Abstract
Objective
Heart failure is currently the most serious complication of muscular dystrophy. The transient receptor potential cation channel, subfamily V, member 2 (TRPV2) is a stretch-sensitive Ca channel. In damaged myocytes or cardiomyocytes, TRPV2 translocates to the cytoplasmic membrane and enhances Ca influx, triggering cell damage. Evidence suggests that the inhibition of TRPV2 may be a new therapeutic target in heart failure. We found that tranilast, which is widely used as an anti-allergic drug, inhibits TRPV2. A pilot study was conducted to assess the safety and efficacy of tranilast in muscular dystrophy patients with cardiomyopathy.
Methods
After obtaining informed consent, two muscular dystrophy patients with advanced heart failure took tranilast (300 mg/day) for three months. Blood tests, echocardiography, electrocardiography (ECG), Holter ECG, analyses of the TRPV2 expression in peripheral mononuclear cells, and circulating micro ribonucleic acid profiling were performed to assess the safety and efficacy of tranilast.
Results
The brain natriuretic peptide levels decreased after treatment. The expression of TRPV2 on the cytoplasmic membrane of peripheral mononuclear cells was enhanced before treatment and was decreased after treatment. Some heart-related micro ribonucleic acids (miR-208a-5p, miR-223-3p) were elevated and then decreased after treatment. Some adverse events, including the potentiation of warfarin, the worsening of renal dysfunction, an increased heart rate and premature ventricular contractions, were observed.
Conclusion
Tranilast can inhibit TRPV2 and can be effective for treating heart failure, even in patients with muscular dystrophy. Although careful attention is needed, the inhibition of TRPV2 can be a new treatment target for cardiomyopathy. A multi-center trial is planned.
Keywords: cardiomyopathy, cardioprotective therapy, muscular dystrophy, tranilast, transient receptor potential cation channel, subfamily V, member 2 (TRPV2)
Introduction
The life expectancy of patients with muscular dystrophy, including those with Duchenne muscular dystrophy (DMD), has been dramatically improved by mechanical ventilation. Now, heart failure is the most important complication in muscular dystrophy (1). Although cardioprotective agents such as angiotensin converting enzyme inhibitors (ACEIs)/angiotensin II receptor blockers (ARBs) and beta-blockers have shown some effects (2), the cardiac function does not fully recover under treatment with these drugs, and the situation remains unsatisfactory for both patients and physicians. Since it is difficult for muscular dystrophy patients to undergo surgical interventions such as ventriculoplasty, artificial heart implantation, and cardiac transplantation, the development of efficient pharmacological treatments is an urgent task.
The transient receptor potential cation channel, subfamily V, member 2 (TRPV2) is a stretch-sensitive Ca channel. It is normally localized in the intracellular membrane compartments but translocates to the cytoplasmic membrane in damaged myocytes or cardiomyocytes and enhances the influx of Ca, which triggers the cell damage process (3). The overexpression of TRPV2 on the cytoplasmic membrane is observed in the cardiac and skeletal muscle of mdx mice and BIO 14.6 hamsters, animal models of muscular dystrophy that contain mutations in dystrophin and δ-sarcoglycan, respectively (4). The overexpression of TRPV2 at the sarcolemma was also observed in the skeletal muscle and cardiomyocytes of muscular dystrophy patients (4, 5) and cardiomyocytes of dilated cardiomyopathy (DCM) patients (5). Transgenic mice with the cardiac-specific overexpression of TRPV2 develop DCM (4).
The inhibition of TRPV2 using mutant TRPV2 and Ca-handling agents blocked the abnormal intracellular Ca influx and ameliorated the muscle pathology and motor function in mdx mice and BIO14.6 hamsters (6-8). In addition, the overexpression of the NT domain of TRPV2 blocked the plasma membrane accumulation of TRPV2 and improved cardiomyopathy in various animal models of DCM (4C30 mice, DOX-induced DCM mice, J2N-k hamsters) (5). We developed a high-throughput screening method and detected some compounds showing the inhibition of TRPV2. Tranilast, which is already approved as an anti-allergic drug, is one such drug and was effective in BIO14.6 hamsters and J2N-k hamsters (5, 6).
These facts suggest that tranilast could be useful in muscular dystrophy patients. Thus, we performed a pilot study in which tranilast was used to treat two muscular dystrophy patients with advanced cardiomyopathy. In both patients, the serum level of brain natriuretic peptide (BNP) decreased after the initiation of tranilast therapy.
Materials and Methods
The study protocol
The aim of this pilot study was to evaluate the safety and efficacy of tranilast in the treatment of heart failure in patients with muscular dystrophy. In addition, since this was the first study of the use of tranilast in the treatment of heart failure in humans, we also gathered data to assess the pharmacological mechanisms. The effects of tranilast on cardiomyopathy in animal models were dose-dependent. Since there had been some clinical trials using tranilast (600 mg/day) (9-11), a dose escalation study, in which tranilast was started at a dose of 300 mg/day and gradually titrated up to 600 mg/day (unless there were adverse events), was initially considered. However, the pharmaceutical company recommended that dose escalation be avoided, since they had seen increased adverse effects, such as urocystitis-like symptoms (1-5%) and hepatic dysfunction (11-23%), at higher doses (9-11). In addition, the ethics review board prescribed the use of the approved dose (300 mg/day). Consequently, the study was designed as an open single-arm study of tranilast (300 mg/day) for three months.
The subjects were muscular dystrophy patients who were >20 years of age with severe cardiac dysfunction [left ventricular dimension in diastole (LVDd)>50 mm, FS<20%]. Patients with severe hepatic dysfunction, renal dysfunction, eosinophilia, leucopenia, or thrombocytopenia were excluded since urocystitis-like symptoms, hepatic dysfunction/jaundice, renal dysfunction, leucopenia, and thrombocythemia are listed as clinically significant adverse reactions (unknown frequency) in the interview form for tranilast.
After providing their informed consent, the participants took tranilast (300 mg/day) for three months. In principle, the doses of drugs that affect the cardiac function, such as ACEIs/ARBs, beta blockers, diuretics, digitalis, inotropic agents, and antiarrhythmic agents, were fixed during the study period. However, the attending physicians could optimize them when they recognized the need based on the patient's physical condition and/or laboratory data. The dose of tranilast was also fixed. However, the attending physicians could also adjust it when they considered that adverse events had occurred in association with the administration of tranilast and that the adjustment of the tranilast dose was necessary.
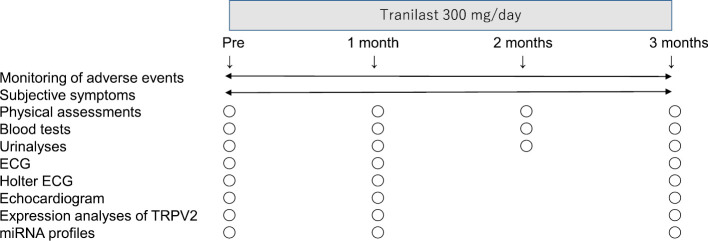
Clinical assessments were performed before and at one and three months after the initiation of tranilast (Fig. 1). Even when tranilast was stopped, the clinical assessments were continued on the same schedule.
Figure 1.
The protocol of this pilot study.
To assess the effects, cardiac functional indices, including echocardiographic parameters (LVDd, FS) and natriuretic peptide levels (human atrial natriuretic peptide: hANP, BNP), and cardiomyocyte damage indices, such as cardiac troponin T (cTnT) and creatine kinase isoform (CK-MB), were examined. The plasma transforming growth factor-beta 1 (TGF-β1) levels were evaluated, the TRPV2 expression in peripheral mononuclear cells (MNCs) was analyzed, and circulating micro ribonucleic acid (miRNA) profiling was performed to assess the pharmacological mechanisms. For the analysis of the TRPV2 expression in MNCs and miRNA profiling, control samples were obtained from four healthy adult volunteers. The participants understood that they were being included as a control in this study and provided their informed consent.
Cardiac events (deterioration of heart failure requiring intravenous inotropic, antiarrhythmic, and/or diuretic agents, cardiac death, total death), subjective symptoms, and vital signs (blood pressure, pulse rate, percutaneous oxygen saturation) were monitored. The New York Heart Association (NYHA) classification was not suitable for these patients because they were bedridden and could not perform daily activities.
To assess the safety, Holter electrocardiography (ECG) [mean heart rate, total number of premature ventricular contractions (PVCs)], blood tests [WBCs, eosinophils (Eo), platelets (Plt)], blood chemistry [cystatin C (CysC), creatinine (Crn), blood urea nitrogen (BUN), uric acid (UA), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (γ-GTP), lactate dehydrogenase (LDH), total bilirubin (T.Bil), C-reactive protein (CRP), Na, K, Cl], the international normalized ratio (INR) of prothrombin time, and a urinalysis (urinary protein, urinary sugar, occult blood) were also performed.
The primary endpoints of this study were LVDd and FS. The secondary endpoints were BNP, cTnT, and cardiac events. In unstable patients, additional assessments were performed to see the detailed changes. With regard to treatment after the study, the plan was to stop tranilast and then provide the best available care. However, if tranilast seemed effective, the participants could choose to continue treatment.
The protocol of this study was in accordance with the Declaration of Helsinki and was approved by the ethics board of National Hospital Organization Toneyama National Hospital (No. 1441). The protocols for the analysis of the TRPV2 expression in MNCs and miRNA profiling were approved by the ethics board of the National Cerebral and Cardiovascular Center (M27-080-2).
The analysis of the TRPV2 expression in MNCs
MNCs were isolated from blood samples gathered into ethylenediaminetetraacetic acid (EDTA)-Na using Lymphoprep™ (Axis-SHIELD PoC AS, Oslo, Norway), and were then centrifuged at 800×g for 30 minutes at room temperature.
For immunostaining, we used affinity-purified polyclonal anti-TRPV2 antibodies (4, 5) which were raised by immunizing rabbits with a glutathione S-transferase (GST) fusion protein containing aa634-756 of mouse TRPV2 protein. Isolated MNCs immobilized on glass slides were fixed with 4% paraformaldehyde for 15 minutes at room temperature, permeabilized with 0.1% Triton™ X-100 (Axis-SHIELD PoC AS), incubated with the antibody (1 : 100 dilution) overnight at 4℃, and then incubated for 30 minutes at room temperature with Alexa Fluor 488 goat anti-rabbit IgG (H+L) (1 : 500 dilution) (Invitrogen, Carlsbad, USA).
Images of MNCs stained with TRPV2 antibodies were analyzed by investigators (who were blinded to the samples), by confocal laser scanning microscopy (FLUOVIEWTM FV1000, Olympus, Tokyo, Japan) mounted on an objective lens (Olympus), using the National Institute of Health Imaging software program. The fluorescence intensity was scanned along lines selected in the center of an MNC. The fluorescence intensity corresponding to the surface region was calculated by subtracting the intensity of the intracellular region from that of the total area. To summarize the data for the membrane localization of TRPV2, the number of MNCs with a strong fluorescence signal at the cell edge (at least three times higher than the average fluorescence in the internal region) was counted (total number of MNCs analyzed: 30-100). We repeated the experiments twice using another affinity purified TRPV2 antibody (anti-human TRPV2 antibody raised by immunizing rabbits with maltose binding protein fusion protein containing aa635-729 of human TRPV2).
RNA extraction and miRNA profiling
Plasma samples were collected from EDTA-Na blood samples by centrifugation at 1,500×g and stored at -80°C. RNA was extracted from the plasma samples using the 3D-GeneⓇ RNA extraction reagent from a liquid sample kit (TORAY, Tokyo, Japan), according to the manufacturer's instructions. The extracted total RNA was labeled with the 3D-GeneⓇ miRNA labeling kit (TORAY). Labeled RNAs were hybridized onto 3D-GeneⓇ Human miRNA Oligo chips (TORAY) (12). The annotation and oligonucleotide sequences of the probes conformed to the miRBase miRNA database (http://www.mirbase.org/). After careful washing, the fluorescence signals were scanned with a 3D-GeneⓇ Scanner (TORAY) and were analyzed using the 3D-GeneⓇ Extraction software program (TORAY).
The raw data of each spot were normalized by substitution with the mean intensity of the background signal (determined by the signal intensities of all of the blank spots' and 95% confidence intervals). The measurements of spots with signal intensities that were greater than two standard deviations of the background signal intensity were considered to be valid. The relative expression level of a given miRNA was calculated by comparing the signal intensities of the valid spots throughout the microarray experiments. The normalized data were globally normalized per array, such that the median signal intensity was adjusted to 25.
Patients
Two muscular dystrophy patients with severe cardiac dysfunction, who agreed with the aims and protocol of this study and wished to participate, were included in the present study.
The first case (Patient 1) was a 45-year-old man with Becker muscular dystrophy (BMD). He had a deletion of exons 3 and 4 in the dystrophin gene, which was detected by multiple ligation-dependent prove amplification (MLPA). He was non-ambulatory but could sit without any support. He had developed cardiac dysfunction and had started ACEI therapy 20 years previously and beta blocker therapy 11 years previously. He was resuscitated from cardiopulmonary arrest due to ventricular tachycardia (VT) and underwent the implantation of a cardioversion-defibrillation and biventricular pacing device 6 years previously. He was then admitted to our hospital due to an exacerbation of heart failure. A stable condition was barely maintained with the best available care, which included ACEI, a beta blocker, a phosphodiesterase 3 inhibitor (PDE3I), diuretics, antiarrhythmic agents, warfarin, and night-time non-invasive ventilation (NIV). However, at the beginning of this study, he still had severe cardiac dysfunction [left ventricular dilated dimension (LVDd), 65 mm; fractional shortening (FS), 4%; BNP, 144.2 pg/mL; cTnT 0.039 ng/mL].
The second case (Patient 2) was a 46-year-old man with unclassified muscular dystrophy. He was initially diagnosed with BMD based on his clinical appearance and the findings of a histological examination of a muscle biopsy specimen in another hospital over 30 years previously, but neither MLPA nor Sanger sequencing detected mutations in the dystrophin gene. He was non-ambulatory and was not able to sit alone. He developed respiratory failure and was started on NIV 19 years previously. Cardiac dysfunction was also detected, and ACEI therapy was started 5 years previously. Non-sustained VT was detected from one year previously. He was strongly advised to take a beta blocker and/or an antiarrhythmic agent but refused because he disliked being admitted to hospital and frequent hospital visits. He developed cardiopulmonary arrest due to an acute exacerbation of heart failure and was successfully resuscitated at his home three months previously and was transferred to our hospital. Although maximal therapy was given, including ACEI, PDE3I, diuretics, antiarrhythmic agents, and full-time tracheal mechanical ventilation, he presented with recurrent multi-organ infection, and still had severe cardiac dysfunction (LVDd 61 mm, FS 6%, BNP 164.3 pg/mL, cTnT 0.016 ng/mL).
Both patients understood the protocol of this study well and participated willingly; both provided their informed consent.
Results
The clinical findings in Patient 1
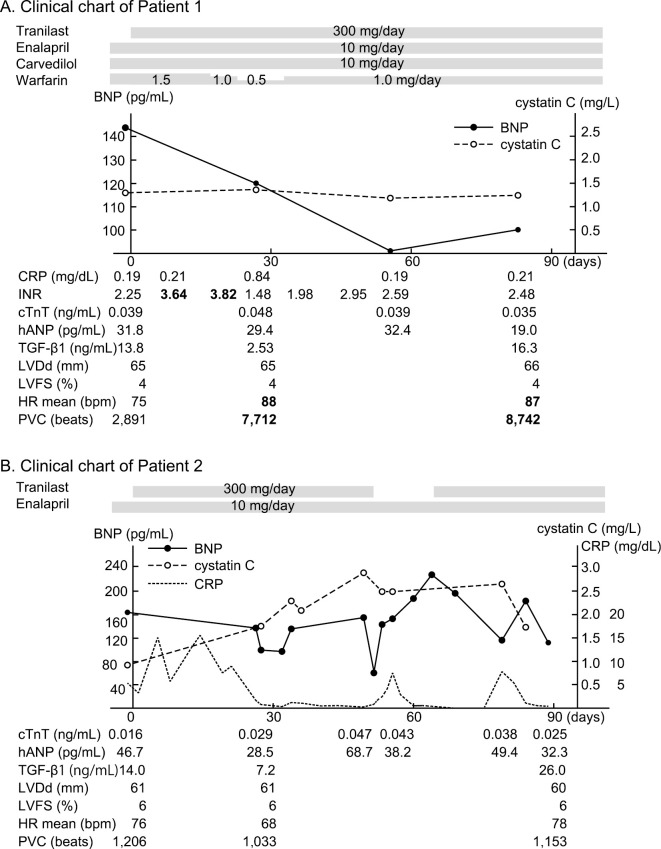
After the initiation of tranilast, the INR increased from 2.25 to 3.64 within one week. The dose of warfarin was adjusted from 1.5 mg/day to 1.0 mg/day according to the INR. He initially had no subjective cardiac symptoms. Although his total energy intake was not greatly changed, his meal portion increased just before starting tranilast because the number of meals supplied was reduced from five (200 Kcal×5/day) to three (350 Kcal×3/day) per day. In addition, his mother's health had deteriorated during the study period. He felt an increased pulse rate, and it was confirmed by Holter ECG (the mean heart rate increased from 75 bpm to 88 bpm at one month, and 87 bpm at three months). The PVC also increased from 2,891 beats/day to 7,712 beats/day at one month and 8,445 beats/day at three months. Nonetheless, the plasma BNP level decreased from 144.2 pg/mL to 119.7 pg/mL at one month and 99.8 pg/mL at three months. The serum UA level decreased from 8.5 mg/dL at the beginning to 4.5 mg/dL at both one and three months. No apparent changes were detected on echocardiography (LVDd, FS) or in cTnT, CysC, or the other indices. There were no other clinically relevant changes (Fig. 2A). All of the laboratory data are presented in Supplementary material 1.
Figure 2.
The clinical courses of both patients.
The clinical findings in Patient 2
The general condition of Patient 2 was serious, with recurrent multi-organ infection. Thus, there were fluctuations in various indices. Subjective symptoms, such as general fatigue were affected by his general condition, and it was difficult to evaluate the effects of tranilast. His BNP level was also unstable, but it decreased from 164.3 pg/mL to 102.6 pg/mL at one month and 64.5 pg/mL at seven weeks. Since his CysC level was elevated from 0.92 mg/L to 2.86 mg/L, it was thought that this might be an adverse effect of tranilast, and tranilast was stopped at 52 days. In addition, his water intake increased simultaneously because the BUN level was elevated (51.4 mg/dL), and dehydration was considered. The BNP then rapidly increased to 228.5 pg/mL at 66 days. After tranilast was restarted, the patient's BNP level decreased to 114.6 pg/mL at 79 days. The serum UA level was 9.8 mg/dL before treatment, 3.5 mg/dL at one month, and 5.5 mg/dL at three months. There were no apparent changes on echocardiography, Holter ECG, or cTnT (Fig. 2B). All of the laboratory data are presented in Supplementary material 2.
The analysis of the TRPV2 expression in MNCs
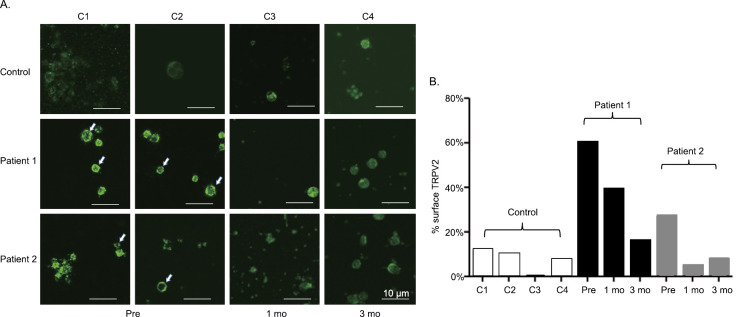
In the control samples, TRPV2 was diffusely expressed in the cytoplasm. In the samples of both patients, TRPV2 was preferentially present on the cytoplasmic membrane (Fig. 3A, arrows). After the initiation of tranilast, the ratios of MNCs expressing TRPV2 on the cytoplasmic membrane were decreased in both cases (Fig. 3A, B). A similar result was obtained by using another TRPV2 antibody.
Figure 3.
The analysis of the TRPV2 expression in the peripheral MNCs. A: The immunohistochemical expression of TRPV2 in the MNCs. B: The ratio of MNCs expressing TRPV2 on the cytoplasmic membrane. Open bar: controls, black bar: Patient 1, gray bar: Patient 2. pre: pre-treatment, 1mo: One month after starting tranilast, 3mo: Three months after starting tranilast
The miRNA expression profiles
The chipset examined over 2,000 miRNAs. However there were only three miRNAs (miR-208a-5p, miR-223-3p, miR-4443) that were expressed at a higher level in comparison to the control samples at the beginning of treatment; they decreased to the control levels at one month after the initiation of tranilast treatment, and were maintained at the same levels at three months after treatment in both patients (Fig. 4). The expression levels of miR-1-3p, miR-1-5p, miR-133a-3p, miR-133b-miRNAs that are known as muscle miRNAs (13)-were not elevated in these patients.
Figure 4.
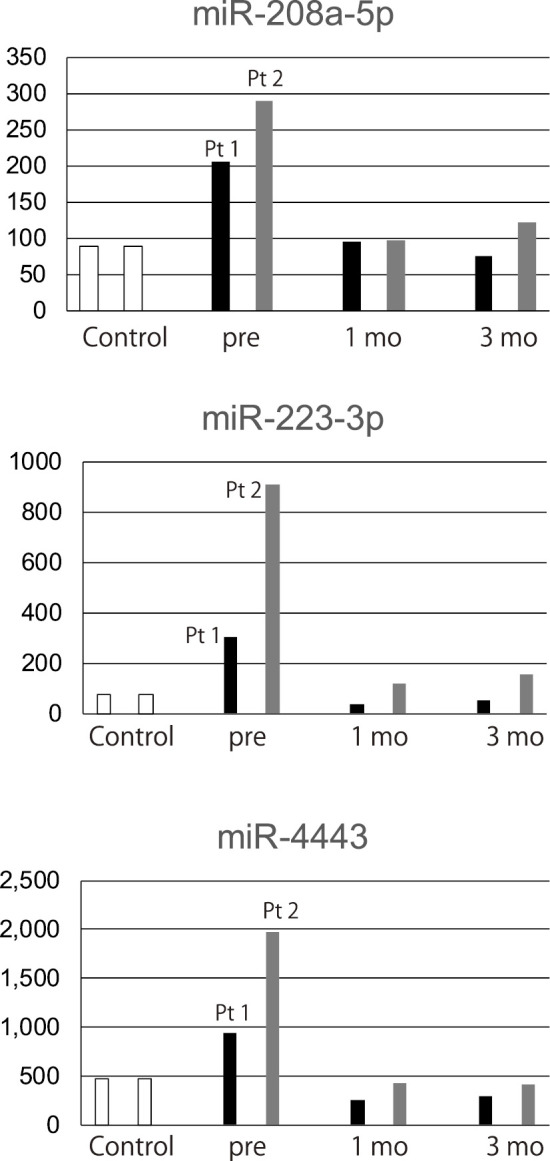
The circulating miRNA profiles. Open bar: controls, black bar: Patient 1, gray bar: Patient 2. Pt1: patient 1, Pt2: patient 2, pre: pre-treatment, 1mo: One month after starting tranilast, 3mo: Three months after starting tranilast
Discussion
Heart failure is a crucial prognostic factor in patients with muscular dystrophy, but there are various problems in the clinical assessment of the cardiac function. First, symptoms of heart failure in these patients are difficult to recognize. Their motor dysfunction is so severe that the cardiac load is extremely low. Heart failure progresses chronically and often concurrently with respiratory failure. Mechanical ventilation also greatly decreases the cardiac load. Physicians familiar with muscular dystrophy treat heart failure from the early asymptomatic stage. Consequently, few patients complain of the symptoms of heart failure, even in the advanced stage. Thus, it is difficult to assess the effects of treatment based on the subjective symptoms.
Second, there are many limitations in the evaluation of the cardiac function. Magnetic resonance imaging is impossible for many patients because of severe deformities or the need for mechanical ventilation. Echocardiography is also difficult in some patients due to narrow echo windows because of the patients' thoracic deformities. BNP frequently remains low, even in the advanced stage of heart failure (14), and 100 pg/mL is known as a critical point for the prognosis (15). A previous study of beta blocker therapy showed improvements in cardiac events, but no obvious changes were detected in the BNP levels (2). Consequently, multiple markers and serial evaluations are needed to assess the cardiac function.
Both of the patients who participated in this pilot study had terminal-stage heart failure. Their cardiac dysfunction was quite severe, despite the provision of the best available treatment. Nonetheless, the BNP levels of both patients decreased after the initiation of tranilast. Notably, in Patient 2, the BNP rapidly increased when he stopped tranilast, and it then decreased after it was restarted. Although the echocardiographic findings showed no obvious changes, these findings suggest that tranilast has beneficial effects on cardiomyopathy in muscular dystrophy patients.
Several assessments of the pharmacological mechanism also supported the efficacy of tranilast. The first assessment was the analysis of the TRPV2 expression. Assessments of the TRPV2 expression in cardiomyocytes before and after treatment are ideal but impractical in human patients. Instead, the TRPV2 expression in peripheral MNCs was analyzed. TRPV2 was preferentially expressed on the cytoplasmic membrane of the peripheral MNCs in both patients. After starting tranilast, the ratio of MNCs expressing TRPV2 on the cytoplasmic membrane decreased to control levels. These data suggest that tranilast can actually inhibit TRPV2, even in humans.
The second assessment was the analysis of the expression of circulating miRNAs. This study showed the elevation of the miR-208a-5p, miR-223-3p, and miR-4443 expression before treatment, with decreased to control levels in both patients after the initiation of tranilast treatment. Among these miRNAs, miR-208a-5p is known to be a regulator of cardiac hypertrophy (16), and its inhibition is reported to improve heart failure (17). In addition, miR-233-3p is known as a marker of heart disease (18, 19). Although there were no changes in the cTnT level, these findings implied that tranilast can ameliorate cardiac damage. These findings not only supported the effects of tranilast, but also suggested that these assessments can be biomarkers in TRPV2 inhibition therapy.
It should be noted that the expression levels of miR-1s and miR-133s were not elevated in these patients. Although these miRNAs have been reported to be elevated in patients with muscular dystrophy (13), the present results seemed natural because both patients were advanced cases with severe muscle atrophy.
With respect to safety, several adverse events occurred during the study period. It is well known that tranilast, which is mainly metabolized by CYP2C9, has synergistic effects with warfarin. Patient 1, who took warfarin, showed an elevated INR and his dose of warfarin required adjustment. Renal dysfunction is also a known adverse effect of tranilast. Based on the interview form, Crn elevation was found in 0.004% of patients who received the approved dose and 9.5% of the patients who received a higher dose (10). In the present study, Patient 2 showed cystatin C elevation. We suspected that dehydration and recurrent infection might also have affected the renal function, and his water intake was increased, but the value remained high. Thus, there was a high possibility that tranilast impaired the renal function. Since renal dysfunction is not rare in muscular dystrophy patients with heart failure (20), it can be a limiting factor. An increased heart rate and PVCs were observed in Patient 1. Tranilast is not known to cause such events. An increase in the food portions and/or mental stress might have been related to the patient's increased heart rate; however, an adverse effect of tranilast could not be ruled out. Although tranilast has been widely used in the general population, careful attention is needed when it is used in the treatment of patients with severe heart failure.
The results obtained from this pilot study suggest that the inhibition of TRPV2 might become a new therapeutic target for heart failure and myopathy. New options for the treatment of heart failure are of great clinical importance. We are planning multi-center clinical trials to clarify the safety and efficacy of tranilast.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This study was partly supported by a Health and Labour Sciences Research Grant for Comprehensive Research on Persons with Disabilities from Japan Agency for Medical Research and Development (15Adk0310043h0002) and an Intramural Research Grant (25-5, 26-6) for Neurological and Psychiatry Disorders of NCNP. The funders had no roles in the study design, data collection and analyses, decision to publish, or the preparation of the manuscript.
Supplementary Materials
Laboratory data of Patient 1
Laboratory data of Patient 2
Acknowledgement
The authors are grateful to Drs. Ryu Nagata and Hideki Ohta of the Japan Agency for Medical Research and Development for their kind advice on the circulating miRNA and metabolome analyses.
References
- 1.Matsumura T, Saito T, Fujimura H, Shinno S, Sakoda S. A longitudinal cause-of-death analysis of patients with Duchenne muscular dystrophy. Rinsho Shinkeigaku 51: 743-750, 2011(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 2.Matsumura T, Tamura T, Kuru S, Kikuchi Y, Kawai M. Carvedilol can prevent cardiac events in Duchenne muscular dystrophy. Intern Med 49: 1357-1363, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Kanzaki M, Zhang YQ, Mashima H, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-1. Nat Cell Biol 1: 165-170, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Iwata Y, Katanosaka Y, Arai Y, Komamura K, Miyatake K, Shigekawa M. A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. J Cell Biol 161: 957-967, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwata Y, Ohtake H, Suzuki O, Matsuda J, Komamura K, Wakabayashi S. Blockade of sarcolemmal TRPV2 accumulation inhibits progression of dilated cardiomyopathy. Cardiovasc Res 99: 760-768, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Iwata Y, Katanosaka Y, Shijun Z, et al. Protective effects of Ca2+ handling drugs against abnormal Ca2+ homeostasis and cell damage in myopathic skeletal muscle cells. Biochem Pharmacol 70: 740-751, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Iwata Y, Katanosaka Y, Arai Y, Shigekawa M, Wakabayashi S. Dominant-negative inhibition of Ca2+ influx via TRPV2 ameliorates muscular dystrophy in animal models. Hum Mol Genet 18: 824-834, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Zanou N, Iwata Y, Schakman O, Lebacq J, Wakabayashi S, Gailly P. Essential role of TRPV2 ion channel in the sensitivity of dystrophic muscle to eccentric contractions. FEBS Lett 583: 3600-3604, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Kato K, Tamai H, Hayakawa H, et al. Clinical evaluation of tranilast on restenosis after percutaneous transluminal coronary angiopathy (PTCA) -a double-blind placebo-controlled comparative study-. Rinshoiyaku 12: 65-85, 1996(in Japanese, Abstract in English). [Google Scholar]
- 10.Itagane H, Haji K, Kodama K, et al. Effects of tranilast on restenosis after percutaneous transluminal coronary angiopathy -a multi center phase III open trial-. Shinnyaku to Rinsho 45: 267-280, 1996(in Japanese, Abstract in English). [Google Scholar]
- 11.Takekoshi N, Kanemitsu S, Kitayama M, et al. Clinical evaluation of tranilast in the prevention of restenosis after percutaneous transluminal coronary angioplasty (PTCA) -a phase III multicenter open trial-. Kiso to Rinsho 30: 593-609, 1996(in Japanese, Abstract in English). [Google Scholar]
- 12.Nagino K, Nomura O, Takii Y, et al. Ultrasensitive DNA chip: gene expression profile analysis without RNA amplification. J Biochem 139: 697-703, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Cacchiarelli D, Legnini I, Martone J, et al. miRNAs as serum biomarkers for Duchenne muscular dystrophy. EMBO Mol Med 3: 258-265, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demachi J, Kagaya Y, Watanabe J, et al. Characteristics of the increase in plasma brain natriuretic peptide level in left ventricular systolic dysfunction, associated with muscular dystrophy in comparison with idopathic dilated cardiomyopathy. Neurmuscul Disord 14: 732-739, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Adachi K, Kawai H, Kimura C, et al. Estimation of the prognosis of patients with Duchenne muscular dystrophy and cardiac failure on their plasma level of natriuretic peptide. Shinkeinaika 1998 49: 532-536, 1998(in Japanese, Abstract in English). [Google Scholar]
- 16.Callis TE, Pandya K, Seok HY, et al. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest 119: 2772-2786, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montgomery RL, Hullinger TG, Semus HM, et al. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation 124: 1537-1547, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickinson BA, Semus HM, Montgomery RL, et al. Plasma microRNAs serve as biomarkers of therapeutic efficacy and disease progression in hypertension-induced heart failure. Eur J Heart Fail 15: 650-659, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Barsanti C, Trivella MG, D'Aurizio R, et al. Differential regulation of microRNAs in end-stage failing hearts is associated with left ventricular assist device unloading. Biomed Res Int 2015(Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumura T, Saito T, Fujimura H, Sakoda S. Renal dysfunction is a frequent complication in patients with advanced stage of Duchenne muscular dystrophy. Rinsho Shinkeigaku 52: 211-217, 2012(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Laboratory data of Patient 1
Laboratory data of Patient 2