Abstract
We report the case of a 61-year-old woman with rapidly progressive interstitial lung disease caused by clinically amyopathic dermatomyositis. Both the serum ferritin and anti-melanoma differentiation-associated gene 5 (MDA5) antibody levels were elevated at the time of admission. Despite intensive treatment with corticosteroids, immunosuppressants, immunoglobulins and polymyxin B direct hemoperfusion, the patient died 75 days after symptom onset. Over the course of treatment, the anti-MDA5 antibody level continually decreased, while the serum ferritin level increased, suggesting that sequential measurements of the serum ferritin level might be useful for evaluating the treatment responsivity, irrespective of the anti-MDA5 antibody level.
Keywords: amyopathic dermatomyositis, anti-clinically amyopathic dermatomyositis 140 antibody, anti-melanoma differentiation-associated gene 5 antibody, ferritin, polymyxin B direct hemoperfusion, rapidly progressive interstitial lung disease
Introduction
Clinically amyopathic dermatomyositis (CADM) is a spectrum of dermatomyositis with typical skin lesions and minimal or absent muscle disease (1, 2). CADM is often complicated by rapidly progressive interstitial lung disease (RP-ILD), leading to a fatal clinical course (3, 4). Combination treatment with pulsed methylprednisolone therapy, intravenous cyclophosphamide and cyclosporin is often implemented for RP-ILD with CADM. Recently, a few reports have shown that multimodal treatment with polymyxin B direct hemoperfusion (PMX-DHP) is associated with favorable outcomes (5, 6).
Several biomarkers have been evaluated for their ability to predict the occurrence, disease activity and outcome of RP-ILD in patients with CADM. Serum ferritin has been reported to be a predictor of the occurrence and prognosis of RP-ILD; in cases of a highly elevated serum ferritin level, intensive treatment is recommended (7-9). Furthermore, some reports have shown that sequential changes in the serum ferritin level correspond to the respiratory condition during the clinical course and reflect the disease activity of RP-ILD (10, 11). In addition to serum ferritin, anti-melanoma differentiation-associated gene 5 (MDA5) antibody was also found to be a strong predictor of RP-ILD by Sato et al. in 2005 (12), and this antibody has been evaluated during the diagnostic process of CADM in many recent case reports (5, 10, 11, 13-18). Moreover, this antibody was also shown to be a useful marker of disease activity (19).
However, few studies have so far reported sequential data regarding the levels of both anti-MDA5 antibody and serum ferritin during the course of treatment for RP-ILD (5). In this study, we measured both of these levels in a patient with RP-ILD as a complication of CADM and found that the level of serum ferritin was associated with the treatment responsivity, irrespective of the anti-MDA5 antibody level.
Case Report
A 61-year-old woman presented to her local hospital with complaints of a dry cough and dyspnea on exertion for 14 days. She had erythema on her face at that time. She was diagnosed with bacterial pneumonia and treated with levofloxacin at 500 mg/day. However, her symptoms gradually worsened, and she was admitted to another hospital at 28 days after onset. A clinical diagnosis of organizing pneumonia was made without pathological confirmation or an assessment of causative factors, and treatment with prednisolone at 1.0 mg/kg/day was initiated. Because her symptoms persisted regardless of the treatment, she was referred to our hospital at 54 days after the onset of her symptoms (day 54). On admission (day 56), she showed a low-grade fever of 37.8℃, moderate tachycardia of 96 beats per minute, tachypnea of 24 breaths per minute and hypoxemia, with an oxygen saturation 88% on ambient air. A physical examination revealed no abnormalities except for fine crackles audible bilaterally in the posterior lower lung fields, erythema on her face (heliotrope rash) and upper chest (V-neck sign), scaly erythematous eruptions on both elbows (Gottron's papules) and hyperkeratosis with slight scaling bilaterally on the fingers (mechanic's hands). Muscle weakness and myalgia were not observed. The manual muscle testing scores were grade 5 of 5 for all extremities.
A serum analysis showed elevated levels of C-reactive protein, lactate dehydrogenase, Krebs von den Lungen-6 and serum ferritin (Table 1). Her creatine kinase level was low, and her surfactant protein-D level was within the normal range. Anti-MDA5 antibody was detected by an enzyme-linked immunosorbent assay, while anti-aminoacyl transfer-RNA synthetase antibody was negative. Infection with acid-fast bacilli, cytomegalovirus or Pneumocystis jirovecii was not detected. Her chest computed tomography (CT) scans showed nonsegmental consolidations and ground-glass attenuations in bilateral lung fields (Fig. 1). Malignancy was not detected by the radiological findings, and her serum tumor marker levels were within the normal ranges. Based on these findings, a diagnosis of RP-ILD with CADM was made.
Table 1.
Laboratory Data.
| Variable | Referenece range | On admission |
|---|---|---|
| Hematocrit (%) | 34-42 | 37.4 |
| Hemoglobin (g/dL) | 12-16 | 12.2 |
| White-cell count (per mm3) | 4,000-8,000 | 10,180 |
| Differential count (%) | ||
| Neutrophils | 48-61 | 92.4 |
| Lymphocytes | 25-45 | 4.9 |
| Monocytes | 4-7 | 2.5 |
| Eosinophils | 1-5 | 0.1 |
| Basophils | 0-1 | 0.1 |
| Platelet count (×104 per mm3) | 13-35 | 22.4 |
| Erythrocyte count (×104 per mm3) | 380-480 | 437 |
| Aspartate aminotransferase (IU/L) | 13-33 | 53 |
| Alanine aminotransferase (IU/L) | 6-27 | 92 |
| γ-glutamyl transpeptidase (IU/L) | 10-47 | 207 |
| Lactate dehydrogenase (IU/L) | 119-229 | 335 |
| Creatine kinase (IU/L) | 45-163 | 33 |
| C-reactive protein (mg/dL) | 0-0.3 | 1.07 |
| Krebs von den lungen-6 (U/mL) | 0-500 | 1,055 |
| Surfactant protein-D (ng/mL) | 0-110 | 75.0 |
| Ferritin (ng/mL) | 3.6-114 | 617 |
| Anti-ARS antibody | negative | negative |
| anti-MDA5 antibody (Unit) | <32 | 75 |
| Arterial blood gas* | ||
| pH | 7.35-7.45 | 7.474 |
| pCO2 (mmHg) | 35.0-45.0 | 33.1 |
| pO2 (mmHg) | 80< | 69.6 |
| HCO3- (mmol/L) | 24-33 | 23.8 |
ARS: aminoacyl transfer-RNA synthetase, MDA5: melanoma differentiation-associated gene 5
*under supplemental oxygen at a rate of 3L/min
Figure 1.
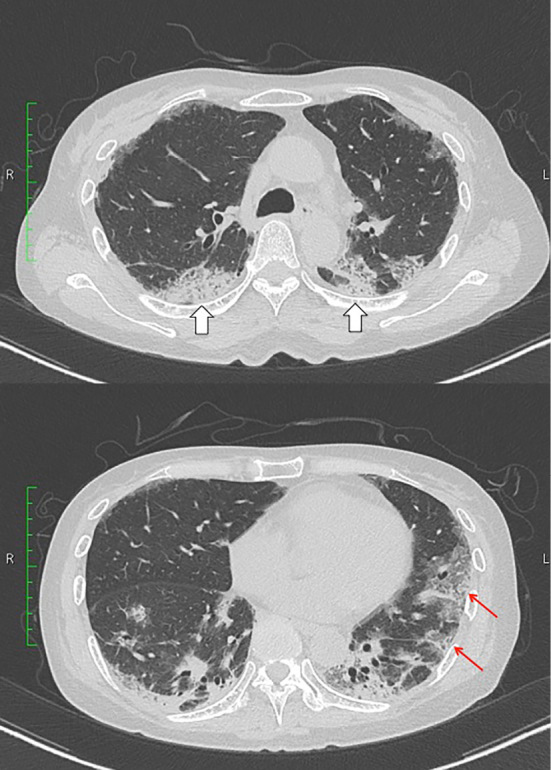
CT scan of the chest on admission showing nonsegmental consolidation (void arrows) and ground-glass attenuation (arrows) in the bilateral lung fields.
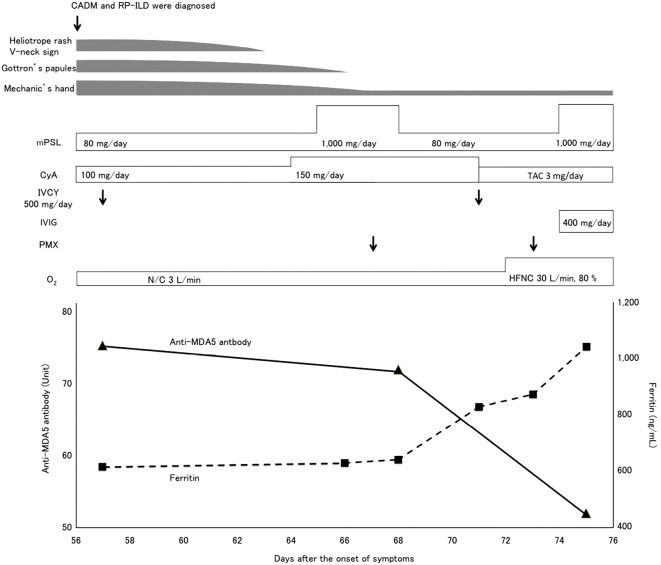
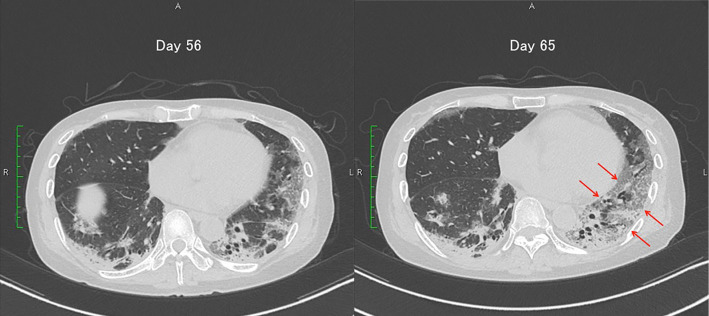
Because we considered RP-ILD with CADM to be a lethal disease, consecutive treatments with methylprednisolone (80 mg/day and pulsed 1,000 mg/day), cyclosporine (100-150 mg/day) and intravenous cyclophosphamide therapy (IVCY, 500 mg, on days 57 and 71) were commenced (Fig. 2). However, no responses to these treatments were observed, and the ground-glass attenuation on her chest CT image expanded on day 65, (Fig. 3). We then conducted an additional treatment with PMX-DHP on day 67. Intravenous immunoglobulin therapy (400 mg/day) starting on day 74 also failed to improve her deteriorating condition. The patient died of respiratory failure due to RP-ILD on day 75.
Figure 2.
Clinical course and changes in the anti-MDA5 antibody and serum ferritin levels. CADM: clinically amyopathic dermatomyositis, CyA: cyclosporin, IVCY: intravenous cyclophosphamide therapy, MDA5: melanoma differentiation-associated gene 5, mPSL: methylprednisolone, N/C: nasal cannula, HFNC: high-flow nasal cannula, PMX: polymyxin B direct hemoperfusion, RP-ILD: rapidly progressive interstitial lung disease, TAC: tacrolimus
Figure 3.
Changes in the findings on chest CT images. Ground grass attenuation was expanded (arrows) on day 65.
The levels of anti-MDA5 antibody slowly decreased from 75.0 U at the time of admission to 71.6 U on day 68; thereafter, the level decreased to 51.6 U on day 75. Conversely, the serum ferritin level continually increased from 642 ng/mL on day 68 to 838 ng/mL on day 71, finally reaching 1,060 ng/mL on day 75. Other inflammatory markers, such as C-reactive protein, white blood cell count and fibrinogen, were not associated with the clinical course.
Discussion
The prognosis of RP-ILD with CADM is poor, and the mortality rates are as high as 45-60% (9, 13, 14). The patient in the present case with RP-ILD had typical skin lesions and elevated serum ferritin and anti-MDA5 antibody levels at the time of admission. Although we administered various treatments, including PMX-DHP along with intensive immunosuppressive drugs, the patient ultimately died. In the present case, sequential changes in the serum ferritin level (642 to 1,060 ng/mL) were inversely correlated with treatment responsivity, irrespective of changes in the anti-MDA5 antibody level (75 to 51.6 U) over a short clinical period.
Studies on the serial measurement of serum ferritin and anti-MDA5 antibody levels in RP-ILD with CADM are summarized in Table 2. A recent report found that monitoring the anti-MDA5 antibody level was helpful in determining the treatment efficacy because the antibody level decreased along with a favorable response to the treatment, and the patient survived (5). In their case, the serum ferritin level also concurrently decreased with the anti-MDA5 antibody level. Other studies have also shown that decreases in the two markers are associated with the remission of ILD or a good prognosis (9, 19-21). However, the measurement intervals in these studies were as long as several weeks or months, and the majority of the studies presented surviving cases, which might introduce a selection bias. Furthermore, the decrease in the antibody level does not always reflect a good prognosis, as the antibody levels have also been shown to decrease after treatment in several patients who did not survive. Variations in the serum ferritin level were assessed over a short clinical course and were associated with the respiratory condition (10, 11). Taken together, these findings suggest that sequential serum ferritin level measurements would be more useful for evaluating the treatment responsivity than would measurements of the anti-MDA5 antibody level. However, because a report showed that the serum ferritin level in surviving cases tended to increase in the early phase and decrease two weeks after IVCY (22), the time course of serum ferritin level measurements warrants further study.
Table 2.
Preceding Reports on Serial Measurement of Serum Ferritin and Anti-MDA5 Antibody.
| Report category | Intervals between points of measurement | Key findings of serum biomarker level and associated factors | Reference | |
|---|---|---|---|---|
| Ferritin | Anti-MDA5 antibody | |||
| Observational study | 2 or 3 months | Decreased and patients survived | Decreased and patients survived | 9 |
| Observational study | 2-13 months | Decreased after remission of ILD | Decreased after remission of ILD | 21 |
| Observational study | 2-40 weeks | Not assessed | Decreased to below cut-off level after improvement of ILD | 19 |
| Observational study | 5-15 years | Not assessed | Decreased to below cut-off level after remission of ILD | 20 |
| Case report | 4-74 days | Decreased and patient survived | Decreased and patient survived | 5 |
| Case report | 10-20 days | Increased during patients needed oxygen supplementation | Not assessed | 10 |
| Case report | 2-27 days | Increased during decreasing of PaO2/FiO2 ratio | Not assessed | 11 |
| Case report | 2-11 days | Increased and the patient died | Decreased, but the patient died | - |
ILD: interstitial lung disease, MDA5: melanoma differentiation-associated gene 5, PaO2/FiO2: arterial partial pressure of oxygen/fraction of inspired oxygen
Intensive treatment, including PMX-DHP, which was initially developed for removing endotoxins, might influence the anti-MDA5 antibody level. On acute exacerbation of ILD or idiopathic pulmonary fibrosis, the absorption of neutrophils (23) and the removal of various inflammatory mediators, such as MMP-9, HMBG-1 and VEGF (23-25), by a PMX fiber column have been reported. Although it remains unclear whether or not PMX-DHP reduces the antibody level, irrespective of treatment efficacy, a recent report showed a decrease in the anti-MDA5 antibody level after multimodal treatment with PMX-DHP (5). In the present case, the antibody level decreased after PMX-DHP, and we posit that the PMX fiber column was able to absorb the antibody. However, some other reports have shown decreases in the antibody level with steroidal and immunosuppressant treatments and without PMX-DHP (9, 19-21). Because the decrease in the anti-MDA5 antibody level was observed after increasing the doses of methylprednisolone and cyclosporine in the present case, this antibody level reduction might be influenced by these medications via the inhibition of B-lymphocyte antibody production. As such, each of these treatments might have the potential to reduce the anti-MDA5 antibody level, irrespective of treatment efficacy; in addition, the reduction would not necessarily reflect the disease activity.
In conclusion, sequential measurements of the serum ferritin level might be useful for evaluating the treatment responsivity in cases of RP-ILD with CADM, irrespective of changes in the anti-MDA5 antibody level.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We would like to thank Dr. Shinji Sato (Division of Rheumatology, Department of Internal Medicine, Tokai University School of Medicine) for performing the anti-MDA5 antibody measurements.
References
- 1. Sontheimer RD. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis siné myositis) as a distinctive subset within the idiopathic inflammatory dermatomyopathies spectrum of clinical illness? J Am Acad Dermatol 46: 626-636, 2002. [DOI] [PubMed] [Google Scholar]
- 2. Gerami P, Schope JM, McDonald L, Walling HW, Sontheimer RD. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis siné myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies. J Am Acad Dermatol 54: 597-613, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Sato S, Kuwana M. Clinically amyopathic dermatomyositis. Curr Opin Rheumatol 22: 639-643, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Fujisawa T, Hozumi H, Kono M, et al. Prognostic factors for myositis-associated interstitial lung disease. PLoS One 9: e98824, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teruya A, Kawamura K, Ichikado K, Sato S, Yasuda Y, Yoshioka M. Successful polymyxin B hemoperfusion treatment associated with serial reduction of serum anti-CADM-140/MDA5 antibody levels in rapidly progressive interstitial lung disease with amyopathic dermatomyositis. Chest 144: 1934-1936, 2013. [DOI] [PubMed] [Google Scholar]
- 6. Ichiyasu H, Horio Y, Tsumura S, et al. Favorable outcome with hemoperfusion of polymyxin B-immobilized fiber column for rapidly progressive interstitial pneumonia associated with clinically amyopathic dermatomyositis: report of three cases. Mod Rheumatol 24: 361-365, 2014. [DOI] [PubMed] [Google Scholar]
- 7. Gono T, Kawaguchi Y, Hara M, et al. Increased ferritin predicts development and severity of acute interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford) 49: 1354-1360, 2010. [DOI] [PubMed] [Google Scholar]
- 8. Gono T, Kawaguchi Y, Satoh T, et al. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford) 49: 1713-1719, 2010. [DOI] [PubMed] [Google Scholar]
- 9. Gono T, Sato S, Kawaguchi Y, et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology (Oxford) 51: 1563-1570, 2012. [DOI] [PubMed] [Google Scholar]
- 10. Gono T, Kawaguchi Y, Ozeki E, et al. Serum ferritin correlates with activity of anti-MDA5 antibody-associated acute interstitial lung disease as a complication of dermatomyositis. Mod Rheumatol 21: 223-227, 2011. [DOI] [PubMed] [Google Scholar]
- 11. Tamai K, Tachikawa R, Otsuka K, Ueda H, Hosono Y, Tomii K. Early pulmonary involvement of anti-CADM-140 autoantibody-positive rapidly progressive interstitial lung disease preceding typical cutaneous symptoms. Intern Med 53: 2515-2519, 2014. [DOI] [PubMed] [Google Scholar]
- 12. Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 52: 1571-1576, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Ichiyasu H, Sakamoto Y, Yoshida C, et al. Rapidly progressive interstitial lung disease due to anti-MDA-5 antibody-positive clinically amyopathic dermatomyositis complicated with cervical cancer: successful treatment with direct hemoperfusion using polymyxin B-immobilized fiber column therapy. Respir Med Case Rep 20: 51-54, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sato S, Kuwana M, Fujita T, Suzuki Y. Amyopathic dermatomyositis developing rapidly progressive interstitial lung disease with elevation of anti-CADM-140/MDA5 autoantibodies. Mod Rheumatol 22: 625-629, 2012. [DOI] [PubMed] [Google Scholar]
- 15. Yamaguchi K, Yamaguchi A, Uchida M, et al. A case of anti-MDA5-positive rapidly progressive interstitial lung disease in a patient with clinically amyopathic dermatomyositis ameliorated by rituximab, in addition to standard immunosuppressive treatment. Mod Rheumatol 27: 536-540, 2017. [DOI] [PubMed] [Google Scholar]
- 16. Horai Y, Isomoto E, Koga T, et al. Early diagnosis and treatment for remission of clinically amyopathic dermatomyositis complicated by rapid progress interstitial lung disease: a report of two cases. Mod Rheumatol 23: 190-194, 2013. [DOI] [PubMed] [Google Scholar]
- 17. Zou J, Li T, Huang X, Chen S, Guo Q, Bao C. Basiliximab may improve the survival rate of rapidly progressive interstitial pneumonia in patients with clinically amyopathic dermatomyositis with anti-MDA5 antibody. Ann Rheum Dis 73: 1591-1593, 2014. [DOI] [PubMed] [Google Scholar]
- 18. Yamaoka T, Doi C, Yokomi A, et al. Anti-MDA5 antibody-positive dermatomyositis with lethal progressive interstitial lung disease and advanced gastric cancer. Eur J Dermatol 24: 490-491, 2014. [DOI] [PubMed] [Google Scholar]
- 19. Sato S, Kuwana M, Fujita T, Suzuki Y. Anti-CADM-140/MDA5 autoantibody titer correlates with disease activity and predicts disease outcome in patients with dermatomyositis and rapidly progressive interstitial lung disease. Mod Rheumatol 23: 496-502, 2013. [DOI] [PubMed] [Google Scholar]
- 20. Muro Y, Sugiura K, Hoshino K, Akiyama M. Disappearance of anti-MDA-5 autoantibodies in clinically amyopathic DM/interstitial lung disease during disease remission. Rheumatology (Oxford) 51: 800-804, 2012. [DOI] [PubMed] [Google Scholar]
- 21. Matsushita T, Mizumaki K, Kano M, et al. Antimelanoma differentiation-associated protein 5 antibody level is a novel tool for monitoring disease activity in rapidly progressive interstitial lung disease with dermatomyositis. Br J Dermatol 176: 395-402, 2017. [DOI] [PubMed] [Google Scholar]
- 22. Nakashima R, Mimori T. [Anti-MDA5 antibody and dermatomyositis with rapidly progressive interstitial pneumonia]. Nihon Rinsho Meneki Gakkai Kaishi (Jpn J Clin Immunol) 36: 71-76, 2013(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 23. Abe S, Seo Y, Hayashi H, et al. Neutrophil adsorption by polymyxin B-immobilized fiber column for acute exacerbation in patients with interstitial pneumonia: a pilot study. Blood Purif 29: 321-326, 2010. [DOI] [PubMed] [Google Scholar]
- 24. Abe S, Hayashi H, Seo Y, et al. Reduction in Serum High Mobility Group Box-1 level by polymyxin B-immobilized fiber column in patients with idiopathic pulmonary fibrosis with acute exacerbation. Blood Purif 32: 310-316, 2011. [DOI] [PubMed] [Google Scholar]
- 25. Oishi K, Mimura-Kimura Y, Miyasho T, et al. Association between cytokine removal by polymyxin B hemoperfusion and improved pulmonary oxygenation in patients with acute exacerbation of idiopathic pulmonary fibrosis. Cytokine 61: 84-89, 2013. [DOI] [PubMed] [Google Scholar]