Abstract
Epicardial adipose tissue (EAT) is associated with the incidence, perpetuation, and recurrence of atrial fibrillation (AF), with elusive underlying mechanisms. We analyzed adipokine expression in samples from 20 patients with sinus rhythm (SR) and 16 with AF. Quantitative real-time PCR showed that connective tissue growth factor (cTGF) expression was significantly higher in EAT than in subcutaneous adipose tissue (SAT) or paracardial adipose tissue (PAT) from patients with AF, and in EAT from patients with SR (P < 0.001). Galectin-3 expression was significantly higher in EAT than in SAT or PAT (P < 0.001), with no significant differences between patients with AF and SR (P > 0.05). Leptin and vaspin expression were lower in EAT than in PAT (P < 0.001). Trichrome staining showed that the fibrosis was much more severe in patients with AF than SR (P < 0.001). We found a linear relationship between cTGF mRNA expression level and collagen volume fraction (y = 1.471x + 27.330, P < 0.001), and logistic regression showed that cTGF level was an independent risk factor for AF (OR 2.369, P = 0.027). In conclusion, highly expressed in EAT, cTGF is associated with atrial fibrosis, and can be an important risk factor for AF.
Introduction
Atrial fibrillation (AF), the most common sustained arrhythmia in the clinic, has raised worldwide concern due to its increasing incidence, morbidity and mortality1. Recent clinical studies have found that epicardial adipose tissue (EAT) is highly associated with the incidence2, perpetuation3 and recurrence4 of AF. The predominant views consider paracrine effects through the release of adipokines, the infiltration of adipocytes into the atrial myocardium, and the source of myofibroblasts producing extracellular matrix as the major mechanisms by which EAT facilitates AF5. However, the precise role that EAT plays in the pathogenesis of AF is not yet been completely understood, and therefore requires further study.
Structural remodeling is a key process in the development and perpetuation of AF, which is manifested by fibrosis6. A study demonstrated that EAT could induce atrial fibrosis via secretion of Activin A, which contributed to the development of AF7. However, whether other adipokines are of importance in the relationship between EAT and AF is still unknown. It was supposed in this study that some adipokines expressed in EAT could facilitate the process of atrial remodeling. We screened previous reports to find potential adipokines that could be expressed in adipose tissue and associated with fibrosis. Connective tissue growth factor (cTGF)8,9, galectin-3 (gal-3)10,11, leptin12,13 and vaspin14,15 were chosen as the candidates of this hypothesis. Previous research studied the link between mRNA level of chemerin16 or omentin-117 in EAT and coronary atherosclerosis, which also demonstrated the feasibility and reliability of mRNA quantification. Hence, quantitative real-time PCR was firstly used to determine the mRNA expression level of the adipokines, while immunochemistry and western blotting were further adopted to validate the results. This study compared the expression of cTGF, gal-3, leptin and vaspin in three types of adipose tissue between AF patients and sinus rhythm (SR) patients undergoing coronary artery bypass graft (CABG) surgery. In addition, the quantitative relationship between adipokine expressions, atrial fibrosis and AF was analyzed.
Results
Patient characteristics
There was no significant differences between SR patients and AF patients in demographical data including age (P = 0.741), sex (P = 0.821), Body Mass Index (BMI) (P = 0.631), or smoking status (P = 0.936). Patients showed no disparity in cardiac function, as indicated by NYHA functional class or left ventricular ejection fraction (LVEF). However, echocardiography showed that the left atrial diameter (LAD) was significantly higher in the AF group than in the SR (P = 0.004). In terms of comorbidity, there was no significant difference between the two groups, including hypertension (P = 0.439), type 2 diabetes mellitus (T2DM) (P = 0.517), stroke (P = 0.418) and chronic obstructive pulmonary disease (COPD) (P = 0.451; see Table 1 for details).
Table 1.
Clinical characteristic of patients in the AF and SR group.
| Variables | SR group (n = 20) | AF group (n = 16) | χ2/t | P |
|---|---|---|---|---|
| Demographics | ||||
| Age(y) | 58.6 ± 9.4 | 57.5 ± 9.1 | 0.333 | 0.741 |
| Gender(%male) | 12 (60.0%) | 9 (56.3%) | 0.051 | 0.821 |
| BMI(kg/m−2) | 23.4 ± 2.1 | 23.7 ± 2.4 | 0.485 | 0.631 |
| Smoking | 6 (30.0%) | 5 (31.3%) | 0.007 | 0.936 |
| NYHA functional class | 1.190 | 0.979 | ||
| I | 1 (5.0%) | 1 (6.3%) | ||
| II | 10 (50.0%) | 8 (50.0%) | ||
| III | 7 (35.0%) | 6 (37.5%) | ||
| IV | 2 (10.0%) | 1 (6.3%) | ||
| Echocardiography | ||||
| LVEF(%) | 58.2 ± 3.9 | 57.7 ± 4.8 | 0.319 | 0.751 |
| LAD (mm) | 38.5 ± 5.4 | 43.3 ± 3.6 | 3.078 | 0.004 |
| Comorbidities | ||||
| Hypertension | 6 (30.0%) | 3 (18.8%) | 0.600 | 0.439 |
| Type II Diabetes | 7 (35.0%) | 4 (25.0%) | 0.419 | 0.517 |
| Stroke | 1 (5.0%) | 2 (12.5%) | 0.655 | 0.418 |
| COPD | 2 (10.0%) | 3 (15.0%) | 0.569 | 0.451 |
Data are presented as n (%) or mean ± SD. BMI, body mass index; LVEF, left ventricular ejection fraction; LAD, left atrial diameter; T2DM, type 2 diabetes mellitus; COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association.
Adipokine mRNA expression
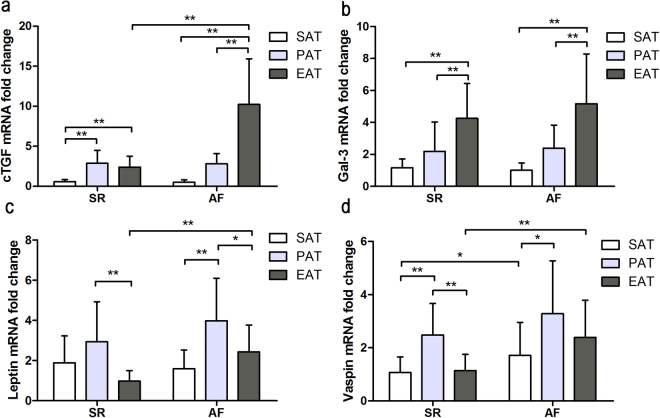
Adipokine mRNA expression is shown in Fig. 1 and Supplemental Table S1. cTGF mRNA expression was much higher in EAT from AF patients, compared to subcutaneous adipose tissue (SAT) and paracardial adipose tissue (PAT), as well as EAT from SR patients (AF EAT vs. AF SAT, 10.23 ± 5.69 vs. 0.49 ± 0.32, P < 0.001; AF EAT vs. AF PAT, 10.23 ± 5.69 vs. 2.80 ± 1.28, P < 0.001; AF EAT vs. SR EAT, 10.23 ± 5.69 vs. 2.39 ± 1.38, P < 0.001).
Figure 1.
Quantitative real-time PCR results of adipokines expression in the adipose tissue of patients with AF (n = 16) and SR (n = 20). (a) Quantitative real-time PCR results of cTGF; (b) Quantitative real-time PCR results of gal-3; (c) Quantitative real-time PCR results of leptin; (d) Quantitative real-time PCR results of vaspin; *P = 0.001, **P < 0.001.
Both groups showed comparable trends in gal-3 mRNA expression, which was manifested in sequential increments in SAT, PAT and EAT (SR F = 17.741, P < 0.001; AF F = 17.831, P < 0.001). However, there was no significant difference in gal-3 mRNA expression between the two groups (SAT, P = 0.306; PAT, P = 0.722; EAT, P = 0.314).
In terms of leptin, the highest expression was observed in PAT from both groups, which was significantly higher than that in SAT or EAT (AF PAT vs. SAT, 3.98 ± 2.12 vs. 1.60 ± 0.93, P < 0.001; AF PAT vs. EAT, 3.98 ± 2.12 vs. 2.43 ± 1.34, P = 0.020; SR PAT vs. SAT, 2.93 ± 1.99 vs. 1.88 ± 1.34, P = 0.068; SR PAT vs. EAT, 2.93 ± 1.99 vs. 0.98 ± 0.52, P < 0.001). Leptin expression in EAT was higher in the AF group than that in the SR group (P < 0.001).
Vaspin expression demonstrated a trend similar to that of leptin expression, with the highest mRNA expression levels in PAT (SR F = 17.820, P < 0.001; AF F = 3.997, P = 0.025), and vaspin expression in SAT and EAT was higher in the AF group than that in the SR group (AF SAT vs. SR SAT, 1.71 ± 1.25 vs. 1.07 ± 0.59, P = 0.049; AF EAT vs. SR EAT, 2.39 ± 1.39 vs. 1.14 ± 0.61, P = 0.001).
Adipokine protein expression
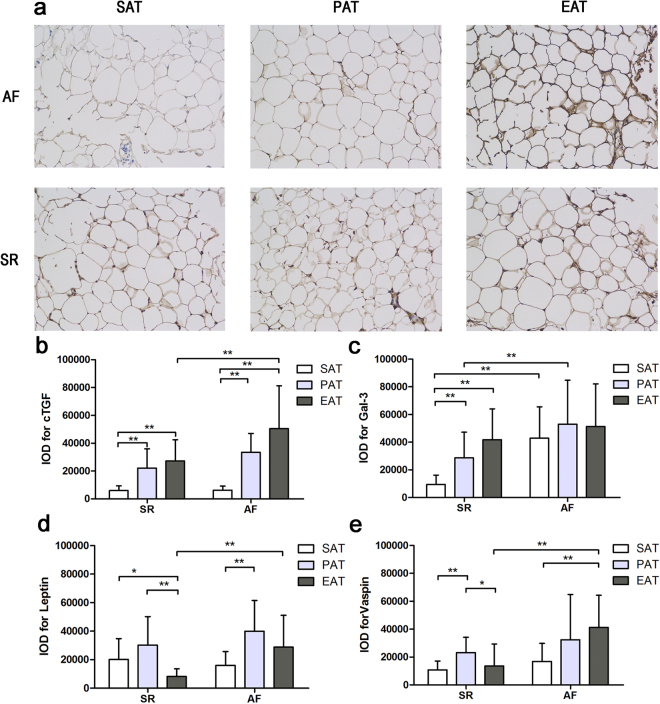
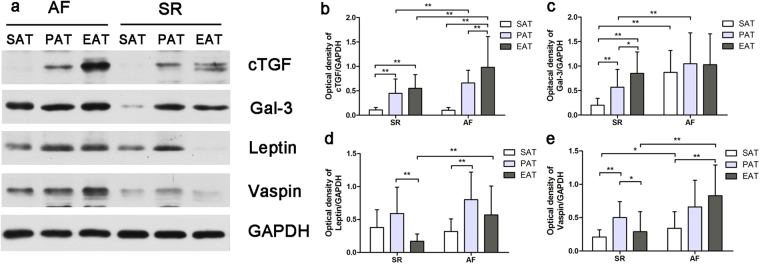
As shown in Fig. 2 and Supplemental Table S2, adipokine protein expression was assayed qualitatively via immunohistochemistry and quantitatively using IOD values. Figure 2a shows representative slides of cTGF staining, which correlate with cTGF mRNA expression. EAT from patients with AF showed much more intense staining than SAT or PAT from the same patient. There were only relatively mild differences between SAT, PAT and EAT from the SR group. As depicted in Fig. 2b, quantitative IOD also confirmed that cTGF expression was significantly higher in EAT from the AF group (AF EAT vs. AF SAT, 50487.00 ± 30750.84 vs. 6240.7 5 ± 3001.37, P < 0.001; AF EAT vs. AF PAT, 50487.00 ± 30750.84 vs. 33461.50 ± 13565.64, P = 0.052; AF EAT vs. SR EAT, 50487.00 ± 30750.84 vs. 27277.85 ± 15141.24, P = 0.006). Western blotting results are shown in Fig. 3 and Supplemental Table S3. Results were consistent with the immunohistochemistry data, where cTGF was highly expressed in EAT from patients with AF (AF EAT vs. AF SAT, 0.98 ± 0.63 vs. 0.10 ± 0.06, P < 0.001; AF EAT vs. AF PAT, 0.98 ± 0.63 vs. 0.66 ± 0.26, P = 0.088; AF EAT vs. SR EAT, 0.98 ± 0.63 vs. 0.55 ± 0.28, P = 0.011).
Figure 2.
Immunohistochemical integrated optical density analysis of adipokines in the adipose tissue of patients with AF (n = 16) and SR (n = 20). (a) Representative sections cTGF expression of SAT, PAT, and EAT in the AF and SR groups (×200); (b) Quantitative results of IOD in sections of cTGF in the AF and SR groups; (c) Quantitative results of IOD in sections of gal-3 in the AF and SR groups; (d) Quantitative results of IOD in sections of leptin in the AF and SR groups; (e) Quantitative results of IOD in sections of vaspin in the AF and SR groups. *P < 0.05, **P < 0.01.
Figure 3.
Western blotting and quantitative results of optical density of adipokines in the adipose tissue of patients with AF (n = 16) and SR (n = 20). (a) Western blotting of representative bands from SR and AF groups (full-length gels and blots are included in the Supplementary Information Fig. 1); (b) Quantitative results of optical density of cTGF; (c) Quantitative results of optical density of gal-3; (d) Quantitative results of optical density of leptin; (e) Quantitative results of optical density of vaspin. *P < 0.05, **P < 0.01.
As for gal-3 expression, the immunohistochemistry and western blotting results were slightly different from the mRNA expression. There was no difference in gal-3 expression between SAT, PAT, and EAT from patients with AF (F = 0.564, P = 0.573) (Figs 2c and 3c). For leptin expression, protein levels showed a similar distribution to mRNA expression in adipose tissues (Figs 2d and 3d). Vaspin expression was higher in EAT from patients with AF than those with SR, which differs from the quantitative real-time PCR results (Figs 2e and 3e).
Correlation between atrial fibrosis levels and adipokine expression
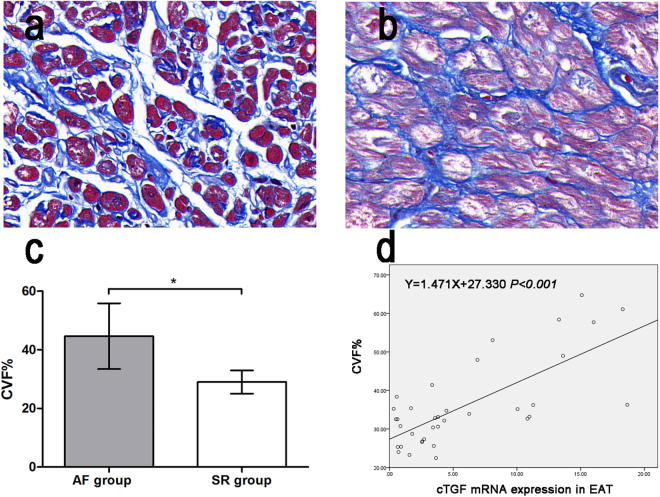
Masson’s-stained sections of the atrial myocardium are shown in Fig. 4a,b. Representative sections indicate a more fibrotic and disordered myocardium in the AF group than in the SR group. As shown in Fig. 4c, the quantitative CVF% in the AF group was significantly higher than that in the SR group (44.64 ± 11.23 vs. 29.02 ± 3.98, t = 5.798, P < 0.001). Univariate linear regression analysis of CVF% and adipokines revealed a linear relationship between cTGF mRNA expression and CVF% (y = 1.471 × + 27.330, P < 0.001), as shown in Fig. 4d. There was no significant linear relationship between CVF% and other adipokines analyzed, including gal-3, leptin and vaspin (Table 2).
Figure 4.
Masson staining and quantitative results of atrial myocardium from patients with AF (n = 16) and SR (n = 20). (a) Masson staining of representative sections from the SR group(×400). (b) Masson staining of representative sections from the AF group(×400). (c) Quantitative results of Masson staining. *P < 0.001. (d) Univariate linear regression analysis of cTGF mRNA expression and the collagen volume fraction (CVF%).
Table 2.
Curve estimation parameters of adipokines expression and CVF%.
| Adipose tissue type | adipokines | Constant | b1 | R2 | F | P |
|---|---|---|---|---|---|---|
| SAT | cTGF | 38.718 | −5.134 | 0.017 | 0.577 | 0.453 |
| gal-3 | 40.666 | −4.321 | 0.039 | 1.367 | 0.250 | |
| vaspin | 33.903 | 1.517 | 0.018 | 0.610 | 0.440 | |
| leptin | 38.055 | −1.190 | 0.016 | 0.539 | 0.468 | |
| PAT | cTGF | 36.754 | −0.278 | 0.001 | 0.045 | 0.834 |
| gal-3 | 37.728 | −0.776 | 0.013 | 0.454 | 0.505 | |
| vaspin | 30.568 | 1.901 | 0.076 | 2.797 | 0.104 | |
| leptin | 33.155 | 0.825 | 0.024 | 0.826 | 0.370 | |
| EAT | cTGF | 27.330 | 1.471 | 0.530 | 38.328 | <0.001 |
| gal-3 | 35.458 | 0.108 | 0.001 | 0.022 | 0.882 | |
| vaspin | 33.214 | 1.619 | 0.030 | 1.059 | 0.311 | |
| leptin | 33.341 | 1.614 | 0.030 | 1.063 | 0.310 |
Multivariate regression analysis
We conducted a multivariate logistic regression analysis, when AF was defined as the dependent variable, while adipokine expression level and demographic data were included as candidates of independent variables. Enter method was used to choose independent varibles for the best model. Results showed cTGF mRNA expression was left in the model, and it was an independent risk factor for the incidence of AF (OR 2.369, 95%CI (1.104–5.087), P = 0.027), as shown in Table 3. A linear regression analysis was also conducted to explore the clinical parameters affecting the secretory profile, which showed that only AF was statistically associated with the mRNA expression of cTGF, leptin and vaspin (Supplemental Table S4).
Table 3.
Multivariate logistic regression of risk factors for incidence of AF.
| Risk factors | OR(95%CI) | P |
|---|---|---|
| Gender male | 1.415 (0.074–23.414) | 0.852 |
| Age | 1.056 (0.892–1.249) | 0.528 |
| Smoking | 6.008 (0.095–380.069) | 0.397 |
| BMI | 1.008 (0.561–1.812) | 0.978 |
| NYHA | 0.174 (0.007–4.178) | 0.281 |
| T2DM | 0.562 (0.026–12.299) | 0.715 |
| Hypertension | 0.116 (0.002–5.955) | 0.284 |
| COPD | 0.776 (0.006–100.081) | 0.919 |
| Stroke | 3.192 (0.034–298.314) | 0.616 |
| cTGF mRNA in EAT | 2.369 (1.104–5.087) | 0.027 |
BMI, body mass index; NYHA, New York Heart Association; T2DM, type 2 diabetes mellitus; COPD, chronic obstructive pulmonary disease.
Discussion
A strong relationship between atrial fibrosis and AF has been reported, and atrial fibrosis is believed to be a key process in structural remodeling of the atrium18. As previously shown, cardiac fibroblasts are activated by cytokines secreted by inflammatory cells, including macrophages, through the TGF-β signalling pathway, causing them to produce collagen I and collagen III, and ultimately leading to atrial fibrosis19. EAT, once thought to have a mere protective and metabolic role in the cardiovascular system, has recently been found to be highly associated with cardiovascular diseases, including coronary artery disease, heart failure20, and AF21. Previous work has suggested that EAT plays a key role in atrial remodeling by infiltrating into the myocardium22 and secreting Activin A, thus predisposing the tissue to atrial fibrosis7. We hypothesized that there were still other adipokines highly expressed and secreted by EAT and associated with atrial fibrosis, which might participate in the development of AF. Our study mainly focused on four potential adipokines, including cTGF, gal-3, leptin and vaspin, and the results showed that cTGF expression level was higher in EAT than other types of adipose tissue, and in EAT of AF patients than SR patients, which correlated well with our hypothesis.
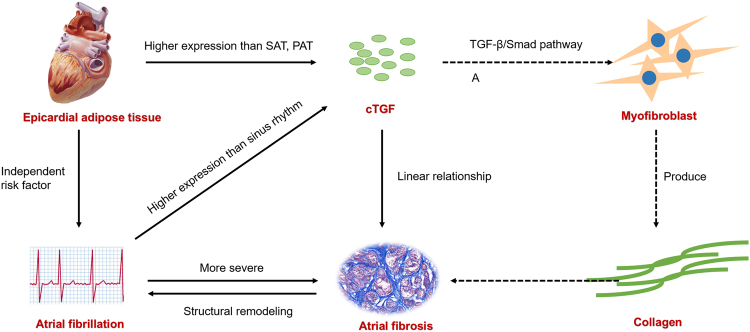
As an versatile cytokines, cTGF is involved in a range of biological processes, including cell growth and differentiation23. A clinical study showed that serum cTGF level was an important predictor of recurrence of AF after the catheter ablation, implying the role of cTGF in atrial remodeling24. An experimental study using rapid pacing canine models also found that cTGF was up-regulated in AF models, providing further evidence that cTGF was associated with AF25. Not only working in circulation system, it is also highly expressed in subcutaneous or epididymal fat pads in an obesity mouse model8. Our study demonstrated that cTGF was highly expressed in EAT, whether with or without AF, suggesting that cTGF might be an important adipokines participating in the regulation of cardiac remodeling. We compared the cTGF expression level in EAT between AF patients and SR patients, and analyzed the risk factors for AF. The results strongly suggested the association between cTGF and AF. Supporting our hypothesis, a strong connection between cTGF and atrial fibrosis was established by linear regression analysis. Other studies also favors our study. One found that cTGF mainly acted in angiotensin II-mediated atrial fibrosis26, while other studies proved that it was regulated by miR-13227 and functioned via TGF-β1/Smad pathway28. Therefore, it is inferred that EAT is an important source of cTGF, which facilitates atrial fibrosis and atrial remodeling and further leads to AF (Illustrated by Fig. 5).
Figure 5.
Visual illustration of the major findings and hypothesis.
About other adipokines investigated in this study, gal-3 showed no difference between AF patients and SR patients, while leptin and vaspin were more expressed in PAT than EAT, which were not in favor of the hypothesis. Gal-3 is a member of the galectin family and is expressed extensively in macrophages, mastocytes and neutrophils29. In recent years, gal-3 has emerged as a promising biomarker associated with atrial fibrosis and AF30. However, its expression in EAT has yet been described. Despite higher gal-3 expression in EAT than SAT and PAT, there was no disparity between AF patients and SR patients. Immunohistochemistry and western blotting yielded slightly different results than for mRNA expression, which could be attributed to differences between mRNA and protein expression or sample error. We attributed the similarity between SR and AF patients in gal-3 expression level to the following potential reasons. On one hand, gal-3 might functioned in atrial fibrosis majorly via circulation system rather than paracrine pathway, which was validated by the higher serum gal-3 level in AF than SR patients. On the other hand, gal-3 expressed in EAT could be a part of inflammation of adipose tissue itself rather than secretory products of EAT31. Leptin was considered one of the most promising candidates for having a role in AF, because previous work verified its strong relationship with AF13 and because of its high expression in EAT32. The present study showed that leptin expression was higher in EAT from patients with AF than those with SR, which correlated with previous studies. However, we found that leptin expression was lower in EAT than PAT, whether for patients with AF or SR. A similar conclusion can be drawn from the vaspin expression results, another adipokine associated with cardiovascular disease15. The higher expression of vaspin and leptin in PAT might be attributed to the association of obesity. A study found that vaspin and leptin level were associated with adipose tissue depot and the insulin resistance, especially for visceral adipose tissue33. Notably, the significant difference in leptin and vaspin expression in EAT between patients with AF and SR suggested that they might still be involved in atrial fibrosis via EAT in a paracrine manner. A recent study showed a higher degree of local tissue hypoxia and up-regulation of leptin expression in the perivascular adipose tissue belonging to EAT, along with increased vascularization, inflammation, and fibrosis, which might contribute to the increased atherosclerotic plaque burden in the coronary arteries34. Similar procedures may happen in peri-atrial adipose tissue, where leptin expression is increased, inflammation and fibrosis was induced, which contributed to atrial remodeling and AF.
Our findings not only add more evidence of the negative role that EAT plays in the development of AF, but demonstrate that cTGF is highly expressed in EAT of AF patients and may facilitate atrial fibrosis, which help elucidate the underlying mechanism of AF and provide more clues for prevention work as well as therapy research. Although a lot of work has been carried out, and some intriguing findings have been acquired, some limitations with the current study should also be noted. Our work revealed differences in expression between SAT, PAT, and EAT from patients with AF and SR; however, further clinical and basic studies will be required to determine the exact role of these adipokines in the pathogenesis of AF. Second, multiple factors cannot be completely eliminated, although most major clinical characteristics were uniform across all groups. Lastly, this study only focused on the four most prominent adipokines, and a more extensive screen will be necessary in order to explore additional potential adipokines.
In conclusion, our study demonstrates that cTGF is highly expressed in EAT from patients with AF and is associated with atrial fibrosis. cTGF expression in EAT may also represent an independent risk factor for AF. Future studies aimed at understanding the role of cTGF in structural and electrical remodeling of the atrium could identify novel targets for the prevention and treatment of AF.
Methods
Human subjects
From March 2016 to April 2017, a total of 68 patients received CABG surgery in Changzheng Hospital affiliated with the Second Military Medical University. Of these, 36 patients were enrolled in this study. Patients were divided into SR group (n = 20) and AF group (n = 16), according to their heart rhythm. All 36 patients were enrolled according to the following inclusion and exclusion criteria: patients aged between 18 to 80 years receiving CABG with SR or AF rhythm were included, while patients with structural heart disease, paroxysmal AF rhythm, severe hepatic or renal dysfunction, metabolic disease, infectious disease, cancer, or aged over 80 years were excluded. Persistent and permanent AF was determined using a 12-lead electrocardiogram. Clinical data from all patients were collected for analysis.
This study was approved by the Committee on Ethics of Biomedicine of the Second Military Medical University. This study also complied with the Declaration of Helsinki, and signed, written informed consent was obtained from all subjects.
Sample acquisition
All patients received a conventional on-pump CABG with a sternal incision. Adipose tissue was acquired during surgery, before cardiopulmonary bypass establishment. Different types of adipose tissue (average 0.5 g each) were collected from each patient, including SAT from the chest incision site, PAT from the pericardium, and EAT from the atrioventricular groove next to the right atrial appendage. Each biopsy was divided into 2 portions; one was frozen immediately at −80 °C for RNA isolation, and the other was immersed in neutralized formalin for immunohistochemistry. Adjacent atrial myocardial tissue (average 0.1 g) from the right atrial appendage tissue was also collected and likewise divided into 2 portions.
RNA isolation and quantitative real-time PCR
TRIzol® reagent (Invitrogen, Carlsbad, CA, USA) was used to extract total RNA. Purity of the isolated RNA was assessed by measuring optical density at 260 nm and 280 nm. Reverse transcription was performed using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA), according to the manufacturer’s instructions. SYBR® Premix Ex Taq™ (Takara Bio, Tokyo, Japan) was used to prepare samples for quantitative real-time PCR. An ABI Prism 7900 Detector System (Applied Biosystems) was used to perform quantitative real-time PCR. Primers were designed using Primer Premier 6.0 software (Premier Biosoft, Palo Alto, CA, USA). Primer sequences were as follows: cTGF (cTGF), forward 5′-ATGCTGCGAGGAGTGGGTGT-3′, reverse 5′-TGGCTCTAATCATAGTTGGGTCT-3′; Gal-3 (LGALS3), forward 5′-GTGAAGCCCAATGCAAACAGA-3′, reverse 5′-AGCGTGGGTTAAAGTGGAAGG-3′; Leptin (LEP), forward 5′-CCTGTGCGGATTCTTGTGG-3′, reverse 5′-GGTGACTTTCTGTTTGGAGGA-3′; Vaspin (SERPINA12), forward 5′-CGGTGAAAGGTCTTCTAAAGCC-3′, reverse 5′- AGCCTAAGTCCATGTTCTGCC-3′; β-actin (ACTB), forward 5′-CATGTACGTTGCTATCCAGGC-3′, reverse 5′-CTCCTTAATGTCACGCACGAT-3′. Relative gene expression was calculated using threshold cycle value (CT) and formula 2−ΔΔCT.
Immunohistochemistry
Paraffin-embedded sections were placed at 60 °C for 1 h, and then dewaxed, rehydrated and rinsed two to three times with PBS, for 3 min each. Sections were incubated in a 3% hydrogen peroxide solution for 8 min at room temperature, then rinsed 3 times with PBS (pH 7.2 to 7.6), for 5 min each. Sections were incubated with primary antibodies (anti-cTGF, anti-Gal-3, or anti-LEP, anti-Vaspin; Abcam, Cambridge, UK) overnight at 4 °C in a moist chamber, then rinsed 3 times with PBS (pH 7.2 to 7.6). Sections were then incubated with appropriate secondary antibodies for 1 h at 4 °C, then rinsed 3 times with PBS. DAB reagent (50–100 μl) was added and sections were observed under a microscope. The integrated optical density (IOD) of positively stained tissue was used to quantify the expression. The IOD of each tissue section was calculated from eight different 400× magnified fields.
Masson’s trichrome staining
Samples were dewaxed and rehydrated using conventional methods, and then washed thoroughly with water. Sections were stained with haematoxylin from the Masson’s staining kit for 5 min, and then differentiated with ethanolic hydrochloric acid and rinsed thoroughly with water. Sections were then rinsed in running water until they turned a blue-black colour. The vermic acid red wine stain was added for 3–8 min, and then, sections were rinsed with distilled water and differentiated with 1% phosphomolybdic acid for 2 min. Finally, sections were stained with the aniline blue complex for 3 min, then differentiated with 1% glacial acetic acid for 1–2 min. Samples were sliced, dehydrated, rendered transparent with xylene and sealed with optical rubber. Sections were observed under a light microscope, and images were collected and used to calculate the volume fraction of collagen (CVF, CVF% = average collagen area/area of total field ×100).
Western blotting
Atrial myocardium samples were weighed and treated with cold Radio-Immunoprecipitation Assay (RIPA) lysis buffer (1 ml/100 mg). Homogenized samples were centrifuged (12 000 rpm, 10 min, 4 °C) and a BCA protein assay kit (Yeason Biotech, Shanghai, China) was used to determine protein concentration. Loading buffer was added to the diluted supernatants to adjust the concentrations and volumes. Samples with equal protein content were then subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis on a 10% polyacrylamide gel, and then transferred to a polyvinylidene difluoride membrane (Millipore, Boston, MA, USA). Membranes were blocked with blocking buffer containing 5% bovine serum albumin for 1 h at room temperature, followed by incubation with antibodies, anti-cTGF, anti-gal-3, anti-leptin, anti-vaspin (Abcam, Cambridge, UK), overnight at 4 °C. Anti-GAPDH was used as an internal control. Membranes were rinsed with TBST 3 times for 10 min each, followed by incubation with secondary antibodies for 1 h, and then washed three times with TBST for 15 min each. Finally, membranes were developed with chemiluminescence solution A and B mixed at a 1:1 ratio, along with the developing substrate. ImageJ software was used to calculate the relative optical density of the bands.
Statistical analysis
Continuous data were expressed as mean ± standard deviation, while categorical data were expressed as percentages. The clinical characteristics of the two groups were compared using a Student’s t-test and chi-square test. Immunohistochemistry IOD and mRNA levels between groups were compared using Student’s t-test, while differences among the three types of adipose tissue were detected via one-way ANOVA with the Bonferroni test for post hoc examinations. The CVF% calculated from the Masson’s staining and the relative optical density of western blotting between the two groups were compared using Student’s t-test. A correlation analysis between mRNA expression and CVF% was conducted using univariate linear regression. A logistic regression model was used to determine risk factors for AF, while a linear regression model was used to explore the clinical parameters affecting the secretory profile of EAT. All data were analysed using SPSS 22.0 software (IBM, Almonte, NY, USA). Differences were considered significant when P < 0.05.
Electronic supplementary material
Acknowledgements
This study was funded by the National Nature Science Foundation of China (NO. 81670299, NO. 81770244), Shanghai Shenkang Medicine Developing Project (SHDC12014107), Shanghai Science and Technology Committee Medicine Leading Project (NO. 15411960100), Shanghai health and family planning commission project (201740225) and Shanghai Leading Talent Program (LJRC-WZN).
Author Contributions
Study conception and design: Q.W., W.X. and L.Y., Sample acquisition and collection of clinical data: Y.G. and J.W., Experiments conduct: H.S. and J.M., Data analysis: Q.W. and J.W., Manuscript writing: H.S. and Q.W., Editing and Reviewing: Y.Z. and Z.W.
Competing Interests
The authors declare no competing interests.
Footnotes
Qing Wang, Wang Xi and Liang Yin contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-21911-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yufeng Zhang, Email: zhyf19810824@163.com.
Zhinong Wang, Email: wangzn007@163.com.
References
- 1.Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation (vol 11, pg 639, 2014) Nature Reviews Cardiology. 2016;13:501–501. doi: 10.1038/nrcardio.2016.114. [DOI] [PubMed] [Google Scholar]
- 2.Al Chekakie MO, et al. Pericardial Fat Is Independently Associated With Human Atrial Fibrillation. Journal of the American College of Cardiology. 2010;56:784–788. doi: 10.1016/j.jacc.2010.03.071. [DOI] [PubMed] [Google Scholar]
- 3.Stojanovska J, et al. Increased epicardial fat is independently associated with the presence and chronicity of atrial fibrillation and radiofrequency ablation outcome. European Radiology. 2015;25:2298–2309. doi: 10.1007/s00330-015-3643-1. [DOI] [PubMed] [Google Scholar]
- 4.Kocyigit D, et al. Periatrial epicardial adipose tissue thickness is an independent predictor of atrial fibrillation recurrence after cryoballoon-based pulmonary vein isolation. Journal of Cardiovascular Computed Tomography. 2015;9:295–302. doi: 10.1016/j.jcct.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Hatem SN, Sanders P. Epicardial adipose tissue and atrial fibrillation. Cardiovascular Research. 2014;102:205–213. doi: 10.1093/cvr/cvu045. [DOI] [PubMed] [Google Scholar]
- 6.Nattel S, Burstein B, Dobrev D. Atrial Remodeling and Atrial Fibrillation Mechanisms and Implications. Circulation-Arrhythmia and Electrophysiology. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 7.Venteclef N, et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. European Heart Journal. 2015;36:795–805A. doi: 10.1093/eurheartj/eht099. [DOI] [PubMed] [Google Scholar]
- 8.Tan JTM, et al. Connective tissue growth factor/CCN-2 is upregulated in epididymal and subcutaneous fat depots in a dietary-induced obesity model. American Journal of Physiology-Endocrinology and Metabolism. 2013;304:E1291–E1302. doi: 10.1152/ajpendo.00654.2012. [DOI] [PubMed] [Google Scholar]
- 9.Lendeckel U, Wolke C, Goette A. Atrial fibrillation and fibrosis: role of connective tissue growth factor. Europace. 2012;14:1079–1080. doi: 10.1093/europace/eus147. [DOI] [PubMed] [Google Scholar]
- 10.de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin-3: a novel mediator of heart failure development and progression. European Journal of Heart Failure. 2009;11:811–817. doi: 10.1093/eurjhf/hfp097. [DOI] [PubMed] [Google Scholar]
- 11.Baek J, et al. Galectin-3 activates PPARγ and supports white adipose tissue formation and high-fat diet-induced obesity. Endocrinology. 2015;156:147–156. doi: 10.1210/en.2014-1374. [DOI] [PubMed] [Google Scholar]
- 12.Baker AR, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovascular diabetology. 2006;5:1–1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ermakov S, et al. The associations of leptin, adiponectin and resistin with incident atrial fibrillation in women. Heart. 2016;102:1354–1362. doi: 10.1136/heartjnl-2015-308927. [DOI] [PubMed] [Google Scholar]
- 14.Kukla M, et al. Serum vaspin may be a good indicator of fibrosis in chronic hepatitis c and is not altered by antiviral therapy. Polish Journal of Pathology. 2012;63:213–220. doi: 10.5114/pjp.2012.32767. [DOI] [PubMed] [Google Scholar]
- 15.Spiroglou S, Kostopoulos C, Varakis J, Papadaki H. Chemerin, vaspin, visfatin and adiponectin expression in human pericoronary and apical epicardial fat: correlation with coronary atherosclerosis. European Heart Journal. 2009;30:60–61. [Google Scholar]
- 16.Gao X, et al. Association of chemerin mRNA expression in human epicardial adipose tissue with coronary atherosclerosis. Cardiovasc Diabetol. 2011;10:87. doi: 10.1186/1475-2840-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du Y, et al. Association between omentin-1 expression in human epicardial adipose tissue and coronary atherosclerosis. Cardiovasc Diabetol. 2016;15:90. doi: 10.1186/s12933-016-0406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burstein B, Nattel S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. Journal of the American College of Cardiology. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 19.Everett TH, Olgin JE. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm. 2007;4:S24–S27. doi: 10.1016/j.hrthm.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parisi V, et al. Increased Epicardial Adipose Tissue Volume Correlates With Cardiac Sympathetic Denervation in Patients With Heart Failure. Circ Res. 2016;118:1244–1253. doi: 10.1161/CIRCRESAHA.115.307765. [DOI] [PubMed] [Google Scholar]
- 21.Al-Rawahi M, Proietti R, Thanassoulis G. Pericardial fat and atrial fibrillation: Epidemiology, mechanisms and interventions. International Journal of Cardiology. 2015;195:98–103. doi: 10.1016/j.ijcard.2015.05.129. [DOI] [PubMed] [Google Scholar]
- 22.Haemers P, et al. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur Heart J. 2017;38:53–61. doi: 10.1093/eurheartj/ehv625. [DOI] [PubMed] [Google Scholar]
- 23.Daniels A, van Bilsen M, Goldschmeding R, van der Vusse GJ, van Nieuwenhoven FA. Connective tissue growth factor and cardiac fibrosis. Acta physiologica (Oxford, England) 2009;195:321–338. doi: 10.1111/j.1748-1716.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- 24.Song Z, Liu X, Zhang D. Connective tissue growth factor: a predictor of recurrence after catheter ablation in patients with nonparoxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2014;37:630–637. doi: 10.1111/pace.12345. [DOI] [PubMed] [Google Scholar]
- 25.Kiryu M, et al. Angiotensin II-mediated up-regulation of connective tissue growth factor promotes atrial tissue fibrosis in the canine atrial fibrillation model. Europace. 2012;14:1206–1214. doi: 10.1093/europace/eus052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko W, et al. Elevated expression of connective tissue growth factor in human atrial fibrillation and angiotensin II-treated cardiomyocytes. Circ. J. 2011;75:1592–1600. doi: 10.1253/circj.CJ-10-0892. [DOI] [PubMed] [Google Scholar]
- 27.Qiao G, Xia D, Cheng Z, Zhang G. miR-132 in atrial fibrillation directly targets connective tissue growth factor. Mol Med Rep. 2017;16:4143–4150. doi: 10.3892/mmr.2017.7045. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, et al. Increased expression of connective tissue growth factor and transforming growth factor-beta-1 in atrial myocardium of patients with chronic atrial fibrillation. Cardiology. 2013;124:233–240. doi: 10.1159/000347126. [DOI] [PubMed] [Google Scholar]
- 29.Henderson NC, Sethi T. The regulation of inflammation by galectin-3. Immunological Reviews. 2009;230:160–171. doi: 10.1111/j.1600-065X.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 30.Takemoto Y, et al. Galectin-3 Regulates Atrial Fibrillation Remodeling and Predicts Catheter Ablation Outcomes. JACC. Basic to translational science. 2016;1:143–154. doi: 10.1016/j.jacbts.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez-Martínez E, et al. Galectin-3 inhibition prevents adipose tissue remodelling in obesity. Int J Obes (Lond) 2016;40:1034–1038. doi: 10.1038/ijo.2016.19. [DOI] [PubMed] [Google Scholar]
- 32.Sweeney G. Cardiovascular effects of leptin. Nature Reviews Cardiology. 2010;7:22–29. doi: 10.1038/nrcardio.2009.224. [DOI] [PubMed] [Google Scholar]
- 33.Genske, F. et al. Abdominal fat deposits determined by magnetic resonance imaging in relation to leptin and vaspin levels as well as insulin resistance in the general adult population. Int J Obes (Lond) (2017). [DOI] [PubMed]
- 34.Drosos I, et al. Differences between perivascular adipose tissue surrounding the heart and the internal mammary artery: possible role for the leptin-inflammation-fibrosis-hypoxia axis. Clin Res Cardiol. 2016;105:887–900. doi: 10.1007/s00392-016-0996-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.