Abstract
We herein report the case of a 25-year-old woman who presented with severe headache and visual field defects after childbirth. Magnetic resonance imaging revealed marked swelling of the pituitary gland, and an endocrinological examination revealed panhypopituitarism and diabetes insipidus. An immunohistological analysis of a transsphenoidal biopsy sample of the pituitary gland showed the significant accumulation of an immunogloblin G4 (IgG4)-positive population, leading to the diagnosis of IgG4-related hypophysitis. The patient was treated with prednisolone, which markedly reduced the swelling of the pituitary gland, in association with recovery of the pituitary function. This is a rare case of biopsy-proven IgG4-related hypophysitis with a postpartum onset.
Keywords: IgG4-related hypophysitis, pituitary gland, postpartum, steroid responsive, pituitary-oriented IgG4-related disease
Introduction
IgG4-related disease is a recently established disease that generally involves multiple systemic organs, including the pancreas, liver, lung, salivary glands, thyroid glands, and pituitary glands. It is characterized by high serum levels of IgG4 and histological evidence for infiltration of IgG4-positive plasma cells into various tissues, including endocrine organs. The following are considered to be comprehensive clinical diagnostic criteria for IgG4-related disease: [1] a clinical examination showing characteristically diffuse/localized swelling or masses in single or multiple organs, [2] a hematological examination showing elevated serum IgG4 concentrations (135 mg/dL), and [3] a histopathological examination showing (a) marked lymphocyte and plasmacyte infiltration and fibrosis and (b) infiltration of IgG4+ plasma cells (ratio of IgG4+/IgG+ cells >40% and >10 IgG4+ cells per high-power field) (1). Clinically, steroid treatment is effective for patients with IgG4-related disease, and serum IgG4 levels decrease after glucocorticoid administration as a result of response to treatment.
IgG4-related hypophysitis was first reported in 2004 in a 66-year-old woman with multiple pseudotumors in the salivary glands, pancreas, and retroperitoneum (2); more extensive cases were subsequently described in 2006 in elderly men with swelling of the salivary or submandibular glands in the presence of marked infiltration by lymphocytes and IgG4-positive plasma cells (3, 4). Thus, IgG4-related hypophysitis was proposed as a new clinical entity, and Leporati et al. determined five criteria to establish a diagnosis of IgG4-related hypophysitis (5). As clinical features, patients reported headache or visual field disturbance due to optic chiasma compression by a swollen pituitary gland and stalk. Although IgG4-related diseases, including hypophysitis, generally appear frequently in middle-aged and elderly men, cases of IgG4-related hypophysitis in young women have also recently been reported (6). In addition, it has been shown that steroid treatment is effective for IgG4-related hypophysitis as well as other forms of multiorgan disease with IgG4 infiltration, although the optimum therapy for IgG4-related hypophysitis is still undefined. Taken together, these observations suggest that further investigation regarding the prevalence and distinct forms of the disease and therapeutic management, including initial and maintenance doses of steroids, are required.
We herein report the case of a 25-year-old woman who presented with headache and the visual field defect of bitemporal hemianopsia after childbirth. Magnetic resonance imaging (MRI) revealed marked swelling of the pituitary gland with compression of the optic chiasma. Consistent with this, endocrine studies showed the presence of panhypopituitarism and diabetes insipidus. Immunohistological staining of a transsphenoidal biopsy sample of the pituitary gland revealed the existence of IgG4-positive plasma cells, consistent with the diagnosis of IgG4-related hypophysitis, despite the absence of serum IgG4 elevation. The patient was treated with prednisolone, which markedly reduced the swelling of the pituitary gland, restored the pituitary function, and eliminated any visual field defects. Unlike lymphocytic hypophysitis, which is known to affect young women, this is a unique and atypical case of postpartum IgG4-related hypophysitis histologically determined by a pituitary biopsy.
Case Report
In January 2013, a 25-year-old woman presented with a severe headache and general fatigue after childbirth, symptoms that gradually worsened. A visual field disturbance appeared about 21 days after delivery, and she was diagnosed with bitemporal hemianopsia by an ophthalmologist (Supplementary material 1A). Brain MRI revealed enlargement of the pituitary gland. At that time, the patient was referred to our hospital for a further examination, particularly with respect to the pituitary function.
The patient had no notable medical history and had one previous uneventful delivery. The patient had no severe bleeding during delivery. At the time of her hospital presentation, she was awake, alert and oriented; she also had a headache, but polyuria was absent. Her vital signs were as follows: blood pressure, 94/55 mmHg; body temperature, 37.2℃; pulse rate, 85/min; height, 150 cm; body weight, 39.5 kg; and body mass index, 17.6 kg/m2.
MRI of the pituitary gland revealed symmetrically marked swelling of the pituitary gland and stalk that was enhanced homogenously after a gadolinium injection. The enlarged stalk was adjacent to the chiasm (Fig. 1A and B). Lymphocytic hypophysitis was initially suspected because of the clinical form of the disease onset and symptoms of bisymmetric pituitary enlargement; however, pituitary adenoma, granulomatous diseases, malignant lymphoma, and other tumorous diseases were also considered as possible diagnoses. No other organs showed signs of enlargement or nodular/hyperplastic lesions.
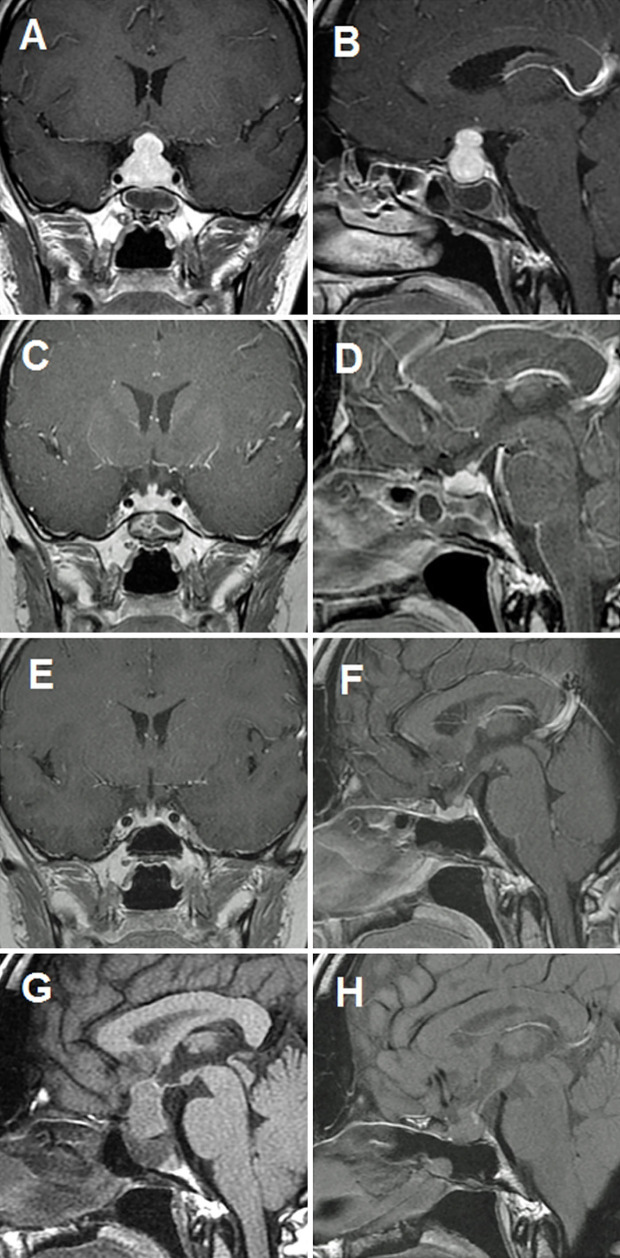
Figure 1.
Before treatment: Coronal (A) and sagittal (B) gadolinium-enhanced MRI showing diffuse enhanced and enlarged pituitary and stalk lesions. Three months after treatment: Coronal (C) and sagittal (D) gadolinium-enhanced MRI showing mild enlargement of the pituitary and stalk lesions, reflecting a significant reduction in pituitary gland size. Nine months after treatment: Coronal (E) and sagittal (F) gadolinium-enhanced MRI showing a normal pituitary gland. Sagittal T1-weighted magnetic resonance images (T1WI MRI) showing disappearance of high-intensity signal (HIS) before treatment (G) and a recovered HIS (H) indicating posterior-pituitary gland activity.
Laboratory blood and urine tests results were as follows: white blood cell count 6,200 /μL (50.2% neutrophils, 13.8% eosinophils, 0.3% basophils, 4.7% monocytes, 31.0% lymphocytes); red blood cell count 3.63×106/μL; hemoglobin 10.5 g/dL; hematocrit 30.8%; platelet count 221×103/μL; renal function: urea nitrogen 12 mg/dL, creatinine 0.54 mg/dL, and creatinine clearance 71.4 mL/min; electrolytes: sodium [Na] 140 mEq/L, potassium [K] 4.3 mEq/L, chloride [Cl] 104 mEq/L, calcium 9.7 mg/dL, and inorganic phosphorus 5.7 mg/dL; glucose metabolism: fasting glucose 107 mg/dL and hemoglobin A1c [HbA1c, National Glycohemoglobin Standardization Program (NGSP)] 4.7%; urine electrolytes: urine-Na 109.2 mEq/d, urine-K 88.2 mEq/d, and urine-Cl 109.2 mEq/d; lipid metabolism: LDL-cholesterol 166 mg/dL, HDL-cholesterol 35 mg/dL, and triglyceride 186 mg/dL; and C-reactive protein 1.13 mg/dL. The levels of dehydroepiandrosterone sulfate (DHEA-S), at 3 μg/dL, were low (reference range: 18-391 μg/dL). Notably, serum IgG4 was within the normal range (55.7 mg/dL). Tests for anti-glutamic acid decarboxylase antibody, anti-thyroid peroxidase antibody and anti-thyroglobulin antibody were all negative. Taken together, these findings pointed to adrenal insufficiency, in light of the findings of eosinophilia and low HbA1c.
Indeed, the patient's basal levels of adrenocorticotropic hormone (ACTH) and cortisol were low at <5.0 pg/mL and <2.0 μg/dL, respectively. The levels of other pituitary and targeting hormones were also relatively low, as follows: thyroid-stimulating hormone (TSH), 0.074 μIU; free triiodothyronine, 2.85 pg/mL; free thyroxin, 0.81 ng/dL; luteinizing hormone (LH), 0.42 mIU/mL; follicle-stimulating hormone (FSH), 5.80 mIU/mL; estradiol (E2) <10 pg/mL; growth hormone (GH), 1.69 ng/mL; insulin-like growth factor 1 (IGF-1), 57 ng/mL (-6.5 SD); and prolactin (PRL), 2.48 pg/dL. The patient's ACTH responded normally to a corticotropin-releasing hormone (CRH) loading test, increasing from <5 to 48.5 pg/mL in 30 minutes, but cortisol showed no response. Her TSH showed a low response, and other anterior pituitary hormones showed delayed responses to a combined anterior pituitary stimulation test (CRH, GRH, TRH, and LHRH) (Table 1). To further examine the hypothalamus pituitary adrenal axis, we carefully performed insulin-tolerance tests. These tests showed no cortisol response to hypoglycemic stimulation (blood sugar; nadir 32 mg/dL), whereas ACTH increased from <5.0 to 66.3 pg/mL (Table 1). Given that the urine cortisol secretion was very low, these findings suggested adrenal insufficiency, possibly due to the impairment of the anterior-pituitary function. In addition, the patient manifested polyuria (over 3,000 ml/day) after hydrocortisone replacement therapy. A water-restriction test showed a disturbance in the ability to concentrate urine (urine osmolality <234 mOsm/kg, even after 6-hour water restriction) with a drastic increase in the urine osmolality after vasopressin administration. The patient was therefore diagnosed as having panhypopituitarism with adrenal insufficiency and masked diabetes insipidus with postpartum onset, possibly related to abnormal aspects of the hypothalamic and pituitary lesion on MRI (Fig. 1A, B and G).
Table 1.
Endocrine Profiles and Pituitary Hormone Responses to Combined Anterior Pituitary Stimulation (CRH, GRH, TRH and LHRH), ITT and GHRP2.
| Normal range | CAPST | ITT | GHRP2 | ||||
|---|---|---|---|---|---|---|---|
| Vor | Peak | Vor | Peak | Vor | Peak | ||
| ACTH (pg/mL) | <46.0 | <5.0 | 48.5 | <5.0 | 66.3 | <5.0 | <5.0 |
| Cortisol (µg/dL) | 5.0–25.0 | <1.0 | <1.0 | <1.0 | <1.0 | <1.0 | <1.0 |
| GH (ng/mL) | 0.04–3.60 | 1.55 | 11.00 | 1.94 | 2.08 | 2.24 | 9.29 |
| TSH (µIU/mL) | 0.35–4.94 | 0.05 | 0.39 | ||||
| PRL (pg/mL) | 5.18–26.53 | 2.32 | 5.84 | ||||
| LH (mIU/mL) | 1.76–10.24 | 0.35 | 2.25 | ||||
| FSH (mIU/mL) | 3.21–14.72 | 5.14 | 8.10 | ||||
| IGF-1 (ng/mL) | 147–358 | 57 | |||||
| IgG4 (mg/dL) | 4.8–105 | 55.7 | |||||
| Responses of pituitary hormones 3 months after treatment | |||||||
| Normal range | CAPST | ITT | GHRP2 | ||||
| Vor | Peak | Vor | Peak | Vor | Peak | ||
| ACTH (pg/mL) | <46.0 | <5.0 | <5.0 | <5.0 | <5.0 | <5.0 | <5.0 |
| Cortisol (µg/dL) | 5.0–25.0 | <1.0 | <1.0 | <1.0 | <1.0 | <1.0 | <1.0 |
| GH (ng/mL) | 0.04–3.60 | 0.58 | 13.20 | 0.91 | 6.27 | 1.29 | 56.50 |
| TSH (pg/mL) | 0.35–4.94 | 1.15 | 9.95 | ||||
| PRL (pg/mL) | 3.46–19.40 | 13.12 | 66.73 | ||||
| LH (µg/dL) | 1.76–10.24 | 1.22 | 7.63 | ||||
| FSH (pg/mL) | 3.21–14.72 | 5.14 | 12.80 | ||||
| IGF-1 (ng/mL) | 147–358 | 150 | |||||
| IgG4 (mg/dL) | 4.8–105 | 19.9 | |||||
CAPST: combined anterior pituitary stimulation test (100 μg CRH: 100 μg GRH: 500 μg TRH: and 100 μg LHRH), ITT: insulin tolerance test, GHRP2: growth hormone-related peptide 2
To clarify the pathogenesis of pituitary enlargement as well as to determine the clinical management of this patient, we carried out an endoscopic transsphenoidal pituitary biopsy after stabilizing her condition. The pathological findings showed massive infiltration of inflammatory cells and storiform fibrosis, with immunoglobulin lambda and kappa, and 219 IgG-positive cells per high-power field (400×) with numerous lymphoid cells in the biopsy-sampled pituitary region (Fig. 2A-E). Importantly, an immunohistochemical analysis revealed that IgG4-positive plasma cells were heterogeneously observed in the pituitary region, with 48 positive cells per high-power field detected at the “hot spot”, meeting the diagnostic criteria for IgG4-related hypophysitis (Fig. 2F) (5). Based on these findings, the patient was diagnosed with IgG4-related hypophysitis.
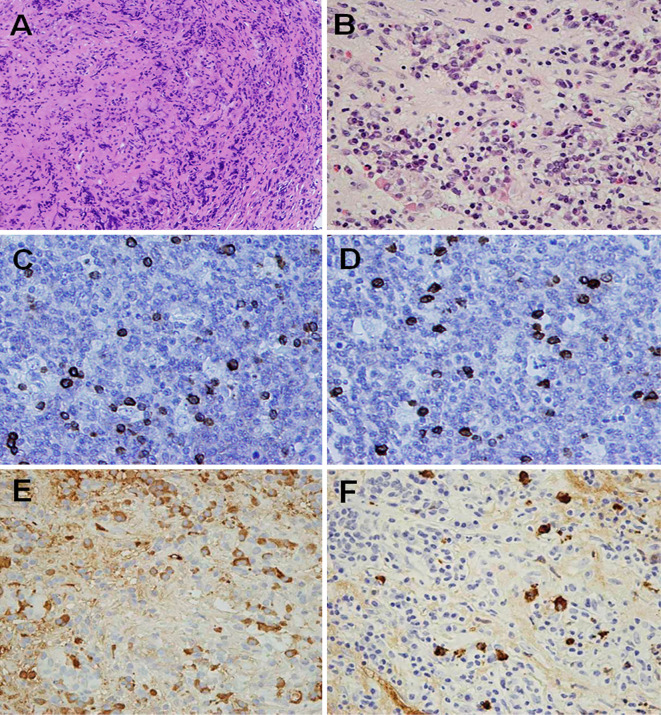
Figure 2.
Hematoxylin and Eosin staining showing infiltration of lymphocytes and plasma cells and storiform fibrosis (A, B) (Magnification: A ×100 and B ×400, respectively), and an immunohistochemical analysis showing the presence of IgG lambda (C) and kappa (D), IgG-positive (E), and IgG4-positive (F) plasma cells and lymphoid cells in the pituitary gland tissue (Magnification: C-F ×400).
Accordingly, prednisolone was administered, starting at 40 mg/day and then gradually decreasing the dose. Prednisolone treatment relieved headache symptoms and visual field defects as a result of a decompression effect (Supplementary material 1B). Excessive thirst and polyuria also disappeared immediately. Vasopressin acetate replacement was discontinued, and symptoms of diabetes insipidus have not been observed thereafter. The urine osmolality and volume were 609 mOsm/kg and around 1,500 mL/day after treatment, respectively. MRI showed that pituitary and stalk enlargement had substantially regressed after 3 months of prednisolone treatment (Fig. 1C and D), revealing a normal pituitary aspect after 9 months (Fig. 1E, F and H). A re-analysis of the patient's pituitary gland function after treatment showed a significant improvement in the TSH, GH, and PRL responses compared with the pre-treatment findings, whereas the LH and FSH responses showed only slight improvement (Table 1). There were still no ACTH or cortisol responses because of the influence of prednisolone at this stage. In contrast, the GH responses to insulin-tolerance tests and growth hormone-releasing peptide 2 loading tests showed a remarkable recovery. IGF-1 also increased after treatment, and levothyroxine sodium was discontinued based on the results of loading tests. The loading tests revealed that, among the anterior pituitary hormones, GH, TSH, and PRL recovered their responses after treatment. E2 was undetectable before treatment, but it was increased to 261 and 1,428 pg/mL at 7 and 14 days after treatment, respectively. Menstruation started again, and the patient has been recovering well with no symptoms or recurrence of pituitary gland swelling, even with low-dose (10 mg/d) hydrocortisone replacement therapy.
Discussion
We report the case of a young woman with IgG4-related hypophysitis with postpartum onset. She presented with a headache and visual field defects caused by marked pituitary swelling, and endocrine studies revealed panhypopituitarism and central diabetes insipidus together with adrenal insufficiency. An immunohistological examination of a transsphenoidal biopsy of the pituitary gland revealed significant accumulation of IgG4-positive plasma cells. This observation is in agreement with the diagnostic criteria for IgG4-related hypophysitis, although the patient atypically exhibited a normal level of serum IgG4 in the absence of other coaffected organs. She was then treated with prednisolone, which drastically reduced the swelling of the pituitary gland and recovered the pituitary function and related symptoms, including visual field defects and headache.
We performed a pituitary biopsy, which proved to be particularly important for making an accurate diagnosis that distinguished the correct diagnosis from among a number of possibilities (e.g. inflammatory change, infectious lesion, adenoma, granulomatous disease and Rathke's cleft cyst), helping us decide on an appropriate therapeutic management, especially for such a young woman. Indeed, the patient was diagnosed with IgG4-related hypophysitis based on a pathological evaluation of the pituitary specimen, and steroid therapy was introduced promptly, contributing to the improvement in pituitary swelling, pituitary dysfunction, and related symptoms, particularly visual defects, as well as remission of diabetes insipidus.
Leporati et al. previously suggested diagnostic criteria for IgG4-related hypophysitis that are widely recognized by endocrinologists (5). Our case met the following three of their five criteria: [I] pituitary histopathology showing mononuclear infiltration of the pituitary gland, with enrichment of lymphocytes and plasma cells, with more than 10 IgG4-positive cells per high-power field; [II] pituitary MRI showing a sellar mass-thickened pituitary stalk; and [III] shrinkage of the pituitary mass and symptom improvement with steroid treatment. It should be noted that the diagnostic significance of the number of IgG4-positive infiltrating cells is still controversial. For instance, a consensus statement on the pathology of IgG4-related disease reported that, for a quantitative assessment, acceptable specificity is achieved if the number of IgG4-positive plasma cells per high-power field exceeds 30. In addition, a finding of 50 or more IgG4-positive plasma cells in diffuse infiltrates is deemed highly specific. The presence of more than 10 IgG4-positive plasma cells is used as one of the comprehensive diagnostic criteria (7). However, the appropriate cut-off point is considered to vary depending on the organ. For example, the presence of 100 or more IgG4-positive plasma cells in the salivary gland is highly suggestive for a diagnosis. Nevertheless, the diagnostic criteria for IgG4-related hypophysitis provided by Leporati et al. are noteworthy because a patient like our pituitary-limited case can be diagnosed when infiltration of more than 10 IgG4 plasma cells per high-power field is detected in the pituitary gland.
According to a previous report, IgG4-positive cells were not present or present in very low numbers among pregnant patients (8). Those patients showed typical features of lymphocytic adenohypophysitis (9, 10) (Table 2). To further document this relation, we performed immunohistochemical analyses for the pituitary biopsy samples of these patients in the same experimental condition as our case. These immunohistochemical analyses showed the presence of CD138-positive cells in patients 1 and 3 and IgG-positive cells in patients 1-3, but no or very few IgG4-positive cells in patients 1-3 (Fig. 3). These results suggest that infiltration of IgG4-positive cells may be very rare in pregnancy-related hypophysitis.
Table 2.
Summary of the Clinical Features of 4 Patients Undergoing Transsphenoidal Pituitary Biopsy with Pregnancy-associated Hypophysitis.
| ID | Ref | Age | Sex | Onset time | CS | Anterior pituitary function | DI | Imaging | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 | 33 | F | Post-partum | HD,PU, VD, | Panhypo-pituitarism | (+) | Symmetric pituitary mass with suprasellar ext | TS biopsy | Symptoms free Replacement therapy |
| 2 | 10 | 40 | F | Late preg (8 ms) | VD | Panhypo-pituitarism | (-) | Symmetric large mass with cyst | TS biopsy | Symptoms free Replacement therapy |
| 3 | 8 | 36 | F | Late preg | HD, VD | Panhypo-pituitarism | (-) | Symmetric pituitary mass with suprasellar ext | TS biopsy followed by steroid pulse Tx (mPSL 1 g × 3 d) | Symptoms free Replacement therapy |
| 4 | Our case | 25 | F | Post-partum | HD, VD | Panhypo-pituitarism | (+) | Symmetric enlargement of the pituitary gland | TS biopsy PSL administration | Symptoms free Low-dose hydrocortisone |
Ref: Reference, F: female, preg: pregnancy, ms: months, CS: Clinical symptoms, HD: Headache, PU: Polyuria, VD: Visual disturbance (bitemporal hemianopia), DI: diabetes insipidus, ext: extension, TS: transsphenoidal surgery, Tx: treatment, mPSL: methyl prednisolone, PSL: prednisolone
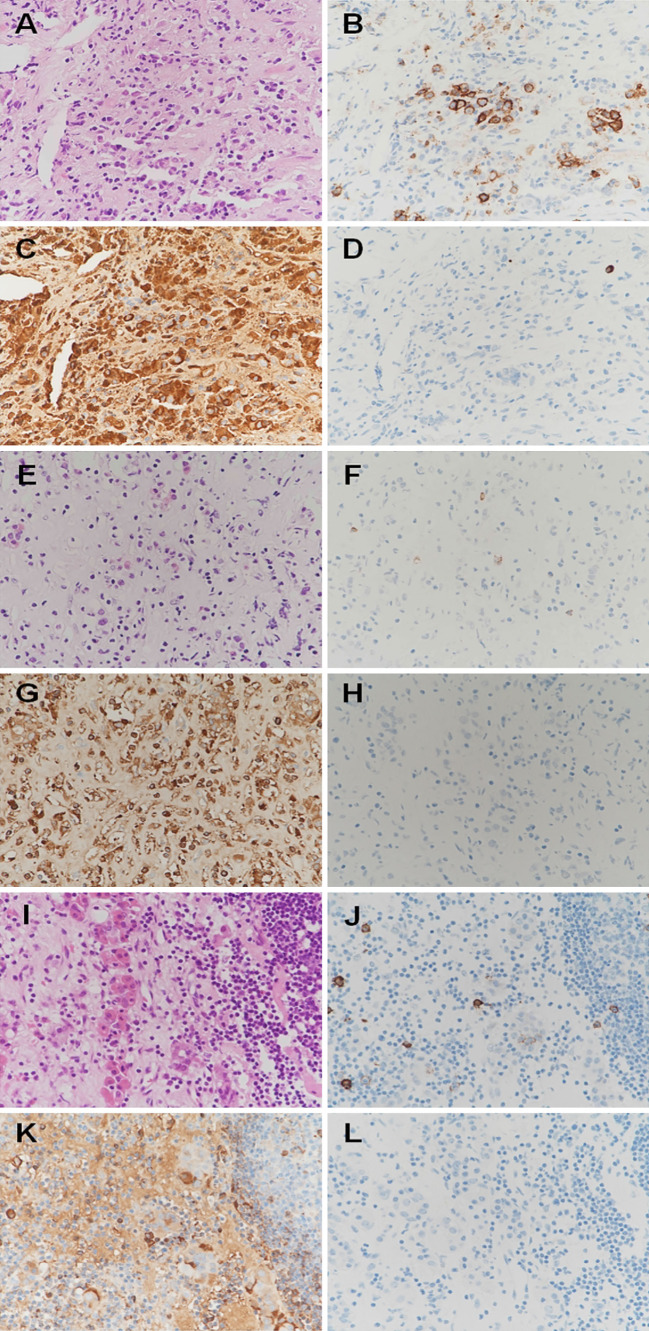
Figure 3.
Pathological findings of pituitary biopsy samples of the patients with pregnancy-related hypophysitis. Patients 1 (A, B, C, D), 2 (E, F, D, H), and 3 (I, J, K, L): Hematoxylin and Eosin staining showing infiltration of lymphocytes and plasma cells (A, E, I), and immunohistochemical analyses showing the presence of CD138-positive cells in Patients 1 and 3 (B, F, J), IgG-positive cells in Patients 1-3 (C, G, K), and no or very few IgG4-positive cells in Patients 1-3 (D, H, L).
It is also interesting that we found a discrepancy between the serum IgG4 level and the infiltration of IgG4-positive plasma cells in the present case. Four other such cases, all of which were diagnosed by a pituitary biopsy without other suspected lesions, were reported previously (11). Khong et al. reported a 33-year-old woman presenting with a swollen pituitary gland on MRI and hypopituitarism indicating hypophysitis with normal serum IgG4 levels. There were more than 100 IgG4-positive plasma-cells per high power field, and over 90% of the plasma cells were stained for IgG4 in the inflamed area of the pituitary tissue (12). Osawa et al. reported a 74-year-old woman with typical hypophysitis appearance and normal serum IgG4 levels showing massive infiltration of inflammatory cells, including abundant plasma cells with moderately positivity for IgG4 in the pituitary sample (13). Furthermore, Leporati et al. reported a 75-year-old man with low serum IgG4 levels overall who showed an increase in the serum IgG4 level during a period of clinical recurrence (5). In our case, there was a possibility that the IgG4 level had approached a peak before a precise examination could be conducted in our hospital, given her response to a small amount of hydrocortisone as replacement therapy. Indeed, her serum IgG4 level was normal before treatment but decreased three months after prednisolone administration (Table 1). Although organ infiltration of IgG4-positive plasma cells is believed to be related to the etiology of IgG4-related disease, an increase in the serum IgG4 is not necessary for the diagnosis of either comprehensive IgG4-related disease or hypophysitis (7). In fact, several lines of evidence have suggested that the serum IgG4 concentration is normal in up to 40% of patients with biopsy-proven IgG4-related disease (14).
In terms of the pathophysiological significance of the infiltration of IgG4-positive plasma cells in localized organs, whether or not this is the cause or a consequence of organ dysfunction remains controversial. Several hypotheses regarding the underlying pathological mechanism of IgG4-related disease have been proposed. Potential triggers of IgG4-related disease, possibly associated with autoimmunity and/or bacterial infections, can activate immune reactions in affected organs in which Th2-cells respond with prominently augmented expression of Th2 cytokines, such as IL-4, IL-5, and IL-13. These cytokines subsequently induce an increase in eosinophils and IgE and IgG4 levels, leading to massive inflammatory changes with IgG4-positive cell infiltration within the affected organs (15-17). Although the pathological and immunological mechanisms of the tissue-specific IgG4-positive cell accumulation are still unclear, these local immune reactions are considered to contribute to the pathological features of IgG4-related disease. Therefore, a tissue biopsy can be invaluable for the diagnosis and treatment of IgG4-related disease, particularly in our case, where the serum IgG4 levels were normal.
We had to make a differential diagnosis among hypophysitis, adenoma, granuloma, and others as possible causes of the pituitary tumor lesion. Simultaneously, a pituitary MRI showed typical findings of hypophysitis, with diffuse thickening of the pituitary stalk, homogeneous contrast enhancement of the gland (which was symmetrically enlarged in gadolinium-enhanced images), and loss of the bright spot of the neurohypophysis in T1-weighted images. Hypophysitis is classified into two types based on its cause: primary hypophysitis, which occurs through an autoimmune mechanism, and secondary hypophysitis, caused by the use of some drugs that produce immunological changes, or by some kinds of tumor lesions, such as Rathke's cleft cyst and adenoma, among others. There was no evidence of a secondary cause of hypophysitis based on the clinical findings such as normal angiotensin-converting enzyme (ACE) levels, medical history, and other images in our patient. However, because infectious disease could not be completely ruled out before treatment with prednisolone, we carried out a pituitary biopsy to clarify the pathogenesis of pituitary enlargement. However, the corticotroph and thyrotroph reserves are the first to be affected in hypophysitis, whereas lactotrophs and somatotrophs are affected later (18). This pattern of typically affected pituitary hormones in lymphocytic hypophysitis was consistent with our case.
An association is recognized between pregnancy and lymphocytic hypophysitis. In 380 biopsy specimens of tumor-related lesions in pituitary glands reported from 1964 to 2010, most pathologies were lymphocytic hypophysitis. Of the 215 women diagnosed with adenohypophysitis at ages between 15 and 45 years, 149 (69%) manifested lymphocytic hypophysitis during late pregnancy or postpartum (19, 20). However, postpartum IgG4-related hypophysitis has not been reported to date, although several studies have reported 40 IgG4-related hypophysitis cases in young women. Because hypophysitis may not be an independent single disease, given the etiology of hypophysitis during postpartum and late pregnancy, it is necessary to collect biopsies as in our case and clarify the commonalities and differences in their characteristics.
Conclusions
We herein reported a rare case of biopsy-proven IgG4-related hypophysitis with postpartum onset. This case highlights the important contribution of an early diagnosis by a pituitary biopsy and subsequent appropriate steroid treatment in the initial stage of the disease to the improvement of the pituitary function.
Informed consent was obtained from the patient for publication of this case report, including any accompanying images.
Supplementary Materials
Visual field test displayed bitemporal hemianopsia before treatment (A) and it recovered completely after treatment (B).
The authors state that they have no Conflict of Interest (COI).
References
- 1. Umehara H, Okazaki K, Masaki Y, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod rheumatol 22: 21-30, 2012. [DOI] [PubMed] [Google Scholar]
- 2. van der Vliet HJ, Perenboom RM. Multiple pseudotumors in IgG4-associated multifocal systemic fibrosis. Ann Intern Med 141: 896-897, 2004. [DOI] [PubMed] [Google Scholar]
- 3. Yamamoto M, Takahashi H, Ohara M, et al. A case of Mikulicz's disease (IgG4-related plasmacytic disease) complicated by autoimmune hypophysitis. Scand J Rheumatol 35: 410-411, 2006. [DOI] [PubMed] [Google Scholar]
- 4. Tanabe T, Tsushima K, Yasuo M, et al. IgG4-associated multifocal systemic fibrosis complicating sclerosing sialadenitis, hypophysitis, and retroperitoneal fibrosis, but lacking pancreatic involvement. Intern Med 45: 1243-1247, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Leporati P, Landek-Salgado MA, Lupi I, Chiovato L, Caturegli P. IgG4-related hypophysitis: a new addition to the hypophysitis spectrum. J Clin Endocrinol Metab 96: 1971-1980, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sosa GA, Bell S, Christiansen SB, et al. Histologically confirmed isolated IgG4-related hypophysitis: two case reports in young women. Endocrinol Diabetes Metab Case Rep 2014: 140062, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol 25: 1181-1192, 2012. [DOI] [PubMed] [Google Scholar]
- 8. Nishioka H, Shibuya M, Haraoka J. Immunohistochemical study for IgG4-positive plasmacytes in pituitary inflammatory lesions. Endocr Pathol 21: 236-241, 2010. [DOI] [PubMed] [Google Scholar]
- 9. Nishioka H, Ito H, Miki T, Akada K. A case of lymphocytic hypophysitis with massive fibrosis and the role of surgical intervention. Surg Neurol 42: 74-78, 1994. [DOI] [PubMed] [Google Scholar]
- 10. Nishioka H, Ito H, Miki T, Wada J, Sano T. Lymphocytic adenohypophysitis associated with Rathke's cleft cyst. Endocr Pathol 6: 337-343, 1995. [DOI] [PubMed] [Google Scholar]
- 11. Bando H, Iguchi G, Fukuoka H, et al. The prevalence of IgG4-related hypophysitis in 170 consecutive patients with hypopituitarism and/or central diabetes insipidus and review of the literature. Eur J Endocrinol 170: 161-172, 2014. [DOI] [PubMed] [Google Scholar]
- 12. Khong P, Enno A, Darwish B. Lymphoplasmacytic hypophysitis associated with immunoglobulin G4. J Clin Neurosc 21: 342-344, 2014. [DOI] [PubMed] [Google Scholar]
- 13. Osawa S, Ogawa Y, Watanabe M, Tominaga T. Hypophysitis presenting with atypical rapid deterioration: with special reference to immunoglobulin G4-related disease-case report. Neurol Med Chir (Tokyo) 49: 622-625, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Sah RP, Chari ST. Serologic issues in IgG4-related systemic disease and autoimmune pancreatitis. Curr Opin Rheumatol 23: 108-113, 2011. [DOI] [PubMed] [Google Scholar]
- 15. Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med 366: 539-551, 2012. [DOI] [PubMed] [Google Scholar]
- 16. Kanari H, Kagami S, Kashiwakuma D, et al. Role of Th2 cells in IgG4-related lacrimal gland enlargement. International archives of allergy and immunology 152 (Suppl 1): 47-53, 2010. [DOI] [PubMed] [Google Scholar]
- 17. Miyake K, Moriyama M, Aizawa K, et al. Peripheral CD4+ T cells showing a Th2 phenotype in a patient with Mikulicz's disease associated with lymphadenopathy and pleural effusion. Mod Rheumatol 18: 86-90, 2008. [DOI] [PubMed] [Google Scholar]
- 18. Thodou E, Asa SL, Kontogeorgos G, Kovacs K, Horvath E, Ezzat S. Clinical case seminar: lymphocytic hypophysitis: clinicopathological findings. J Clin Endocrinol Metab 80: 2302-2311, 1995. [DOI] [PubMed] [Google Scholar]
- 19. Caturegli P, Newschaffer C, Olivi A, Pomper MG, Burger PC, Rose NR. Autoimmune hypophysitis. Endocr Rev 26: 599-614, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Caturegli P, Lupi I, Landek-Salgado M, Kimura H, Rose NR. Pituitary autoimmunity: 30 years later. Autoimmun Rev 7: 631-637, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Visual field test displayed bitemporal hemianopsia before treatment (A) and it recovered completely after treatment (B).