Abstract
Introduction
The effect of parathyroidectomy on the incidence of recurrent stone formation is uncertain. We aimed to compare the biochemistry and recurrence rate of urolithiasis in patients with primary hyperparathyroidism (pHPT) and stone formation (SF) and non-stone formation (NSF) with idiopathic stone formers (ISF).
Methods
Patients with pHPT and SF (Group 1) were identified from a prospective database. pHPT patients and NSF (Group 2) and ISFs (Group 3) were randomly selected from respective databases to form three equal groups. Preoperative and postoperative biochemical data were analysed and recurrent urolithiasis diagnosed if present on follow-up radiology. Out-of-area patients were asked about recurrence via telephone.
Results
From July 2002 to October 2011, 640 patients had parathyroidectomy for pHPT. Of these, 66 (10.3%) had a history of renal colic; one was lost to follow-up. Patient demographics were similar across all three groups. Three months post-parathyroidectomy, Groups 1 and 2 had significantly reduced serum calcium concentrations (p<0.01). Group 1 had lower urinary calcium excretion after parathyroidectomy (p<0.01), but estimated glomerular filtration rate did not change following surgery. During median follow-up of 4.33 years (0.25–9 years) in Groups 1 and 2 and 5.08 years (0.810–8 years) in Group 3, one patient (1.5%) in Group 1 and 16 patients (25%) in Group 3 had recurrent urolithiasis (p<0.01). No Group 2 patients developed stones.
Conclusion
Curative parathyroidectomy confers a low recurrence rate for urolithiasis, but does not prevent recurrence in all patients. Further research should aim to identify the risk factors for continued SF in these patients.
Keywords: Hyperparathyroidism, Primary, Parathyroidectomy, Urolithiasis, Kidney Calculi, Hypercalciuria
Renal SF is a significant health problem with an increasing prevalence – the lifetime risk of urolithiasis is 12% in men and 6% in women.1 Furthermore, a number of systemic factors are now understood to play a role in renal stone formation (Table 1).1–6 Hypercalciuria secondary to primary hyperparathyroidism (pHPT) is associated with an increased risk of urolithiasis, but the underlying reasons why only a proportion of patients with pHPT will form renal stones are not clearly understood. With a trend toward earlier diagnosis of pHPT and the subsequent treatment of milder disease,6 the number of patients with pHPT and concurrent renal SF is falling,7 but there is still an estimated 7% of patients with pHPT who suffer from urolothiasis.8
Table 1.
Factors known to influence renal SF (1–6)
| Promote SF | Inhibit SF |
| Family history | |
| Urinary calcium | |
| Urinary tract infection | ↑Fluid intake |
| Oxalate | Citrate |
| Urate | Urinary macromolecules* |
| Cystine | –Uromodulin |
| Poor urine flow | –Nephrocalcin |
| ↓Urinary pH | –Trefoil factor |
| Obesity | ↑Urinary pH |
| Systemic disorders: | Female gender |
| Diabetes Gout Crohn’s disease | |
| Primary hyperparathyroidsm |
These molecules generally bind cations and anions in the urine to increase the point at which it becomes supersaturated and precipitation may occur
The effect of parathyroidectomy on recurrent stone formation is uncertain. In several studies, the risk of recurrent stone disease has been shown to decrease following parathyroidectomy,9–12 but other investigators have demonstrated no discernable benefit in reducing recurrent episodes.13–15 There is also evidence that the risk of recurrent SF, although significantly reduced following surgery, remains higher than that of a similar population of non-pHPT controls for up to 10 years postoperatively.16 Outside the setting of pHPT, prior history of idiopathic ISF is also a risk factor for further SF, with early reports suggesting that without intervention, recurrence rates could be as high as 50% in the first 5 years.13 More recently however, the quoted expected recurrence rate for ISF is closer to 30%.11,13,16
The aim of this study is to compare the serum and urinary biochemical markers of pHPT patients with SF SF and no stone formation NSF undergoing parathyroidectomy with ISF to identify any biochemical differences that may be responsible for SF. We also aim to determine the recurrence rate of symptomatic renal stone disease in all three groups during follow-up.
Methods
Study population
From July 2002 to October 2011, 640 patients underwent parathyroidectomy for primary hyperparathyroidism (pHPT) under 2 consultant endocrine surgeons. Of these, 66 (10.3%) patients with a history of renal SF (pHPT and SF) were identified from an endocrine surgery database (Group 1). The database is prospectively maintained by consultant endocrine surgeons at our centre and includes demographic data on all patients who have undergone endocrine surgery since 2002. The data include preoperative and intraoperative biochemistry and histology results, as well as relevant clinical presentations such as renal stones, depression and family history.
Seventeen (26%) of the 66 pHPT and SF patients had travelled from out-of-area for their surgery, one of which was lost to follow-up (n=65). Of the remaining patients (pHPT and NSF), an equal number were selected from the endocrine surgery database at random (every ninth patient), to form a comparable cohort of patients (Group 2). A third group of equal size was formed by a selection at random (every ninth patient) from a prospectively maintained database of ISFs attending a specialist metabolic stone clinic at the same centre (Group 3). The diagnosis of pHPT had been excluded in these patients. This database is prospectively maintained by the consultant clinical biochemist at our centre, and includes demographic data on all patients referred with renal stones, biochemical serum and urine results, date of diagnosis of SF/recurrent as well as any available results on stone composition.
Data collection and follow-up
Baseline serum and urine biochemical data were obtained for patients from all three groups via their respective databases following collection as part of routine preoperative assessments or during referral to clinic. Estimated glomerular filtration rate (eGFR) was calculated using the abbreviated modification of diet in renal disease (MDRD) equation (186 × (serum creatinine/88.4)–1.154 × (age)–0.203 × (0.742 if female) × (1.210 if Afro-Caribbean in ethnic origin). Results greater than 90mL/min/1.73m2 were simply recorded as 90 for the purposes of analysis. For Groups 1 and 2, postoperative serum biochemistry samples were collected in the outpatient clinic at a median period of 7.5 months (3–12 months) after surgery. Urine biochemical analysis, where available, was collected at a median period of 4.5 years (1–10 years) post-parathyroidectomy.
Electronic case files were retrospectively reviewed and recurrence of renal stones was defined as a patient having new stones observed on any follow-up radiology. Patients who travelled from out of area to undergo surgery were contacted by telephone to enquire about recurrence of symptoms of renal stone disease. Follow-up for renal SF was a median of 4.33 years (0.25–9 years) for Groups 1 and 2 and a median of 5.08 years (0.8–10.8 years) for Group 3. Statistical significance was defined as p≤ 0.05.
Results
The three groups were similar in terms of patient demographics and the histopathological description of parathyroid glands removed was similar in pHPT patients with and without SF (Groups 1 and 2, Table 2).
Table 2.
Comparison of demographics (Groups 1, 2 and 3) and postoperative histopathology of excised parathyroid tissue (Groups 1 and 2 only)
| Variable | Group 1 pHPT and SF | Group 2 pHPT and NSF | Group 3 ISF | p value |
| n | 65 | 65 | 65 | |
| Median age, y (range)* | 54(18-89) | 57(26-81) | 59(19-88) | 0.27 |
| M:F ratio** | 34F:31M | 35F:30M | 35F:30M | 0.98 |
| Parathyroid | ||||
| Pathology | 58 adenoma | |||
| 60 adenoma | ||||
| 6 hyperplasia | 5 hyperplasia | |||
| 1 normal |
Kruskal Wallis ANOVA,
X2 = 0.041, 2 degrees of freedom
Baseline serum biochemical analysis of mean/median serum concentrations of calcium, PTH and vitamin D in patients from Groups 1 and 2 revealed no significant differences in the severity of hypercalcaemia or elevation in parathyroid hormone secretion that might account for the difference in previous renal SF in Group 1 (Table 3). Patients in all three groups displayed similar mean serum creatinine and eGFR preoperatively and, as expected, Groups 1 and 2 showed higher serum calcium and PTH concentrations and lower serum phosphate when compared with Group 3. However, following parathyroidectomy, the serum levels in Groups 1 and 2 became similar to Group 3 (Table 3).
Table 3.
Comparison of serum biochemistry before and after parathyroidectomy for Groups 1, 2 and 3
| Variable | Group 1 hPTH and SF | n | Group 2 hPTH and NSF | n | Group 3 ISF | n | ANOVA p value |
| Mean baseline calcium ±SD mmol/L | 2.81±0.22 | 65 | 2.88±0.26 | 65 | 2.37±0.11† | 62 | <0.0001 |
| Mean postoperative calcium ±SD mmol/L | 2.36±0.11** | 65 | 2.38±0.22** | 65 | N/D | ||
| Median baseline PTH (range) pmol/L | 12.6 (2.3–54) | 65 | 14.1 (5.7–115) | 65 | 3.30 (1.7–6.3)† | 9 | <0.0001 |
| Median postoperative PTH (range) pmol/L | 5.65 (0.9– 16.2)** | 55 | 4.9 (1.7– 11.9)** | 53 | N/D | ||
| Mean baseline vitamin D ±SD µg/L | 18.21±10.92 | 44 | 15.84±6.45 | 51 | 28.08±14.72† | 4 | 0.02 |
| Mean baseline phosphate ±SD mmol/L | 0.77±0.19† | 65 | 0.89±0.18† | 65 | 1.03±0.24† | 40 | <0.0001 |
| Mean postoperative phosphate ±SD mmol/L | 1.01±0.26* | 38 | 1.08±0.21* | 56 | N/D | ||
| Mean baseline creatinine ±SD µmol/L | 83.34±17.17 | 65 | 87.98±21.46 | 65 | 86.2±21.22 | 65 | 0.412 |
| Mean postoperative creatinine ±SD µmol/L | 81.43±18.48 | 65 | 89.57±20.08 | 65 | N/D | ||
| Mean baseline eGFR ±SD mL/min/1.73m2 | 76.88±12.83 | 65 | 71.38±17.98 | 65 | 75.92±15.50 | 65 | 0.102 |
| Mean postoperative eGFR ±SD mL/min/1.73m2 | 77.82±13.82 | 65 | 70.95±15.22 | 65 | N/D |
One-way analysis of variance used, apart from PTH which was assessed using the Kruskal-Wallis one-way ANOVA test. Post-hoc analysis was performed using Tukey’s Test.
p<0.05 versus equivalent baseline value
p<0.01 versus equivalent baseline value
Significantly different from baseline values in other groups
Interestingly, patients with hyperparathyroidism (Groups 1 and 2) had lower serum vitamin D levels than ISF (Group 3) (p=0.02, ANOVA). Similarly, serum phosphate concentrations varied significantly between all three groups, and were significantly lower in Group 1 compared with both Group 2 and Group 3 (p<0.0001, ANOVA).
Twenty-four hour urine calcium excretion was available in a subgroup of patients, and this revealed elevated concentrations in Groups 1 and 2 preoperatively (Table 4) (p=0.15, ANOVA). Repeat samples from Group 1 obtained in the postoperative period revealed a statistically significant decrease from a mean of 8.74 to 4.14 (p<0.01, Student’s t-test) following surgery.
Table 4.
Comparison of urine biochemistry of patients for groups 1, 2 and 3
| Variable | Group 1 hPTH and SF mean (range) | n | Group 2 hPTH and NSF mean (range) | n | Group 3 ISF mean (range) | n | ANOVA p value |
| Mean baseline 24-hour calcium ±SD mmol/L | 8.74±2.21 | 12 | 8.46±4.96 | 21 | 6.14±2.85 | 15 | 0.15 |
| Mean postoperative 24-hour calcium (±SD) mmol/L | 4.14±2.00* | 9 | N/D | 0 | N/D |
p<0.01 compared with baseline figure
During follow-up, one patient (1.5%) from Group 1 developed proven recurrence of renal stones seen on follow-up imaging after an acute admission. No patients from Group 2 developed new stones, whereas 16 patients with ISF (25%) developed recurrent stone disease (p<0.01 c2=18.654, 2 degrees of freedom), with 4 (6%) patients experiencing more than one episode of recurrent urolithiasis. No patients from out-of-area went on to experience further symptoms from or imaging for renal stones. Kaplan-Meier analysis demonstrated that the median five-year recurrence of symptomatic stone disease in ISF was just over 20% compared with 1.5% in SF with pHPT undergoing surgery (p<0.001, logrank test, Fig 1).
Figure 1.
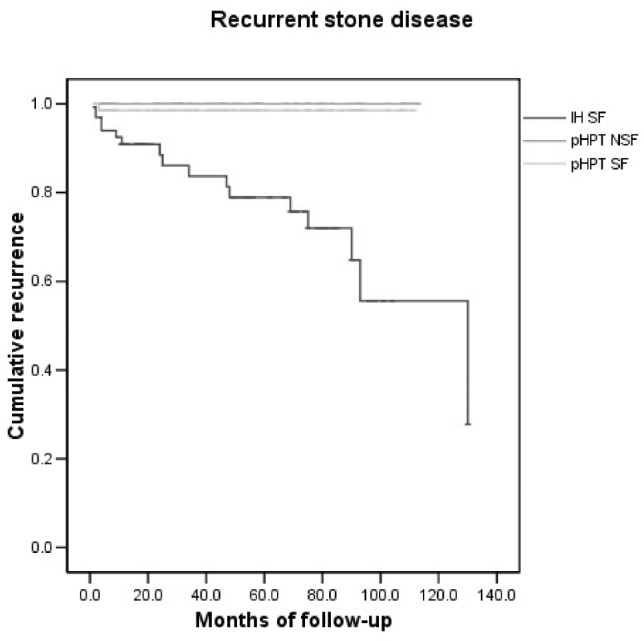
Kaplan-Meier analysis of time from initial treatment until clinically recurrent renal stone disease in Groups 1, 2 and 3 (p<0.001, logrank test)
Discussion
In this study we observed a low incidence (1.5%) of symptomatic recurrent renal stone disease following parathyroidectomy in patients with a prior history of renal SF, when compared to ISFs, of whom 25% experienced recurrence during follow-up. This rate is in keeping with the previously suggested figure of 30% recorded in the literature.
As previously described, there were no discernible differences in the standard serum or urinary biochemistry when patients with pHPT and SF were compared with pHPT and NSF, with the exception of serum phosphate, which was significantly lower in SF with pHPT compared with NSF. Two authors18,19 have previously reported this finding, and one possible explanation would be that higher renal excretion of phosphate in these patients combined with hypercalciuria could cause precipitation in renal tubules. No patients in our study underwent urinary phosphate analysis, but further work to evaluate the role of phosphaturia is required to determine this.
The finding of significantly higher serum levels of Vitamin D in Group 3 compared with Groups 1 and 2 is noted, and desirable Vitamin D levels in our centre is 30–100µg/L, showing that even ISF were borderline Vitamin D deficient in this study. However, caution must be made over the significance of this finding, with only 4/65 patients in Group 3 having Vitamin D assays available for comparison and in the presence of such a wide-ranging standard deviation.
Urinary calcium excretion fell significantly postoperatively in patients with a history of SF – a finding in keeping with a number of other studies14,20,21. However one patient, in spite of this, went on to experience recurrent SF. This patient (Group 1) was male and younger than the median age, in keeping with a previous study that identified these features as risk factors for recurrent SF after successful parathyroidectomy.16 This paper also reported that risk of recurrent SF remained higher than a sex and age-matched normal population for up to 10 years postoperatively. In our centre, the only recurrence was identified at 94 days following successful parathyroidectomy (serum calcium concentration and urine calcium excretion fell appropriately postoperatively) a finding would support the theory that there is a period of time following successful parathyroidectomy during which patients remain at an increased risk of forming renal stones. However, the two study populations may not be directly comparable as the 2002 paper by Mollerup et al16 demonstrated a higher incidence of preoperative renal SF in their patients with pHPT (25% compared with 10.3% in our study) despite similar-sized study populations (674 versus 640).
Overall, our reporting of a cohort of patients who show only a 1.5% recurrence rate of renal SF postoperatively falls into an existing body of evidence on this topic, which as yet has failed to reach a consensus on whether patients do benefit from a lower incidence of recurrent urolithiasis following parathryoidectomy. There are several possible explanations for this, not least the lack of a randomised study to evaluate the benefit of surgery versus conservative treatment. The findings of one author16 that the relative risk of SF may remain elevated for a period of time could suggest why previous studies have found conflicting results, perhaps dependent on their length of follow-up. Similarly, there is a disparity in modes of determining recurrent disease, with some patients undergoing radiological investigations to determine new SF, which could be asymptomatic; and others determining outcomes by symptomatic recurrence or presentation to hospital, which could potentially underestimate the true recurrence of renal calculi.
There may also be an argument that with the trend toward treating milder pHPT disease,6 the number of patients with pHPT and concurrent renal SF is falling over time.7 This could lead to more modern evaluations of the effectiveness of parathyroidectomy being misleading, as patients with a history of SF are known to be more at risk of recurrence than those who have never previously formed stones.
One possibility to explain the inconsistency in outcomes following parathyroidectomy is that the formation of renal stones is a systemic disorder, or at least the manifestation of a large number of potential risk factors (Table 1). Certainly, as we have demonstrated, the occurrence of urolithiasis in only a minority of those with pHPT, does not seem to correlate solely with the degree of hypercalciuria observed, since quite profound hypercalciuria was observed in several NSF with pHPT. Similarly, this may explain why previous investigators have shown little discernable benefit to some patients, and yet studies such as ours can seemingly show considerable advantages, as none of the existing literature evaluates all the risk factors for the formation of stones. These data would therefore also support the argument that urinary calcium is a contributing factor, but not the only mechanism by which pHPT patients develop renal stones, and may suggest that in some patients, despite successful parathyroidectomy, their overall risk of SF remains increased from other co-existing factors.
There is now evidence to suggest an association between SF and the presence within certain individuals of specific genetic haplotypes within several genes. These include polymorphisms in genes involved in calcium homeostasis, eg calcium-sensing receptor (CaSR)22–24 calcium transport in the kidney and gut (TRPV5 and TRPV6)25–29 and in genes that encode the urinary macromolecules that raise the metastability of renal tubular fluid and urine (eg osteopontin, trefoil factor).4,29,30 It therefore seems likely that the presence of hypercalciuria induced by pHPT tips the balance toward SF in some patients who may have a reduced ability to prevent stones because of other such susceptibilities.
The limitations of our study include its retrospective design and a medium term follow-up of up to five years, with some data suggesting longer periods may be required to observe true trends in renal stone recurrence. This was largely due to the endocrine surgery database formation in 2002. Similarly, the low number of patients who had recorded data on their urinalysis, in particular for the postoperative follow-up on patients undergoing surgery, was also a limitation. This means that we have been unable to establish the potential change in urinary calcium for patients with pHPT NSF undergoing parathryoidectomy. This would make for an interesting follow-on study.
In addition, our study used only symptomatic recurrence rates, ie those patients returning to hospital for further radiological imaging of the renal tract for any reason, as opposed to scheduled routine follow-up imaging to detect recurrence. As a reduction in symptoms is what is clinically relevant to patients, we concede that this may represent an underestimate of true stone recurrence. However, it likely that those returning to present with renal colic symptoms and a history of stone disease would almost certainly undergo further investigation. The benefit of this patient-centered, pragmatic approach of investigating only the clinically significant symptoms is that it also acts to protect patients from ionizing radiation, particularly when computed tomography is now the goldstandard investigation for urolithiasis, and serial follow-up imaging would not be without risk.
Conclusion
We have shown that in the short to medium term, successful parathyroidectomy confers a low recurrence rate for urolithiasis in patients with pHPT and a previous history of renal stones, when compared with the recurrence rates experienced by ISFs and, to that end, urolithiasis should remain a strong indication for surgery.
Our data confirm that postoperatively, serum and urinary calcium in these patients falls significantly, but as other investigators have reported higher incidence of stone recurrence with similar biochemical results, this suggests that these changes are unlikely to be the only causative factors in urolithiasis for patients with pHPT. Low serum phosphate levels with a potentially higher urinary excretion in some patients warrants further investigation. With renal SF understood to be a complex multifactorial condition, future research could perhaps concentrate on risk-stratifying patients, or evaluation of the role of genetic haplotypes, to identify those patients who may continue to experience SF.
References
- 1.Curhan GC. Epidemiology of stone disease. Urol Clin N Am 2007; 34: 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest 2005; 115: 2,598–2,608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rampoldi L, Scolari F, Amoroso A, Ghiggeri G, Devuyst O. The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int 2011; 80: 338–347. [DOI] [PubMed] [Google Scholar]
- 4.Thongboonkerd V, Chutipongtanate S, Semangoen T, Malasit P. Urinary trefoil factor 1 is a novel potent inhibitor of calcium oxalate crystal growth and aggregation. J Urol 2008; 179: 1,615–1,619. [DOI] [PubMed] [Google Scholar]
- 5.Bihl G, Meyers A. Recurrent renal stone disease – advances in pathogenesis and clinical management. Lancet 2001; 358: 651–656. [DOI] [PubMed] [Google Scholar]
- 6.Fraser WD. Hyperparathyroidism. Lancet 2009; 374: 145–158. [DOI] [PubMed] [Google Scholar]
- 7.Rejnmark L, Vestergaard P, Mosekilde L. Nephrolithiasis and renal calcifications in primary hyperparathyroidism. J Clin Endocrinol Metab 2011; 96: 2,377–2,385. [DOI] [PubMed] [Google Scholar]
- 8.Suh JM, Cronan JJ, Monchik JM. Primary hyperparathyroidism: is there an increased prevalence of renal stone disease? AJR Am J Roentgenol 2008; 191: 908–911. [DOI] [PubMed] [Google Scholar]
- 9.Jabbour N, Corvilain J, Fuss M, Kinnaert P, Van Geertruyden J. The natural history of renal stone disease after parathyroidectomy for primary hyperparathyroidism. Surg Gynecol Obstet 1991; 172: 25–28. [PubMed] [Google Scholar]
- 10.Valle Díaz de la Guardia F, Arrabal Martín M et al. . Renal lithiasis in patients with primary hyperparathyroidism. Evolution and treatment. Arch Esp Urol. 2010; 63: 32–40. [PubMed] [Google Scholar]
- 11.Deaconson TF, Wilson SD, Lemann J. The effect of parathyroidectomy on the recurrence of nephrolithiasis. Jr Surgery 1987; 102: 910–913. [PubMed] [Google Scholar]
- 12.McGeown MG. Effect of parathyroidectomy on the incidence of renal calculi. Lancet 1961; 1: 586–587. [DOI] [PubMed] [Google Scholar]
- 13.Mollerup CL, Lindewald H. Renal stones and primary hyperparathyroidism: natural history of renal stone disease after successful parathyroidectomy. World J Surg 1999; 23: 173–175. [DOI] [PubMed] [Google Scholar]
- 14.Frøkjaer VG, Mollerup CL. Primary hyperparathyroidism: renal calcium excretion in patients with and without renal stone disease before and after parathyroidectomy. World J Surg 2002; 26: 532–535. [DOI] [PubMed] [Google Scholar]
- 15.Posen S, Clifton-Bligh P, Reeve TS, Wagstaffe C, Wilkinson M. Is parathyroidectomy of benefit in primary hyperparathyroidism? Q J Med 1985; 54: 241–251. [PubMed] [Google Scholar]
- 16.Mollerup CL, Vestergaard P, Frøkjaer VG, Mosekilde L, Christiansen P, Blichert-Toft M. Risk of renal stone events in primary hyperparathyroidism before and after parathyroid surgery: controlled retrospective follow up study. BMJ 2002; 325: 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson CM, Wilson DM, O’Fallon WM, Malek RS, Kurland LT. Renal stone epidemiology: a 25-year study in Rochester, Minnesota. Kidney Int 1979; 16: 624–631. [DOI] [PubMed] [Google Scholar]
- 18.Söreide JA, van Heerden JA, Grant CS, Lo CY, Ilstrup DM. Characteristics of patients surgically treated for primary hyperparathyroidism with and without renal stones. Surgery 1996; 120: 1,033–1,037; discussion 1,037–1,038. [DOI] [PubMed] [Google Scholar]
- 19.Odvina CV, Sakhaee K, Heller HJ, Peterson RD, Poindexter JR, Padalino PK, Pak CY. Biochemical characterization of primary hyperparathyroidism with and without kidney stones. Urol Res 2007; 35: 123–128. [DOI] [PubMed] [Google Scholar]
- 20.Berger AD, Wu W, Eisner BH, Cooperberg MR, Duh QY, Stoller ML. Patients with primary hyperparathyroidism – why do some form stones? J Urol 2009; 181: 2,141–2,145. [DOI] [PubMed] [Google Scholar]
- 21.Rao DS, Phillips ER, Divine GW, Talpos GB. Randomized controlled clinical trial of surgery versus no surgery in patients with mild asymptomatic primary hyperparathyroidism. J Clin Endocrinol Metab 2004; 89: 5,415–5,422. [DOI] [PubMed] [Google Scholar]
- 22.Scillitani A, Guarnieri V, Battista C, De Geronimo S, Muscarella LA, Chiodini I, Cignarelli M, Minisola S, Bertoldo F, et al. . Primary hyperparathyroidism and the presence of kidney stones are associated with different haplotypes of the calcium-sensing receptor. J Clin Endocrinol Metab 2007; 92: 277–283. [DOI] [PubMed] [Google Scholar]
- 23.Vezzoli G, Terranegra A, Arcidiacono T, Gambaro G, Milanesi L, Mosca E, Soldati L on behalf of GENIAL network (Genetics and Environment in Nephrolithiasis Italian Alliance) . Calcium kidney stones are associated with a haplotype of the calcium-sensing receptor gene regulatory region. Nephrol Dial Transplant 2010; 25: 2,245–2,252. [DOI] [PubMed] [Google Scholar]
- 24.Vezzoli G, Tanini A, Ferrucci L, et al. . Influence of calcium-sensing receptor gene on urinary calcium excretion in stone-forming patients. J Am Soc Nephrol 2002; 13: 2,517–2,523. [DOI] [PubMed] [Google Scholar]
- 25.de Groot T, Lee K, Langeslag M, et al. . Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J Am Soc Nephrol 2009; 20: 1,693–1,704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Abel M, Huybers S, Hoenderop JG, van der Kemp AW, van Leeuwen JP, Bindels RJ. Age-dependent alterations in Ca2+ homeostasis: role of TRPV5 and TRPV6. Am J Physiol Renal Physiol 2006; 291: F1177–1183. [DOI] [PubMed] [Google Scholar]
- 27.Lieben L, Carmeliet G. The involvement of TRP channels in bone homeostasis. Front Endocrinol (Lausanne) 2012; 3: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki Y, Pasch A, Bonny O, Mohaupt MG, Hediger MA, Frey FJ. Gain-of-function haplotype in the epithelial calcium channel TRPV6 is a risk factor for renal calcium stone formation. Hum Mol Genet 2008; 17. [DOI] [PubMed] [Google Scholar]
- 29.Hirose M, Tozawa K, Okada A, et al. . Role of osteopontin in early phase of renal crystal formation: immunohistochemical and microstructural comparisons with osteopontin knock-out mice. Urol Res 2012; 40: 121–129. [DOI] [PubMed] [Google Scholar]
- 30.Chutipongtanate S, Nakagawa Y, Sritippayawan S, et al. . Identification of human urinary trefoil factor 1 as a novel calcium oxalate crystal growth inhibitor. J Clin Invest 2005; 115: 3,613–3,622. [DOI] [PMC free article] [PubMed] [Google Scholar]


