NPR-9, is a galanin-like GPCR and expressed exclusively in AIB interneurons.23 NPR-9 may potentially regulate multiple biological processes such as fat storage and local search behaviour in nematodes.23 In the present study, we further investigated the neuronal control of innate immunity by NPR-9 and the underlying mechanisms of this regulation in C. elegans. Our findings not only aid our understanding of the neuronal basis of innate immunity in nematodes but will also aid our understanding the complex biological functions of GRPRs in animals and humans.
MATERIALS AND METHODS
C. elegans strains
Some of the nematode strains used in the present study were obtained from the Caenorhabditis Genetics Center, which is funded by the NIH Office of Research Infrastructure Program (P40 OD010440). Nematodes were maintained on nematode growth medium (NGM) plates seeded with Escherichia coli OP50 at 20 °C as described.24
P. aeruginosa PA14 pathogenesis assay
Age-synchronized populations of L4-larvae were prepared and exposed to the P. aeruginosa strain, PA14. PA14 was cultured in Luria broth, then seeded on killing plates containing modified NGM (0.35% instead of 0.25% peptone). PA14 was incubated for 24 h at 37 °C, then for 24 h at 25 °C. The infection was started by adding 60 animals to each plate at 25 °C. Full-lawn PA14 killing plates were prepared. Given that npr-9 mutants are defective in local search behaviour,23 we performed the infection of nematodes on full-lawn PA14 plates.
Behavioural analysis
The methods were performed as described previously.25, 26, 27 Pharyngeal pumping was counted for 1 min under DIC optics using a Zeiss axioscope. Thirty nematodes were examined per treatment. Three replicates were performed. To assay mean defecation cycle length, individual animals were examined for a fixed number of cycles. A cycle period was defined as the interval between initiations of two successive posterior body-wall muscle contractions. Thirty nematodes were examined per treatment. Three replicates were performed. To assay brood size, the number of offspring at all stages beyond the egg stage was counted. Twenty nematodes were examined per treatment. Three replicates were performed. To further determine the fitness in C. elegans, the population growth assay was conducted as described.28 The number of eggs, young larvae (L1–L3), L4 and adults were counted to evaluate the population size and composition of each plate. Twenty replicates were performed.
After PA14 infection for 24 h, locomotion behaviour was examined. Each nematode examined was transferred to an agar plate, atop 50 μl of K medium. After a 1-min recovery period, body bend was used to assess locomotion behaviour. A body bend was counted as a change in the direction of the part of the nematode corresponding to the posterior bulb of the pharynx along the y axis, assuming that nematode was travelling along the x axis. Twenty nematodes were examined per treatment. Three replicates were performed.
Lifespan assay
Lifespan assays were performed as described.29 During PA14 infection, nematodes were scored as being either dead or live every 12 h. Animals were scored as dead if no response was detected after prodding with a platinum wire. The hermaphrodites were transferred daily at 20 °C for the first 4 days of adulthood. For lifespan assays, graphs are representative of three trials. The survival curves were considered significantly different from the control when the P-values were <0.05. The lifespan data were statistically analysed using the log-rank test.
C. elegans bacterial colony-forming unit analysis
After infecting animals with PA14 for 24 h, 6 replicates of 10 nematodes each were transferred to M9 solution containing 25 mM levamisole to paralyze nematodes and stop pharyngeal pumping. Nematodes were transferred to a NGM plate containing ampicillin (1 mg/ml) and gentamicin (1 mg/ml) for 15 min to eliminate P. aeruginosa that was stuck to the body of the animals. Nematodes were transferred to a new NGM plate containing ampicillin (1 mg/ml) and gentamicin (1 mg/ml) for 30 min to remove any residual P. aeruginosa. Nematodes were lysed with a motorized pestle, and the lysates were serially diluted in M9 solution and plated on Luria-Bertani plates containing rifampicin (100 μg/ml) to select for PA14. After overnight incubation at 37 °C, the colonies were counted to determine the colony-forming unit (CFU) per nematode.
Quantitative real-time PCR
After PA14 infection for 24 h, total RNA was extracted using the RNeasy Mini Kit (Qiagen, Chatsworth, CA, USA). Total nematode RNA (~1 μg) was reverse-transcribed using a cDNA Synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA). Quantitative reverse transcription–PCR (RT-PCR) was run at the optimized annealing temperature of 58 °C. The relative quantification of targeted genes was calculated by comparing expression of the target gene to the reference, tba-1 gene, which encodes for tubulin. The final results were expressed as the relative expression ratio between the targeted gene and reference gene. The primers used for the target genes and reference, tba-1 gene are shown in Supplementary Table S1. Three replicates were performed.
Construct of transgenes
To generate an entry vector carrying the npr-9 promoter sequence, the npr-9 promoter (1848 bp, PstI/BamHI) used for AIB-specific expression was amplified by PCR from C. elegans genomic DNA. The npr-9 promoter was then inserted into a pPD95_77 vector in the sense orientation. npr-9, pkc-1/F57F5.5a1 or human GRPR complementary DNA (cDNA) was amplified by PCR, and verified by sequencing. The resulting cDNA was then inserted into the corresponding entry vector, behind the npr-9 promoter. To construct Pnpr-9-ChR2 for optical activation, the npr-9 promoter fragment was inserted into the HindIII/PstI site of the 95-75-ChR2 vector. Transgenic nematodes were generated as described by co-injecting test DNA at a concentration of 10–40 μg/ml and marker DNA (Pdop-1::rfp) at a concentration of 60 μg/ml, into the gonad of nematodes.30
Optical genetic assay
As optogenetic pharmacology is an important tool for the study of neuronal function,31 we performed optogenetic manipulation of AIB interneurons to determine whether AIB interneurons directly regulate the innate immune response of nematodes to the P. aeruginosa strain, PA14. Channelrhodopsin-2 (ChR2), which is expressed in cells of certain organisms, enables light to control electrical excitability.31 The npr-9 promoter was used for AIB-specific expression. Nematodes expressing ChR2 in AIB interneurons were grown on OP50-seeded NGM agar plates containing 50 μM of chromophore, all-trans retinal (ATR). Plates were seeded on day 0. Nematodes were transferred to OP50-seeded plates containing 50 μM of ATR on day 1, in the dark. Nematodes were transferred to PA14-seeded NGM plates containing 50 μM of ATR for 24 h from day 2. During the assay, whole-field illumination was performed, and ChR2 was excited by a round blue light, 9.5 cm in diameter, sourced from a LED array (460–470 nm, ~0.5 mW/mm2) constructed in a LED light source (Chenyufanli Trading Co. Ltd., Nanjing, China). Light intensity measured at the sample was 5 mW/mm2 of 465 nm light. For optogenetic experiments, light intensity was monitored using an optical power metre (PM100, Thorlabs, Newton, NJ, USA). All work was carried out under low-illumination conditions to prevent the preactivation of ChR2-expressing neurons. Nematodes without ATR treatment served as the control population. Ten replicates were performed. Previous study has suggested that optogenetic activation of AIB interneurons would significantly affect reversal frequency in nematodes.32 Activation of AIB interneurons was confirmed by the identification of a significant alteration in reversal frequency among nematodes.
RNA interference
RNA interference (RNAi) was performed by feeding nematodes with E. coli strain HT115 (DE3) expressing double-stranded RNA that was homologous to the target gene as described.33 E. coli HT115 (DE3) grown at 37 °C, overnight, in LB broth containing ampicillin (100 μg/ml) was plated onto NGM containing ampicillin (100 μg/ml) and isopropyl 1-thio-β-D-galactopyranoside (5 mM). L2 larvae were placed on RNAi plates for 2 days at 20 °C until nematodes became gravid. Gravid adults were transferred to fresh RNAi-expressing bacterial lawns to lay eggs for 2 h to obtain the second generation of RNAi population. Eggs were then allowed to develop in young adults at 20 °C for use in subsequent assays.
Statistical analysis
All data in this article were expressed as the mean±s.d. Graphs were generated using Microsoft Excel (Microsoft Corp., Redmond, WA, USA). Statistical analysis was performed using SPSS 12.0 (SPSS Inc., Chicago, IL, USA). Differences between groups were determined using analysis of variance. Probability levels of 0.05 and 0.01 were considered statistically significant.
RESULTS
Mutation of npr-9 enhanced innate immunity in C. elegans
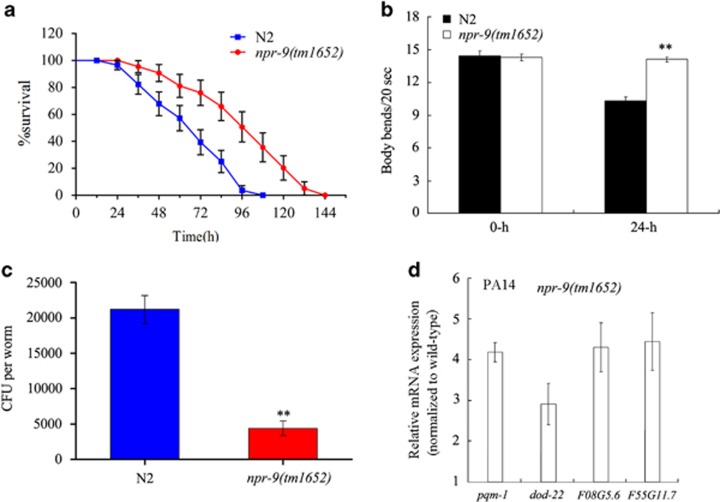
In C. elegans, npr-9(tm1652) is a loss-of-function mutant.23 npr-9(tm1652) showed a significant resistance to killing by the Pseudomonas aeruginosa strain PA14 (Figure 1a). In addition to effects on lifespan, we also analysed the changes in locomotive behaviour using body bend as an endpoint. Following PA14 infection for 24 h, we observed a significant decrease in the body bend frequency among wild-type N2 nematodes; however, there was no discernible impact of PA14 infection on the frequency of body bend among npr-9(tm1652) mutants (Figure 1b). We next quantified the PA14 CFU after transferring the infected nematodes to nonpathogenic E. coli OP50. Compared with the wild-type N2 animals, npr-9(tm1652) exhibited significantly decreased CFU (Figure 1c), implying that the loss-of-function mutation of npr-9 gene may suppress PA14 colonization in the body of nematodes.
Figure 1.
npr-9 mutants were resistant to P. aeruginosa infection. (a) Comparison of survival plots between wild-type N2 and npr-9 mutants exposed to P. aeruginosa PA14. A statistical comparison of the survival plots indicates that survival of the mutant animals was significantly different from that of wild-type N2 animals (P<0.0001). (b) Comparison of body bend between wild-type N2 and npr-9 mutants exposed to P. aeruginosa PA14 for 24 h. Bars represent mean±s.d. **P<0.01 vs N2. (c) Comparison of CFU between wild-type N2 and npr-9 mutants exposed to P. aeruginosa PA14. Bars represent the mean±s.d. **P<0.01 vs N2. (d) Quantitative real-time PCR analysis of expression patterns for immunity-related genes in npr-9 mutants exposed to P. aeruginosa PA14. Normalized expression is presented relative to wild-type expression; bars represent the mean±s.d.
To assess the relative fitness of npr-9 mutants, we compared the lifespan of npr-9(tm1652) mutants with that in wild-type N2 animals. The lifespan of npr-9(tm1652) mutant worms was equivalent to that in wild-type N2 animals (Supplementary Figure S1a). There were no differences in brood size, pumping rate or mean defecation cycle length between npr-9(tm1652) mutants and wild-type N2 animals (Supplementary Figures S1b–d). In addition, both wild-type N2 and npr-9(tm1652) mutants were inoculated with a similar number of OP50 E. coli (data not shown). Moreover, the npr-9(tm1652) mutants displayed a similar population growth of eggs, young larvae (L1–L3), L4 and adults to wild-type nematodes (Supplementary Figure S2). Taken together, these data suggest that the increased resistance of the npr-9(tm1652) mutants to P. aeruginosa PA14 infection is not attributable to diminished fitness, defective egg-laying behaviour, impaired feeding or impaired defecation.
To examine whether the resistance of npr-9 mutants was due to an enhanced immune function, we measured the expression of four immunity-related genes (pqm-1, dod-22, F08G5.6 and F55G11.7) with antimicrobial activity in both wild-type N2 and npr-9(tm1652) mutants exposed to P. aeruginosa PA14 for 12 h (to measure the infection-induced expression). PA14 exposure has been shown to induce the expression of these antimicrobial genes in wild-type N2 nematodes.14, 34 After exposure to P. aeruginosa PA14, the expression levels of these antimicrobial genes were higher in npr-9(tm1652) mutants compared with wild-type N2 animals (Figure 1d). On plates fed with OP50, the expression levels of these antimicrobial genes in npr-9(tm1652) mutants were similar to those in wild-type N2 (Supplementary Figure S3). These data suggest that the npr-9(tm1652) mutants have elevated immune competence, which may account for the enhanced resistance to both colonization and killing of P. aeruginosa PA14.
NPR-9 activity in AIB interneurons contributed to the innate immune response of nematodes to P. aeruginosa PA14
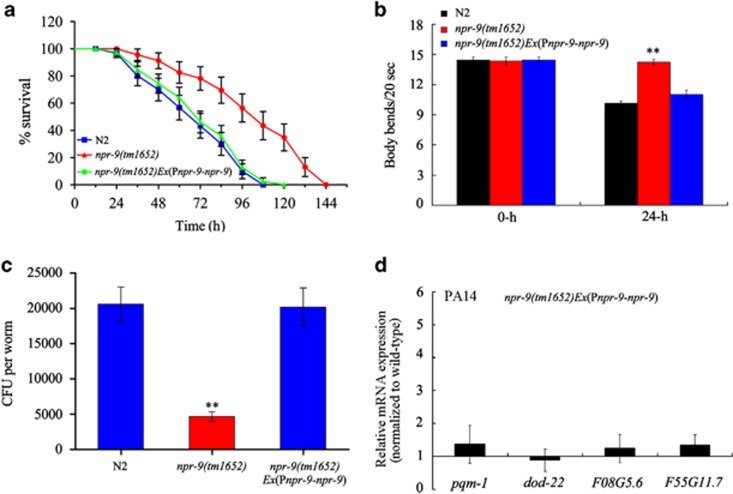
To further explore the function of NPR-9 in regulating innate immunity, we used the endogenous npr-9 promoter to drive npr-9 gene expression in npr-9(tm1652) mutants. After exposure to P. aeruginosa PA14, npr-9(tm1652) mutants expressing npr-9 showed similar survival and locomotion behaviour as wild-type N2 animals (Figures 2a and b, Supplementary Table S2). After infected nematodes were transferred to a lawn of nonpathogenic E. coli OP50, npr-9(tm1652) mutants expressing npr-9 gene exhibited a similar frequency of CFU as infected wild-type N2 animals (Figure 2c). Moreover, after exposure to P. aeruginosa PA14, expression patterns of the antimicrobial genes pqm-1, dod-22, F08G5.6 and F55G11.7 were similar between npr-9(tm1652) mutants expressing npr-9 and wild-type N2 animals (Figure 2d). Expression of these antimicrobial genes was also similar between npr-9(tm1652) mutants expressing npr-9 and wild-type N2 animals fed OP50 (Supplementary Figure S3). Because NPR-9 is expressed exclusively in AIB interneurons, NPR-9 activity in AIB interneurons may modulate the innate immune response of nematodes exposed to P. aeruginosa PA14.
Figure 2.
NPR-9 expression in AIB interneurons contributed to the innate immune response of nematodes exposed to P. aeruginosa PA14. (a) Effects of npr-9 expression in AIB interneurons on the survival curve for npr-9(tm1652) mutants exposed to P. aeruginosa PA14. A statistical comparison of the survival plots indicates that survival of npr-9(tm1652)Ex(Pnpr-9-npr-9) animals was not significantly different from that of the wild-type, N2 strain (P=0.9234). (b) Effects of npr-9 expression in AIB interneurons on body bend in npr-9(tm1652) mutants exposed to P. aeruginosa PA14. (c) Effects of npr-9 expression in AIB interneurons on CFU formation in npr-9(tm1652) mutants exposed to P. aeruginosa PA14. (d) Effects of npr-9 expression in AIB interneurons on expression patterns of several immunity-related genes in npr-9(tm1652) mutants exposed to P. aeruginosa PA14. Normalized expression is presented relative to wild-type expression. Bars represent mean±s.d. **P<0.01 vs N2. CFU, colony-forming unit.
Optogenetic manipulations of AIB interneurons affected the innate immune response in nematodes
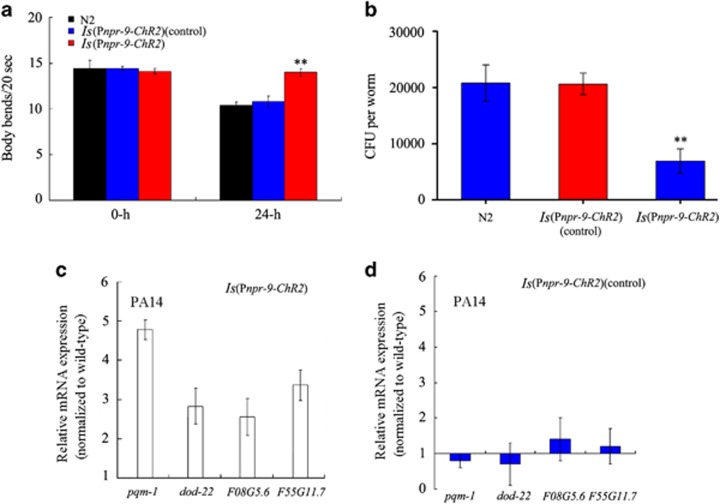
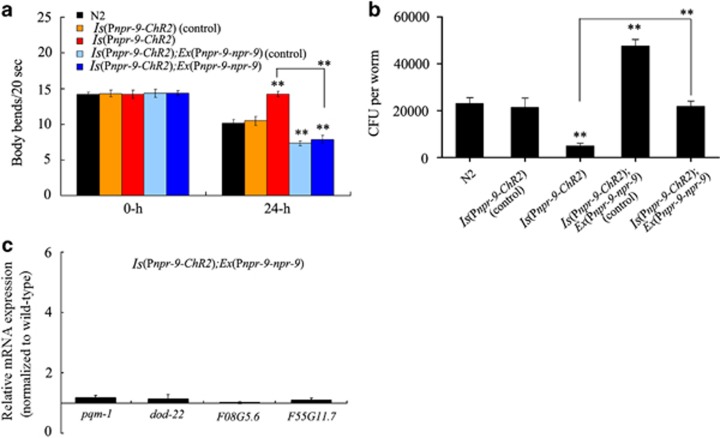
We found that ChR2-mediated activation of AIB interneurons enhanced the resistance of nematodes to P. aeruginosa PA14 (Figure 3a). After exposure to P. aeruginosa PA14, nematodes with ChR2-mediated AIB interneuron activation exhibited significantly increased locomotion behaviour compared with wild-type N2 animals (Figure 3a).
Figure 3.
The effect of Optogenetic manipulations of AIB interneurons on the regulation innate immunity. (a) Effects of ChR2-mediated activation of AIB interneurons on body bend of nematodes exposed to P. aeruginosa PA14 for 24-h. Bars represent mean±s.d. **P<0.01 vs N2. (b) Effects of ChR2-mediated activation of AIB interneurons on CFU of nematodes exposed to P. aeruginosa PA14. Bars represent mean±s.d. **P<0.01 vs N2. (c) Effects of ChR2-mediated activation of AIB interneurons on the expression patterns of immunity-related genes in nematodes exposed to P. aeruginosa PA14. Normalized expression is presented relative to wild-type expression. Control: without ATR. Bars represent mean±s.d. CFU, colony-forming unit.
After exposure to P. aeruginosa PA14, nematodes with ChR2-mediated AIB interneuron activation showed a significantly decreased frequency of CFU compared with wild-type N2 animals (Figure 3b). Considering the fact that AIB interneurons may modulate the starvation response in nematodes,35 we further examined the effect of ChR2-mediated AIB interneuron activation on PA14::GFP expression in the intestine of nematodes. ChR2-mediated AIB interneuron activation significantly inhibited the relative fluorescence intensity of PA14::GFP in the nematode intestine (Supplementary Figure S4). These data suggest that optogenetic manipulations of AIB interneurons may influence the colonization of P. aeruginosa PA14 in nematodes.
Interestingly, after exposure to P. aeruginosa PA14, the four immunity-related genes examined (that is, pqm-1, F08G5.6, and F55G11.7) showed increased expression in nematodes with ChR2-mediated AIB interneuron activation when compared with wild-type N2 animals (Figure 3c). Control transgenic nematodes without ATR showed a similar pattern of expression as it pertains to immunity-related genes (Figure 3c). Expression of these antimicrobial genes in nematodes with ChR2-mediated AIB interneuron activation, and control transgenic nematodes without ATR, was similar to that in wild-type N2 animals on plates fed with OP50 (Supplementary Figure S3). Together these observations suggest that activation of AIB interneurons may regulate the innate immune response of nematodes to P. aeruginosa PA14.
In C. elegans, nematodes with ChR2-mediated AIB interneuron activation showed similar locomotion behaviour to wild-type N2 animals (Figure 3a). Nematodes with ChR2-mediated AIB interneuron activation also displayed a similar brood size, pumping rate and mean defecation cycle length to that of wild-type N2 animals (Supplementary Figure S5).
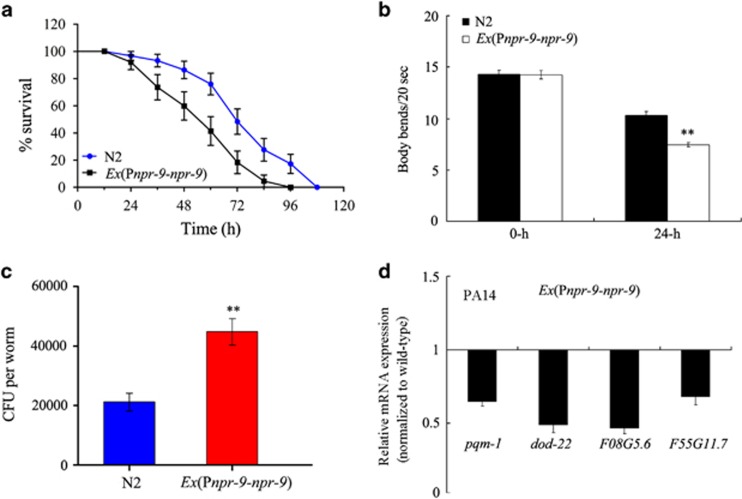
Nematodes overexpressing npr-9 were susceptible to infection by P. aeruginosa PA14
We next investigated the effects of npr-9 overexpression in AIB interneurons on innate immunity in nematodes. Overexpression of npr-9 in transgenic strains was confirmed by qRT-PCR (Supplementary Figure S6). After exposure to P. aeruginosa PA14, nematodes overexpressing npr-9 in AIB interneurons showed significantly decreased lifespan or locomotion behaviour compared with wild-type N2 (Figures 4a and b, and Supplementary Table S3). After exposure to P. aeruginosa PA14, overexpression of npr-9 in AIB interneurons caused a significant increase in CFU in nematodes compared with wild-type N2 animals (Figure 4c). Moreover, after exposure to P. aeruginosa PA14, nematodes overexpressing npr-9 in AIB interneurons exhibited decreased expression of several immunity-related genes (that is, pqm-1, dod-22, F08G5.6 and F55G11.7) compared with wild-type N2 animals (Figure 4d), suggesting that NPR-9 may negatively regulate the innate immune response in nematodes. Nematodes overexpressing npr-9 in AIB interneurons showed similar patterns of expression for immunity-related genes as wild-type N2 animals fed with OP50 (Supplementary Figure S3).
Figure 4.
The effects of npr-9 overexpression on innate immunity. (a) Effects of npr-9 overexpression on the survival curve of nematodes exposed to P. aeruginosa PA14. A statistical comparison of the survival plots indicates that survival of nematodes overexpressing NPR-9 was significantly different from that of the wild-type N2 strain (P<0.0001). (b) Effects of npr-9 overexpression on body bend in nematodes exposed to P. aeruginosa PA14. Bars represent the mean±s.d. **P<0.01 vs N2. (c) Effects of npr-9 overexpression on CFU in nematodes exposed to P. aeruginosa PA14. Bars represent the mean±s.d. **P<0.01 vs N2. (d) Effects of npr-9 overexpression on expression patterns immunity-related genes in nematodes exposed to P. aeruginosa PA14. Normalized expression is presented relative to wild-type expression. Bars represent the mean±s.d. CFU, colony-forming unit.
In C. elegans, overexpression of npr-9 in AIB interneurons did not significantly alter lifespan or locomotion behaviour (Figure 4b and Supplementary Figure S3a). In addition, overexpression of npr-9 in AIB interneurons also did not significantly influence brood size, pumping rate or mean defecation cycle length (Supplementary Figures S3b–d).
NPR-9 might regulate innate immunity by antagonizing the function of AIB interneurons
The above observations indicate that NPR-9 and AIB interneurons have opposing functions in regulating innate immunity. We next determined whether NPR-9 regulates innate immunity by antagonizing the function of AIB interneurons in nematodes. npr-9 was expressed in AIB interneurons in nematodes with ChR2-mediated activation in AIB interneurons. Interestingly, after exposure to P. aeruginosa PA14, expression of npr-9 in AIB interneurons significantly suppressed locomotion behaviour of nematodes with ChR2-mediated activation in AIB interneurons (Figure 5a), implying that NPR-9 may antagonize the functions of AIB interneurons to regulate the response of nematodes to P. aeruginosa PA14 infection.
Figure 5.
NPR-9 antagonized the function of AIB interneurons to regulate innate immunity. (a) Effects of npr-9 overexpression on body bend of nematodes with ChR2-mediated activation in AIB interneurons exposed to P. aeruginosa PA14. (b) Effects of npr-9 overexpression on CFU in nematodes with ChR2-mediated AIB interneuron activation exposed to P. aeruginosa PA14. (c) Effects of npr-9 overexpression on expression patterns for immunity-related genes in nematodes with ChR2-mediated activation of AIB interneurons exposed to P. aeruginosa PA14. Normalized expression is presented relative to wild-type expression. Bars represent the mean±s.d. **P<0.01 vs N2 if not specially indicated. CFU, colony-forming unit.
We further observed that, after exposure to P. aeruginosa PA14, expression of npr-9 in AIB interneurons significantly increased the frequency of CFU in nematodes with ChR2-mediated activation in AIB interneurons (Figure 5b). This observation suggests that NPR-9 may also antagonize the functions of AIB interneurons in the process of regulating colonization of P. aeruginosa PA14 in nematodes.
In addition, after exposure to P. aeruginosa PA14, expression of NPR-9 in AIB interneurons induced similar patterns of expression of immunity-related genes (pqm-1, dod-22, F08G5.6 and F55F11.7) in nematodes with ChR2-mediated activation of AIB interneurons as it did in wild-type N2 animals (Figure 5c). In contrast, after exposure to P. aeruginosa PA14, expression patterns of these immunity-related genes in the transgenic strain, Is(Pnpr-9-ChR2);Ex(Pnpr-9-npr-9), in the absence of optogenetic manipulation, were similar to those in nematodes overexpressing npr-9 (data not shown). Transgenic strains of Ex(Pnpr-9-npr-9), exhibiting AIB interneuron activation, had similar patterns of expression for immunity-related genes as wild-type N2 animals fed with OP50 (Supplementary Figure S3). Taken together, our data are consistent with the hypothesis that NPR-9 may regulate the innate immune response in nematodes exposed to P. aeruginosa PA14 in part by antagonizing the functions of AIB interneurons.
NPR-9 influenced the innate immune response in nematodes expressing pkc-1(gf) in AIB interneurons upon infection with P. aeruginosa PA14
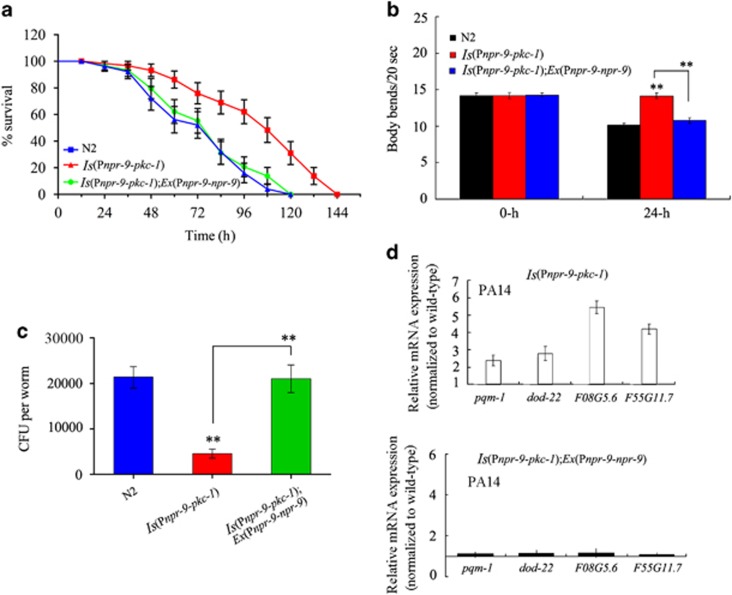
In C. elegans, synaptic transmission can be potentiated by expression of the active protein kinase C homologue (pkc-1(gf)).36, 37 Activation of AIB interneurons, by expressing a constitutively active protein kinase C homologue (pkc-1(gf)), in nematodes resulted in a number of functional differences. Expression of pkc-1(gf) in AIB interneurons caused nematodes to become resistant to infection by P. aeruginosa PA14 (Figures 6a and b and Supplementary Table S4). After exposure to P. aeruginosa PA14, both the lifespan and body bend frequency in nematodes expressing pkc-1(gf) in AIB interneurons was significantly increased when compared with that of wild-type N2 animals (Figures 6a and b and Supplementary Table S4). After exposure to P. aeruginosa PA14, nematodes expressing pkc-1(gf) in AIB interneurons also exhibited significantly reduced CFU formation and PA14::GFP expression in the intestine compared with that of wild-type N2 animals (Figure 6c and Supplementary Figure S4). Moreover, after exposure to P. aeruginosa PA14, we observed elevated expression of several immunity-related genes (pqm-1, dod-22, F08G5.6 and F55G11.7) in nematodes expressing pkc-1(gf) in AIB interneurons compared with that in wild-type N2 animals (Figure 6d). Nematodes expressing pkc-1(gf) in AIB interneurons showed similar patterns of expression for these immunity-related genes as wild-type N2 animals fed with OP50 (Supplementary Figure S3). In C. elegans, expression of pkc-1(gf) in AIB interneurons did not significantly alter lifespan, brood size, pumping rate and mean defecation cycle length (Supplementary Figure S8).
Figure 6.
NPR-9 inhibited the effects of a gain-of-function protein kinase C mutation, (pkc-1(gf)), on innate immunity in AIB interneurons. (a) Effects of npr-9 overexpression on the survival curve of nematodes exposed to P. aeruginosa PA14, expressing gain-of-function protein kinase C (pkc-1(gf)) mutations in their AIB interneurons. A statistical comparison of the survival plots indicates that survival of nematodes expressing gain-of-function, protein kinase C (pkc-1(gf)) mutations in AIB interneurons was significantly different from that of wild-type N2 animals (P<0.0001), whereas the survival plots indicate that the survival of Ex(Pnpr-9-pkc-1);Ex(Pnpr-9-npr-9) nematodes was not significantly different from that of wild-type N2 animals (P=0.945). (b) Effects of npr-9 overexpression on body bend in nematodes exposed to P. aeruginosa PA14 and expressing gain-of-function protein kinase C (pkc-1(gf)) mutations in their AIB interneurons. Bars represent the mean±s.d. **P<0.01 vs N2 (if not specially indicated). (c) Effects of npr-9 overexpression on CFU of nematodes expressing gain-of-function protein kinase C (pkc-1(gf)) mutations in AIB interneurons in animals exposed to P. aeruginosa PA14. Bars represent the mean±s.d. **P<0.01 vs N2 if not specially indicated. (d) Effects of npr-9 overexpression on the expression patterns of immunity-related genes in nematodes expressing gain-of-function protein kinase C (pkc-1(gf)) mutations in AIB interneurons and exposed to P. aeruginosa PA14. Normalized expression is presented relative to wild-type expression. Bars represent the mean±s.d. CFU, colony-forming unit.
Again, we examined the effects of NPR-9 on the innate immune response of nematodes with synaptic activation in AIB interneurons by expressing pkc-1(gf) in the context of P. aeruginosa PA14 infection. After exposure to P. aeruginosa PA14, expression of npr-9 noticeably inhibited lifespan or locomotion behaviour of nematodes expressing pkc-1(gf) in AIB interneurons (Figures 6a and b and Supplementary Table S4). In addition, after exposure to P. aeruginosa PA14, expression of NPR-9 significantly increased the frequency of CFU in nematodes expressing pkc-1(gf) in AIB interneurons (Figure 6c). Expression of npr-9 resulted in similar patterns of expression for several immunity-related genes (that is, pqm-1, dod-22, F08G5.6 and F55G11.7) in nematodes expressing pkc-1(gf) in AIB interneurons compared with wild-type N2 animals (Figure 6d). The transgenic strain, Is(Pnpr-9-pkc-1);Ex(Pnpr-9-npr-9), also exhibited a similar patterns of expression for these immunity-related genes as wild-type N2 animals fed with OP50 (Supplementary Figure S3). These results provide further evidence for an antagonistic role of NPR-9 in AIB interneurons as it pertains to the regulation of colonization of P. aeruginosa PA14 and in the nematode immune response.
The effects of human GRPR on the immune response of nematodes infected with of P. aeruginosa PA14
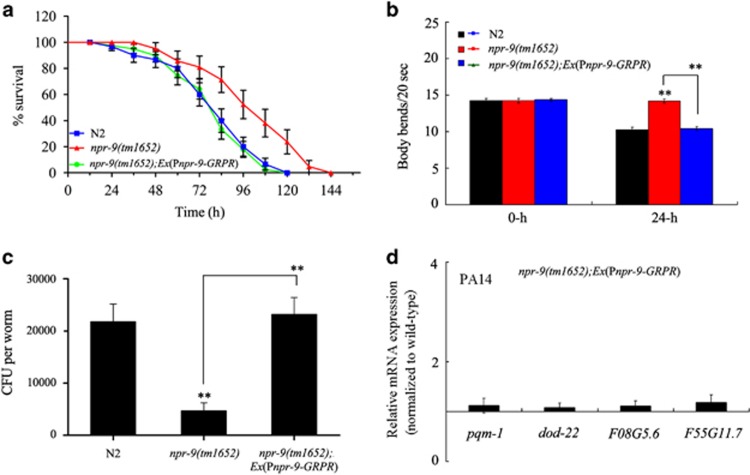
The human homologue of NPR-9, GRPR, which has been shown to regulate numerous biological processes.22, 23 To examine potentially conserved functions between C. elegans NPR-9 and its human homologue, human GRPR was expressed in AIB interneurons in npr-9(tm1652) mutant nematodes. After exposure to P. aeruginosa PA14, npr-9(tm1652) mutants expressing human GRPR exhibited a similar lifespan and body bend frequency as wild-type N2 animals (Figures 7a and b and Supplementary Table S5). Moreover, after exposure to P. aeruginosa PA14, npr-9(tm1652) mutants, expressing the human GRPR gene, showed a similar frequency of CFU as wild-type N2 animals (Figure 7c). Furthermore, after exposure to P. aeruginosa PA14, npr-9(tm1652) mutants expressing human GRPR gene had a similar pattern of as patterns of several immunity-related genes (pqm-1, dod-22, K08G5.6 and F55G11.7) observed among wild-type N2 animals (Figure 7d). npr-9(tm1652) mutants expressing the human GRPR gene showed similar patterns of expression for immunity-related genes as wild-type N2 animals fed OP50 (Supplementary Figure S3). These results suggest that the function of C. elegans NPR-9 in regulating innate immune response is at least, in part, conserved with the human homologue, GRPR.
Figure 7.
Effects of human GRPR on nematode innate immunity. (a) Effects of expressing human GRPR in AIB interneurons on the survival curve of npr-9(tm1652) mutant nematodes exposed to P. aeruginosa PA14. A statistical comparison of the survival plots indicates that survival of npr-9(tm1652) mutant nematodes expressing human GRPR in AIB interneurons was not significantly different from that of wild-type N2 animals (P=0.948). (b) Effects of expressing human GRPR in AIB interneurons on body bend in npr-9(tm1652) mutant nematodes exposed to P. aeruginosa PA14. (c) Effects of expressing human GRPR in AIB interneurons on CFU of nematodes exposed to P. aeruginosa PA14. (d) Effects of expressing human GRPR in AIB interneurons on expression patterns of immunity-related genes in nematodes exposed to P. aeruginosa PA14. Normalized expression is presented relative to wild-type expression. Bars represent the mean±s.d. **P<0.01 vs N2 if not specially indicated. CFU, colony-forming unit; GRPR, gastrin-releasing peptide receptor.
Genetic interaction of npr-9 with daf-16, pmk-1 or dbl-1 in regulating innate immunity
In C. elegans, the daf-16 gene encodes a FOXO transcription factor in the insulin signalling pathway, the pmk-1 gene encodes a MAPK in the p38 MAPK signalling pathway, and the dbl-1 gene encodes a TGF-β ligand in the TGF-β signalling pathway. Genetic interaction assays indicate that, after exposure to P. aeruginosa PA14, RNAi knockdown of daf-16 or pmk-1 resulted in reduced lifespan in npr-9(tm1652) mutants; however, RNAi knockdown of the dbl-1 gene did not significantly affect the lifespan in npr-9(tm1652) mutants (Supplementary Figure S9).
DISCUSSION
In the present study, we provide several lines of evidence supporting a crucial role of NPR-9 in regulating innate immunity in C. elegans. First, npr-9(tm1652) mutants showed resistance to infection by P. aeruginosa PA14, as indicated by increased lifespan and locomotion behaviour (Figures 1a and b). Second, the expression levels of several immunity-related genes were higher in npr-9(tm1652) mutants exposed to P. aeruginosa PA14 than in wild-type N2 nematodes exposed to P. aeruginosa PA14 (Figure 1d). In contrast to the increased resistance phenotype of npr-9(tm1652) mutants, nematodes overexpressing npr-9 exhibited increased susceptibility to P. aeruginosa PA14 infection compared with wild-type animals, as indicated by their decreased lifespan and locomotion behaviour (Figures 4a and b and Supplementary Table S3) and the decreased expression of several immunity-related genes observed in P. aeruginosa PA14-exposed nematodes overexpressing NPR-9 (compared with those in P. aeruginosa PA14-exposed wild-type N2 animals; Figure 4d). Third, NPR-9 regulated the colonization of P. aeruginosa PA14 in the intestine of nematodes. The npr-9(tm1652) mutants had a decreased frequency of CFU compared with wild-type N2 animals (Figure 1c). In contrast, nematodes overexpressing NPR-9 showed an increased frequency of CFU compared with wild-type N2 animals (Figure 4c). Therefore, the GPCR, NPR-9, is required for the control of both the innate immune response and P. aeruginosa colonization in nematodes. Previous studies have suggested that NPR-9 is involved in the control of multiple biological processes including fat storage, local search behaviour and octopamine inhibition.23, 38 Our study provides support for the complex biological functions of GRPRs.
In nematodes, a subset of sensory neurons has been identified that have important roles in the control of innate immunity. For example, the nematode strains lacking AQR, PQR and URX sensory neurons showed increased survival in the presence of P. aeruginosa, indicating that AQR, PQR and URX neurons may suppress innate immunity.18 In the current study, our data suggest that interneurons, such as AIB interneurons, can also participate in the regulation of innate immunity in nematodes. ChR2-mediated activation of AIB interneurons induced the resistance of nematodes to P. aeruginosa PA14 infection (Figure 3a). In addition, after exposure to P. aeruginosa PA14, several immunity-related genes were induced in nematodes with ChR2-mediated AIB interneuron activation (Figure 3c). Moreover, nematodes with ChR2-mediated AIB interneuron activation showed a decreased frequency of CFU (Figure 3b), indicating that both sensory neurons and interneurons can participate in the control of innate immunity in nematodes. Because the previously identified sensory neurons, including AQR, PQR and URX, cannot form synaptic connections with AIB interneurons,39 the detailed sensory inputs for AIB interneurons during the regulation of innate immunity in nematodes remain to be identified. nlp-5 and nlp-6 are prime candidate genes as they encode neuropeptides that are released and are capable of binding to NPR-9 in AIB interneurons in nematodes,23, 40 which may provide some clues for the elucidation of the related neuronal circuit. Both nlp-5 and nlp-6 are expressed in ASI sensory neurons, and ASI sensory neurons can provide sensory inputs for AIB interneurons in nematodes.23, 39
Our data further suggest that NPR-9 can participate in neuronal circuit(s) that integrate(s) the related input information to coordinate an appropriate immune response. After exposure to P. aeruginosa PA14, npr-9(tm1652) mutants expressing npr-9 in AIB interneurons showed a similar survival curve (Figure 2a and Supplementary Table S2), locomotion behaviour (Figure 2b), frequency of CFU (Figure 2c) and expression pattern for several immunity-related genes (Figure 2d) as wild-type N2 animals, suggesting that NPR-9 activity in AIB interneurons may contribute to the control of the innate immune response in nematodes exposed to P. aeruginosa PA14.
With respect to the underlying mechanisms by which NPR-9 regulates innate immunity, we hypothesize that NPR-9 may regulate the innate immune response by antagonizing the function of AIB interneurons. Expression of NPR-9 in AIB interneurons of nematodes exposed to P. aeruginosa PA14 suppressed lifespan, locomotion behaviour (Figures 5a and 6a, b and Supplementary Table S4), the frequency of CFU (Figures 5b and 6c) and expression of several immunity-related genes (Figures 5c and 6d) in the context of either ChR2-mediated activation in AIB interneurons or pkc-1(gf) expression in AIB interneurons. These findings suggest that NPR-9 may antagonize the functions of AIB interneurons to regulate the innate immune response of nematodes upon infection by P. aeruginosa PA14.
In this study, we further showed that GRPR, the human homologue of C. elegans NPR-9,22, 23 can largely mimic the function of NPR-9 to regulate innate immunity in nematodes. After exposure to P. aeruginosa PA14, npr-9(tm1652) mutants expressing the human GRPR gene showed a similar lifespan (Figure 7a and Supplementary Table S5), locomotion behaviour (Figure 7b), frequency of CFU (Figure 7c) and induced expression of immunity-related genes (Figure 7d) as wild-type N2 animals. In humans, mutations of GRPR are associated with the development of several diseases, including autism,41 suggesting a 'double-edged sword' function for GRPR/NPR-9 during development. On one hand, mutations of GRPR/NPR-9 may contribute to the development of certain diseases; however, GRPR/NPR-9 mutation may also have beneficial effects in humans or animals by conferring resistance to pathogenic infection.
In C. elegans, the insulin signalling pathway, the p38 MAPK signalling pathway, and the TGF-β signalling pathway have important roles in regulating innate immunity.3, 8, 9 Genetic interaction assays demonstrated that NPR-9 may genetically act through the functions of insulin and p38 MAPK signalling pathways to regulate innate immunity. One possibility is that NPR-9 may regulate innate immunity through insulin signalling or other unknown signalling mechanisms in neurons. Another possibility is that NPR-9 may regulate innate immunity through signalling pathways, such as the p38 MAPK signalling pathway, in the intestine.
In conclusion, our data suggest that the GRPR homologue, NPR-9, can regulate the immune response of nematodes to P. aeruginosa PA14 infection. NPR-9 expression, specifically in AIB interneurons, contributed to the control of innate immunity. In AIB interneurons, NPR-9 may antagonize the functions of AIB interneurons to regulate the immune response of nematodes infected with P. aeruginosa PA14. Our study implies that GRPR/NPR-9 is a key regulator of complex biological functions during development in both animals and humans.
Acknowledgments
Several nematode strains used in this study were provided by the CGC, which is funded by NIH Office of Research Infrastructure Program (P40 OD010440).
Footnotes
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website (http://www.nature.com/cmi)
The authors declare no conflict of interest.
Supplementary Material
References
- Kurz CL, Ewbank JJ. Caenorabditis elegans: an emerging genetic model for the study of innate immunity. Nat Rev Genet 2003; 4: 380–390. [DOI] [PubMed] [Google Scholar]
- Sifri CD, Begun J, Ausubel FM. The worm has turned-microbial virulence modeled in Caenorhabditis elegans. Trends Microbiol 2005; 13: 119–127. [DOI] [PubMed] [Google Scholar]
- Kim DH, Feinbaum R, Alloing G, Emerson EF, Garsin DA, Inoue H et al. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 2002; 297: 623–626. [DOI] [PubMed] [Google Scholar]
- Liberati NT, Fitzgerald KA, Kim DH, Feinbaum R, Golenbock DT, Ausubel FM. Requirement for a conserved Toll/interleukin-1 resistance domain protein in the Caenorhabditis elegans immune response. Proc Natl Acad Sci USA 2004; 101: 6593–6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillault C, Pujol N, Reboul J, Sabatier L, Guichou J, Kohara Y et al. TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nat Immunol 2004; 5: 488–494. [DOI] [PubMed] [Google Scholar]
- Liang B, Moussaif M, Kuan C, Gargus JJ, Sze JY. Serotonin targets the DAF-16/FOXO signaling pathway to modulate stress responses. Cell Metab 2006; 4: 429–440. [DOI] [PubMed] [Google Scholar]
- Irazoqui JE, Ng A, Xavier RJ, Ausubel FM. Role of β–catenin and HOX transcription factors in Caenorhabditis elegans and mammalian host epithelial-pathogen interactions. Proc Natl Acad Sci USA 2008; 105: 17469–17474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EA, Kawli T, Tan M. Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans. PLoS Pathog 2008; 4: e1000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge FA, Gravato-Nobre MJ, Hodgkin J. Signal transduction pathways that function in both development and innate immunity. Dev Dyn 2010; 239: 1330–1336. [DOI] [PubMed] [Google Scholar]
- Richardson CE, Kooistra T, Kim DH. An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature 2010; 463: 1092–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AF, Gumienny TL, Gleason RJ, Wang H, Padgett RW. Regulation of genes affecting body size and innate immunity by the DBL-1/MBP-like pathway in Caenorhabditis elegans. BMC. Dev Biol 2010; 10: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irazoqui JE, Urbach JM, Ausubel FM. Evolution of host innate defense: insights from C. elegans and primitive invertebrates. Nat Rev Immunol 2010; 10: 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukkila-Worley R, Ausubel FM. Immune defense mechanisms in the Caenorhabditis elegans intestinl epithelium. Curr Opin Immunol 2012; 24: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawli T, Tan M. Neuroendocrine signals modulate the innate immunity of Caenorhabditis elegans through insulin signaling. Nat Immunol 2008; 9: 1415–1424. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang Y. Neural-immune communication in Caenorhabditis elegans. Cell Host Microbiol 2009; 5: 425–429. [DOI] [PubMed] [Google Scholar]
- Anyanful A, Easley KA, Benian GM, Kalman D. Conditioning protects C. elegans from the lethal effects of enteropathogenic E. coli through activation of genes that regulate lifespan and innate immunity. Cell Host Microbe 2009; 5: 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivers RP, Kooistra T, Chu SW, Pagano DJ, Kim DH. Tissue-specific activities of an immunity signaling module regulate physiological responses to pathogenic and nutritional bacteria in C. elegans. Cell Host Microbe 2009; 6: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styer KL, Singh V, Macosko E, Steele SE, Bargmann CI, Aballay A. Innate immunity in Caenorhabditis elegans is regulated by neurons expressing NPR-1/GPCR. Science 2008; 322: 460–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Aballay A. Endoplasmic reticulum stress pathway required for immune homeostasis is neurally controlled by Arrestin-1. J Biol Chem 2012; 287: 33191–33197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JR, Kim DH, Ausubel FM. The G protein-coupled receptor FSHR-1 is required for the Caenorhabditis elegans innate immunity response. Proc Natl Acad Sci USA 2009; 106: 2782–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Singh V, Kajino-Sakamoto R, Aballay A. Neuronal GPCR controls innate immunity by regulating noncanonical unfold protein response genes. Science 2011; 332: 729–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R, Gundlach AL, Kofler B. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol Ther 2007; 115: 177–207. [DOI] [PubMed] [Google Scholar]
- Bendena WG, Boudreau JR, Papanicolaou T, Maltby M, Tobe SS, Chin-Sang ID A. Caenorhabditis elegans allatostatin/galanin-like receptor NPR-9 inhibits local search behavior in response to feeding cues. Proc Natl Acad Sci USA 2008; 105: 1339–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics 1974; 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q-L, Qu Y-Y, Li X, Wang D-Y. Chromium exhibits adverse effects at environmental relevant concentrations in chronic toxicity assay system of nematode Caenorhabditis elegans. Chemosphere 2012; 87: 1281–1287. [DOI] [PubMed] [Google Scholar]
- Nouara A, Wu Q-L, Li Y-X, Tang M, Wang H-F, Zhao Y-L et al. Carboxylic acid functionalization prevents the translocation of multi-walled carbon nanotubes at predicted environmental relevant concentrations into targeted organs of nematode Caenorhabditis elegans. Nanoscale 2013; 5: 6088–6096. [DOI] [PubMed] [Google Scholar]
- Zhao Y-L, Wu Q-L, Tang M, Wang D-Y. The in vivo underlying mechanism for recovery response formation in nano-titanium dioxide exposed Caenorhabditis elegans after transfer to the normal condition. Nanomedicine 2014; 10: 89–98. [DOI] [PubMed] [Google Scholar]
- Diaz SA, Mooring EQ, Rens EG, Restif O. Association with pathogenic bacteria affects life-history traits and population growth in Caenorhabditis elegans. Ecol Evolut 2015; 5: 1653–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D-Y, Cao M, Dinh J, Dong Y-Q. Methods for creating mutations in C. elegans that extend lifespan. Methods Mol Biol 2013; 1048: 65–75. [DOI] [PubMed] [Google Scholar]
- Mello C, Fire A. DNA transformation. Methods Cell Biol 1995; 48: 451–482. [PubMed] [Google Scholar]
- Kramer RH, Mourot A, Adesnik H. Optogenetic pharmacology for control of native neuronal signaling proteins. Nat Neurosci 2013; 16: 816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocabas A, Shen C, Guo ZV, Ramanathan S. Controlling interneuron activity in Caenorhabditis elegans to evoke chemotactic behaviour. Nature 2012; 490: 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath RK, Martinez-Campos M, Zipperlen P, Fraser AG, Ahringer J. Effectiveness of specific RNA-mediated interference through ingested double stranded RNA in C. elegans. Genome Biol 2001; 2: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper S, McBride SJ, Lackford B, Freedman JH, Schwartz DA. Specificity and complexity of the Caenorhabditis elegans innate immune response. Mol Cell Biol 2007; 27: 5544–5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, Avery L. Systemic regulation of starvation response in Caenorhabditis elegans. Genes Dev 2009; 23: 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth D, Ch’ng Q, Dybbs M, Tavazoie M, Kennedy S, Wang D et al. Systematic analysis of genes required for synapse structure and function. Nature 2005; 436: 510–517. [DOI] [PubMed] [Google Scholar]
- Sieburth D, Madison JM, Kaplan JM. PKC-1 regulates secretion of neuropeptides. Nat Neurosci 2007; 10: 49–57. [DOI] [PubMed] [Google Scholar]
- Mills H, Wragg R, Hapiak V, Castelletto M, Zahratka J, Harris G et al. Monoamines and neuropeptides interact to inhibit aversive behaviour in Caenorhabditis elegans. EMBO J 2012; 31: 667–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 1986; 314: 1–340. [DOI] [PubMed] [Google Scholar]
- Nathoo AN, Moeller RA, Westlund BA, Hart AC. Identification of neuropeptide-like protein gene families in Caenorhabditis elegans and other species. Proc Natl Acad Sci USA 2001; 98: 14000–14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa-Brush Y, Powell JF, Bolton P, Miller AP, Francis F, Willard HF et al. Autism and multiple exostoses associated with an X;8 translocation occurring within the GRPR gene and 3’ to the SDC2 gene. Hum Mol Genet 1997; 6: 1241–1250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.