Abstract
An 85-year-old woman underwent endoscopic retrograde cholangiopancreatography (ERCP) for obstructive jaundice. Selective bile duct cannulation was unsuccessful because of periampullary diverticula (PAD). A pancreatic spontaneous dislodgement stent (PSDS) (5F diameter, 3 cm, straight type) was inserted to prevent post-ERCP pancreatitis. Three days after ERCP, she complained of abdominal pain, and computed tomography revealed retroperitoneal perforation because of PSDS migration to the PAD. If the papillary orifice is observed at the diverticular rim or in the diverticula, a pigtailed PSDS on the duodenal side or flanged stent on the pancreatic ductal side should be inserted in order to prevent this rare adverse event.
Keywords: pancreatic spontaneous dislodgement stent, periampullary diverticula, retroperitoneal perforation, pancreatic stent migration, ERCP complication
Introduction
Pancreatitis after endoscopic retrograde cholangiopancreatography (ERCP) is the most common and potentially the most serious complication of ERCP. Insertion of a pancreatic spontaneous dislodgement stent (PSDS) is considered an effective and safe method for preventing pancreatitis after ERCP, and PSDS use has increased in recent years (1, 2). Although periampullary diverticula (PAD) sometimes occur in patients who undergo ERCP, major complications following PSDS insertion in patients with PAD have not been reported. In this case report, we present a rare case of retroperitoneal perforation caused by PSDS migration to PAD in a patient.
Case Report
An 85-year-old previously healthy Japanese woman presented to our hospital with a 1-week history of anorexia and general fatigue. She had been treated with digestive enzymes for one week with no clinical improvement. On admission, she complained of fatigue and anorexia. She denied experiencing abdominal pain, a fever, nausea, vomiting, diarrhea, or weight loss. Her medical history was otherwise unremarkable. On a physical examination, she was icteric but showed no signs of chronic liver disease and no lymphadenopathy. The remainder of the physical examination findings and her vital signs were normal. Her laboratory data showed elevated levels of alkaline phosphatase (ALP, 2,950 IU/L), transaminases (AST, 335 IU/L, ALT, 223 IU/L), and bilirubin (T-bil, 4.3 mg/dL; D-bil, 2.7 mg/dL). Contrast-enhanced computed tomography (CT) and magnetic resonance cholangiopancreatography revealed a 3.0-cm hepatic hilar mass with a corresponding intrahepatic biliary obstruction, suspected of being hilar cholangiocarcinoma (Fig. 1).
Figure 1.
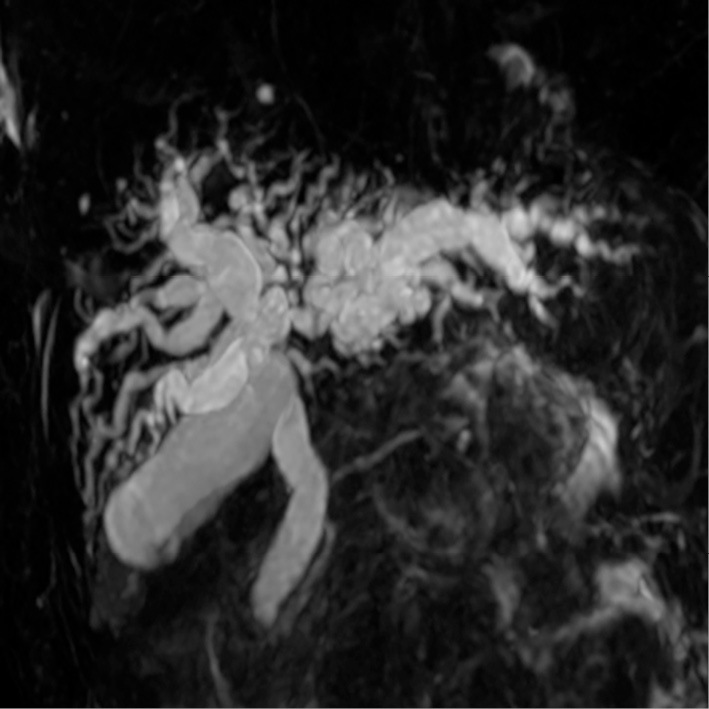
Magnetic resonance cholangiopancreatography (MRCP) image. MRCP identified a hepatic hilar mass with a corresponding intrahepatic biliary obstruction.
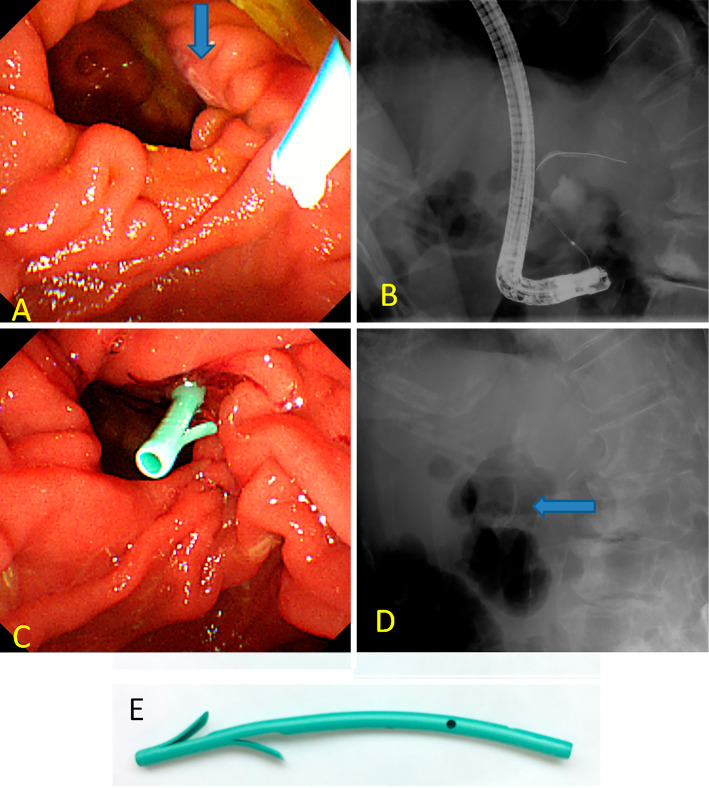
The patient underwent ERCP for obstructive jaundice due to hilar cholangiocarcinoma, with the intention of receiving bile duct brushing and stent placement at the intra bile duct. On ERCP, the papilla were identified at the rim of the duodenal diverticula (Fig. 2A). Selective bile duct cannulation was unsuccessful, although pancreatic guide-wire cannulation was performed simultaneously (Fig. 2B). PSDS was inserted to prevent post-ERCP pancreatitis due to repeated insertion of the cannula and guide-wire (Fig. 2C and D). The stent used was a polyethylene 5F diameter, 3 cm in length, straight-type stent, unflanged on the pancreatic ductal side with 2 flanges on the duodenal side (Fig. 2E).
Figure 2.
Endoscopic (A, C) and fluoroscopic (B, D) images during endoscopic retrograde cholangiopancreatography. (A) The papillary orifice (blue arrow) was seen at the right side of the diverticular rim. (B) Selective bile duct cannulation was unsuccessful, although pancreatic guide-wire cannulation was performed simultaneously. (C, D) A pancreatic spontaneous dislodgement stent (blue arrow) was inserted to prevent post-ERCP pancreatitis. (E) The stent used was a polyethylene 5F diameter, 3 cm in length, straight-type stent, unflanged on the pancreatic ductal side with 2 flanges on the duodenal side (GPDS-5-3; Cook Japan, Tokyo, Japan).
The patient progressed favorably after ERCP; however, she complained of right lower quadrant pain and developed a high-grade fever three days later. Contrast-enhanced CT revealed the retroperitoneal collection of fluid and migration of the PSDS to the PAD through a perforation in the duodenal wall (Fig. 3). She was immediately started on broad-spectrum antibiotics, and after surgical consultation, she immediately underwent surgery. Intraoperative findings revealed retroperitoneal bile leakage, and micro perforation of the PAD was noted (Fig. 4). The PSDS had not completely penetrated the wall of the PAD, so we removed the PSDS via intraoperative duodenoscopy with an endoscopic forceps (Fig. 5). We repaired the micro perforation in the PAD using part of the round ligament of the liver. In addition, debridement and drainage of the right retroperitoneal area were performed. The postoperative course was uneventful. Her condition steadily improved following treatment, and she was discharged on the 45th hospital day. Two weeks later, she was readmitted to our hospital for the progression of obstructive jaundice. She and her family did not wish for ERCP a second time. She underwent percutaneous transhepatic biliary drainage (PTBD) and was able to be discharged again a week after PTBD. However, she died of hilar cholangiocarcinoma that developed four months after PTBD.
Figure 3.
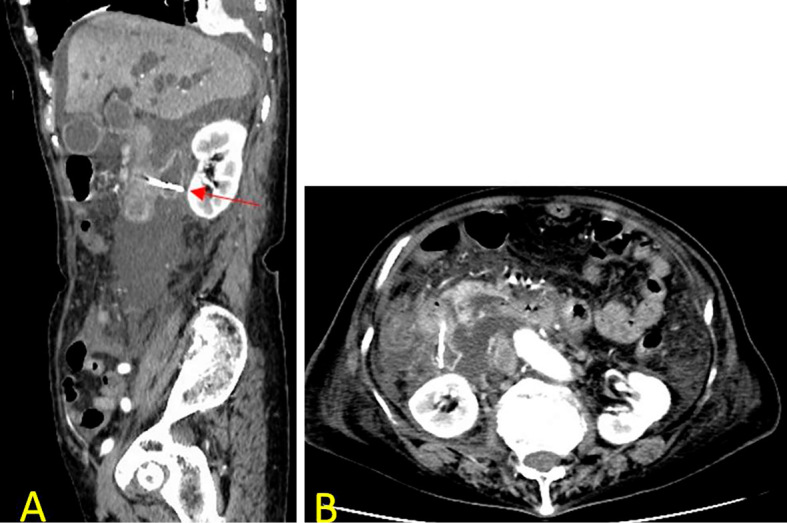
Computed tomography image. Sagittal (A) and axial (B) computed tomography revealed the retroperitoneal collection of fluid and migration of the pancreatic spontaneous dislodgement stent to the periampullary diverticula by perforating through the duodenal wall (red arrow).
Figure 4.
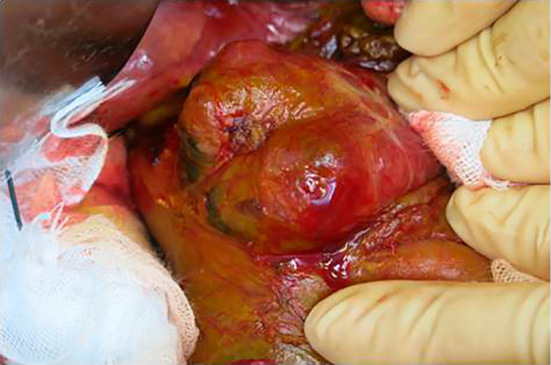
Intraoperative picture of periampullary duodenal diverticula. Intraoperative findings revealed retroperitoneal bile leakage and micro perforation that were not identified on the macroscopic evaluation of the periampullary diverticula.
Figure 5.
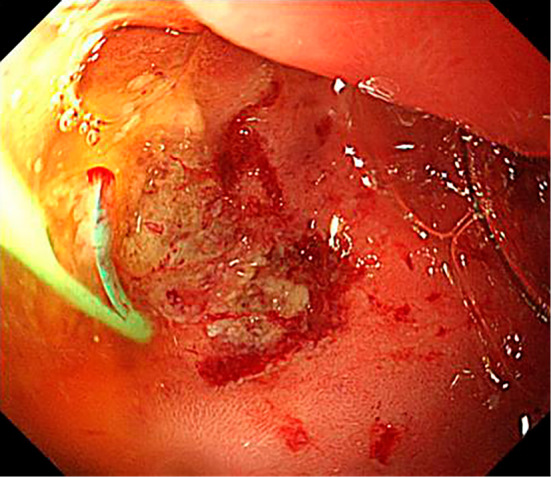
Intraoperative endoscopic image. The pancreatic spontaneous dislodgement stent migrated to the periampullary diverticula, causing erosion at the periampullary diverticula wall.
Discussion
Prophylactic pancreatic stent insertion has been frequently used to prevent post-ERCP pancreatitis in recent years (1, 2). With the increasing use of prophylactic pancreatic stents including PSDS, various complications have been reported (2-5). According to previous studies, overall prophylactic pancreatic stent-related complications [infection (3.0%), bleeding (2.5%), cholangitis and cholecystitis (3.1%), necrosis (0.4%), pancreatic duct perforation (0.8%), and stent migration (4.9%) and occlusion (7.9%)] occurred in 4.4% of patients (2). A rare complication of PSDS migration to the appendix has been reported, which might result in obstructive appendicitis (3). Although there have been several reports of intestinal perforation associ¬ated with migrating biliary stents (5-8), serious complications that require surgical operation secondary to PSDS migration are very rare (4). To our knowledge, major complications from PSDS insertion in patients with PAD have not been reported.
The overall incidence of PAD ranges from 9% to 31.7% according to the diagnostic approach and is known to increase with age (9, 10). The formation of PAD is related to the progression of duodenal motility disorders. Furthermore, increased intraduodenal pressure and progressive weakening of intestinal smooth muscles are known as the main underlying etiologies for PAD formation (9). The size of the diverticula and the position of the papilla in relation to the diverticula are variable. Therefore, it is reasonable to anticipate difficulty with selective bile duct cannulation and a high risk of complications in patients with PAD. Ampullary cannulation is successful in 62.4% of patients with PAD and in 92.7% of patients without PAD (9). However, more recent studies have shown that PAD cause no technical difficulties during ERCP nor do they increase the risk of complications (11, 12). The European Society of Gastrointestinal Endoscopy (ESGE) suggests pancreatic duct stent placement followed by precut sphincterotomy or needle-knife fistulotomy as suitable options for achieving cannulation in patients with PAD or difficult cannulation. The organization also suggests the selection of the most suitable technique in accordance with patient anatomy and operator experience (13). As we had limited experience with such techniques as precut sphincterotomy and needle-knife fistulotomy, we used pancreatic guide-wire cannulation in the present case. This technique might be effective for cases in whom biliary cannulation is difficult because of PAD, but such patients must be closely monitored after the placement of pancreatic stents. Despite the rare occurrence of retroperitoneal perforations because of pancreatic stent migration to the PAD, it is important to recognize that the outcome is potentially fatal and that prompt initiation of treatment is required.
In our case, selective bile duct cannulation was unsuccessful because of PAD, and a PSDS was inserted to prevent post-ERCP pancreatitis. Consequently, PSDS migration to the PAD occurred with retroperitoneal perforation. The papillary orifice was observed at the right side of the diverticular rim, which can cause the pancreatic duct to face toward the orifice in PAD. In addition, the PSDS gradually fell out of the pancreatic duct; therefore, duodenal peristalsis can cause PSDS migration to PAD. The mechanical force exerted by the tip of the PSDS against the duodenal mucosa of the PAD can lead to necrosis of the wall, which was exposed to bile and pancreatic juice. The previous case of intestinal perforation due to PSDS (5F, 4 cm in length, unflanged on the pancreatic ductal side, and pigtailed on the duodenal side) migration involved a patient with pancreatic cancer who had adhesion between the jejunum due to peritonitis carcinomatosa (4). The relationship between the type of pancreatic stent (flanged or unflanged stent on the pancreatic ductal side, long and pigtailed or straight on the duodenal side) and the potential for PAD migration and intestinal perforation is still unknown. The likelihood of intestinal perforation due to pigtailed biliary stent migration is expected to be lower than that with straight biliary stent migration because the mechanical force exerted by the tip of pigtailed stents against the intestinal mucosa is lower than that exerted by straight stents due to the rounded shape (7). There are more reported cases of intestinal perforation resulting from the migration of straight biliary stents than reports of perforation due to pigtailed stent migration (7, 8). There is a possibility that the risk of intestinal perforation is lower with a pigtailed PSDS on the duodenal side than with a straight PSDS. In our case, the PSDS gradually fell out of the pancreatic duct and slipped into the PAD. Although flanged stents should still be removed on the pancreatic ductal side with duodenoscopy after a few days, there is a possibility that the risk of migration to the PAD is lower with the flanged stent on the pancreatic ductal side than with the PSDS. If the papillary orifice is observed at the diverticular rim or in the diverticula, prophylactic pancreatic stent insertion should be performed carefully in order to reduce the possibility of migration to the diverticula, and pigtailed stents on the duodenal side or flanged stents on the pancreatic ductal side should be used. In cases with large PAD or intradiverticular papilla, endoscopic nasal pancreatic drainage (ENPD) tube insertion might be better than prophylactic pancreatic stent insertion because of the low risk of migration to the diverticula. Intestinal perforation is assumed to be rare during the migration of pancreatic stents, which are shorter and more flexible than biliary stents. However, endoscopists should be mindful of this potential complication after both pancreatic stent insertion and biliary stent insertion in patients with gastrointestinal obstruction, such as intra-abdominal adhesions and diverticulosis.
In conclusion, we herein described a rare case of retroperitoneal perforation caused by PSDS migration to the PAD. Since prophylactic pancreatic stent insertion is being performed increasingly frequently to prevent post-ERCP pancreatitis and PAD are not uncommon in patients who undergo ERCP, careful attention must be paid to this serious complication in patients with PAD presenting with abdominal pain after pancreatic stent insertion. The mean duration from placement to dislodgment of the pancreatic stent is 1.8 days (1). Endoscopists should plan to remove the PSDS with duodenoscopy if it takes longer than expected to be dislodged. Abdominal CT might be useful for visualizing the exact location and extraluminal extension of the stent. Despite the rarity of this complication, the outcome is potentially fatal, and prompt treatment is required.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Sofuni A, Maguchi H, Mukai T, et al. . Endoscopic pancreatic duct stents reduce the incidence of post-endoscopic retrogradecholangiopancreatography pancreatitis in high risk patients. Clin Gastroenterol Hepatol 9: 851-858, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Mazaki T, Masuda H, Takayama T. Prophylactic pancreatic stent placement and post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy 42: 842-853, 2010. [DOI] [PubMed] [Google Scholar]
- 3. Takano S, Fukasawa M, Sato T, et al. . Migration of pancreatic spontaneous dislodgement stent to the appendix. Dig Endosc 24: 481, 2012. [DOI] [PubMed] [Google Scholar]
- 4. Harada R, Kawamoto H, Fukatsu H, et al. . Perforation of jejunum induced by the deployment of a temporary prophylactic pancreatic stent in the patient with peritonitis carcinomatosa. Clin J Gastroenterol 1: 80-82, 2008. [DOI] [PubMed] [Google Scholar]
- 5. Seerden TC, Moreels TG, Salgado RA, et al. . Intestinal bowel perforation and bacterial peritonitis secondary to migrated biliary and pancreatic stents. Endoscopy 40 (Suppl 2): E25, 2008. [DOI] [PubMed] [Google Scholar]
- 6. Akimboye F, Lloyd T, Hobson S, et al. . Migration of endoscopic biliary stent and small bowel perforation within an incisional hernia. Surg Laparosc Endosc Percutan Tech 16: 39-40, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Hokimoto N, Ichikawa K, Fujiwara S, et al. . A case of duodenal perforation due to deviation of an endoscopic naso-biliary drainage tube. Nihon Rinsho Geka Gakkai Zasshi (J Jpn Surg Assoc) 69: 2537-2541, 2008(in Japanese, Abstract in English). [Google Scholar]
- 8. Namdar T, Raffel AM, Topp SA, et al. . Complications and treatment of migrated biliary endoprostheses: a review of the literature. World J Gastroenterol 13: 5397-5399, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lobo DN, Balfour TW, Iftikhar SY. Periampullary diverticula: consequences of failed ERCP. Ann R Coll Surg Engl 80: 326-331, 1998. [PMC free article] [PubMed] [Google Scholar]
- 10. Egawa N, Anjiki H, Takuma K, et al. . Juxtapapillary duodenal diverticula and pancreatobiliary disease. Dig Surg 27: 105-109, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Tham TC, Kelly M. Association of periampullary duodenal diverticula with bile duct stones and with technical success of endoscopic retrograde cholangiopancreatography. Endoscopy 36: 1050-1053, 2004. [DOI] [PubMed] [Google Scholar]
- 12. Panteris V, Vezakis A, Filippou G, et al. . Influence of juxtapapillary diverticula on the success or difficulty of cannulation and complication rate. Gastrointest Endosc 68: 903-910, 2008. [DOI] [PubMed] [Google Scholar]
- 13. Testoni PA, Mariani A, Aabakken L, et al. . Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 48: 657-683, 2016. [DOI] [PubMed] [Google Scholar]