Abstract
The intragastric balloon (IGB) has been used for decades as a temporary measure for weight reduction in the morbidly obese. Serious complications related to the device remain rare. We present the case of a 23-year-old Caucasian man who developed signs of bowel obstruction following spontaneous partial deflation of an air filled IGB with subsequent migration and impaction in the jejunum. We discuss the role of the IGB in the treatment of obesity, side effects and the serious complications that can occur.
Keywords: Obesity, Obstruction, Gastric balloon
Obesity is on the rise and is likely to be one of the major global epidemics of the 21st century. It results in increased chronic health problems, reduced life expectancy and increased financial pressure on healthcare systems. Treatment of obesity can include lifestyle modifications and pharmacotherapy. Invasive treatments can be surgical or non-surgical. The intragastric balloon (IGB) is a medical device that is placed endoscopically in the stomach and inflated either with air or, more commonly, saline. Radioopaque markers allow for easy detection of the device. It is used primarily to achieve weight loss prior to bariatric surgery or in those patients who are not deemed fit enough for surgery. The IGB has been used extensively since the 1980s and many studies have confirmed its safety and efficacy as a temporary treatment.1,2 However, a number of rare, serious complications have been reported with its use.
We present a case of spontaneous partial deflation and subsequent migration of an air filled Heliosphere® device (Helioscopie, Vienne, France), with resultant entrapment in the jejunum and failed attempts to remove it endoscopically as well as by percutaneous deflation under fluoroscopy. The IGB was eventually retrieved by laparotomy.
Case history
A 23-year-old Caucasian man presented to the medical assessment unit with acute onset vomiting, diarrhoea and abdominal pain following an alcohol binge 2 days prior to the onset of his symptoms. He had undergone endoscopic insertion of a Heliosphere® air filled IGB seven months previously in Belgium as treatment for obesity. He had no other significant past medical history. On admission, he had mild periumbilical tenderness but no signs of acute peritonitis. Blood tests including serum amylase and inflammatory marker levels were normal. The abdominal x-ray on admission showed the presence of the IGB in the stomach. The chest x-ray was normal. He was treated initially for viral gastroenteritis with intravenous fluids and antispasmodics for pain relief.
Over the next three days the patient continued to have persistent, mild abdominal pain. As a result, he underwent an oesophagogastroduodenoscopy (OGD) with intention to remove the IGB. However, at the time of the OGD it was noted that the IGB was no longer present. An urgent abdominal x-ray revealed the location of the IGB in the mid jejunum with proximal dilatation of the bowel loops (Fig 1). A repeat endoscopic attempt to retrieve it on the same day using a paediatric colonoscope failed. Following this, he underwent percutaneous deflation under fluoroscopy and the IGB was deflated successfully (Fig 2). Computed tomography of the abdomen confirmed the deflated state of the IGB in the jejunum (Fig 3). He was kept under close observation for the next 12 hours and it was anticipated that the device would pass naturally.
Figure 1.
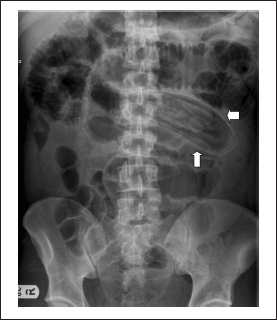
Plain abdominal x-ray showing the entrapped intragastric balloon (arrows) in the small bowel
Figure 2.
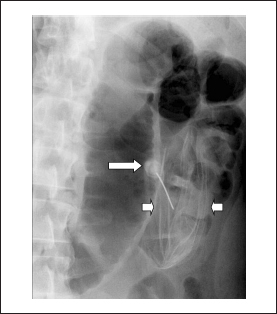
Fluoroscopy showing the partially deflated intragastric balloon (small arrows) after percutaneous needle (long arrow) puncture
Figure 3.
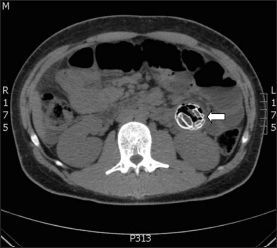
Computerised tomography showing the deflated intragastric balloon (arrow) in the small bowel
Unfortunately, the patient developed signs of intestinal obstruction and an emergency mini-laparotomy was undertaken. At laparotomy, the IGB was located in the jejunum. The overlying omentum was found to be thickened, suggesting localised peritonitis. The IGB was milked down to a lower level so as not to interfere with the acute gut wall inflammation and it was then retrieved by an enterotomy. Post-operative recovery was complicated by nosocomial pneumonia, which was treated successfully. He was discharged on the sixth post-operative day.
Discussion
The IGB is used as a temporary weight loss measure in morbidly obese patients who are awaiting bariatric surgery or who are not deemed suitable for it. They are reported to work by inducing early satiety. Their use has remained controversial with early studies showing no measurable benefit. More recent evidence suggests that the IGB is an effective method of achieving sustained weight loss either as an adjunct prior to a more definitive solution in bariatric surgery or as a minimally invasive endoscopic therapy in combination with diet and physical activity.2,3
Associated side effects include nausea, vomiting, abdominal pain, gastro-oesophageal reflux disease, peptic ulcer disease and gastric stasis. Side effects can be intolerable and may necessitate early removal of the IGB.3
Serious complication rates appear to be rare, with a risk of approximately 0.2% for gastric perforation and intestinal obstruction in the fluid filled BioEnterics® IGB device (Allergan, Irvine, CA, US).3 There has been one reported case of oesophageal perforation after gastric balloon extraction.4 IGBs are also known to deflate spontaneously, either partially or fully.5
Manufacturers of the IGB recommend that it should be removed approximately six months after insertion because of the risk of spontaneous balloon deflation, resulting in migration of the balloon. The risk is significantly higher when balloons are left in place longer than the recommended period.
One double blind comparative study evaluated the efficacy and safety of the air filled Heliosphere® and the saline filled BioEnterics® device.6 The study found both were equally effective in achieving weight loss although the Heliosphere® was more likely to migrate and therefore require extraction by rigid oesophagoscopy or surgery (p=0.02). The authors concluded that the Heliosphere® device had severe technical problems that needed resolving before it could be recommended.
Conclusions
Even though serious complications with IGBs are rare, our case report highlights what can potentially go wrong. Patients need to be thoroughly counselled on the benefits and risks, including rare complications such as intestinal obstruction.
References
- 1.Evans JT, DeLegge MH. Intragastric balloon therapy in the management of obesity: why the bad wrap? Parenter Enteral Nutr 2011; 35: 25–31. [DOI] [PubMed] [Google Scholar]
- 2.Farina MG, Baratta R, Nigro A et al. Intragastric balloon in association with lifestyle and/or pharmacotherapy in the long-term management of obesity. Obes Surg 2011. September 7 [Epub ahead of print.] [DOI] [PubMed]
- 3.Dumonceau JM. Evidence-based review of the Bioenterics intragastric balloon for weight loss. Obes Surg 2008; 18: 1,611–1,617. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz D, Vranas K, Robinson DA et al. Esophageal perforation after gastric balloon extraction. Obes Surg 2009; 19: 257–260. [DOI] [PubMed] [Google Scholar]
- 5.Forestieri P, De Palma GD, Formato A et al. Heliosphere Bag in the treatment of severe obesity: preliminary experience. Obes Surg 2006; 16: 635–637. [DOI] [PubMed] [Google Scholar]
- 6.De Castro ML, Morales MJ, Del Campo V et al. Efficacy, safety, and tolerance of two types of intragastric balloons placed in obese subjects: a double-blind comparative study. Obes Surg 2010; 20: 1,642–1,646. [DOI] [PubMed] [Google Scholar]


