Abstract
Introduction
Despite the developments in modern medicine, acute renal injury is still a challenging and common health problem. It is well known that ischaemia and reperfusion takes place in pathological mechanisms. Efforts to clarify the pathophysiology and interventions to improve outcomes are essential. Our study aimed to investigate whether the prophylactic use of paricalcitol is beneficial in renal ischaemia/reperfusion (I/R) injury.
Methods
Twenty-four Wistar albino rats were assigned randomly to four groups. Right nephrectomies were performed at the time of renal arterial clamping. Sham surgery was performed on the rats in group 1. For the rats in group 2, the left renal artery was clamped for 45 minutes. The rats in group 3 received paricalcitol for seven days (0.2µg/kg/day); following this, a right nephrectomy and left renal arterial clamping were not performed. The rats in group 4 received paricalcitol for seven days (0.2µg/ kg/day); following this, a right nephrectomy and left renal arterial clamping for 45 minutes were performed. Tissue thiobarbituric acid reactive substances (TBARS), superoxide dismutase, sulfhydryl groups as well as nitric oxide metabolites, serum urea and creatinine levels were measured for all four groups.
Results
In group 4, there were some improvements in terms of TBARS, nitrite, nitrate, superoxide dismutase and creatinine levels. In the histopathological evaluation, paricalcitol therapy improved tubular necrosis and medullar congestion but there was no significant difference in terms of tubular cell swelling, cellular vacuolisation or general damage. Immunohistopathological examination revealed lower scores for vascular endothelial growth factor in the group 4 rats than in group 2.
Conclusions
Paricalcitol therapy improved renal I/R injury in terms of serum and histopathological parameters. These potential beneficial effects need to be further investigated.
Keywords: Renal ischaemia reperfusion, Paricalcitol, Vascular endothelial growth factor, Rat model
Acute kidney injury is an important healthcare problem that is more common in hospitalised patients, especially in intensive care units.1 Cardiopulmonary surgery and major surgical interventions are the leading causes in these patients.2,3
In renal transplantation, the graft is exposed to ischaemia/reperfusion (I/R) injury. The degree of the injury is associated with graft failure and delayed graft function. Prevention of I/R injury is thought to improve graft functions and survival.4–6 It seems reasonable to perform preconditioning before major surgical interventions and kidney transplantation.
Acute kidney injury mediated by I/R injury is based on oxidative stress and infiltration of inflammatory cells. Besides neutrophil infiltration, T cells and cytokines are also important mediators. It is generally assumed that endothelial and tubular damage and prolonged recovery is associated with the presence of inflammation.7 It has been demonstrated that neutrophil infiltration is present 2–4 hours after ischaemia.8
Paricalcitol is a third-generation and more specific vitamin D receptor activator.9 It has been shown that paricalcitol suppresses parathyroid hormone secretion with no or minor elevations in serum calcium and phosphorus levels.10–12 Several studies have also demonstrated that paricalcitol improves proteinuria and kidney injury,13 renal inflammation,14 interstitial fibrosis15 and proteinuria in diabetic nephropathy.16 The aim of this study was to evaluate any beneficial effect of paricalcitol in an experimental model of renal I/R injury.
Methods
Our study included 24 Wistar albino rats. They were 12 months old and weighed 200–230g. Ethical approved was obtained from the local animal studies ethics committee. The experimental animals were kept at 18–22°C under a 12h light/12h dark cycle. They had free access to standard pellet diet for rats and tap water ad libitum throughout the study. All of the surgical procedures were performed under xylazine/ketamine anaesthesia in sterile conditions. All rats were sacrificed after the completion of the experimental procedures.
The rats were randomised into four groups. Group 1 rats did not receive any specific treatment. They underwent a laparotomy on the seventh day for a right nephrectomy. The left kidney was left at the laparotomy position for 45 minutes without any intervention.
Group 2 rats did not receive any specific treatment. They underwent a laparotomy on the seventh day for a right nephrectomy. The left renal arteries were clamped in order to perform renal ischaemia for 45 minutes.
Group 3 rats received 0.2µg/kg/day paricalcitol (Zemplar®; Abbott, Maidenhead, UK) for seven days. They underwent a laparotomy on the seventh day for a right nephrectomy. The left kidney was left at the laparotomy position for 45 minutes without any intervention.
Group 4 rats received 0.2µg/kg/day paricalcitol for seven days. They underwent a laparotomy on the seventh day for a right nephrectomy. The left renal arteries were clamped in order to perform renal ischaemia for 45 minutes.
Biochemical measurements
Thiobarbituric acid reactive substances (TBARS), superoxide dismutase (SOD), sulfhydryl (SH) groups and nitric oxide (NO) metabolites were measured from the homogenised kidney tissue of the rats. On the day of the measurements, samples were weighed and then homogenised in a cold potassium phosphate buffer using a Heidolph Diax 900 homogeniser (Sigma-Aldrich, Gillingham, Dorset, UK). SOD analyses were performed using an original Ransel commercial kit (Randox, Crumlin, County Antrim, UK). TBARS measurements were performed using a spectrofluorometric method developed by Wasowitcz et al.17 Blood urea nitrogen (BUN) and creatinine levels were measured from the blood samples obtained from the inferior vena cava.
NO metabolite levels (nitrite and nitrate) were measured with the enzymatic method modified by Smárason et al.18 Concentrations were measured with a UV-1208 spectro-photometer (Shimadzu, Tokyo, Japan). SH group measurements were studied using Sedlak and Lindsay’s method.19
Histopathological evaluation
Histopathological evaluations were performed with light microscopy (haematoxylin and eosin stain). Analysis was carried out in sections selected randomly from the most sensitive zone for ischaemic injury (outer stripe of the outer medulla) by a pathologist who was blinded to the identity of the treatment groups. I/R injury was evaluated in terms of cellular swelling, cellular vacuolisation, medullar congestion, presence of necrosis and general damage to the cellular structure as described previously.20
For immunohistopathological staining, paraffin embedded sections were stained with anti-vascular endothelial growth factor (VEGF) (Santa Cruz Biotechnology, Dallas, TX, US) at 1:200 dilution. Cytoplasmic VEGF staining was measured semiquantitatively as minimal (score = 1), mild (score = 2), moderate (score = 3) or heavy (score = 4).
Statistical analysis
Multiple comparisons among groups were carried out by one-way analysis of variance with post-hoc Tukey test correction (SPSS® version 15.0; SPSS, Chicago, IL, US). Comparisons between two groups were performed using a t-test. The level of statistical significance was p<0.05. Chi-squared and Kruskal–Wallis tests were used to compare non-parametric data of the groups.
Results
Biochemical measurements
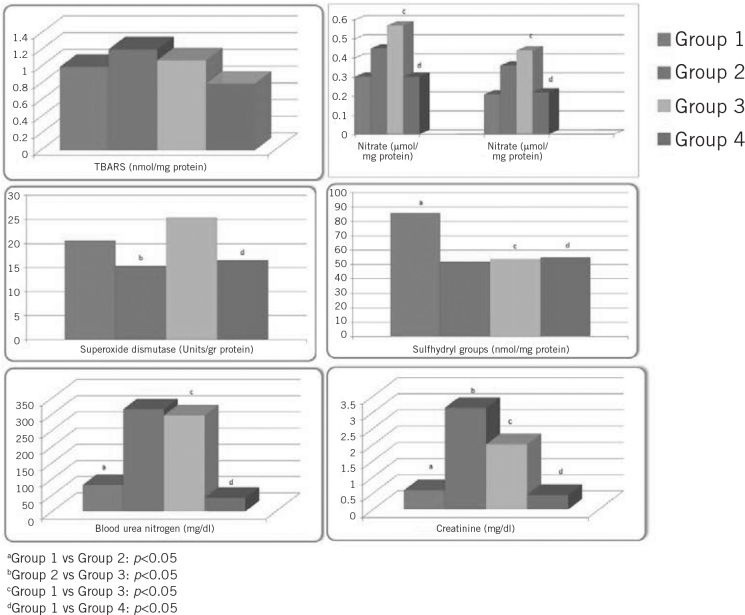
All of the 24 rats completed the study. TBARS, nitrite and nitrate levels were significantly higher in group 2, and SH groups and SOD levels were significantly lower in group 2 than in the other groups. When the groups were compared in terms of TBARS levels, groups 3 and 4 had significantly lower levels than group 2 (p<0.05). When groups 2 and 4 were compared in terms of nitrite, nitrate, SH groups and SOD levels, group 4 had significantly higher values (p<0.05). Serum urea and creatinine levels were significantly higher in groups 2 and 4 compared with groups 1 and 3 (p<0.05). Serum urea and creatinine levels were significantly lower in group 4 than in group 2 (p<0.05). The results of the biochemical evaluation of the tissue levels and the serum of the rats are listed in Table 1 and Figure 1.
Table 1.
Mean biochemical measurement results
| Group 1 (n=6) | Group 2 (n=6) | Group 3 (n=6) | Group 4 (n=6) | p-value | |
| TBARS (nmol/mg protein) | 1.00 (SD: 0.19) | 1.21 (SD: 0.15) | 0.80 (SD: 0.21) | 1.08 (SD: 0.40) | 0.867 |
| Nitrite (µmol/mg protein) | 0.30 (SD: 0.04) | 0.35 (SD: 0.12) | 0.30 (SD: 0.07)c | 0.57 (SD: 0.27)d | <0.01 |
| Nitrate (µmol/mg protein) | 0.21 (SD: 0.04) | 0.26 (SD: 0.10) | 0.22 (SD: 0.08)c | 0.44 (SD: 0.23)d | <0.01 |
| SOD (u/g protein) | 20.62 (SD: 1.84) | 17.45 (SD: 4.42)b | 16.49 (SD: 2.48) | 25.50 (SD: 5.06) | <0.05 |
| SH groups (nmol/mg protein) | 85.75 (SD: 18.91)a | 52.33 (SD: 16.19) | 55.23 (SD: 23.22)c | 53.82 (SD: 20.20)d | <0.01 |
| Blood urea nitrogen (mg/dl) | 81.33 (SD: 25.16)a | 314.83 (SD: 14.49) | 41.8 (SD: 6.11)c | 296.25 (SD: 35.70)d | <0.01 |
| Creatinine (mg/dl) | 0.59 (SD: 0.10)a | 3.14 (SD: 0.31)b | 0.45 (SD: 0.04)c | 2.01 (SD: 0.36)d | <0.01 |
TBARS = thiobarbituric acid reactive substances; SOD = superoxide dismutase; SH = sulfhydryl; SD = standard deviation
Group 1 vs Group 2: p<0.05
Group 2 vs Group 3: p<0.05
Group 1 vs Group 3: p<0.05
Group 1 vs Group 4: p<0.05
Figure 1.
Comparison of the study groups in terms of mean thiobarbituric acid reactive substances (TBARS), nitrite, nitrate, superoxide dismutase, sulfhydryl, blood urea nitrogen and creatinine levels
Histopathological evaluation
All of the groups were evaluated in terms of tubular cell swelling, cellular vacuolisation, medullar congestion, tubular necrosis and general damage. Immunohistopathological evaluation was performed by anti-VEGF staining.
In groups 1 and 3, normal cytoarchitecture was observed. In group 2, the mean tubular cell swelling, cellular vacuolisation, medullar congestion, medullar congestion, tubular necrosis, general damage and VEGF scores were 1.33 (standard deviation [SD]: 0.51), 1.33 (SD: 0.51), 1.33 (SD: 0.51), 1.33 (SD: 0.51), 1.33 (SD: 0.51) and 4.00 (SD: 0.00) respectively. In group 4, the mean tubular cell swelling, cellular vacuolisation, medullar congestion, medullar congestion, tubular necrosis, general damage and VEGF scores were 1.75 (SD: 0.50), 1.75 (SD: 0.50), 2.00 (SD: 0.00), 0, 1.75 (SD: 0.50) and 1.25 (SD: 0.50) respectively.
When the histopathological results were compared between groups 1 and 2, group 2 had higher cellular swelling, cellular vacuolisation, medullar congestion, tubular necrosis and general damage scores than group 1 (p<0.05). The mean medullar congestion score was higher in group 3 than in group 1.
Group 4 had significantly higher scores of cellular swelling, cellular vacuolisation, medullar congestion, tubular necrosis and general damage but lower VEGF scores than group 1. Group 4 also had higher scores of medullar congestion, tubular necrosis and lower VEGF scores than group 2.
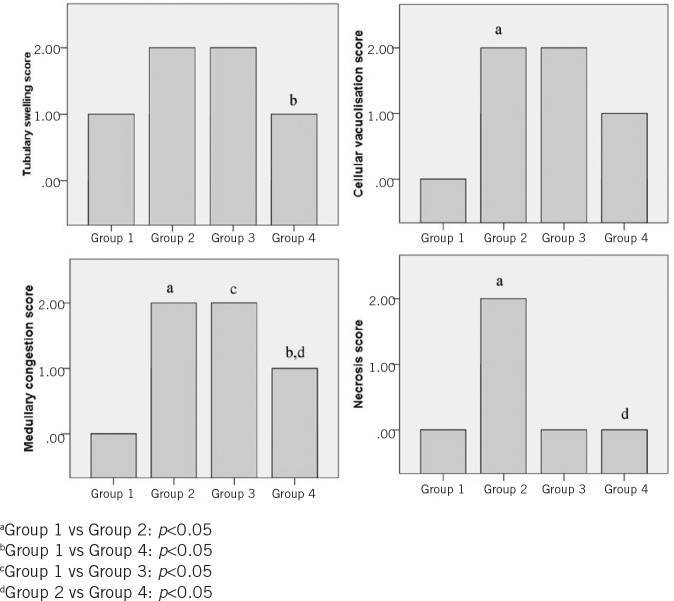
The results of the histopathological evaluation of the rats are listed in Table 2. Light microscopy images of the groups and light microscopy-based scores of the groups are shown in Figures 2 and 3. Immunohistopathological images and the scores of the groups are shown in Figures 4 and 5.
Table 2.
Mean histopathological scores
| Group 1 (n=6) | Group 2 (n=6) | Group 3 (n=6) | Group 4 (n=6) | p-value | |
| Tubular cell swelling | 0.50 (SD: 0.54) | 1.33 (SD: 0.51) | 0.66 (SD: 0.51) | 1.75 (SD: 0.50)c | 0.108 |
| Cellular vacuolisation | 0 | 1.33 (SD: 0.51)a | 0.66 (SD: 0.51) | 1.75 (SD: 0.50)c | 0.554 |
| Medullar congestion | 0 | 1.33 (SD: 0.51)a | 0.83 (SD: 0.40)b | 2.00 (SD: 0.00)c,d | 0.727 |
| Tubular necrosis | 0 | 1.33 (SD: 0.51)a | 0 | 0d | <0.01 |
| VEGF | 4.00 (SD: 0.00) | 4.00 (SD: 0.00) | 3.33 (SD: 0.81) | 1.25 (SD: 0.50)c,d | <0.01 |
| General damage | 0 | 1.33 (SD: 0.51)a | 0 | 1.75 (SD: 0.50)c | 0.108 |
VEGF = vascular endothelial growth factor; SD = standard deviation
Group 1 vs Group 2: p<0.05
Group 1 vs Group 3: p<0.05
Group 1 vs Group 4: p<0.05
Group 2 vs Group 4: p<0.05
Figure 2.
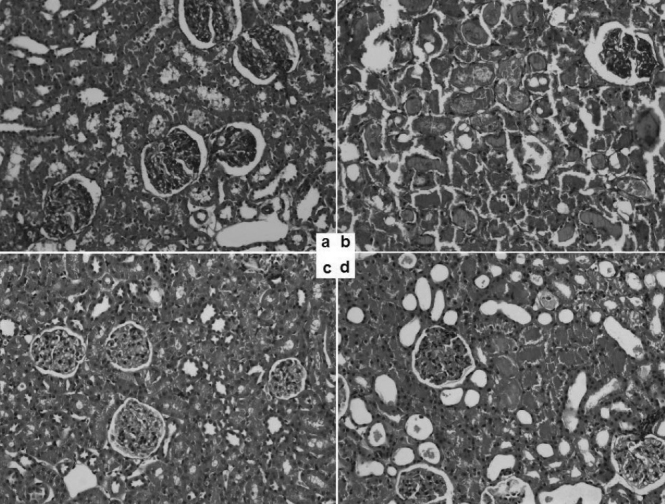
Light microscopy (haematoxylin and eosin stain, 20x magnification) of the four rat groups: a) group 1 (control); b) group 2 (ischaemia/reperfusion); c) group 3 (paricalcitol control); d) group 4 (ischaemia reperfusion plus paricalcitol)
Figure 3.
Comparison of the study groups based on pathological evaluation of mean tubulary swelling, cellular vacuolisation, medullary congestion and necrosis scores
Figure 4.
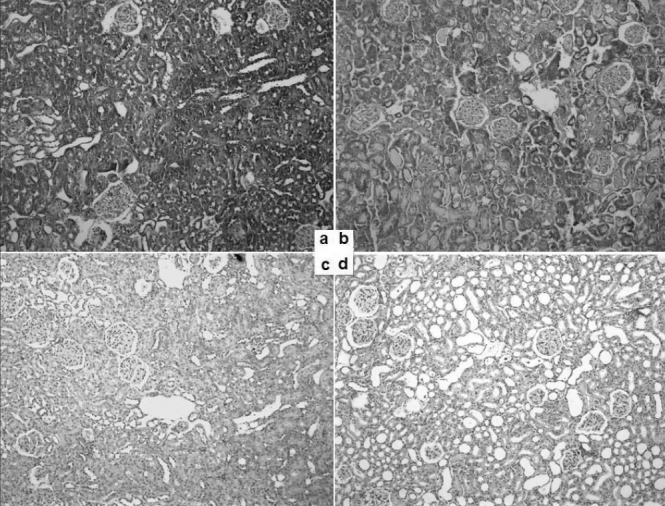
Immunohistopathology (vascular endothelial growth factor, 10x magnification) of the four rat groups: a) group 1 (control); b) group 2 (ischaemia/reperfusion); c) group 3 (paricalcitol control); d) group 4 (ischaemia reperfusion plus paricalcitol)
Figure 5.
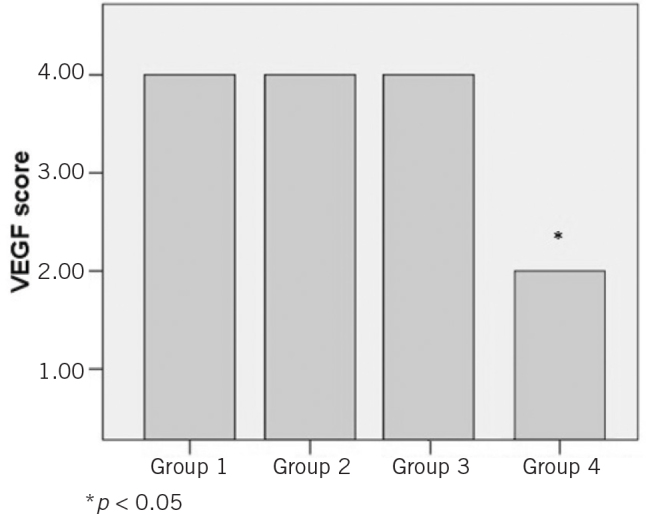
Comparison of the study groups based on immunohistopathological evaluation of mean vascular endothelial growth factor (VEGF) score
Discussion
The mechanism of the renal damage that is caused by ischaemia followed by reperfusion is still not clear. In recent trials, some favourable renal endpoints have been demonstrated by the use of vitamin D. In our study, we aimed to evaluate the effect of the selective vitamin D receptor activator paricalcitol on I/R injury.
It has been shown that 45 minutes of ischaemia and at least 2 hours of reperfusion increase BUN and creatinine levels significantly compared with controls.21 In our study, we performed 24 hours of reperfusion, and our results confirm that I/R injury increases BUN and creatinine levels.
Tan et al showed that 1,25-dihydroxyvitamin D3 treatment has protective effects on I/R injury.14 Their results demonstrate that this beneficial effect is based on the increment of heat shock protein 70 levels. It has been reported in the literature that paricalcitol may have an improving effect in cases of obstructive nephropathy, gentamicin and cyclosporine induced nephrotoxicity.22–24
It has been demonstrated that paricalcitol takes part in proinflammatory responses by targeting nuclear factor kappa B (NF-κB). Paricalcitol binds to the p65 subunit of NF-κB, preventing its interaction with the deoxyribonucleic acid and suppressing the expression of the target genes.25 This leads to the idea that paricalcitol treatment may improve inflammatory renal injury.
NO is synthesised from the endothelium, which has cytoprotective, regulatory and also cytotoxic effects. NO is thought to be a potential protective agent in renal I/R injury. This renal beneficial effect is probably mediated by the vasodilatory effect against vasoconstrictive effects of angiotensin, catecholamine and endothelin.26 Unal et al showed that increased NO levels has protective effects in renal I/R injury in rats.27 In our study, we found that paricalcitol significantly increased NO levels in group 4 compared with group 2.
Kiriş et al evaluated renal injury in an infrarenal aortic ischaemia reperfusion model.28 After the rats we exposed to 30 minutes of ischaemia and 60 minutes of reperfusion, tissue malondialdehyde and antioxidative enzyme levels were increased significantly. Our findings show that group 4 had lower levels of tissue TBARS than group 2.
SOD, catalase and glutation enzyme systems defend the organism against oxidative damage. These systems reduce hydrogen peroxide to water and oxygen. SOD plays a large role in protecting the organism against the deleterious effects of superoxide.26 In our study, we found the SOD levels in group 2 significantly lower than in groups 1 and 4 (p<0.05).
It is known that glutation reductase is consumed during tissue ischaemia.27 Our results showed no significant difference between the groups in terms of tissue SH levels.
In our groups, there was also no significant difference in terms of swelling of tubular cells. Cellular vacuolisation was observed to a higher degree in all groups when compared with controls. Furthermore, no beneficial effect of paricalcitol was demonstrated. Medullar congestion scores were also significantly lower in group 4 than in group 2. Similarly, necrosis scores were significantly lower in group 4 than in group 2 but when the general damage was evaluated, the scores between the groups did not show any significant difference.
It has been demonstrated in a renal ischaemia reperfusion study that VEGF (particularly VEGF receptor 2 [VEG-FR2]) expressions are increased.28 In our study, there was no difference between the groups in terms of VEGF except lower scores in the group 3 rats. This may be caused by the inability to show VEGFR2 using immunohistopathological staining.
Conclusions
Reperfusion followed by ischaemia in order to protect the tissue may actually worsen the damage caused by ischaemia.29 If this collateral damage is averted, it may be possible to ameliorate the degree of the injury of ischaemia and of that following reperfusion. Our results show that the vitamin D receptor analogue paricalcitol may have beneficial effects in terms of preventing renal I/R injury.
References
- 1.Brady HR, Singer GG. Acute renal failure. Lancet 1995; 346: 1,533–1,540. [DOI] [PubMed] [Google Scholar]
- 2.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med 1996; 334: 1,448–1,460. [DOI] [PubMed] [Google Scholar]
- 3.Nolan CR, Anderson RJ. Hospital-acquired acute renal failure. J Am Soc Nephrol 1998; 9: 710–718. [DOI] [PubMed] [Google Scholar]
- 4.Grinyo JM. Role of ischemia-reperfusion injury in the development of chronic renal allograft damage. Transplant Proc 2001; 33: 3,741–3,742. [DOI] [PubMed] [Google Scholar]
- 5.Shoskes DA. Nonimmunologic renal allograft injury and delayed graft function: clinical strategies for prevention and treatment. Transplant Proc 2000; 32: 766–768. [DOI] [PubMed] [Google Scholar]
- 6.Tilney NL, Guttmann RD. Effects of initial ischemia/reperfusion injury on the transplanted kidney. Transplantation 1997; 64: 945–947. [DOI] [PubMed] [Google Scholar]
- 7.Brady HR. Leukocyte adhesion molecules and kidney diseases. Kidney Int 1994; 45: 1,285–1,300. [DOI] [PubMed] [Google Scholar]
- 8.Rabb H, Daniels F, O’Donnell M et al. . Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol 2000; 279: F525–F531. [DOI] [PubMed] [Google Scholar]
- 9.Goldenberg MM. Paricalcitol, a new agent for the management of secondary hyperparathyroidism in patients undergoing chronic renal dialysis. Clin Ther 1999; 21: 432–441. [DOI] [PubMed] [Google Scholar]
- 10.Cozzolino M, Brancaccio D. Emerging role for the vitamin D receptor activator (VDRA), paricalcitol, in the treatment of secondary hyperparathyroidism. Expert Opin Pharmacother 2008; 9: 947–954. [DOI] [PubMed] [Google Scholar]
- 11.Lindberg J, Martin KJ, González EA et al. . A long-term, multicenter study of the efficacy and safety of paricalcitol in end-stage renal disease. Clin Nephrol 2001; 56: 315–323. [PubMed] [Google Scholar]
- 12.Martin KJ, González EA, Gellens M et al. . 19-Nor-1-alpha-25-dihydroxyvitamin D2 (paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol 1998; 9: 1,427– 1,432. [DOI] [PubMed] [Google Scholar]
- 13.He W, Kang YS, Dai C, Liu Y. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 2011; 22: 90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan X, Wen X, Liu Y. Paricalcitol inhibits renal inflammation by promoting vitamin D receptor-mediated sequestration of NF-kappaB signaling. J Am Soc Nephrol 2008; 19: 1,741–1,752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan X, Li Y, Liu Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol 2006; 17: 3,382–3,393. [DOI] [PubMed] [Google Scholar]
- 16.de Zeeuw D, Agarwal R, Amdahl M et al. . Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 2010; 376: 1,543–1,551. [DOI] [PubMed] [Google Scholar]
- 17.Wasowicz W, Nève J, Peretz A. Optimized steps in fluorometric determination of thiobarbituric acid-reactive substances in serum: importance of extraction pH and influence of sample preservation and storage. Clin Chem 1993; 39: 2,522–2,526. [PubMed] [Google Scholar]
- 18.Smárason AK, Allman KG, Young D, Redman CW. Elevated levels of serum nitrate, a stable end product of nitric oxide, in women with pre-eclampsia. Br J Obstet Gynaecol 1997; 104: 538–543. [DOI] [PubMed] [Google Scholar]
- 19.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 1968; 25: 192–205. [DOI] [PubMed] [Google Scholar]
- 20.Sener G, Sehirli AO, Keyer-Uysal M et al. . The protective effect of melatonin on renal ischemia-reperfusion injury in the rat. J Pineal Res 2002; 32: 120–126. [DOI] [PubMed] [Google Scholar]
- 21.Sehirli AO, Sener G, Satiroglu H, Ayanoğlu-Dülger G. Protective effect of N-acetylcysteine on renal ischemia/reperfusion injury in the rat. J Nephrol 2003; 16: 75–80. [PubMed] [Google Scholar]
- 22.Slawsky MT, Colucci WS, Gottlieb SS et al. . Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Circulation 2000; 102: 2,222–2,227. [DOI] [PubMed] [Google Scholar]
- 23.Salvemini D, Cuzzocrea S. Superoxide, superoxide dismutase and ischemic injury. Curr Opin Investig Drugs 2002; 3: 886–895. [PubMed] [Google Scholar]
- 24.McCord JM, Fridovich I. Superoxide dismutase. J Biol Chem 1969; 244: 6,049–6,055. [PubMed] [Google Scholar]
- 25.Meister A. Glutathione metabolism and its selective modification. J Biol Chem 1988; 263: 17,205–17,208. [PubMed] [Google Scholar]
- 26.Liu F, Lou YL, Wu J et al. . Upregulation of microRNA-210 regulates renal angiogenesis mediated by activation of VEGF signaling pathway under ischemia/perfusion injury in vivo and in vitro. Kidney Blood Press Res 2012; 35: 182–191. [DOI] [PubMed] [Google Scholar]
- 27.Unal D, Yeni E, Erel O et al. . Antioxidative effects of exogeneus nitric oxide versus antioxidant vitamins on renal ischemia reperfusion injury. Urol Res 2002; 30: 190–194. [DOI] [PubMed] [Google Scholar]
- 28.Kiriş İ, Okutan H, Savaş Ç et al. . Deneysel aortik iskemi reperfüzyon modelinde renal hasara gadolinyum klorürün etkisi. Turk J Vasc Surg 2005; 14: 13–18. [Google Scholar]
- 29.Kaneshiro Y, Ichihara A, Sakoda M et al. . Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol 2007; 18: 1,789–1,795. [DOI] [PubMed] [Google Scholar]