Abstract
Objective
The purpose of this study was to present the recent clinical profiles and the real-world management of infective endocarditis (IE).
Methods
All medical records of patients with IE were reviewed retrospectively for their clinical data, including clinical presentation, laboratory results, blood cultures, echocardiographic findings, treatments and complications. Using the clinical data collected, we calculated the EuroSCORE II, the European risk score for adult cardiac surgery, the Charlson Comorbidity Index as a surrogate of comordibity, and the Katz Index as a surrogate of frailty.
Results
Thirty-eight patients were identified as having IE (24 men, age: 71.8±13.1 years). Congestive heart failure occurred in 16 patients (42%), stroke in 14 (50%), and systemic embolism in 5 (13%). The EuroSCORE II and Charlson Comorbidity Index were high (7.7±5.8% and 5.5±2.8%, respectively). The Katz Index was fair (5.5±1.4) before the onset but deteriorated to 2.8±2.7 at the time of establishing the diagnosis of IE (p<0.001). Early surgery was performed in 22 cases (61%). In-hospital death occurred in 10 cases (26%). A EuroSCORE II ≥9%, Staphylococcus aureus etiology, and a Charlson Comorbidity Index were suggested as determinants of in-hospital death (hazard ratios: 173.60, 9.31, 1.57, respectively). In contrast, early surgery was suggested as a determinant of the survival (hazard ratio: 0.04). The Charlson Comorbidity Index was also suggested as a determinant for selecting conservative management (odds ratio: 1.40).
Conclusion
Comorbidity may influence the treatment selection and outcome of elderly patients with IE.
Keywords: infective endocarditis, cardiac surgery, comorbidity, frailty
Introduction
Infective endocarditis (IE) has shown increased morbidity and mortality and has become the third-most common life-threatening infection syndrome (1). Although the overall incidence of IE is relatively low and remains stable, the characteristics of IE patients have changed. The incidence of IE caused by Staphylococcus aureus has increased, while the spectrum of IE patients has shifted towards an older population with a higher frequency of healthcare contact, prosthetic valves and other implantable cardiac devices and a lower rate of rheumatic heart disease. In addition to these epidemiological changes, new diagnostic and therapeutic challenges have been developed (2). Although the role of early surgical intervention in patients with IE has been controversial in the past (3-5), the latest IE guidelines have clearly defined the criteria indicating early surgery (2). One multicenter cohort survey found that more than 50% of patients with active IE undergo valve surgery during initial hospitalization, before completing a full therapeutic course of antibiotics, even though cardiac surgery carries significant risk for the patient (6, 7). However, only one small, randomized trial has been conducted to examine the role of early valve surgery in the management of IE (8). In addition, the definition of “early surgery” differs among studies. It is therefore important for the management of a potentially fatal disease such as IE to be based on scientific guidelines that are constantly updated with new evidence.
Japan is one of the world's most industrialized countries and is confronting issues arising from a declining birthrate and an aging society; however, investigations of the incidence and characteristics of IE are relatively limited (9-12), and the most recent nationwide registry of IE was published in 2013 (13). In combination with aging, patient comorbidity and frailty are emerging clinical factors that have been associated with refusing surgical treatment (14, 15) or poorer outcomes after cardiac surgery (16-19).
Therefore, in the present study, we reviewed all medical records of IE in the past decade at a community hospital and collected data concerning the Charlson Comorbidity Index, which can predict the 10-year survival in patients with multiple comorbidities (20, 21), and the Katz Index of activities of daily living (ADL), a widely accepted measure of frailty in elderly individuals (22). We herein report the most recent clinical profiles of IE and its real-world management.
Materials and Methods
The medical records of adult patients with IE (over 18 years of age) hospitalized between January 2006 and December 2016 at the Japan Self-Defense Forces Central Hospital were reviewed retrospectively. The Japan Self-Defense Forces Central Hospital is a community-based hospital with 300 beds and outpatient care services authorized to treat patients with health insurance coverage in Japan and serves as a secondary emergency designated hospital located in the southern Tokyo Metropolitan area. This institute was founded to admit both Japan Self-Defense Forces personnel and citizens, including local residents, and has taken in patients referred by Mishuku Hospital, a neighboring community-based hospital with 250 beds, for cardiac surgery in accordance with agreements made between the Ministry of Defense and the National Public Officers Mutual Aid Association Federation headquarters.
Patients with suspected IE were managed in a coordinated manner by one of several clinical departments, including general internal medicine, cardiology, infectious disease and cardiovascular surgery. Clinical interventions including cardiac surgery were introduced after discussions at a multi-disciplinary conference, taking into account the patient's symptoms, hemodynamic conditions, results of laboratory and imaging examinations, comorbidities, frailty, intervention risks and personal wishes, in accordance with the latest IE guidelines by the European Heart Society revised in 2004 (23) and 2015 (24), the American Heart Association guidelines revised in 2005 (25) and 2015 (2), and the Japanese Circulation Society guidelines revised in 2008. Surgical indications were based on the above-mentioned guidelines, and surgery was performed for patients with the following: congestive heart failure (>moderate) despite the introduction of standard heart failure therapy; persistent infection with positive blood cultures after one week of antibiotic therapy or infection with highly resistant organisms; recurrent embolic events with persistent vegetation; valve dehiscence, perforation, rupture or fistula, or a large perivalvular abscess; and vegetation (>10 mm) or persistent vegetation after systemic embolization.
We collected clinical data, including clinical presentation, laboratory results, blood cultures, echocardiographic findings, treatments (antibiotic therapy and/or surgical interventions) and complications (congestive heart failure, stroke, systemic embolization and in-hospital death). We identified IE cases according to the modified Duke criteria of “definite” (26) or “probable” with pathological confirmation by surgical specimens or autopsy. Definitions of the clinical variables were based on published reports by the International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators (6, 27). Systemic embolization was defined as embolism to any major arterial vessel, excluding stroke. Persistent positive blood cultures were defined as blood cultures positive after 72 h of adapted antibiotic therapy. Healthcare-associated IE consisted of either nosocomial or non-nosocomial acquired infection, where nosocomial healthcare-associated IE was defined as IE occurring in a patient hospitalized for >48 h and non-nosocomial healthcare-associated IE was defined as signs or symptoms consistent with IE developing before hospitalization in patients with extensive out-of-hospital contact with healthcare interventions. Surgery (valve replacement and/or valve repair) was defined as “early”, if the surgical procedure was required during the initial hospitalization to minimize the chance of further clinical deterioration based on the previously reported study (6). Patients who had not been selected for early surgery were considered to have received conservative management, namely a full course (six weeks) of intravenous antibiotic therapy and additional elective surgery if required.
We calculated the time interval from the initial manifestation to the establishment of the diagnosis, the time interval from the diagnosis to the surgery, the number of transthoracic echocardiographic examinations required to establish the diagnosis of active IE, and the hospitalization period. The cause of death was inquired about for each patient who died during the index hospitalization. The EuroSCORE II, a European risk scoring system for adult cardiac surgery that includes the presence of IE as one of its clinical variables (28), as well as the Society of Thoracic Surgeons Endocarditis Score (STS-IE) (29), was calculated based on the clinical information at the time of establishing the diagnosis described in the medical records in order to estimate the operative mortality (≥9: extra high risk, 6-8: high risk, 3-5: medium risk, 0-2: low risk). Similarly, we calculated the updated Charlson Comorbidity Index at the time of establishing the diagnosis as the measure of comorbidity (≥3: high-risk of death from comorbid disease in 10 years) (21), the Sequential organ failure assessment (SOFA) score at the time of establishing the diagnosis as a surrogate of the severity of organ failure by sepsis (>11: severe) (30) and the Katz Index before the initial manifestation of IE as the measure of frailty (6: excellent, 3-5: fair, 0-2: poor) (22). In addition, we repeated the calculation of the Katz Index at the time of establishing the diagnosis and at the time of discharge if the patient survived in order to evaluate any change in the patient's functional status as a consequence of interventions.
The study protocol was approved by the Institutional Review Board of the Japan Self-Defense Forces Central Hospital. The study conformed to the principles outlined in the Declaration of Helsinki.
Data are shown as the mean±standard deviation. For categorical variables, the chi-squared test or Fisher's exact test was performed. For continuous variables, an unpaired Student t-test was performed for comparisons between two groups, and a paired Student's t-test was performed for repeated measures. A Cox's proportional hazard model analysis was performed to assess the determinant factors of in-hospital death using a p value of <0.1 as the cut-off. Cumulative survival probability plots were generated using the Kaplan-Meier method. A logistic regression analysis (forward selection) was performed to assess the determinant factors of conservative management using a p value of <0.1 as the cut-off. All statistical analyses were conducted using the SPSS 11.0J software program (SPSS, Chicago, USA), and p values of <0.05 were considered statistically significant.
Results
Demographic characteristics
First, we screened patients with a diagnosis of IE among the discharge records between January 2006 and December 2016. Then, by reviewing medical records, 33 patients were identified as being “definite” IE patients based on positive echocardiographic findings and blood culture results (32 patients) or positive echocardiographic findings and negative blood culture results accompanied by more than three IE symptoms (one patient). There were no “definite” IE patients with positive blood culture results and negative echocardiographic findings accompanied by more than three IE symptoms. Finally, by reviewing pathology reports, an additional five patients were identified as IE patients with pathological evidence (four by surgical specimens, one by autopsy). Therefore, in total, 38 cases were identified as having active IE and are summarized in Table 1.
Table 1.
Demographic Characteristics.
| Age, years | 71.8±13.1 (44-92) |
| Male gender | 24 (63%) |
| Predisposing background | |
| Decayed teeth/periodontitis | 4 (11%) |
| Chronic kidney disease | 5 (13%) |
| Diabetes mellitus requiring medication | 5 (13%) |
| Chronic liver disease | 8 (21%) |
| Cancer | 7 (18%) |
| Valvular heart disease | 15 (40%) |
| Cerebral vascular disease | 10 (26%) |
| Declined cognitive function | 11 (29%) |
| Healthcare-associated infection | 4 (11%) |
| Prosthetic valve infection | 5 (13%) |
| Intracardiac device infection | 4 (11%) |
| Symptoms at the onset | |
| Fever | 29 (76%) |
| Stroke | 4 (11%) |
| Systemic embolization | 2 (5%) |
| Congestive heart failure | 1 (3%) |
| Asymptomatic | 2 (5%) |
| Time interval from the onset to diagnosis, days# | 8 (19) |
| Less than 2 weeks | 23 (61%) |
| More than 3 months | 2 (5%) |
| EuroSCORE II2 | 7.7±5.8 |
| Charlson Comorbidity Index2 | 5.5±2.8 |
| Katz Index1 | 5.5±1.4 |
| Katz Index2 | 2.8±2.7* |
| Katz Index3 | 4.7±1.9† |
| Sequential organ failure assessment (SOFA) score2 | 7.0±2.8 |
| Duration of the hospitalization, days | 54.0±49.4 |
1before the onset, 2at the time of establishing the diagnosis, 3at the time of discharge (including only survived patients).
*p<0.05 vs. before the onset, †p<0.05 vs. at the time of establishing the diagnosis, #median value (interquartile range).
There were 24 men and 14 women a mean 71.8±13.1 years of age (range 44 to 92 years). The median time interval from the onset to the diagnosis was 8.0 days (interquartile range, 19). Twenty-three cases (61%) were diagnosed within two weeks after the onset, whereas in two cases, the diagnosis was made more than three months after the onset. The EuroSCORE II at the time of establishing the diagnosis was high (7.7±5.8%). The Charlson Comorbidity Index at the time of establishing the diagnosis was also high (5.5±2.8%). The SOFA score at the time of establishing the diagnosis was moderate (7.0±2.8). The Katz Index, which was 5.5±1.4 before the onset, deteriorated to 2.8±2.7 at the time of establishing the diagnosis of IE (p<0.0001) and recovered to 4.7±1.9 at the time of discharge if the patient survived (p=0.005). There was a discrepancy between the severity of sepsis measured by the SOFA score and the functional status measured by the Katz Index. Although the SOFA score at the time of establishing the diagnosis was not significantly different between elderly patients (≥80 years, 14 patients) and younger patients (<80 years, 26 patients) (7.7±2.2 vs. 6.6±3.0, p=0.249), the Katz Index at the time of establishing the diagnosis was significantly different between these two groups (1.4±2.1 vs. 3.5±2.8, p=0.019). This suggests that elderly IE patients were more vulnerable to sepsis and tended to become dependent in their daily living.
Microbiological and echocardiographic variables
The results of the microbiological and echocardiographic studies are shown in Table 2. The major causative organisms were S. aureus in 12 cases (32%) and viridans streptococci in seven cases (18%), followed by coagulase-negative staphylococci in 16 cases (16%).
Table 2.
Microbiological and Echocardiographic Variables.
| Microbiological study | |
| Coagulase-negative staphylococci | 6 (16%) |
| Staphylococcus aureus | 12 (32%) |
| MSSA | 10 (26%) |
| MRSA | 2 (5%) |
| Enterococcus Faecalis | 2 (5%) |
| Viridians Streptococci | 7 (18%) |
| HACEK group | 0 (0%) |
| Others | 5 (13%)* |
| Fungi | 0 (0%) |
| Culture negative | 6 (16%) |
| Echocardiographic study | |
| Left ventricular ejection fraction, % | 64.2±9.2 |
| Vegetation presentation | 31(82%) |
| Size of vegetation, mm | 12.1±4.9 |
| Size of vegetation >10 mm | 21 (55%) |
| Adhesion sites of vegetation | |
| Aortic valve | 11 (29%)† |
| Mitral valve | 18 (47%) |
| Tricuspid valve | 1 (3%) |
| Left ventricular wall | 2 (5%) |
| Pacemaker lead | 1 (3%) |
| Abscess formation | 7 (18%) |
| Perforation | 3 (8%) |
| Dehiscence | 3 (8%) |
| Fistula | 0 (0%) |
| Number of times of TTE required to establish the diagnosis | 1.4±0.7 |
| TEE performance | 28 (74%) |
*including one case with Streptococcus bovis, †including three bicuspid aortic valve cases.
MSSA: methicillin-sensitive Staphylococcus aureus, MRSA: methicillin-resistant Staphylococcus aureus, HACEK: Haemophilus species, Aggregatibacter species, Cardiobacteriumhominis, Eikenella corrodens and Kingella species, TTE: transthoracic echocardiography, TEE: transesophageal echocardiography
Transthoracic and/or transesophageal echocardiography detected the presence of vegetations in 31 cases (82%), abscess formation in seven cases (18%), valvular perforation in three cases (8%) and dehiscence in three cases (8%). The number of transthoracic echocardiographic examinations required for establishing the diagnosis was 1.4±0.7. In two cases, transthoracic echocardiography could not detect the pathological lesions. Transesophageal echocardiography was performed in 74% of all patients (100% of patients with surgery and 38% of patients without surgery).
Management and complications
The choices of antibiotic therapy, surgical intervention and the complications during hospitalization are shown in Table 3. In 16% of patients, the results of blood cultures were repeatedly negative; thus, empiric antibiotic therapy was indicated. In all survivors among the patients who underwent both early surgery and conservative management, a full course (six weeks) of intravenous antibiotic therapy was completed during hospitalization and was followed by oral antibiotic therapy, if necessary. Early surgery was selected in 58% of patients (22 cases including four cases with prosthetic valve). Indications for early surgery were moderate to severe congestive heart failure despite the introduction of standard heart failure therapy (eight cases), persistent positive blood cultures (one case), recurrent emboli with persistent vegetation (one case), larger vegetations (>10 mm) (eight cases), abscess formation (five cases) and dehiscence (three cases). There were only six patients without any surgical indications (16%). In other words, in most patients (84%), surgical intervention was indicated. Even among patients treated using conservative management (16 cases), 63% (10 cases) had surgical indications. The median time interval from the diagnosis to early surgery was two days (interquartile range, 5.3).
Table 3.
Management and Complications.
| Management | |
| Antibiotic treatment | |
| Empiric therapy | 6 (16%) |
| Absence of evident surgical indications | 6 (16%) |
| Surgical interventions | |
| Early surgery | 22 (58%) |
| Conservative management with elective surgery | 1 (3%) |
| Conservative management without surgery | 15 (40%) |
| Indications for early surgery | |
| Congestive heart failure >moderate | 8 |
| Persistent positive blood cultures after 1 week antibiotics | 1 |
| Recurrent emboli with persistent vegetation | 1 |
| Abscess formation | 5 |
| Dehiscence of the prosthesis | 3 |
| Size of vegetation >10 mm | 8 |
| Time interval from the diagnosis to the surgery, days# | 2.0 (5.3) |
| Complications | |
| Congestive heart failure | 16 (42%) |
| Stroke | 14 (37%)† |
| Systemic embolization | 5 (13%) |
| Coronary artery | 1 (3%) |
| Liver and spleen | 4 (11%) |
| Lower extremities | 1 (3%) |
| Persistent positive blood cultures | 4 (11%)* |
| In-hospital mortality | 10 (26%) |
| Cerebral embolism | 2 |
| Cerebral hemorrhage | 1* |
| Renal failure | 1 |
| Pneumonia | 4* |
| Congestive heart failure | 1 |
| Myocardial infarction | 1 |
*including one case after early surgery, †including one cerebral hemorrhage, #median value (interquartile range).
The most common complication was congestive heart failure in 16 patients (42%), followed by stroke in 14 (37%) and systemic embolism in five (13%). In one case, acute myocardial infarction due to coronary embolization occurred and induced fatal ventricular fibrillation. After early surgery, newly developed stroke (cerebral hemorrhaging) was observed in only one patient (3%) but was fatal. In-hospital death occurred in 10 patients, and the overall mortality rate was high (26%). However, there was a significant difference in the in-hospital mortality between patients undergoing early surgery (two patients with in-hospital death) and those receiving conservative management (eight patients with in-hospital death) (9% vs. 50%, p=0.006). The causes of death were pneumonia in four patients (11%), stroke in three (8%), congestive heart failure in one (3%), renal failure in one (3%) and myocardial infarction in one (3%).
Determinants of the in-hospital death or survival from IE and of conservative management
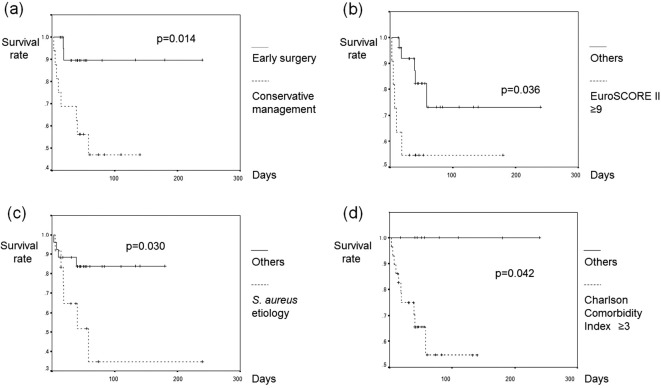
Table 4 shows the results of the univariate analysis and Cox's proportional hazard model analysis for predicting the in-hospital mortality. Only the variables with p<0.1 by a univariate analysis are shown. An extremely high EuroSCORE II (≥9%), S. aureus etiology and a Charlson Comorbidity Index were suggested as determinants of in-hospital mortality (hazard ratios: 173.60, 9.31, 1.57; p=0.002, 0.023, 0.007; respectively). Early surgery was suggested as a determinant of the survival from IE (hazard ratio: 0.04, p=0.035). Figure shows the results of Kaplan-Meier cumulative survival probability plots with a log-rank test, which demonstrated a significantly worse survival in those in the EuroSCORE II extra-high-risk category (≥9%) and Charlson Comorbidity Index high-risk category (≥3) and with S. aureus etiology (p=0.036, 0.042, 0.030, respectively) and a better survival in those undergoing early surgery (p=0.014).
Table 4.
Univariate Analysis and Cox’s Proportional Hazard Model Analysis for Determining In- hospital Mortality.
| Death (n=10) | Survival (n=28) | p value | HR | 95% CI | p value | |
|---|---|---|---|---|---|---|
| Cancer | 5 (50%) | 2 (7%) | 0.027 | |||
| Chronic liver disease | 5 (50%) | 5 (18%) | 0.010 | |||
| Healthcare-associated IE | 3 (30%) | 1 (4%) | 0.019 | |||
| EuroSCORE II1 ≥9% | 5 (50%) | 6 (21%) | 0.087 | 173.60 | 6.74-4475.27 | 0.002 |
| Charlson Comorbidity Index1 | 7.6±2.5 | 4.7±2.7 | 0.004 | 1.57 | 1.06-2.31 | 0.023 |
| Katz Index1 | 1.0±1.9 | 3.4±2.7 | 0.015 | |||
| SOFA score1 | 8.4±3.2 | 6.5±2.5 | 0.060 | |||
| Staphylococcus . aureus etiology | 6 (60%) | 6 (21%) | 0.024 | 9.31 | 1.86-46.70 | 0.007 |
| Vegetation presentation | 10 (100%) | 21 (75%) | 0.080 | |||
| Early surgery | 2 (20%) | 20 (71%) | 0.005 | 0.04 | 0.002-0.80 | 0.035 |
1at the time of establishing the diagnosis.
HR: hazard ratio, CI: confidence interval, IE: infective endocarditis, SOFA: sequential organ failure assessment
Figure.
(a) The survival according to the management selection (early surgery vs. conservative management). (b) The survival according to EuroSCORE II ≥9. (c) The survival according to the presence of Staphylococcus aureus etiology. (d) The survival according to Charlson Comorbidity Index ≥3.
Table 5 shows the results of the univariate analysis and forward logistic regression analysis for predicting conservative management. Only the variables with p<0.1 by a univariate analysis are shown. The Charlson Comorbidity Index was suggested as the only significant determinant for the selection of conservative management (odds ratio: 1.40, p=0.031).
Table 5.
Univariate Analysis and Logistic Regression Analysis for Selection of Conservative Management.
| Conservative (n=16) | Early surgery (n=22) | p value | OR | 95% CI | p value | |
|---|---|---|---|---|---|---|
| Age | 77±12 | 68±13 | 0.034 | |||
| Diabetes mellitus requiring medication | 0 (0%) | 5 (23%) | 0.041 | |||
| Cancer | 5 (31%) | 2 (9%) | 0.082 | |||
| Declined cognitive function | 7 (44%) | 4 (18%) | 0.086 | |||
| Charlson Comorbidity Index2 | 6.8±3.0 | 4.6±2.4 | 0.016 | 1.40 | 1.03-1.91 | 0.031 |
| Katz Index1 | 5.0±2.1 | 5.9±0.5 | 0.055 | |||
| Katz Index2 | 1.7±2.6 | 3.5±2.6 | 0.036 | |||
| Empiric therapy of antibiotics | 0 (0%) | 6 (27%) | 0.023 | |||
| Absence of evident surgical indications | 6 (37%) | 0 (0%) | 0.002 |
1before the onset, 2at the time of establishing the diagnosis.
OR: odds ratio, CI: confidence interval
Discussion
By reviewing medical records of IE in the past decade at a community-based hospital, we presented the most recent profile and issues regarding the management of IE. This study has three main findings. First, unique characteristics were observed in the study cohort, including old age, high operative risk, comorbidity and a fair but easily deteriorating Katz Index. Second, early surgery was associated with a lower in-hospital mortality rate than conservative management. Third, EuroSCORE II ≥9%, S. aureus etiology, comorbidity measured by the Charlson Comorbidity Index and conservative management without early surgery were suggested as determinants of in-hospital death in this IE cohort. Furthermore, comorbidity was suggested as a significant determinant for the selection of conservative management.
According to a recent large French observational study, elderly patients (age >70) made up 38.7% of all IE cases, and the incidence in men peaked between 75 and 79 years of age (31). Peculiarities of IE in the elderly, including predisposing background, symptoms, microbiological and echocardiographic studies, management and outcomes, are discussed in several studies from European countries (32-37). However, very few data have been published regarding IE in the elderly population of Japan. Nakatani et al. reported a Japanese nationwide registry (CADRE-IE) in 2013 (13). The Japanese cohort in CADRE-IE had a mean age of 59.5±13.1 years (about 11 years younger than our patients), a predominance of tooth decay or periodontitis as a background condition (31%), a predominance of viridans streptococci as the causative organism (38%) and the aortic valve as most commonly affected (59%). In contrast, the IE cohort in our study had a less common background of tooth decay or periodontitis (11%) and a predominance of S. aureus etiology (32%), mitral valve involvement (47%) and comorbidities such as a decreased cognitive function (29%), cerebral vascular disease (26%), chronic liver disease (21%) and cancer (18%), which was concordant with the findings in the previously-mentioned large-scale studies with elderly patients (32-37). Therefore, it can be said that the profile of elderly patients with IE in this study tends to be similar to those in European countries.
Aging itself is not a contraindication for surgery (32). In our study, surgical treatment was performed in 60% of the total cohort, and early surgery was performed in 58%. The in-hospital mortality rate of early surgery was lower than in patients receiving conservative management (9% vs. 50%). Furthermore, early surgery was an independent predictor of the survival from IE. Thus far, only a few studies evaluating surgical therapy in elderly patients with IE have been reported, showing lower rates of surgery and worse clinical outcomes than in younger patients (38, 39). In those studies, the rate of performing surgery was significantly lower in elderly patients with surgical indications than in younger patients with surgical indications (41% vs. 76%) (39). Therefore, there may be a potential selection bias in elderly patients who were operated on with a resultant high mortality rate (48%). Furthermore, surgery was performed within a median 12 to 14 days following the establishment of the diagnosis. In our study, surgery was performed more frequently (72%) in patients with evident indications (22 early surgery, one elective surgery) within a median two days after the establishment of the diagnosis, which was a shorter period than in the prior study. In addition, the proportion of S. aureus-caused IE was not markedly different between patients with early surgery and those with conservative management. These facts might have contributed to the relatively good surgical outcomes seen in our study.
Recently, Wang et al. reported that the perioperative prognostic value of the EuroSCORE II for IE patients was similar to that of STS-IE (40). In that study, the patients' mean age was 48.8 years, and the in-hospital mortality was 6.8%, with an EuroSCORE II of 9.1%. The in-hospital mortality of patients undergoing early surgery in our study was slightly higher (9.1%) than in Wang's study, and the EuroSCORE II was slightly lower at 7.4%. However, these differences seem to be relatively negligible, considering the very high in-hospital mortality rate of conservative management (50%) in our elderly IE cohort. Thus, the outcomes of early surgery in our study may be considered acceptable and encouraging.
Making the decision to operate has resulted in several issues specific to elderly patients, due to the increase in mortality rates seen following surgical interventions for the cardiovascular system (41, 42). Bach et al. reported that intervention was not performed in 41% of elderly patients with severe aortic valve stenosis, despite the existence of guideline-recommended indications (43). Comorbidities in elderly patients, such as neurological dysfunction, chronic renal insufficiency and respiratory infection, are reported to be associated with refusal of surgical treatment (14, 15). As mentioned above, in our study, surgery was performed in 72% of patients with evident indications, with the rest (28%) of the patients not receiving surgical intervention. Comorbidities, as measured using the Charlson Comorbidity Index (rather than the operative risk and frailty, measured using the EuroSCORE II and Katz Index, respectively), seem to have strongly influenced the treatment selection for each patient. These critical decisions were made during a multi-disciplinary conference that included expert specialists in our study. Instead of evaluating only a few factors peculiar to the elderly patients, such as neuro-mental problems, the total evaluation of comorbidities seems to have been considered.
Frailty, which can be quantified as a patient's functional status using the Katz Index, has been associated with relatively poor outcomes after cardiac surgery (16-18). There is only one study that evaluated frailty in elderly patients who were scheduled to undergo cardiac sugery (19). That study showed the predictive value for frailty in perioperative risk scoring, but it excluded IE cases. In our study, patients who were selected for conservative management originally had poor frailty, whereas those who were selected for early surgery had fair frailty as measured by the Katz Index based on the functional status before the onset of IE. However, the Katz Index before the onset failed to show the prediction of both the survival rate and selection for conservative management. Beyond this result, a significant deterioration in the functional status in elderly IE patients and its recovery by surgical intervention were observed by the application of the Katz index. Furthermore, a discrepancy between the severity of sepsis and the functional status was observed. These findings may offer new insight into the value of the Katz Index in the management of IE, especially with elderly patients whose condition tends to deteriorate in the acute phase of IE.
In the cohort of this study, 22 patients (58%) were hospitalized within two weeks after the onset, although in two patients, hospitalization occurred more than three months after the onset. These findings are higher than those observed in the CADRE-IE registry, in which 40% of the patients were hospitalized within two weeks after the onset and 9% more than three months after the onset. A recent IE study in Japan by infectious disease specialists criticized the delay in the diagnosis of IE due to the inappropriate use of antibiotics (12). In that study, the median time interval from the onset to the diagnosis was reported as 14 days, which was longer than in our study (eight days). These facts ensure the level of quality of healthcare in our study and may strengthen the scientific basis of this single-institutional study.
Several limitations associated with the present study warrant mention. First, we enrolled only definite IE patients as per the Duke criteria or pathology and excluded probable IE patients. Due to the retrospective study design, the identification of definite IE patients by the review of medical records was more feasible and might be more accurate than a review of probable patients. Although the size of the cohort that contained only definite patients in this study might have been smaller than that containing both definite and probable patients, the collected data in the former cohort might be more reliable. Consequently, our cohort was associated with a very high rate of surgical indication (86%). Therefore, our cohort might have mostly comprised patients with more advanced or “severe” stages of this disease than other clinical studies. Second, transthoracic echocardiogram (TTE) missed detecting pathological lesions in only two patients (5%) in our cohort, which was a very low rate compared with other studies. Logically, there are two possibilities for this: 1) missing enrollment of IE patients with less evident TTE findings that require serial TTEs and TEE for confirmation or 2) the inability to diagnose such IE patients. In cases with a more complex and longer diagnostic process to reach the final judgment, it was sometimes difficult to locate the key descriptions for the IE diagnosis during the medical records review. This might have caused patient identification and enrollment to have been missed. However, we probably could not have avoided these missing IE patients because of the limitation of our retrospective reviewing method. Finally, the sample size of the cohort of this study was small, and the data were based on the clinical database of a single center. For this reason, it is likely that institutional biases influenced our results. Furthermore, due to the small sample size, the results from multivariate analyses, such as the Cox's proportional hazard model and logistic regression analyses, might not be perfectly reliable (sample-size bias). However, as the cohort and the clinical variables were carefully selected, we believe that sample-size bias might be minimized in this study. Because of the above-mentioned limitations, the results and conclusions from this study may not be generalizable to other institutions. Despite these limitations, however, the findings of this study can be used to guide larger and/or multicenter studies in the future.
Conclusion
Currently, IE is becoming more common in the Japanese elderly population, which has features similar to those in European countries. Comorbidities may influence the treatment selection and outcomes of elderly patients with IE.
The authors state that they have no Conflict of Interest (COI).
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional review board) and with the Helsinki Declaration of 1964 and later versions.
Acknowledgement
The authors wish to thank Dr. Yoshiaki Tanaka, the vice president of Saitama-Eastern Cardiovascular Hospital, for his enormous contribution to our patients' management.
References
- 1.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2197-2223, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Baddour LM, Wilson WR, Bayer AS, American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals from the American Heart Association. Circulation 132: 1435-1486, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Hoen B, Duval X. Infective endocarditis. N Engl J Med 368: 1425-1433, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Delahaye F. Is early surgery beneficial in infective endocarditis? A systematic review. Arch Cardiovasc Dis 104: 35-44, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Tleyjeh IM, Kashour T, Zimmerman V, Steckelberg JM, Wilson WR, Baddour LM. The role of valve surgery in infective endocarditis management: a systematic review of observational studies that included propensity score analysis. Am Heart J 156: 901-909, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Lalani T, Cabell CH, Benjamin DK, International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators, et al. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 121: 1005-1013, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiefer T, Park L, Tribouilloy C, et al. Association between valvular surgery and mortality among patients with infective endocarditis complicated by heart failure. JAMA 306: 2239-2247, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 366: 2466-2473, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Nakatani S, Mitsutake K, Hozumi T, Committee on Guideline for Prevention and Management of Infective Endocarditis, Japanese Circulation Society, et al. Current characteristics of infective endocarditis in Japan: an analysis of 848 cases in 2000 and 2001. Circ J 67: 901-905, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Ako J, Ikari Y, Hatori M, Hara K, Ouchi Y. Changing spectrum of infective endocarditis: review of 194 episodes over 20 years. Circ J 67: 3-7, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Hanai M, Hashimoto K, Mashiko K, et al. Active infective endocarditis: management and risk analysis of hospital death from 24 years' experience. Circ J 72: 2062-2068, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Fukuchi T, Iwata K, Ohji G. Failure of early diagnosis of infective endocarditis in Japan-a retrospective descriptive analysis. Medicine (Baltimore) 93: e237, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakatani S, Mitsutake K, Ohara T, Kokubo Y, Yamamoto H, Hanai S, CADRE Investigators . Recent picture of infective endocarditis in Japan-lessons from Cardiac Disease Registration (CADRE-IE). Circ J 77: 1558-1564, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2714-2720, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Hu K, Wan Y, Hong T, et al. Therapeutic decision-making for elderly patients with symptomatic severe valvular heart diseases. Int Heart J 57: 434-440, 2016. [DOI] [PubMed] [Google Scholar]
- 16.Edwards FH, Peterson ED, Coombs LP, et al. Prediction of operative mortality after valve replacement surgery. J Am Coll Cardiol 37: 885-892, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Afilalo J, Eisenberg MJ, Morin JF, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol 56: 1668-1676, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Lee DH, Buth KJ, Martin BJ, Yip AM, Hirsch GM. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation 121: 973-978, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Afilalo J, Mottillo S, Eisenberg MJ, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes 5: 222-228, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40: 373-383, 1987. [DOI] [PubMed] [Google Scholar]
- 21.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 173: 676-682, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The Index of ADL: a standardized measure of biological and psychosocial function. JAMA 185: 914-919, 1963. [DOI] [PubMed] [Google Scholar]
- 23.Horstkotte D, Follath F, Gutschik E, Task Force Members on Infective Endocarditis of the European Society of Cardiology, ESC Committee for Practice Guidelines (CPG), Document Reviewers, et al. Guidelines on prevention, diagnosis and treatment of infective endocarditis executive summary; the task force on infective endocarditis of the European society of cardiology. Eur Heart J 25: 267-276, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Habib G, Lancellotti P, Antunes MJ, Document Reviewers, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 36: 3075-3128, 2015. [DOI] [PubMed] [Google Scholar]
- 25.Baddour LM, Wilson WR, Bayer AS, Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association, Infectious Diseases Society of America, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111: e394-e434, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 96: 200-209, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Fowler VG Jr, Miro JM, Hoen B, ICE Investigators, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293: 3012-3021, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg 41: 734744, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Gaca JG, Sheng S, Daneshmand MA, et al. Outcomes for endocarditis surgery in North America: a simplified risk scoring system. J Thorac Cardiovasc Surg 141: 98-106. e1-e2, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira FL, Bota DP, Bross A, AEPEI Study Group, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 286: 1754-1758, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Selton-Suty C, Célard M, Le Moing V, AEPEI Study Group, et al. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis 54: 1230-1239, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Forestier E, Fraisse T, Roubaud-Baudron C, Selton-Suty C, Pagani L. Managing infective endocarditis in the elderly: new issues for an old disease. Clin Interv Aging 11: 1199-1206, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhawan VK. Infective endocarditis in elderly patients. Curr Infect Dis Rep 5: 285-292, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Di Salvo G, Thuny F, Rosenberg V, et al. Endocarditis in the elderly: clinical, echocardiographic, and prognostic features. Eur Heart J 24: 1576-1583, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Gregoratos G. Infective endocarditis in the elderly: diagnosis and management. Am J Geriatr Cardiol 12: 183-189, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Remadi JP, Nadji G, Goissen T, Zomvuama NA, Sorel C, Tribouilloy C. Infective endocarditis in elderly patients: clinical characteristics and outcome. Eur J Cardiothorac Surg 35: 123-129, 2009. [DOI] [PubMed] [Google Scholar]
- 37.López J, Revilla A, Vilacosta I, et al. Age-dependent profile of left-sided infective endocarditis: a 3-center experience. Circulation 121: 892-897, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Durante-Mangoni E, Bradley S, Selton-Suty C, International Collaboration on Endocarditis Prospective Cohort Study Group, et al. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 168: 2095-2103, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Ramírez-Duque N, García-Cabrera E, Ivanova-Georgieva R, Grupo para el Estudio de las Infecciones Cardiovasculares de la Sociedad Andaluza de Enfermedades Infecciosas (SAEI), et al. Surgical treatment for infective endocarditis in elderly patients. J Infect 63: 131-138, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Wang TK, Oh T, Voss J, Gamble G, Kang N, Pemberton J. Comparison of contemporary risk scores for predicting outcomes after surgery for active infective endocarditis. Heart Vessels 30: 227-234, 2015. [DOI] [PubMed] [Google Scholar]
- 41.Freeman WK, Schaff HV, O'Brien PC, Orszulak TA, Naessens JM, Tajik AJ. Cardiac surgery in the octogenarian: perioperative outcome and clinical follow-up. J Am Coll Cardiol 18: 29-35, 1991. [DOI] [PubMed] [Google Scholar]
- 42.Bouma BJ, van den Brink RBA, van der Meulen JHP, et al. To operate or not on elderly patients with aortic stenosis: the decision and its consequences. Heart 82: 143-148, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bach DS, Siao D, Girard SE, Duvernoy C, McCallister BD Jr, Gualano SK. Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk. Circ Cardiovasc Qual Outcomes 2: 533-539, 2009. [DOI] [PubMed] [Google Scholar]