Abstract
A 67-year-old man was admitted to our hospital complaining of dry cough. Chest computed tomography showed diffuse infiltrates and ground-glass opacities in the bilateral lung fields. Transbronchial lung biopsy specimens showed alveoli filled with yeast-like fungi. With a diagnosis of pneumocystis pneumonia (PCP), he was given oral sulfamethoxazole/trimethoprim, to which he responded well. However, seven months later, PCP relapsed. Analyses revealed a low bronchoalveolar lavage fluid CD4/CD8 ratio of 0.04 and CD4+ lymphocytopenia (250/μL). Despite intensive work-up, we were unable to detect the underlying cause of CD4+ lymphocytopenia; therefore, a final diagnosis of idiopathic CD4+ T-lymphocytopenia was made.
Keywords: pneumocystis pneumonia, human immunodeficiency virus, idiopathic CD4+ T-lymphocytopenia, low CD4/CD8 ratio
Introduction
Idiopathic CD4+ T-lymphocytopenia (ICL) is a disease that predisposes individuals to develop opportunistic infections and other immunodeficiency diseases associated with CD4+ T-lymphocytopenia (1). The diagnosis is made by the demonstration of a low CD4+ lymphocyte count (<300 cells/μL or <20% of the total lymphocytes) in the absence of human immunodeficiency virus (HIV) infection or any other immune-deficient condition. Pneumocystis pneumonia (PCP) is a typical opportunistic infection that affects patients with a cellular immunodeficient condition. However, little is known about the features of PCP patients with ICL.
We herein report a case of ICL in a patient with recurrent PCP.
Case Report
A 67-year-old man presented at our hospital with a chief complaint of cough that had lasted for more than a month. He was a former smoker with a 60 pack-year history. His medical, social, and family histories were unremarkable.
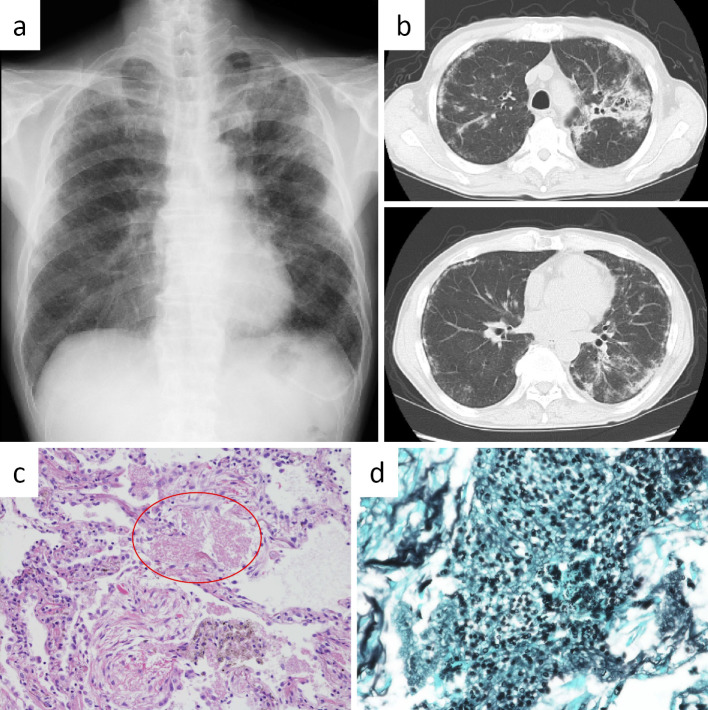
On a physical examination, the patient had a healthy general appearance and was afebrile. Percutaneous oxygen saturation was 96%. Chest auscultation revealed fine crackles on both sides. Chest X-ray showed diffuse ground-glass opacities and infiltrates in both lung fields (Figure a). Chest computed tomography (CT) showed diffuse ground-glass opacities, reticular shadows in both lungs, and consolidation with traction bronchiectasis in the apicoposterior segment of the left upper lobe (Figure b). Laboratory findings were as follows: white blood cell count 8,700/μL (neutrophils: 60.6%, lymphocytes: 29.2%, eosinophils: 1.7%, monocytes: 7.9%, basophils: 0.6%), hemoglobin 13.0 g/dL, platelet count 17.7×104/μL, lactic dehydrogenase 266 IU/L, C-reactive protein 0.63 mg/dL, β-D glucan 599 pg/mL, sialylated carbohydrate antigen Krebs von den Lungen-6 at 4,559 U/mL, and serum surfactant protein-D 874 ng/mL.
Figure.
a: Chest X-ray obtained on admission showed diffuse ground-glass opacity and infiltrates in both lung fields. b: Chest CT on admission showed diffuse ground-glass opacities and reticular shadows in both lung and consolidation with traction bronchiectasis in left S1+2. c: The histological findings revealed foamy exudates in the alveolar spaces (Hematoxylin and Eosin staining, ×100). d: Groccot stain (×400) showed that the alveoli were filled with yeast-like fungi.
Histological findings obtained by a transbronchial lung biopsy revealed foamy exudates in the alveolar spaces (Figure c; Hematoxylin and Eosin staining), and Groccot staining showed yeast-like fungi filling the alveoli (Figure d). The patient was given a diagnosis of PCP and started on oral sulfamethoxazole/trimethoprim (TMP-SMX) therapy, to which he responded well. Further extensive work-up to detect an underlying immunodeficiency disease was made. An elevated serum immunoglobulin A level and the presence of a monoclonal protein on serum immunoelectrophoresis led to a diagnosis of monoclonal gammopathy of undetermined significance (MGUS). There was no specific hematologic disease, malignancy, or immunodeficiency disease, such as HIV infection.
Seven months later, the patient returned to our hospital because of recurrent coughing. Chest X-ray and CT showed diffuse ground-glass opacities; the serum β-D glucan level was elevated at 257 pg/mL. Bronchoalveolar lavage (BAL) of the lateral segment of the right middle lobe was performed and recovered 44% (66/150 mL) of the instilled fluid. The total cell count was elevated, with an increase in the numbers of lymphocytes and neutrophils; a lymphocyte subset analysis showed a marked decrease in the CD4/CD8 ratio (0.04). Pneumocystis jirovecii DNA polymerase chain reaction was positive in the BAL fluid (BALF), suggesting a diagnosis of recurrent PCP. A blood analysis revealed a decreased CD4+ lymphocyte count (257/μL; 14.6% of the total lymphocytes) (Table). Because the lymphocytopenia had persisted for over one year since the first analysis and no apparent cause had been determined, the diagnosis of ICL was made by exclusion. A therapeutic dose of oral TMP-SMX was administered, followed by a chemoprophylactic dose. No further recurrence of PCP has occurred to date.
Table.
Analysis of BALF and Peripheral Blood Lymphocyte.
| BALF | Peripheral blood | ||||
|---|---|---|---|---|---|
| Total cell counts | 20×105 | /mL | WBC | 5,700 | /μL |
| Neutrocyte | 24 | % | Lymphocyte | 30.9 | % |
| Eosinophil | 1 | % | CD4 | 14.6 | % |
| Lymphocyte | 37 | % | CD8 | 42.5 | % |
| Macrophage | 38 | % | CD4/CD8 | 0.34 | |
| CD4 | 3.1 | % | |||
| CD8 | 87.8 | % | CD4+ lymphocyte | 257 | /μL |
| CD4/CD8 | 0.04 | ||||
| P. jirovecii PCR | positive | ||||
BALF: bronchoalveolar lavage fluid, P. jirovecii: Pneumocystis jirovecii, PCR: polymerase chain reaction, WBC: white blood cell
Discussion
ICL is a clinical syndrome defined by persistent CD4+ T cell lymphocytopenia in the absence of HIV infection or any other cause of immunodeficiency (1). ICL shares many features with acquired immunodeficiency syndrome (AIDS) based on the presence of CD4+ lymphocytopenia in both types of patients. The clinical presentation of patients with PCP differs among those with and without AIDS. However, few studies have described the features of PCP patients with ICL.
PCP patients without AIDS typically present with a more abrupt onset of symptoms, more severe respiratory failure, higher mortality rate, and more neutrophils and fewer pneumocystis organisms in the alveoli than those with AIDS (2). This case had a course that was typical of PCP in patients with AIDS, implying that the infection was secondary to the CD4+ lymphocytopenia and that MGUS was only complicated by CD4+ lymphocytopenia. We considered the MGUS to be unrelated to the CD4+ lymphocytopenia because MGUS is a B-cell disorder, and in this patient, there were few monoclonal plasma cells in the bone marrow. After the first episode of PCP in this case, chemoprophylaxis was not recommended because there no predisposing illness had been identified (3). In hindsight, we probably should have screened the blood lymphocyte profile at the first diagnosis of PCP.
The features of PCP patients with ICL have not been investigated in detail because ICL is a rare disease (4). Differences in the radiologic features of PCP according to basic immunodeficiency diseases have been reported. For example, in PCP patients with AIDS, cystic lesions are more common, but diffuse ground glass lesions are less frequently observed than in those without AIDS (5). Another study reported that consolidation along the bronchovascular bundle was more common in non-AIDS patients than in AIDS patients (6). The chest CT findings of this case mainly showed diffuse ground-glass opacities and consolidation with no cystic lesions. This suggests that the chest CT findings of PCP patients with ICL may be similar to those without AIDS; however, this hypothesis needs further investigation. Given the similar etiologies between ICL and AIDS, the duration of TMP-SMX therapy in PCP patients with ICL should be three weeks. In cases complicated by respiratory failure, the addition of corticosteroids is needed (7).
The BALF findings were also distinctive in this case. Iriart et al. compared the BALF findings between PCP patients with and without AIDS (8). They found that the CD4+ lymphocyte counts in the BALF and peripheral blood were significantly correlated in PCP patients with AIDS, but not in those without AIDS. We therefore deduced that the CD4+ lymphocyte counts in BALF and peripheral blood were also correlated in patients with ICL. In addition, the median BALF CD4/CD8 ratio in PCP patients with AIDS was significantly lower than that in PCP patients without AIDS [0.05 (range, 0.02-0.08) vs. 1.19 (0.66-2.23)]. This might explain the markedly low BALF CD4/CD8 ratio in the present PCP patient with ICL.
ICL and AIDS are clinically similar in many aspects; however, the pathologic and immunologic characteristics of ICL have not been sufficiently clarified. For example, patients with ICL and AIDS commonly suffer from opportunistic infections, but their causative agents are not similar. In a review of 258 patients with ICL, the most common infection was cryptococcal (69 patients, 26.6%), followed by mycobacterial (44 patients, 17.0%); PCP affected only 20 patients (7.7%) (9). In contrast, in a cohort with AIDS-defining opportunistic illnesses in the United States in the pre-antiretroviral therapy era, cytomegalovirus infections were most common (33.0%), followed by PCP (29.9%); cryptococcal infections affected only 2.6% of patients (10). In AIDS patients, the acquisition of opportunistic infections is generally in proportion to the CD4+ cell count in peripheral blood. PCP occurs most frequently when the CD4+ cell count is <200 cells/mm3 (11). The above-mentioned review reported that the mean initial CD4+ count in ICL was <150/mm3 and that patients with that level of CD4+ count often have PCP (9). These data may involve many biases, but there is a possibility that the immune function of ICL patients is different from that of patients with AIDS.
In summary, ICL and AIDS have a lot in common because of the presence of CD4+ lymphocytopenia in both patient pools. However, PCP patients with ICL may differ from those with AIDS or with other immunodeficiency disease in some important ways.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Dr. Keisuke Goto and Dr. Yu Asao for their helpful discussions and assistance.
References
- 1. Unexplained CD4+ T-lymphocyte depletion in persons without evident HIV infection--United States. MMWR Morb Mortal Wkly Rep 41: 541-545, 1992. [PubMed] [Google Scholar]
- 2. Kovacs JA, Hiemenz JW, Macher AM, et al. . Pneumocystis carinii pneumonia: a comparison between patients with the acquired immunodeficiency syndrome and patients with other immunodeficiencies. Ann Intern Med 100: 663-671, 1984. [DOI] [PubMed] [Google Scholar]
- 3. Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med 325: 1313-1315, 1991. [DOI] [PubMed] [Google Scholar]
- 4. Duncan RA, von Reyn CF, Alliegro GM, Toossi Z, Sugar AM, Levitz SM. Idiopathic CD4+ T-lymphocytopenia--four patients with opportunistic infections and no evidence of HIV infection. N Engl J Med 328: 393-398, 1993. [DOI] [PubMed] [Google Scholar]
- 5. Hardak E, Brook O, Yigla M. Radiological features of Pneumocystis jirovecii pneumonia in immunocompromised patients with and without AIDS. Lung 188: 159-163, 2010. [DOI] [PubMed] [Google Scholar]
- 6. Tasaka S, Tokuda H, Sakai F, et al. . Comparison of clinical and radiological features of pneumocystis pneumonia between malignancy cases and acquired immunodeficiency syndrome cases: a multicenter study. Intern Med 49: 273-281, 2010. [DOI] [PubMed] [Google Scholar]
- 7. Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 58: 1-207; quiz CE1-4, 2009. [PubMed] [Google Scholar]
- 8. Iriart X, Witkowski B, Cassaing S, et al. . Alveolar and blood T lymphocyte profiles in Pneumocystis jirovecii-positive patients: effects of HIV status. J Infect Dis 204: 544-553, 2011. [DOI] [PubMed] [Google Scholar]
- 9. Ahmad DS, Esmadi M, Steinmann WC. Idiopathic CD4 lymphocytopenia: spectrum of opportunistic infections, malignancies, and autoimmune diseases. Avicenna J Med 3: 37-47, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buchacz K, Baker RK, Palella FJ Jr, et al. . AIDS-defining opportunistic illnesses in US patients, 1994-2007: a cohort study. AIDS 24: 1549-1559, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Stansell JD, Osmond DH, Charlebois E, et al. . Predictors of Pneumocystis carinii pneumonia in HIV-infected persons. Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med 155: 60-66, 1997. [DOI] [PubMed] [Google Scholar]