Abstract
Acquired coagulation factor inhibitor is a rare coagulation disorder. We herein report a patient with acquired factor V inhibitor showing a decrease in multiple coagulation factor activities. A high titer of factor V inhibitor presumably led to a marked inhibition of factor V activity in the specific factor-deficient plasma used in coagulation factor activity assays based on either an activated partial thromboplastin time (APTT) or prothrombin time (PT) clotting assay, resulting in false low values of the coagulation activity. We re-examined the coagulation factor activity using several dilutions of the patient's plasma and confirmed that the high factor V inhibitor titer had caused an apparent decrease in multiple coagulation factor activities.
Keywords: acquired factor V inhibitor, high titer inhibitor, APTT or PT based clotting assay
Introduction
Factor V is a plasma cofactor that plays a pivotal role in the blood coagulation cascade. Acquired factor V inhibitor is an uncommon bleeding disorder that can arise from various conditions and diseases, especially exposure to bovine thrombin or fibrin glue, the use of antibiotics, blood transfusion, autoimmune disorders, and malignancies (1). A diagnosis of factor V inhibitor is usually confirmed by measuring the factor V activity and the inhibitor titer, which are determined using a clotting assay and the Bethesda assay, respectively. We herein present a patient with acquired factor V inhibitor who showed a decrease in the activity of multiple coagulation factors because of a very high factor V inhibitor titer.
Case Report
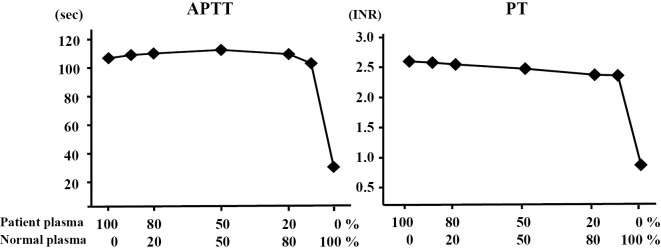
A 72-year-old Japanese woman was referred to our hospital by her general practitioner because of fatigue and nausea. She had a history of chronic kidney disease (CKD) due to hypertensive nephrosclerosis. Under the management of her local hospital, she was on treatment with a β-blocker, warfarin, diuretics, phosphate binders, and activated vitamin D for hypertension, atrial fibrillation, and CKD. Although she had stage 5 CKD, her renal function was stable with a serum creatinine level of about 5.5 mg/dL. On admission, her blood pressure was 148/108 mmHg. Non-contrast abdominal computed tomography (CT) revealed a subcapsular crescent-shaped high-density area in the left kidney that was consistent with a subcapsular renal hematoma (Fig. 1). She had no history of renal trauma and no evidence of any renal neoplasm, so we considered that the subcapsular renal hematoma was likely a bleeding complication due to warfarin therapy (2). Laboratory tests showed an elevation of serum C-reactive protein (CRP) and creatinine to 20.08 mg/dL and 7.49 mg/dL, respectively. The platelet count was 134×109/L. Routine coagulation tests revealed a prolongation of the prothrombin time to 20.8 seconds (international normalized ratio: 1.72), but the activated partial thromboplastin time (32.1 seconds) was within the normal range (24.0-35.0 seconds). The plasma levels of fibrin/fibrinogen degradation products and D-dimer were slightly elevated to 11.5 μg/mL and 3.7 μg/mL, respectively. Blood culture was performed on admission, and it was positive for Klebsiella pneumoniae. Urine culture also yielded Klebsiella pneumoniae (>100.000 colonies) with the same antibiotic sensitivity profile. Intravenous ceftriaxone was initiated for the treatment of sepsis and urinary tract infection due to Klebsiella pneumoniae. The subcapsular renal hematoma was thought to be due to the prolongation of the prothrombin time by her warfarin therapy, and she was treated conservatively by radiological follow-up without suspending the warfarin dosage. She responded well to antibiotic therapy and CRP decreased rapidly. The subcapsular renal hematoma was stable. Two weeks after admission, routine coagulation tests revealed a prolongation of activated partial thromboplastin time (APTT) in addition to prothrombin time (PT). As a result, warfarin was stopped and Vitamin K was started, however, her clotting parameters thereafter became worse. One month after admission, APTT significantly increased to 106.6 seconds and international normalized ratio (INR) remained at 2.64 (Table 1). She did not show any clinical evidence of bleeding, except for the subcapsular renal hematoma. The presence of an acquired coagulation factor inhibitor was suspected, so a cross-mixing test was performed using plasma from the patient and from a healthy volunteer. In this test, both the APTT and PT curves demonstrated an upwardly convex shape (Fig. 2). Serological tests for anticardiolipin antibodies and anti-β2 glycoprotein-I antibodies were negative. Serum protein electrophoresis did not show any abnormalities. We checked the full coagulation factor activity profile, including factor XIII (Table 2). The activity of factors XII, XI, IX, and VIII involved in the intrinsic coagulation pathway was undetectable, and the factor VII activity (extrinsic pathway) was found to have decreased to 20%. Factors X, V, and II (common coagulation pathway) had also markedly decreased, with factor V being undetectable. On the other hand, the factor XIII activity was normal. While the factor XIII activity was measured by a synthetic substrate, the other coagulation factors were measured by APTT- or PT-based clotting assays. We thought that a high titer of an inhibitor for one coagulation factor might also completely inhibit that factor in the specific factor-deficient plasma used for APTT- or PT-based clotting assays, leading to false low levels of coagulation factor activity. In that case, if the patient's plasma was sufficiently diluted, then the effect of the inhibitor would be reduced and the coagulation factor activity would thus be corrected. To confirm our hypothesis, we re-examined the coagulation factor activity using three dilutions of the patient's plasma (5-, 10-, and 15-fold) (Table 3). Factor V activity remained undetectable with all dilutions, but the activity of the other coagulation factors increased along with the dilution ratio. In addition, an inhibitor assay (Bethesda assay) detected an extremely high titer of factor V inhibitor (102 Bethesda U/mL), while the titers of the inhibitors for the other factors were low (3-11 Bethesda U/mL) (Table 4). As the Bethesda assay is also an APTT- or PT-based test, the low titers of other inhibitors were thought to be false results caused by the same mechanism as in the coagulation factor activity assays. Based on these findings, we concluded that the patient had acquired factor V inhibitor and that a very high titer of this inhibitor had interfered with the assays of the other coagulation factors. We performed the hepaplastin test (HPT) and the thrombo test (TT) for confirmation. The results were in the normal range (104% and 126%, respectively), indicating that she did not have any deficiency of factors II, VII, and X.
Figure 1.
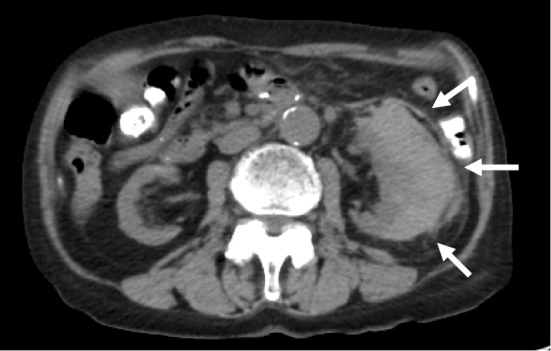
Computed tomography revealed a subcapsular renal hematoma.
Table 1.
Serial changes in the laboratory values after admission
| Warfarin stopped/Vitamin K started | ||||||
|---|---|---|---|---|---|---|
| Time (days) | 1 | 16 | ↓ | 17 | 22 | 33 |
| PT (sec) | 20.8 | 20.1 | 35.8 | 32.5 | 31.6 | |
| INR | 1.72 | 1.66 | 2.99 | 2.71 | 2.64 | |
| APTT (sec) | 32.1 | 39.2 | 74.2 | 97.7 | 106.6 | |
| Platelet (109/L) | 134 | 110 | 120 | 123 | ||
| Fib (mg/dL) | 724.6 | 439.9 | ||||
| FDP (µg/mL) | 11.5 | 3.0 | ||||
| D-dimer (µg/mL) | 3.7 | 1.9 | 1.4 | 1.1 | ||
| CRP (mg/dL) | 20.08 | 4.95 | 0.57 | 0.35 | ||
PT: prothrombin time, INR: international normalized ratio, APTT: activated partial thromboplastin time, FDP: fibrin/fibrinogen degradation products, CRP: C-reactive protein
Figure 2.
Cross-mixing tests. The APTT and PT values showed upwardly convex curves, indicating the presence of an inhibitor. APTT: activated partial thromboplastin time, PT: prothrombin time
Table 2.
Coagulation Factor Activities in Our Patient.
| Coagulation factor | Activity (%) | Method |
|---|---|---|
| XII | <3 | APTT-based clotting assay |
| XI | <3 | |
| IX | <1 | |
| VIII | <1 | |
| VII | 20 | PT-based clotting assay |
| X | 11 | |
| V | <3 | |
| II | 7 | |
| XIII | 107 | Synthetic substrate assay |
APTT: activated partial thromboplastin time, PT: prothrombin time
Table 3.
Coagulation Factor Activities Assayed with Various Dilutions of Patient Plasma.
| X1 | X5 | X10 | X15 | |
|---|---|---|---|---|
| XII | <3% | <3% | 4% | 8% |
| XI | <3% | <3% | 6% | 7% |
| IX | <1% | <1% | 11% | 16% |
| VIII | <1% | 2% | 13% | 22% |
| VII | 15% | 17% | 29% | 33% |
| X | 6% | 9% | 13% | 14% |
| V | <3% | <3% | <3% | <3% |
| II | 4% | 4% | 9% | 10% |
Table 4.
Coagulation Factor Inhibitors in Our Patient.
| Coagulation factor | Inhibitor (BU/mL) |
|---|---|
| XII | 7 |
| XI | 7 |
| IX | 7 |
| VIII | 7 |
| VII | 3 |
| X | 4 |
| V | 102 |
| II | 11 |
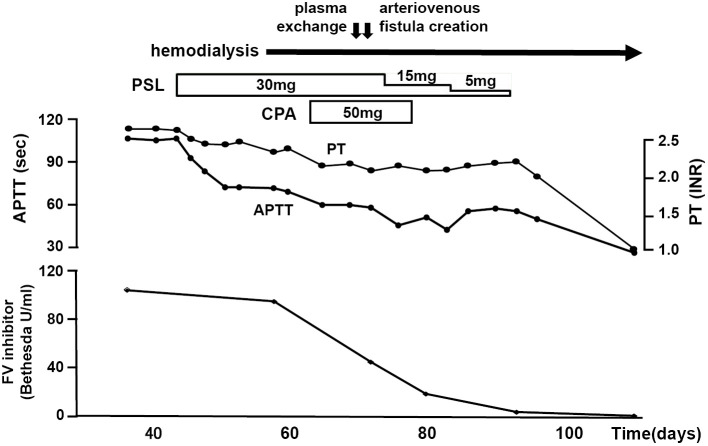
The patient did not have any apparent cutaneous or mucosal bleeding, such as petechial hemorrhage. Although the inhibitor could be expected to disappear within a period of months without inhibitor elimination therapy (1, 3, 4), the initiation of hemodialysis was required due to advanced renal failure, which necessitated the creation of an arteriovenous fistula. To reduce the risk of bleeding associated with surgery, we considered that inhibitor elimination therapy was required (5). Treatment with oral prednisolone was started at 30 mg/day, but the response was insufficient. We added oral cyclophosphamide (50 mg/day) and also performed one session of plasma exchange before surgery, which was performed without any bleeding complications (6). Subsequently, prednisolone was tapered as the factor V inhibitor level declined (Fig. 3).
Figure 3.
Clinical course of our patient with an acquired factor V inhibitor.
Discussion
Coagulation factor activity other than factor XIII is usually evaluated by APTT-based (VIII, IX, XI, and XII) or PT-based (II, V, VII, and X) one-stage clotting assays. In these assays, the coagulation factor activity is measured by assessing the ability of patient plasma to normalize the prolonged clotting time of specific factor-deficient plasma (7). For example, if patient plasma is added to factor VIII-deficient plasma and the APTT remains prolonged, this is taken to indicate that the patient has a factor VIII deficiency. However, there is a potential pitfall in the interpretation of such coagulation factor tests. If an extremely high titer of a coagulation factor inhibitor is present in the plasma (factor V inhibitor in the present patient), this large excess of such an inhibitor will completely inhibit factor V in plasma that is specifically deficient for other factors and the prolonged clotting time will not be corrected, leading to a misdiagnosis of deficiency even though the activity of the other factors is normal. When coagulation factor activity assays are performed, patient plasma is usually diluted 10-fold before being added to specific factor-deficient plasma in order to avoid this phenomenon. However, the plasma of our patient had an extremely high factor V inhibitor titer and the inhibitor still blocked factor V in specific factor-deficient plasma after 10-fold dilution, and, as a result, the various assays gave false low results.
To avoid this problem, another method can be used to determine the specific coagulation factor activities based on the cleavage of specific chromogenic substrates by activated factors (7). Chromogenic substrate assays are available for several coagulation factors (including factors VIII, IX, and X), but it is difficult or impossible to obtain these for all of the coagulation factors.
Our patient showed a marked decrease in the activity of multiple coagulation factors. We concluded that an excessively high titer of factor V inhibitor had blocked the factor V activity in various specific factor-deficient plasma samples, thereby preventing the normalization of the prolonged clotting time, although the patient actually had a normal activity of other coagulation factors. To confirm this, we performed the one-stage clotting assay after further diluting our patient's plasma and the findings demonstrated that the factor V inhibitor had caused a reduction of multiple coagulation factor activities.
To the best of our knowledge, there have been only two case reports of patient with acquired coagulation factor inhibitor showing a decrease in the activity of multiple coagulation factors, and the mechanisms underlying such abnormal laboratory findings have not been verified (8, 9). In contrast, we clearly demonstrated that performing coagulation factor activity assays by carrying out several dilutions of patient plasma could provide more accurate results. Both of the previously reported patients also had high titers of acquired factor V inhibitor. An inhibitor of factor V will affect both APTT and PT, and a profound inhibition of factor V can influence the assay for any coagulation factor, except factor XIII. There is also another interesting aspect to consider. In patients with factor V inhibitor, the bleeding tendency is characteristically mild and some patients do not show any bleeding symptoms (3). This could be due to the fact that factor V is not only found in the plasma, but is also stored in the alpha granules of platelets (10). Thus, platelet-derived factor V might play a local hemostatic role at sites of vascular injury. Despite having a very high titer of factor V inhibitor, our patient did not develop any bleeding symptoms throughout the clinical course. It is possible that the lack of bleeding allowed the inhibitor titer to rise to such a high level.
However, there have been some reports of patients with a high inhibitor titer who did not develop a pseudo-deficiency of multiple coagulation factors (11). Coagulation factor inhibitors are classified as type 1 or type 2 based on reaction kinetics. An inhibitor with type 1 kinetics completely inactivates the target coagulation factor and there is a linear relationship between the logarithm of the residual coagulation factor activity and the inhibitor concentration. On the other hand, an inhibitor with type 2 kinetics does not completely inactivate the target coagulation factor and there is a nonlinear relationship between the residual coagulation factor activity and the antibody concentration (12, 13). Even in the presence of a high titer of a type 2 inhibitor, minimal coagulation factor activity might persist. This difference of inhibitor properties might be related to whether or not the patients develop a pseudo-deficiency of multiple coagulation factors.
In the present case, the HPT and TT were useful for confirming the presence of a factor V deficiency. The HPT and TT comprehensively show the activity of factors II, X, and VII, while the PT evaluates factors II, V, X, VII, and I (fibrinogen). Therefore, a prolonged PT together with normal values for fibrinogen, HPT, and TT indicates a factor V deficiency. Combining these coagulation screening tests is thus considered to be useful for diagnosing factor V deficiency (14).
The reason why our patient developed factor V inhibitor remains unclear. We confirmed that she had not been exposed to bovine thrombin by a careful review of her medical history. She also had no indicators or symptoms of malignancy or autoimmune disease. It is possible that ceftriaxone had played a causative role in the development of our patient's inhibitor. Because our patient needed shunt surgery, she was treated with immunosuppressive therapy and plasma exchange despite the absence of any bleeding symptoms, eventually resulting in the disappearance of the acquired inhibitor.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Franchini M, Lippi G. Acquired factor V inhibitors: a systematic review. J Thromb Thrombolysis 31: 449-457, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Mao Y, De Oliveira IS, Hedgire S, Prapruttam D, Harisinghani M. Aetiology, imaging features, and evolution of spontaneous perirenal haemorrhage. Clin Radiol 72: 175 e119-175 e126, 2017. [DOI] [PubMed] [Google Scholar]
- 3. Ang AL, Kuperan P, Ng CH, Ng HJ. Acquired factor V inhibitor. A problem-based systematic review. Thromb Haemost 101: 852-859, 2009. [PubMed] [Google Scholar]
- 4. Kinjo Y, Yoshimura K, Suzuki T, et al. . Development of asymptomatic acquired factor V inhibitor after administration of antibiotics. Rinsho Ketsueki (Jpn J Clin Hematol) 55: 2311-2315, 2014. [PubMed] [Google Scholar]
- 5. Coppola A, Windyga J, Tufano A, Yeung C, Di Minno MN. Treatment for preventing bleeding in people with haemophilia or other congenital bleeding disorders undergoing surgery. Cochrane Database Syst Rev CD009961, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baron BW, Mittendorf R, Baron JM. Presurgical plasma exchange for severe factor V deficiency. J Clin Apher 16: 29-30, 2001. [DOI] [PubMed] [Google Scholar]
- 7. Potgieter JJ, Damgaard M, Hillarp A. One-stage vs. chromogenic assays in haemophilia A. Eur J Haematol 94 (Suppl 77): 38-44, 2015. [DOI] [PubMed] [Google Scholar]
- 8. Shimizu I, Ichikawa N, Yotsumoto M, Sumi M, Ueno M, Kobayashi H. High-titer idiopathic acquired factor V inhibitor patient showing decreased activities of multiple coagulation factors. Rinsho Ketsueki (Jpn J Clin Hematol) 49: 413-416, 2008. [PubMed] [Google Scholar]
- 9. Yamada Y, Miyakawa Y, Sawano M, Okano Y. Successful treatment of severe lung hemorrhage caused by acquired factor V inhibitor with rituximab. Intern Med 53: 1083-1085, 2014. [DOI] [PubMed] [Google Scholar]
- 10. Huang JN, Koerper MA. Factor V deficiency: a concise review. Haemophilia 14: 1164-1169, 2008. [DOI] [PubMed] [Google Scholar]
- 11. Bayani N, Rugina M, Haddad-Vergnes L, Lelong F. High-titer acquired factor V inhibitor responsive to corticosteroids and cyclophosphamide in a patient with two malignant tumors. Am J Hematol 71: 33-36, 2002. [DOI] [PubMed] [Google Scholar]
- 12. Gawryl MS, Hoyer LW. Inactivation of factor VIII coagulant activity by two different types of human antibodies. Blood 60: 1103-1109, 1982. [PubMed] [Google Scholar]
- 13. Ling M, Duncan EM, Rodgers SE, et al. . Classification of the kinetics of factor VIII inhibitors in haemophilia A: plasma dilution studies are more discriminatory than time-course studies. Br J Haematol 114: 861-867, 2001. [DOI] [PubMed] [Google Scholar]
- 14. Nakagoshi R, Takamiya O, Nakata S, et al. . A case of coagulation factor V inhibitor. Rinsho Byori (Jpn J Clin Pathol) 47: 971-975, 1999(in Japanese, Abstract in English). [PubMed] [Google Scholar]