Innate lymphoid cells (ILCs) represent a heterogeneous population, including both effectors and regulators of innate immunity, inflammation and tissue modeling.1 ILCs are categorized into three subgroups, ILC1s, ILC2s and ILC3s, based on similarities in phenotypic, ontogenetic and functional characteristics.2 ILC3s intrinsically require the transcription factor retinoic acid receptor-related orphan receptor γt (ROR γt) for their development and function.3 In response to specific stimuli, ILC3s produce IL-17 and IL-22 and play a critical role in intestinal mucosal protection, inflammation and innate responses 4 accompanied by a series of pathophysiological changes involving large-scale genetic upregulation and downregulation.5, 6 However, a key challenge remains in understanding the molecular mechanisms underlying the development and maintenance of ILC3s. Recently, progress has been made in identifying a critical factor, long noncoding RNA (lncRNA) lncKdm2b, for the regulation of ILC3 function. Liu et al.7 have published their findings in the latest issue of Nature Immunology. The identification of lncKdm2b along with a comprehensive evaluation of interactions among lncKdm2b, the chromatin organizer Satb1, the nuclear remodeling factor complex NURF and Zfp292 has improved our understanding of how this lncRNA is involved in transcriptional programs that modulate ILC3s (Figure 1).7
Figure 1.
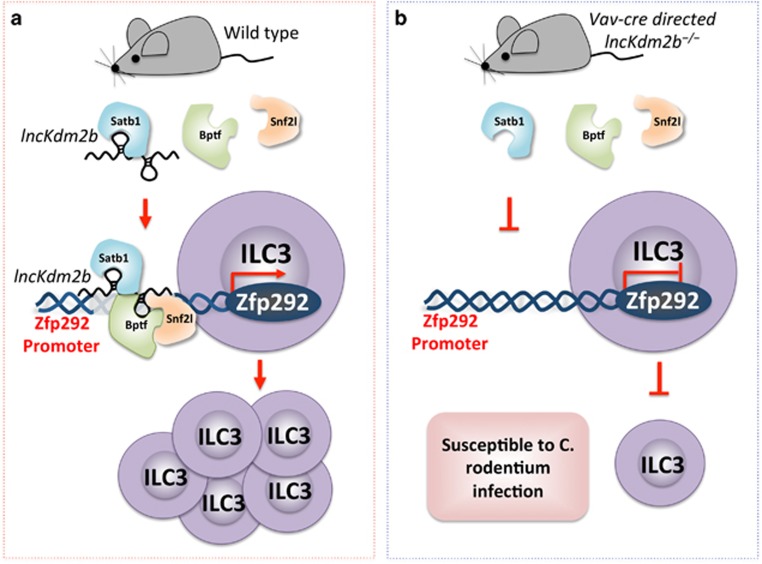
A novel mechanism of lncKdm2b-mediated transcriptional regulation implicated in maintaining ILC3 proliferation and function. (a) lncKdm2b is required for the activation of Zfp292 expression. lncKdm2b directly binds to Satb1, which subsequently recruits the NURF complex comprising Bptf and Snf2l. This RNA/protein complex further binds to the Zfp292 promoter to activate Zfp292 expression in ILC3s of wild-type mice. (b) Loss of lncKdm2b abrogates the recruitment of Satb1 and NURF complex comprising Bptf and Snf2l on the Zfp292 promoter, resulting in impaired number and function of ILC3s. Consequently, Vav-Cre-directed lncKdm2b conditional knockout mice are susceptible to Citrobacter rodentium infection compared to wild-type mice.
lncRNAs are largely uncharacterized non-protein-coding transcripts longer than 200 nucleotides in length that are implicated in the regulation of a variety of genes and genome activity at multiple levels, including epigenetic mechanisms and nuclear organization, as well as RNA processing, stability and translation.8 Increasing evidence has indicated that lncRNAs are important factors associated with a wide range of biological processes, including cell proliferation, cell differentiation, apoptosis and self-renewal.9 lncKdm2b, identified by Liu et al. is a previously uncharacterized lncRNA that is highly expressed in murine bone marrow, embryo and intestinal lamina propria lymphocytes. Importantly, high expression levels of lncKdm2b were observed in ILC3 populations (defined as Lin−CD45+RORγt+), which was further validated using RNA fluorescence in situ hybridization (RNA-FISH) and lncKdm2b reporter mice. Given that homozygous lncKdm2b deficiency is lethal, lncKdm2bflox/flox mice were crossed with Vav-Cre mice to generate a conditional deletion of lncKdm2b in hematopoietic cells and their progeny. The data obtained from this model indicate that the expression level of lncKdm2b is associated with the proliferation and effector functions of ILC3s without affecting their development and apoptosis.
IL-22 produced by ILC3s is one of the critical effector molecules for mucosal defense against bacterial invasion.10 As expected, lncKdm2b conditional knockout mice, which exhibit reduced numbers of IL-22-producing cells, are very susceptible to Citrobacter rodentium infection. In addition to IL-22-mediated protective immune responses, there are other effector molecules, including IL-17 and IL-23, responsible for the maintenance of gut homeostasis and protection against gastrointestinal invasions.11 Therefore, the production of these effector molecules may be impaired in lncKdm2b conditional knockout mice.
lncRNAs play critical roles in spatially orchestrating gene expression through epigenetic, transcriptional and posttranscriptional mechanisms.12 lncRNAs act as decoy molecules, competing with microRNAs and RNA-binding proteins via target proteins and influence neighboring gene expression in cis or trans.13 To address the contribution and mechanism of lncKdm2b on the genetic regulatory network in ILC3s, transcriptome microarray and bioinformatics analyses of lncKdm2b+/+ ILC3s versus lncKdm2b−/− ILC3s were performed. An uncharacterized transcription factor, Zfp292, was identified, which was downregulated by 5-fold in lncKdm2b−/− ILC3s compared to lncKdm2b+/+ ILC3s. As Zfp292 is an ultimate regulator of lncKdm2b in ILC3s, its overexpression offers a feasible and remedial approach to significantly promote ILC3 proliferation and increased resistance to Citrobacter rodentium infection. Clearly, there are many regulatory factors upstream of Zfp292. The chromatin organizer Satb1 and the nuclear remodeling factor complex (NURF) are considered to form an indispensable ‘bridge’ between lncKdm2b and Zfp292 in ILC3s. lncKdm2b is one of many divergent lncRNAs that are transcribed in the opposite direction relative to their neighboring genes, and thus they influence the expression of neighboring protein-coding genes in cis. Interestingly, the neighboring genes of lncKdm2b are not influenced in lncKdm2b−/− ILC3s. Mechanistically, lncKdm2b was found to directly bind to the Satb1 protein and regulate Satb1 expression in trans (regulating genes at distal loci) without affecting the neighboring genes in the nuclei of ILC3s. Satb1 is a tissue-specific matrix attachment region-binding protein, which participates in higher order chromatin compaction and tissue-specific gene expression, and it plays a vital role in the regulation of gene expression through chromatin remodeling, histone acetylation and methylation.14 In ILC3s, Satb1 can regulate Zfp292 expression by recruiting subunits of the NURF chromatin remodeling complex (Bptf and Snf2l) to the Zfp292 promoter. An in-depth study reported that lncKdm2b, acting as a scaffold and an amplifier, could recruit and interact with Satb1 and the NURF remodeling complex via distinct binding domains and then bridge these functionally related protein complexes to localize to the Zfp292 promoter to initiate transcription in ILC3s, consistent with the hypothesis of Guttman and Rinn.15
These findings reveal a novel lncKdm2b-mediated regulatory network involved in the modulation of intestinal homeostasis through the initiation of Zfp292 expression and maintenance of ILC3s, suggesting that lncKdm2b may be therapeutically harnessed to regulate ILC3 function during mucosal immune responses and intestinal homeostasis. Taken together, Liu et al. have pioneered prospective lncRNA studies by defining how lncKdm2b binds or sequesters proteins to activate gene expression in trans but not in cis. This work highlights two uncharacterized molecules, lncRNAs and Zfp292, in ILC3s and proposes a novel regulatory mechanism underlying ILC3 maintenance. By unraveling the structural and functional relationships and biological relevance, targeting lncRNAs may become a promising approach for treatment.
Footnotes
The authors declare no conflict of interest.
References
- Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol 2016; 17: 765–774. [DOI] [PubMed] [Google Scholar]
- Kwon B. Regulatory roles of dermal type 2 innate lymphoid cells. Cell Mol Immunol 2013; 10: 453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxatto D, Micheletti A, Montaldo E, Orecchia P, Loiacono F, Canegallo F et al. Group 3 innate lymphoid cells regulate neutrophil migration and function in human decidua. Mucosal Immunol 2016; 9: 1372–1383. [DOI] [PubMed] [Google Scholar]
- Melo-Gonzalez F, Hepworth MR. Functional and phenotypic heterogeneity of group 3 innate lymphoid cells. Immunology 2017; 150: 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajuddin SM, Schick UM, Eicher JD, Chami N, Giri A, Brody JA et al. Large-scale exome-wide association analysis identifies loci for white blood cell traits and pleiotropy with immune-mediated diseases. Am J Hum Genet 2016; 99: 22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koues OI, Collins PL, Cella M, Robinette ML, Porter SI, Pyfrom SC et al. Distinct gene regulatory pathways for human innate versus adaptive lymphoid cells. Cell 2016; 165: 1134–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Ye B, Yang L, Zhu X, Huang G, Zhu P, Du Y et al. Long noncoding RNA lncKdm2b is required for ILC3 maintenance by initiation of Zfp292 expression. Nat Immunol 2017; 18: 499–508. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cao X. Long noncoding RNAs in innate immunity. Cell Mol Immunol 2016; 13: 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, York SR, Chen JY, Pondick JV, Motola DL, Chung RT et al. Long noncoding RNAs expressed in human hepatic stellate cells form networks with extracellular matrix proteins. Genome Med 2016; 8: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratsimandresy RA, Indramohan M, Dorfleutner A, Stehlik C. The AIM2 inflammasome is a central regulator of intestinal homeostasis through the IL-18/IL-22/STAT3 pathway. Cell Mol Immunol 2017; 14: 127–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiec Z, Cyman M, Slebioda TJ. Cells of the innate and adaptive immunity and their interactions in inflammatory bowel disease. Adv Med Sci 2017; 62: 1–16. [DOI] [PubMed] [Google Scholar]
- Knauss JL, Sun T. Regulatory mechanisms of long noncoding RNAs in vertebrate central nervous system development and function. Neuroscience 2013; 235: 200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Mo X, Fu L, Xiao B, Guo J. Molecular mechanisms of long noncoding RNAs on gastric cancer. Oncotarget 2016; 7: 8601–8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottimukkala KP, Jangid R, Patta I, Sultana DA, Sharma A, Misra-Sen J et al. Regulation of SATB1 during thymocyte development by TCR signaling. Mol Immunol 2016; 77: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature 2012; 482: 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]