Abstract
Introduction
Antimicrobial prophylaxis remains the most powerful tool used to reduce infection rates in orthopaedics but the choice of antibiotic is complex. The aim of this study was to examine trends in antimicrobial prophylaxis in orthopaedic surgery involving the insertion of metalwork between 2005 and 2011.
Methods
Two questionnaires (one in 2008 and one in 2011) were sent to all National Health Service trusts in the UK using the Freedom of Information Act.
Results
In total, 87% of trusts that perform orthopaedic surgery responded. The use of cefuroxime more than halved between 2005 and 2011 from 80% to 36% and 78% to 26% in elective surgery and trauma surgery respectively. Combination therapy with flucloxacillin and gentamicin rose from 1% to 32% in elective and 1% to 34% in trauma surgery. Other increasingly popular regimes include teicoplanin and gentamicin (1% to 10% in elective, 1% to 6% in trauma) and co-amoxiclav (3% to 8% in elective, 4% to 14% in trauma). The majority of changes occurred between 2008 and 2010. Over half (56%) of the trusts stated that Clostridium difficile was the main reason for changing regimes.
Conclusions
In 2008 a systematic review involving 11,343 participants failed to show a difference in surgical site infections when comparing different antimicrobial prophylaxis regimes in orthopaedic surgery. Concerns over C difficile and methicillin resistant Staphylococcus aureus have influenced antimicrobial regimes in both trauma and elective surgery. Teicoplanin would be an appropriate choice for antimicrobial prophylaxis in both trauma and elective units but this is not reflected in its current level of popularity.
Keywords: Antimicrobial prophylaxis, Infection, Orthopaedics
Infection prevention in orthopaedic surgery is a multifactorial process. Strict theatre protocols,1 antimicrobial use systemically or in bone cement, ultraclean air/laminar flow theatres,2,3 body exhaust suits and bacteriologically occlusive gowning4 are all methods used to reduce infection rates. This is particularly the case in arthroplasty surgery. Given the low incidence of prosthetic joint infections, it has been difficult to prove effectiveness of any of these measures5 but evidence suggests that antimicrobial prophylaxis is the most powerful method for preventing infection.6,7
Antimicrobial prophylaxis has been used in orthopaedic surgery since the 1950s but it was not until 1970, when Fogelberg et al demonstrated a reduction in postoperative infections, that clinical evidence supported the accepted theory.8 This clinical evidence was further substantiated by two randomised double blinded clinical trials proving that antimicrobial prophylaxis, with sodium nafcillin9 or cephaloridine,10 reduced postoperative infection rates in orthopaedic surgery when compared directly with placebos. More recently, a systematic review involving 26 randomised controlled trials (11,343 participants) failed to show superiority of any particular antimicrobial prophylaxis regime.11
Cephalosporins remain the first choice of many international guidelines for antimicrobial prophylaxis in orthopaedic surgery. This has been due to their safety profile, broad spectrum, tissue penetration, price and their early proven effectiveness in clinical trials.12,13 Some have cited marketing and habit as reasons for widespread use of cephalosporins.13,14 The American Academy of Orthopaedic Surgeons recommends either cefuroxime or cefazolin as the first line antimicrobial for patients undergoing arthroplasty procedures.15 Cephalosporins are active against many Gram positive and anaerobic organisms with subsequent generations expanding its Gram negative coverage. Their broad range of microbial cover includes the most common microorganisms encountered in orthopaedic surgery.
A survey by Leach and Wilson in 1990 revealed that 86% of Scottish orthopaedic surgeons were using cephalosporins as prophylaxis for elective hip replacements.16 A similar survey of Canadian orthopaedic surgeons during 2004–2005 revealed that 97% of respondents employed a first generation cephalosporin in total joint arthroplasty.17 The aim of this study was to examine trends in antimicrobial prophylaxis regimes in elective and trauma orthopaedic surgery (involving the insertion of metalwork) from 2005 to 2011 in the UK, and to identify factors (if any) that precipitated changes in prophylactic regimes.
Methods
Using the Freedom of Information Act, two separate questionnaires were sent to all 195 acute care National Health Service (NHS) trusts in the UK. The first was distributed during 2008 and the second during 2011. The questions included were:
Which antimicrobial regime is used for routine prophylaxis in trauma orthopaedic surgery? Please specify dose(s) and time(s) of administration.
Which antimicrobial regime is used for routine prophylaxis in elective orthopaedic surgery? Please specify dose(s) and time(s) of administration.
- Has there been any change in antibiotic or regimen used in the last three years? If ‘yes’:
-
>When was it changed?
-
>Why was it changed?
-
>What was the previous regimen?
-
>
Which antimicrobial regime is used if the patient is allergic to penicillin? Please specify dose(s) and time(s) of administration.
This information was used to create a timeline of which antimicrobial prophylaxis regimes were used between 2005 and 2011 across all UK trusts conducting elective and trauma orthopaedic surgery.
Results
Information collected was divided to define regimes used across a seven-year period encompassing three checkpoints (2005, 2008 and 2011). Of all the UK trusts conducting elective orthopaedic surgery, defined regimes were identified for 152/172 (88%) in 2005, 157/172 (91%) in 2008 and 136/173 (79%) in 2011. For trusts conducting trauma orthopaedic surgery, responses were 151/166 (91%) in 2005, 155/166 (93%) in 2008 and 135/166 (81%) in 2011. The cumulative response rate was 87% of UK trusts.
Elective orthopaedic surgery
Table 1 shows all elective orthopaedic regimes used during 2005, 2008 and 2011. In 2005, 15 different regimes were employed. The top three regimes used were cefuroxime alone (79.6%), other cephalosporins (5.3%) and co-amoxiclav (2.6%). At this time, regimes using flucloxacillin or teicoplanin were in the minority. Two trusts had no formal antimicrobial prophylaxis regime defined in 2005. In 2008, the number of different potential regimes used increased to 18. The top three were cefuroxime alone (59.9%), flucloxacillin plus gentamicin (15.9%) and co-amoxiclav (5.1%). In 2011, 18 different regimes were used. The top three regimes were cefuroxime alone (36.0%), flucloxacillin plus gentamicin (32.4%) and teicoplanin plus gentamicin (9.6%).
Table 1.
The antimicrobial prophylaxis regimes used by UK NHS trusts for elective orthopaedic surgery (involving prosthetic joint insertion/insertion of metalwork)
| Antimicrobial regime used | Number of trusts | ||
| 2005 | 2008 | 2011 | |
| Cefuroxime | 121 (79.6%) | 94 (59.9%) | 49 (36.0%) |
| Cefuroxime + gentamicin | 2 (1.3%) | 1 (0.6%) | 1 (0.7%) |
| Cefuroxime + teicoplanin | 1 (0.7%) | 1 (0.6%) | 1 (0.7%) |
| Cefuroxime +/- teicoplanin | – | 1 (0.6%) | 1 (0.7%) |
| Cefuroxime + metronidazole | 2 (1.3%) | 1 (0.6%) | – |
| Other cephalosporins | 8 (5.3%) | 6 (3.8%) | 2 (1.5%) |
| Flucloxacillin | 1 (0.7%) | 2 (1.3%) | 3 (2.2%) |
| Flucloxacillin + gentamicin | 2 (1.3%) | 25 (15.9%) | 44 (32.4%) |
| Flucloxacillin + benzylpenicillin + gentamicin | – | 1 (0.6%) | 1 (0.7%) |
| Teicoplanin | 1 (0.7%) | 1 (0.6%) | 1 (0.7%) |
| Teicoplanin + gentamicin | 2 (1.3%) | 5 (3.2%) | 13 (9.6%) |
| Co-amoxiclav | 4 (2.6%) | 8 (5.1%) | 11 (8.1%) |
| Co-amoxiclav + gentamicin | 1 (0.7%) | 3 (1.9%) | 2 (1.5%) |
| Ertapenem | – | 1 (0.6%) | 1 (0.7%) |
| Vancomycin | – | – | 1 (0.7%) |
| Gentamicin | – | – | 1 (0.7%) |
| Vancomycin + gentamicin | 1 (0.7%) | 1 (0.6%) | – |
| Cefuroxime or vancomycin | 1 (0.7%) | 1 (0.6%) | 1 (0.7%) |
| Cefuroxime or flucloxacillin | 1 (0.7%) | 1 (0.6%) | 1 (0.7%) |
| Cefuroxime or co-amoxiclav | 2 (1.3%) | 2 (1.3%) | 1 (0.7%) |
| Nil formal | 2 (1.3%) | 2 (1.3%) | 1 (0.7%) |
| Total responses | 152 | 157 | 136 |
Fifty-eight trusts did not change their elective orthopaedic antimicrobial prophylaxis regime across the seven-year period. Between 2005 and 2011, elective antimicrobial prophylaxis regimes changed a total of 88 times (Table 2). The most popular regime adopted was flucloxacillin plus gentamicin (55.7%) while 12.5% changed to teicoplanin plus gentamicin and 11.4% changed to co-amoxiclav alone. The majority of the trusts that changed regimes moved away from cefuroxime (63/88, 71.6%).
Table 2.
Antimicrobial prophylactic regimes that UK NHS trusts changed to during 2005–2011 for orthopaedic surgery (involving prosthetic joint insertion/metalwork insertion)
| Antimicrobial regime | Elective | Trauma |
| Flucloxacillin + gentamicin | 49 (55.7%) | 50 (50.0%) |
| Teicoplanin + gentamicin | 11 (12.5%) | 6 (6.0%) |
| Co-amoxiclav | 10 (11.4%) | 18 (18.0%) |
| Co-amoxiclav + gentamicin | 3 (3.4%) | 4 (4.0%) |
| Cefuroxime | 3 (3.4%) | 1 (1.0%) |
| Flucloxacillin | 2 (2.3%) | 5 (5.0%) |
| Teicoplanin or flucloxacillin | 2 (2.3%) | 1 (1.0%) |
| Cefuroxime +/- teicoplanin | 1 (1.1%) | 1 (1.0%) |
| Cefuroxime + gentamicin | 1 (1.1%) | 1 (1.0%) |
| Vancomycin | 1 (1.1%) | 1 (1.0%) |
| Ertapenem | 1 (1.1%) | 1 (1.0%) |
| Flucloxacillin + benzylpenicillin + gentamicin | 1 (1.1%) | 1 (1.0%) |
| Teicoplanin | 1 (1.1%) | 1 (1.0%) |
| Cefuroxime or flucloxacillin + gentamicin | 1 (1.1%) | 1 (1.0%) |
| Cefuroxime + teicoplanin | 1 (1.1%) | – |
| Piperacillin/tazobactam + gentamicin | – | 1 (1.0%) |
| Vancomycin + gentamicin | – | 1 (1.0%) |
| Amoxicillin + gentamicin | – | 1 (1.0%) |
| Cefuroxime + metronidazole | – | 1 (1.0%) |
| Flucloxacillin + benzylpenicillin | – | 1 (1.0%) |
| Teicoplanin + metronidazole | – | 1 (1.0%) |
| Cefradine + teicoplanin | – | 1 (1.0%) |
| Cefuroxime or teicoplanin | – | 1 (1.0%) |
| Total responses | 88 | 100 |
During the study period, 2008 proved to be the year that had the most changes of elective antimicrobial prophylaxis regimes, with 27 trusts (31%) changing their antimicrobial prophylaxis policy (Fig 1). In 2010, 19 trusts (22%) changed their policy. Owing to poor response quality, it was unclear when five of the trusts changed their regime.
Figure 1.
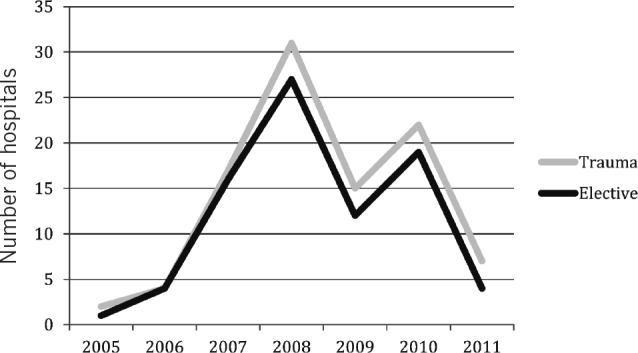
The number of hospitals in the UK that changed antimicrobial prophylaxis regimes for orthopaedic surgery each year
Trauma orthopaedic surgery
Table 3 shows all trauma orthopaedic surgery antimicrobial prophylaxis regimes used during 2005, 2008 and 2011. In 2005, 15 different regimes were used. The top three regimes used were cefuroxime alone (77.5%), other cephalosporins (4.6%) and co-amoxiclav (4.0%). During this time, antimicrobial prophylaxis using flucloxacillin or teicoplanin was infrequent. Two trusts had no formal antimicrobial prophylaxis regime defined during 2005.
Table 3.
Antimicrobial prophylaxis regimes used by UK NHS trusts for trauma orthopaedic surgery (involving prosthetic joint insertion/insertion of metalwork)
| Antimicrobial regime used | Number of trusts | ||
| 2005 | 2008 | 2011 | |
| Cefuroxime | 117 (77.5%) | 83 (53.5%) | 35 (25.9%) |
| Cefuroxime + gentamicin | 3 (2.0%) | 1 (0.6%) | 1 (0.7%) |
| Cefuroxime +/- teicoplanin | – | 1 (0.6%) | 1 (0.7%) |
| Cefuroxime + metronidazole | 3 (2.0%) | 3 (1.9%) | – |
| Cefuroxime + teicoplanin + metronidazole | 1 (0.7%) | 1 (0.6%) | – |
| Other cephalosporins | 7 (4.6%) | 5 (3.2%) | 1 (0.7%) |
| Flucloxacillin | 1 (0.7%) | 3 (1.9%) | 5 (3.7%) |
| Flucloxacillin + gentamicin | 2 (1.3%) | 24 (15.5%) | 46 (34.1%) |
| Flucloxacillin + benzylpenicillin | – | – | 1 (0.7%) |
| Flucloxacillin or teicoplanin | – | – | 1 (0.7%) |
| Flucloxacillin + teicoplanin + metronidazole | 1 (0.7%) | 1 (0.6%) | 1 (0.7%) |
| Flucloxacillin + benzylpenicillin + gentamicin | – | 1 (0.6%) | 1 (0.7%) |
| Flucloxacillin + gentamicin + metronidazole | – | 1 (0.6%) | 1 (0.7%) |
| Teicoplanin | – | – | 1 (0.7%) |
| Teicoplanin + gentamicin | 2 (1.3%) | 3 (1.9%) | 8 (5.9%) |
| Teicoplanin + metronidazole | – | – | 1 (0.7%) |
| Teicoplanin + cefradine | – | 1 (0.6%) | – |
| Co-amoxiclav | 6 (4.0%) | 15 (9.7%) | 19 (14.1%) |
| Co-amoxiclav + gentamicin | 1 (0.7%) | 4 (2.6%) | 3 (2.2%) |
| Amoxicillin + gentamicin | 1 (0.7%) | 1 (0.6%) | 1 (0.7%) |
| Piperacillin/tazobactam + gentamicin | – | – | 1 (0.7%) |
| Ertapenem | – | 1 (0.6%) | 1 (0.7%) |
| Vancomycin | – | – | 1 (0.7%) |
| Vancomycin + gentamicin | 1 (0.7%) | 2 (1.3%) | – |
| Cefuroxime or flucloxacillin | 1 (0.7%) | 1 (0.6%) | 1 (0.7%) |
| Cefuroxime or flucloxacillin + gentamicin | – | – | 1 (0.7%) |
| Cefuroxime or teicoplanin | – | – | 1 (0.7%) |
| Cefuroxime or co-amoxiclav | 2 (1.3%) | 2 (1.3%) | 1 (0.7%) |
| Flucloxacillin or vancomycin or co-amoxiclav | – | – | 1 (0.7%) |
| Nil formal | 2 (1.3%) | 1 (0.6%) | – |
| Total responses | 151 | 155 | 135 |
The total number of different antimicrobial prophylaxis regimes increased in 2008 to 20. The top regimes were cefuroxime alone (53.5%), flucloxacillin plus gentamicin (15.5%) and co-amoxiclav (9.7%).
In 2011, 25 different regimes were used. The top three regimes were flucloxacillin plus gentamicin (34.1%), cefuroxime alone (25.9%) and co-amoxiclav (14.1%).
Forty-one trusts did not change their trauma orthopaedic antimicrobial prophylaxis regime across the seven-year period. During this time, the use of cefuroxime or other cephalosporins alone reduced from 82.1% to 26.6%. The use of flucloxacillin plus gentamicin rose from 1.3% in 2005 to 34.1% in 2011 and was the most popular antimicrobial prophylaxis regime.
Trauma orthopaedic surgical antimicrobial prophylaxis regimes changed a total of 100 times during the study period (Table 2). The most popular regime adopted was flucloxacillin plus gentamicin (50.0%) while 18.0% changed to coamoxiclav alone and 6.0% to teicoplanin plus gentamicin. The majority of trusts that changed regimes moved away from cephalosporins (83/100, 83.0%).
As for elective surgery regimes, 2008 also proved to be the year that had the most changes of trauma antimicrobial prophylaxis regimes. Thirty-one trusts (31.0%) changed regimes (Fig 1). This was followed by 2010 (22/100, 22.0%). The fewest changes took place in 2005 (2/100, 2.0%). Owing to poor response quality, it was unclear when two trusts changed their policy.
Reasons for changing
Reasons for changing orthopaedic antimicrobial prophylaxis regimes varied across elective and trauma units (Table 4). The most common reported reason was association/fear of Clostridium difficile. This was reported by 55.5% of elective and 56.0% of trauma units. The desire to reduce cephalosporin use (11.4% elective, 10.0% trauma) and advice from microbiology departments (8.0% elective, 8.0% trauma) were the second and third most common reasons.
Table 4.
Reasons given by hospitals for a change in orthopaedic surgery antimicrobial prophylaxis regimes
| Reasons | Elective | Trauma |
| Clostridium difficile | 48 (54.5%) | 56 (56.0%) |
| Reduce use of cephalosporins | 10 (11.4%) | 10 (10.0%) |
| Microbiology advice | 7 (8.0%) | 8 (8.0%) |
| Antibiotic policy review | 6 (6.8%) | 6 (6.0%) |
| Unsure why changed | 5 (5.7%) | 7 (7.0%) |
| Scottish Intercollegiate Guidelines Network | 3 (3.4%) | 5 (5.0%) |
| Supply shortages | 2 (2.3%) | 1 (1.0%) |
| Surveillance/resistance patterns | 2 (2.3%) | 1 (1.0%) |
| Department of Health advice | 1 (1.1%) | 2 (2.0%) |
| Methicillin resistant Staphylococcus aureus | 1 (1.1%) | 1 (1.0%) |
| New regime started | 1 (1.1%) | 1 (1.0%) |
| Trust board advice | 1 (1.1%) | 1 (1.0%) |
| Reduce spectrum of coverage | 1 (1.1%) | – |
| Ease of use | – | 1 (1.0%) |
| Total responses | 88 | 100 |
Penicillin allergy
Table 5 shows all the antimicrobial prophylaxis regimes used in 2008 and 2011 for patients with penicillin allergy. In 2008 in elective surgery, the most popular regime was teicoplanin plus gentamicin (21.2%). This increased to 41.5% in 2011. In trauma surgery, a similar pattern was evident with teicoplanin plus gentamicin used most frequently in 2008 (20.8%) and in 2011 (39.4%). Vancomycin and cefuroxime use reduced over this time period. In 2011 teicoplanin alone or in combination with gentamicin constituted 69.2% of elective and 63.8% of trauma antimicrobial prophylaxis regimes.
Table 5.
The antimicrobial prophylaxis regimes used in orthopaedic surgery (involving the insertion of metalwork) in patients with penicillin allergy
| Antimicrobial regime | Number of elective trusts | Number of trauma trusts | ||
| 2008 | 2011 | 2008 | 2011 | |
| Vancomycin | 25 (17.1%) | 12 (9.2%) | 25 (17.4%) | 11 (8.7%) |
| Vancomycin + gentamicin | 6 (4.1%) | – | 4 (2.8%) | – |
| Teicoplanin | 26 (17.8%) | 36 (27.7%) | 24 (16.7%) | 31 (24.4%) |
| Teicoplanin + gentamicin | 31 (21.2%) | 54 (41.5%) | 30 (20.8%) | 50 (39.4%) |
| Teicoplanin + gentamicin + metronidazole | – | – | 1 (0.7%) | 2 (1.6%) |
| Erythromycin | 11 (7.5%) | 4 (3.1%) | 11 (7.6%) | 4 (3.1%) |
| Clarithromycin | 2 (1.4%) | 5 (3.8%) | 2 (1.4%) | 3 (2.4%) |
| Clarithromycin + gentamicin | – | – | – | 1 (0.8%) |
| Clindamycin | 2 (1.4%) | 2 (1.5%) | 2 (1.4%) | 3 (2.4%) |
| Clindamycin + gentamicin | 1 (0.7%) | 2 (1.5%) | 1 (0.7%) | 3 (2.4%) |
| Gentamicin | 8 (5.5%) | 4 (3.1%) | 8 (5.6%) | 4 (3.1%) |
| Ertapenem | 1 (0.7%) | 1 (0.8%) | 1 (0.7%) | 1 (0.8%) |
| Cefuroxime | 24 (16.4%) | 4 (3.1%) | 25 (17.4%) | 5 (3.9%) |
| Cefuroxime + gentamicin | 1 (0.7%) | – | 1 (0.7%) | – |
| Ceftriaxone + gentamicin | 2 (1.4%) | – | 2 (1.4%) | – |
| Aztreonam + vancomycin + metronidazole | – | – | – | 1 (0.8)% |
| Gentamicin + metronidazole | – | – | – | 1 (0.8%) |
| Clarithromycin or teicoplanin | 1 (0.7%) | – | 1 (0.7%) | – |
| Vancomycin or gentamicin | 1 (0.7%) | – | 1 (0.7%) | – |
| Vancomycin or teicoplanin | 1 (0.7%) | – | 1 (0.7%) | – |
| Clindamycin or teicoplanin | – | 1 (0.8%) | – | 1 (0.8%) |
| Teicoplanin or erythromycin | – | 1 (0.8%) | – | 1 (0.8%) |
| Microbiology advice | 2 (1.4%) | 2 (1.5%) | 3 (2.1%) | 3 (2.4%) |
| Surgeon's choice | 1 (0.7%) | 2 (1.5%) | 1 (0.7%) | 2 (1.6%) |
| Total responses | 146 | 130 | 144 | 127 |
Discussion
During the late 1990s and up to the early 2000s, 86–97% of hospitals across the developed world were using cephalosporins as antimicrobial prophylaxis for elective orthopaedic surgery.16–19 This study shows a gradual decline in the use of cephalosporins in the UK, in both elective orthopaedics (85% to 38%) and trauma orthopaedic surgery (82% to 27%).
Systematic reviews of randomised controlled trials have not shown any difference in prosthetic joint infection rates when comparing cephalosporin, teicoplanin and penicillin derivatives as antimicrobial prophylaxis.11,20 These may be essential findings to justify the use of new antimicrobial prophylaxis regimes in arthroplasty surgery but issues including resistance patterns, methicillin resistant Staphylococcus aureus (MRSA) and C difficile infections may also affect antibiotic choice. Studies have highlighted cost and local availability as other key concerns when selecting an antimicrobial prophylaxis regime.11,20
Concluding studies have advised against single centre comparisons of orthopaedic antimicrobial prophylaxis given the large number of patients required to show any significant difference in surgical site infection (SSI) rates.11 For example, to demonstrate a reduction in infection rate from 2% to 1% with a power of 90%, at the 95% confidence interval, a study would need over 3,000 patients per group.
MRSA
Patients colonised with MRSA have a higher risk of SSI than other orthopaedic admissions.21 Cephalosporins are of little use in the prevention of MRSA infections22 and the American Academy of Orthopaedic Surgeons recommendations advise the addition of vancomycin in patients who are known to be colonised with MRSA or in response to an MRSA outbreak.15
Over the past decade, an increase in acquired MRSA skin and soft tissue infections has been demonstrated in American communities.23,24 In contrast, according to European Antimicrobial Resistance Surveillance Network reports, the proportion of MRSA infections has remained static across Europe since 2009.25,26 The UK Health Protection Agency’s microorganism breakdown also shows that over two separate time periods the percentage of MRSA prosthetic joint infections has remained static.27 In the UK between 2004 and 2008, 26.6% of prosthetic joint infections were due to MRSA, and between 2008 and 2010 the incidence was 26.0%. This suggests that infection control measures, in hospitals and in the community, have successfully controlled the growth of MRSA infections. However, given the morbidity and mortality associated with infection, MRSA remains a significant pathogen against which orthopaedic surgeons must remain vigilant.
There have been doubts regarding the accuracy of prescreening for MRSA in elective admissions. One study showed that almost 10% of elective orthopaedic patients who were MRSA negative at preassessment were in fact MRSA positive on admission to the ward.28 In the trauma setting, its has been shown that up to 86% of neck of femur fracture patients reach the operating theatre before MRSA screening results are obtained.29
Clostridium difficile
It was during the 1980s that an association between antimicrobial prophylaxis in orthopaedic surgery and the development of C difficile associated diarrhoea was noted.30–32 Higher numbers of C difficile infections were noted in institutes using clindamycin or cephalosporins as antimicrobial prophylaxis.33 The widespread use of these antibiotics was criticised by the microbiology community and their use was restricted in hospital settings. Studies revealed that the use of cephalosporins was an important predisposing factor for the development of C difficile infections and the restriction of cephalosporins has reduced C difficile outbreaks.33,34
The overall incidence of C difficile infection in orthopaedic trauma patients has been reported as being 0.6%35 but incidences of 4–6% have been reported in the fractured neck of femur cohort.36,37 C difficile is far less of a problem among elective orthopaedic patients than trauma orthopaedic patients, with reported incidences of 0.1–0.17%.35,38 Jenkins et al concluded that, given the low incidence of C difficile in arthroplasty patients, cephalosporins were a safe option as antimicrobial prophylaxis in elective surgery.38
C difficile continues to be an important cause of morbidity and mortality in orthopaedic trauma patients and measures must be put in place to reduce risk factors.36 We have demonstrated previously how trusts changing away from cephalosporins reduced their C difficile infection rates in orthopaedic surgery significantly.35 Al-Obaydi et al mirrored this message after a change away from cefuroxime in a single unit reduced C difficile infections.39
Teicoplanin
Teicoplanin is a glycopeptide antimicrobial agent that offers high soft tissue and bone penetration. Concentration of teicoplanin in bone reaches 65% of serum concentration, with a peak occurring between 0.5 and 6 hours after intravenous administration.40
Four randomised controlled trials have compared joint infection rates in patients undergoing total hip and knee arthroplasty who received teicoplanin versus a cephalosporin (cefazolin, cefamandole or cefuroxime).41–44 No individual trial showed a significant difference in infection rates and a summarising study that pooled data also showed there was no significant difference in infection rates.40 Furthermore, the regional use of teicoplanin in total knee arthroplasty following tourniquet inflation has been proven to be effective.45
We have shown that the use of teicoplanin alone or in combination with gentamicin increased from 2.0% to 10.3% in elective surgery and from 1.3% to 6.7% in trauma surgery between 2005 and 2011. This regime is not as popular as one may have envisaged given the ease of use, effectiveness against SSI, coverage of common orthopaedic pathogens and benefits in treating MRSA. These clinical advantages are also mirrored with a financial change with a reduction in the price of teicoplanin from £52.40 in 199546 to £6.00 in 2011.47
Study limitations
Our study was based on a national questionnaire distributed, via the Freedom of Information Act, to all UK NHS trusts. Consequently, the information presented is dependent on the quality and accuracy of responses received. The average response rate across the three checkpoint years was 87%. Failure of some hospitals to respond does reduce the accuracy of our results but we feel that an 87% response rate from across the UK provides an acceptable representation of current UK practice. The trends in antimicrobial prophylaxis are apparent and potential future issues can be drawn from these changes.
Conclusions
Over the past seven years, the use of cephalosporin as orthopaedic antimicrobial prophylaxis has declined in the UK. This is likely to be due to multiple factors but in orthopaedic trauma surgery concerns over C difficile appear to have driven this change. MRSA and antimicrobial resistance patterns will continue to affect antimicrobial prophylaxis regimes in the future. Flucloxacillin or teicoplanin based regimes are becoming more popular, with teicoplanin having the added advantage of MRSA coverage. No regime has been proven to reduce SSI over any other and this may be a target for future research to provide evidence for the ideal prophylaxis regime.
References
- 1.Dickson GH. Deterioration of theatre discipline during total joint replacement: have theatre protocols been abandoned? Ann R Coll Surg Engl 2001; 83: 296. [PMC free article] [PubMed] [Google Scholar]
- 2.Charnley J. Postoperative infection after total hip replacement with special reference to air contamination in the operating room. Clin Orthop Relat Res 1972; 87: 167–187. [DOI] [PubMed] [Google Scholar]
- 3.Lidwell OM, Elson RA, Lowbury EJ et al. . Ultraclean air and antibiotics for prevention of postoperative infection. A multicenter study of 8,052 joint replacement operations. Acta Orthop Scand 1987; 58: 4–13. [DOI] [PubMed] [Google Scholar]
- 4.Gulihar A, Taub NA, Taylor GJ. A randomised prospective comparison of Rotecno versus new Gore occlusive surgical gowns using bacterial air counts in ultraclean air. J Hosp Infect 2009; 73: 54–57. [DOI] [PubMed] [Google Scholar]
- 5.Evans RP. Current concepts for clean air and total joint arthroplasty: laminar airflow and ultraviolet radiation. Clin Orthop Relat Res 2011; 469: 945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lidwell OM, Lowbury EJ, Whyte W et al. . Effect of ultraclean air in operating rooms on deep sepsis in the joint after total hip or knee replacement: a randomised study. Br Med J (Clin Res Ed) 1982; 285: 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lidwell OM, Lowbury EJ, Whyte W et al. . Infection and sepsis after operations for total hip or knee-joint replacement: influence of ultraclean air, prophylactic antibiotics and other factors. J Hyg 1984; 93: 505–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fogelberg EV, Zitzmann EK, Stinchfield FE. Prophylactic penicillin in orthopaedic surgery. J Bone Joint Surg Am 1970; 52: 95–98. [PubMed] [Google Scholar]
- 9.Boyd RJ, Burke JF, Colton T. A double-blind clinical trial of prophylactic antibiotics in hip fractures. J Bone Joint Surg Am 1973; 55: 1,251–1,258. [PubMed] [Google Scholar]
- 10.Paval A, Smith RL, Ballard A, Larsen IJ. Prophylactic antibiotics in clean orthopaedic surgery. J Bone Joint Surg Am 1974; 56: 777–782. [PubMed] [Google Scholar]
- 11.AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br 2008; 90: 915–919. [DOI] [PubMed] [Google Scholar]
- 12.Hill C, Flamant R, Mazas F, Evrard J. Prophylactic cefazolin versus placebo in total hip replacement. Lancet 1981; 1: 795–796. [DOI] [PubMed] [Google Scholar]
- 13.McEniry DW, Gorbach SL. Cephalosporins in surgery. Prophylaxis and therapy. Drugs 1987; 34: 216–239. [DOI] [PubMed] [Google Scholar]
- 14.Gorbach SL. The role of cephalosporins in surgical prophylaxis. J Antimicrob Chemother 1989; 23: 61–70. [DOI] [PubMed] [Google Scholar]
- 15.American Academy of Orthopaedic Surgeons Information Statement: Recommendations for the Use of Intravenous Antibiotic Prophylaxis in Primary Total Joint Arthroplasty. Rosemont, IL: AAOS; 2004. [Google Scholar]
- 16.Leach WJ, Wilson NI. Trends in infection prophylaxis in orthopaedics. J R Coll Surg Edinb 1992; 37: 265–266. [PubMed] [Google Scholar]
- 17.de Beer J, Petruccelli D, Rotstein C et al. . Antibiotic prophylaxis for total joint replacement surgery: results of a survey of Canadian orthopaedic surgeons. Can J Surg 2009; 52: E229–234. [PMC free article] [PubMed] [Google Scholar]
- 18.Engesaeter LB, Lie SA, Espehaug B et al. . Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0–14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand 2003; 74: 644–651. [DOI] [PubMed] [Google Scholar]
- 19.Peel TN, Cheng AC, Buising KL, Choong PF. The microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother 2012; 56: 2,386–2,391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glenny A, Song F. Antimicrobial prophylaxis in total hip replacement: a systematic review. Health Technol Assess 1999; 3: 1–57. [PubMed] [Google Scholar]
- 21.Murphy E, Spencer SJ, Young D et al. . MRSA colonisation and subsequent risk of infection despite effective eradication in orthopaedic elective surgery. J Bone Joint Surg Br 2011; 93: 548–551. [DOI] [PubMed] [Google Scholar]
- 22.Fluit AC, Wielders CL, Verhoef J, Schmitz FJ. Epidemiology and susceptibility of 3,051 Staphylococcus aureus isolates from 25 university hospitals participating in the European SENTRY study. J Clin Microbiol 2001; 39: 3,727–3,732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fridkin SK, Hageman JC, Morrison M et al. . Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med 2005; 352: 1,436–1,444. [DOI] [PubMed] [Google Scholar]
- 24.Hota B, Ellenbogen C, Hayden MK et al. . Community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections at a public hospital: do public housing and incarceration amplify transmission? Arch Intern Med 2007; 167: 1,026–1,033. [DOI] [PubMed] [Google Scholar]
- 25.European Centre for Disease Prevention and Control Antimicrobial Resistance Surveillance in Europe 2009. Stockholm: ECDC; 2010. [Google Scholar]
- 26.European Centre for Disease Prevention and Control Antimicrobial Resistance Surveillance in Europe 2010. Stockholm: ECDC; 2011. [Google Scholar]
- 27.Health Protection Agency Sixth Report of the Mandatory Surveillance of Surgical Site Infection in Orthopaedic Surgery. London: HPA; 2010. [Google Scholar]
- 28.Walley G, Orendi J, Bridgman S et al. . Methicillin resistant Staphylococcus aureus (MRSA) is not always caught on the orthopaedic ward. Acta Orthop Belg 2009; 75: 245–251. [PubMed] [Google Scholar]
- 29.Bryson DJ, Gulihar A, Aujla RS, Taylor G. The hip fracture best practice tariff: early surgery and the implications for MRSA screening and antibiotic prophylaxis. Inj Extra 2012; 43: 78–79. [DOI] [PubMed] [Google Scholar]
- 30.Sankarankutty M, McGeorge D, Galasko CS. Pseudomembranous colitis following cephradine prophylaxis. Postgrad Med J 1982; 58: 726–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts AP, Hughes AW. Complications with antibiotics used prophylactically in joint replacement surgery: a case report of cephradine-induced pseudomembranous colitis. Int Orthop 1985; 8: 299–302. [DOI] [PubMed] [Google Scholar]
- 32.Cannon SR, Dyson PH, Sanderson PJ. Pseudomembranous colitis associated with antibiotic prophylaxis in orthopaedic surgery. J Bone Joint Surg Br 1988; 70: 600–602. [DOI] [PubMed] [Google Scholar]
- 33.McNulty C, Logan M, Donald IP et al. . Successful control of Clostridium difficile infection in an elderly care unit through use of a restrictive antibiotic policy. J Antimicrob Chemother 1997; 40: 707–711. [DOI] [PubMed] [Google Scholar]
- 34.Nelson DE, Auerbach SB, Baltch AL et al. . Epidemic Clostridium difficile-associated diarrhea: role of second- and third-generation cephalosporins. Infect Control Hosp Epidemiol 1994; 15: 88–94. [DOI] [PubMed] [Google Scholar]
- 35.Aujla RS, Peysakhova E, Gulihar A, Taylor GJ. Orthopaedic antimicrobial prophylaxis in the United Kingdom. Eur J Orthop Surg Traumatol 2011; 21: 21–25. [Google Scholar]
- 36.Gulihar A, Nixon M, Taylor G. Reducing Clostridium difficile infection and mortality in fracture neck of femur patients. J Bone Joint Surg Br 2010; 92 Supp 2: 314–315. [Google Scholar]
- 37.Kakwani R, Chakrabarti D, Katam K, Wahab K. C difficile infection: morbidity and mortality following fracture neck of femur. J Bone Joint Surg Br 2011; 93 Supp 1: 40–41. [Google Scholar]
- 38.Jenkins PJ, Teoh K, Simpson PM et al. . Clostridium difficile in patients undergoing primary hip and knee replacement. J Bone Joint Surg Br 2010; 92: 994–998. [DOI] [PubMed] [Google Scholar]
- 39.Al-Obaydi W, Smith CD, Foguet P. Changing prophylaxis antibiotic protocol for reducing Clostridium difficile-associated diarrhoeal infections. J Orthop Surg 2010; 18: 320–323. [DOI] [PubMed] [Google Scholar]
- 40.Periti P, Mini E, Mosconi G. Antimicrobial prophylaxis in orthopaedic surgery: the role of teicoplanin. J Antimicrob Chemother 1998; 41: 329–340. [DOI] [PubMed] [Google Scholar]
- 41.Mollan RA, Webb CH, Haddock M. A comparison of teicoplanin vs cefamandole in orthopaedic surgical prophylaxis. In: Program and Abstracts of the 7th European Congress of Clinical Microbiology and Infectious Diseases, Vienna: 1995. p147. [Google Scholar]
- 42.Periti P, Stringa G, Mini E. Comparative multicenter trial of teicoplanin versus cefazolin for antimicrobial prophylaxis in prosthetic joint implant surgery. Eur J Clin Microbiol Infect Dis 1999; 19: 113–119. [DOI] [PubMed] [Google Scholar]
- 43.Suter F, Avai A, Fusco U et al. . Teicoplanin versus cefamandole in the prevention of infection in total hip replacement. Eur J Clin Microbiol Infect Dis 1994; 13: 793–796. [DOI] [PubMed] [Google Scholar]
- 44.Wall R, Klenerman L, McCullough C, Fyfe I. A comparison of teicoplanin and cefuroxime as prophylaxis for orthopaedic implant surgery: a preliminary report. J Antimicrob Chemother 1998; 21: 141–146. [DOI] [PubMed] [Google Scholar]
- 45.de Lalla F, Viola R, Pellizzer G et al. . Regional prophylaxis with teicoplanin in monolateral or bilateral total knee replacement: an open study. Antimicrob Agents Chemother 2000; 44: 316–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davey PG, South R, Malek M. Impact of glycopeptide therapy after hospital discharge on inpatient costs: a comparison of teicoplanin and vancomycin. J Antimicrob Chemother 1996; 37: 623–633. [DOI] [PubMed] [Google Scholar]
- 47.Joint Formulary Committee British National Formulary 62. London: Pharmaceutical Press; 2011. [Google Scholar]


