The increasing incidence of type 1 diabetes mellitus (T1DM) in children represents one of the major worldwide health concerns. It is widely thought that T1DM is caused by the autoimmune destruction of insulin-producing β cells in the pancreas due to an aberrant T-cell immune response. To date, there are no effective therapeutic strategies to prevent T1DM, as its clinical symptoms become apparent only when most β cells have already been destroyed. Furthermore, clinical trials for the prevention of T1DM in children at risk have all been unsuccessful to date. Intriguingly, recent studies have implicated alterations in the gut microbial flora (that is, microbiota) as a potential etiological factor for T1DM, raising the possibility that interfering with the microbes populating our body could represent a promising approach to prevent or manage T1DM. New evidence strongly supporting this hypothesis comes from a milestone study by Mariño et al.,1 which shows that a diet capable of modulating the activity of the gut microbiota can indeed inhibit the disease onset in a mouse model of T1DM. Overall, these findings pave the way for the development of food-based dietary approaches for preventing high-risk children from developing T1DM.
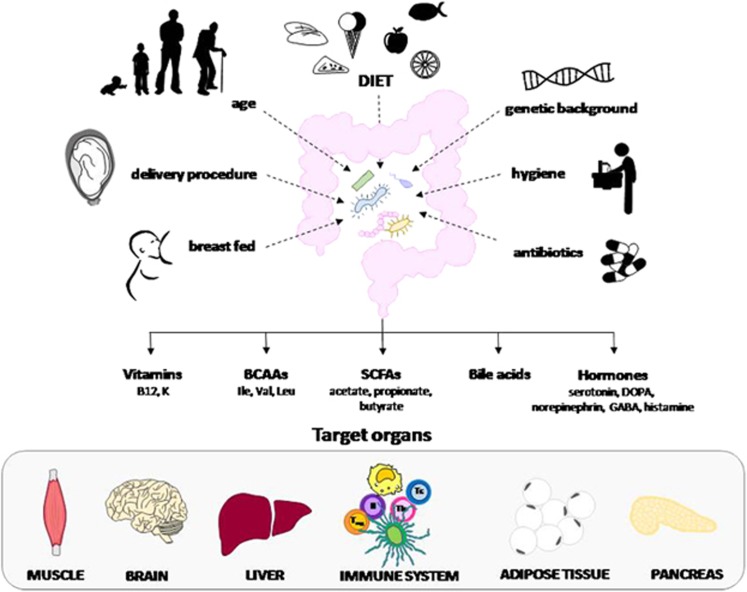
T1DM is a multifactorial disease that is triggered by environmental factors acting on a complex, predisposing genetic background that involves the cooperation of multiple genes, among which human leukocyte antigen (HLA) alleles seem to play a major role. In the past two decades, the incidence of T1DM has been steadily increasing at a rate of ~2–4% per year worldwide. More recently, an increase in the incidence of T1DM has been reported in children younger than 5 years in both Europe and the United States, a trend that correlates with the increasing number of individuals lacking the predisposing HLA alleles.2, 3 In this scenario, the observation that migrants tend to acquire the same risk of developing T1DM as that of long-term residents has led to the hypothesis that the environment and lifestyle are key factors for T1DM development.4 Thus, in recent years, there has been an ongoing research effort aimed at determining which environmental factors affecting an individual’s prenatal life and early life are indeed involved in the pathogenesis of T1DM. Intriguingly, a number of maternal and hygiene factors, as well as antibiotic treatment, have been shown to influence the composition and activity of the gut microbiota in infants and children, suggesting that T1DM development may arise due to an alteration of the intestinal flora (Figure 1).
Figure 1.
The gut microbiota is influenced by several factors, including maternal nutrition, type of delivery, age, genetic background, hygienic conditions, antibiotic treatments and precocious diet factors. It influences the mucosal immune response and the integrity of the mucosal barrier, with effects on systemic immunity and inflammation. This is partly due to the production of signaling metabolites targeting endocrine, neuronal and immune cells of the gut and liver and influences of lipid and glucose metabolism. BCAAs, branched-chain amino acids; SCFAs, short-chain fatty acids.
The gut microbiota represents a complex, symbiotic ecological community that is involved in several physiological activities that are shared with the host. Moreover, it can influence lipid and glucose metabolism and the mucosal immune response, as well as the integrity of the mucosal barrier, thereby affecting systemic immunity and inflammation. This is partly due to the production of signaling metabolites that target the endocrine, neuronal and immune cells of the gut and liver. These may include short-chain fatty acids (SCFAs), vitamins, amino acids, bile acids and hormones.5, 6 Although several recent studies have proposed that alterations in commensal microbiota (that is, dysbiosis) can modulate the risk of T1DM, they have failed to detect the mechanisms involved.
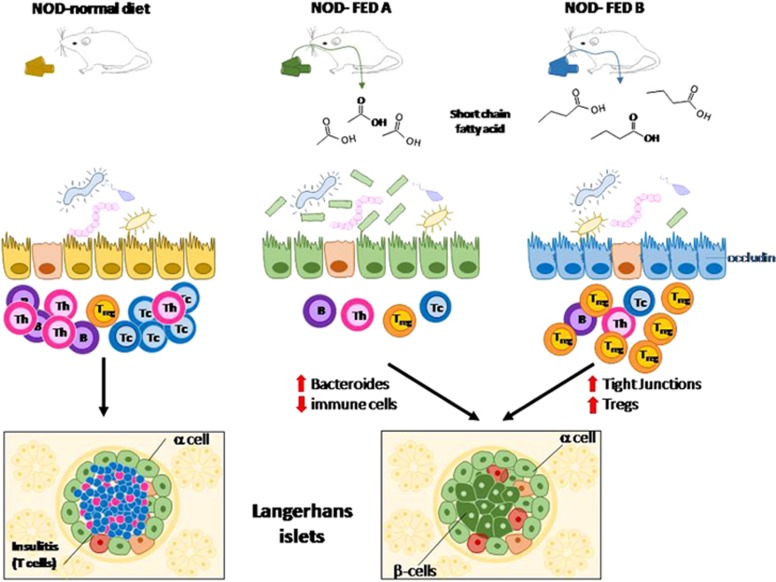
In a paper featured in the May issue of Nature Immunology, Mariño and co-workers demonstrate that this can be achieved through a diet-based approach in non-obese diabetic (NOD) mice, a common animal model of T1DM. These mice develop T1DM at 10–20 weeks of age, with a higher incidence in females (60–80%) than in males (20–30%), and disease onset is preceded by abnormal lymphocyte infiltration of the pancreatic islets (that is, insulitis). The article in question shows that feeding these mice diets containing high amounts of two SCFAs, namely acetate and butyrate, significantly inhibits disease onset, with a maximum effect obtained when the two supplements are given simultaneously.
The study by Mariño et al. begins with the observation that NOD mice that are deficient in a protein (that, MyD88) involved in sensing microbes are protected from T1DM development and display higher levels of acetate and butyrate in the blood compared to conventional NOD mice. Interestingly, these levels tend to be higher in males, which is in good agreement with the lower predisposition of male NOD mice for developing T1DM compared to females. To directly test whether diet supplementation with acetate and/or butyrate could boost acetate and butyrate-mediated protection from T1DM, the NOD mice were fed a diet enriched with 15% acetylated (FED-A) or butylated (FED-B) high-amylose maize starch, which, upon bacterial fermentation, releases high amounts of acetate or butyrate into the gut, respectively (Figure 2). The results show that both diets can increase the levels of acetate or butyrate in the feces, cecal content and blood while protecting mice from developing insulitis and T1DM. Intriguingly, mice that are fed a diet containing both supplements are completely protected against diabetes, suggesting that these compounds act partly through different mechanisms. In this regard, the acetate-enriched diet seems to act predominantly by inhibiting the expansion of the autoimmune effector T cells. This effect might be ascribed to the decreased proliferation or increased apoptosis of autoimmune T cells.7, 8, 9 Furthermore, these mice are characterized by a reduced number of B cells that are no longer able to work as antigen-presenting cells, which further enhances autoimmune T-cell expansion. Finally, FED-A mice show a significant increase of the Bacteroides genus in the gut microbiota, which, once transferred into germ-free NOD mice, protects these mice against T1DM. In contrast, the aforementioned effects are minimal or absent from FED-B mice, which instead display an expansion of regulatory T cells (Tregs), and this is undetectable in FED-A mice. Interestingly, the butyrate-enriched diet seems to have a higher impact than the acetated-enriched one in protecting the integrity of the mucosal barrier, as judged by its ability to increase the expression of occludin, a membrane protein located at the tight junctions in the colon.
Figure 2.
Non-obese diabetic (NOD) mice are an animal model of T1DM. The disease is influenced by the gut microbiota. Disease onset is preceded by the development of inflammatory infiltration, which is rich with T cells, in the pancreatic islets (insulitis). Diets that release high amounts of two SCFAs, acetate (FED-A) or butyrate (FED-B), in the gut substantially prevent the onset of disease in NOD mice. The acetate-enriched diet predominantly acts by decreasing activation of the autoimmune effector T cells, and it is associated with an increase in Bacteroides. The above effects are minimal or absent from the FED-B mice. By contrast, these mice show an expansion of Treg cells, which protect the integrity of the mucosal barrier, by increasing the expression of occludin in tight junctions in the colon.
Previous work has proposed that the microbiota may influence the immune response by SCFAs produced through the fermentation of diet fibers. These may act through at least two mechanisms: (1) binding to metabolite-sensing G-protein-coupled receptors expressed by immune cells, which are responsible for delivering anti-inflammatory signals, and (2) inhibiting histone deacetylase activity, which modulates gene transcription. In this report, Mariño et al. declare that both mechanisms may be involved in their animal model. The authors also submit that the gut microbiota may be particularly relevant for T1DM because of the proximity of the pancreas and draining peripheral lymph nodes to the gut. Accordingly, dietary metabolites seem to be particularly effective in treating gastrointestinal disorders such as colitis, food allergies and colon cancer. On the other hand, it is worth noting that dysbiosis has been implicated in the etiology of other autoimmune diseases that attack organs that are by no means close to the gut. For instance, alteration of the gut microbiota can influence both T-helper cell polarization and autoimmune attacks in experimental autoimmune encephalomyelitis, a model of multiple sclerosis in which the autoimmune targets are represented by the myelin sheaths of the central nervous system. In this and other settings, the microbiota can act by activating T cells, influencing the local antigen-presenting cells and releasing immunoreactive metabolites.10
So what is the impact of this study on diabetes? The economic burden of T1DM has been constantly increasing due to an ever-increasing number of children and adults suffering from this disease. As a result, large trials focusing on environmental factors that protect against islet autoimmunity and T1DM should generate invaluable information to prevent or delay disease onset. Several experts suggest that the primary prevention of T1DM should target the general childhood population using vaccines or in some way altering the gut microbiota. The latter could be achieved through fecal microbiota transplantation from tested donors or delivery of bacterial consortia with a known genetic content, metabolic output and antibiotic susceptibility. An alternative approach may be represented by the oral administration of well-characterized probiotic strains that can be readily produced and easily monitored. However, the efficacy of these probiotic strains still remains to be assessed due to the complexity of the interplay among the dominant phyla.11 In this scenario, the paper by Mariño et al. proposes a much simpler approach that is based on the introduction of medicinal foods in the diet of children at high risk of developing T1DM, as a way of hampering T-cell-dependent destruction of insulin-producing β cells. This strategy does not come as a total surprise given that a diet-based approach has represented, for quite some time, the cornerstone treatment for celiac disease, an autoimmune disease frequently associated with T1DM. Thus, in theory, food industries should already have all of the requirements and expertise to produce modified foods that are able to alter the gut microbiota, thereby preventing the pathogenesis of T1DM in childhood.
Although the findings by Mariño et al. open exciting new perspectives in terms of T1DM prevention, further data analysis and novel markers are clearly needed to determine whether a diet-based approach can be effective during the early life when the gut microbiota heavily influences gut immune homeostasis.12, 13 In future years, regardless of its beneficial effects against T1DM, medicinal food has the potential to become a revolutionary drug-free approach with which to modulate the immune systems of children for whom drug-safety considerations are mandatory. So, guys, are you ready for healthy meals?
Footnotes
The authors declare no conflict of interest.
References
- Mariño E, Richards JL, McLeod KH, Stanley D, Yap YA, Knight J et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol 2017; 18: 552–562. [DOI] [PubMed] [Google Scholar]
- Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, EURODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet 2009; 373: 2027–2033. [DOI] [PubMed] [Google Scholar]
- Insel R, Dunne JL. JDRF's vision and strategy for prevention of type 1 diabetes. Pediatr Diabetes 2016; 17 (Suppl 22): 87–92. [DOI] [PubMed] [Google Scholar]
- Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet 2016; 387: 2340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: gut microbiota: the neglected endocrine organ. Mol Endocrinol 2014; 28: 1221–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husted AS, Trauelsen M, Rudenko O, Hjorth SA, Schwartz TW. GPCR-mediated signaling of metabolites. Cell Metab 2017; 25: 777–796. [DOI] [PubMed] [Google Scholar]
- Dianzani U, Chiocchetti A, Ramenghi U. Role of inherited defects decreasing Fas function in autoimmunity. Life Sci 2003; 72: 2803–2824. [DOI] [PubMed] [Google Scholar]
- DeFranco S, Bonissoni S, Cerutti F, Bona G, Bottarel F, Cadario F et al. Defective function of Fas in patients with type 1 diabetes associated with other autoimmune diseases. Diabetes 2001; 50: 483–488. [DOI] [PubMed] [Google Scholar]
- Comi C, Fleetwood T, Dianzani U. The role of T cell apoptosis in nervous system autoimmunity. Autoimmun Rev 2012; 12: 150–156. [DOI] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 2011; 108 (Suppl 1): 4615–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne JL, Triplett EW, Gevers D, Xavier R, Insel R, Danska J et al. The intestinal microbiome in type 1 diabetes. Clin Exp Immunol 2014; 177: 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio J, Honda K. Immunoregulation by the gut microbiota. Cell Mol Life Sci 2012; 69: 3635–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Wang Z, Zhou Z. miRNAs: novel regulators of autoimmunity-mediated pancreatic β-cell destruction in type 1 diabetes. Cell Mol Immunol 2017; 14: 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]