Abstract
A 79-year-old woman with a history of Guillain-Barré syndrome (GBS) developed somnolence and tetraparesis after pneumonia. Based on clinical and laboratory findings, she was diagnosed with complications of acute inflammatory demyelinating polyneuropathy (AIDP) and acute disseminated encephalomyelitis (ADEM). Anti-galactocerebroside (Gal-C) IgG antibodies were detected in her serum. Cases of recurrent GBS in patients who are positive for this antibody are extremely rare. The anti-Gal-C IgG antibodies likely played an important role in the pathogenesis of the AIDP and ADEM.
Keywords: Guillain-Barré syndrome, acute disseminated encephalomyelitis, encephalomyeloradiculoneuropathy, anti-galactocerebroside antibody, acute inflammatory demyelinating polyneuropathy
Introduction
Guillain-Barré syndrome (GBS) is an acute, post-infectious, immune-mediated polyradiculoneuropathy involving the peripheral nervous system (PNS). While it generally shows a monophasic course, 2-5% of GBS patients relapse after an asymptomatic period of several months to years (1). Furthermore, rare cases of GBS with simultaneous central nervous system (CNS) lesions have been reported, mainly in children (2-4).
Some autoantibodies to the peripheral nerve epitopes, many of which target gangliosides, are thought to play an important role in the pathogenesis of GBS, (1). Galactocerebroside (Gal-C) is a type of neutral glycosphingolipid that is localized in both the CNS and PNS as a component of myelin (5, 6). The antibody to Gal-C is detected in the serum of some GBS patients, and it is also detected following post-infectious encephalopathy, especially that caused by Mycoplasma pneumoniae (7, 8). We herein report case of recurrent GBS in a patient who was seropositive for anti-Gal-C antibodies.
Case Report
In 2003, a 66-year-old, right-handed woman was admitted to hospital due to the development of progressive weakness of the extremities. Approximately 3 weeks before admission, she had a cough and throat pain, which lasted for approximately one week. Three days before admission, she developed fever and malaise. On the following day, she developed progressive weakness that affected all four limbs. She had no notable medical history. Her son had a history of encephalitis, which caused headache and behavioral abnormalities for several months in adolescence.
A neurological examination showed severe motor weakness associated with distal-dominant paresthesia and hyperpathia of the four limbs. The weakness was prominent in the patient's upper limbs. Her deep tendon reflexes (DTRs) were decreased. Blood tests were unremarkable. The patient's cerebrospinal fluid (CSF) showed 59 leukocytes/μL (97% mononuclear cells and 3% polymorphs), protein 96 mg/dL, and glucose 78 mg/dL. A nerve conduction study (NCS) showed that the compound muscle action potential (CMAP) amplitudes of the right median and tibial nerves were decreased and a conduction block between the wrist and elbow of the right median nerve. The sensory nerve action potential (SNAP) amplitude of the right median nerve was also decreased. Head CT showed no abnormalities. The patient was diagnosed with GBS. After treatment with intravenous immunoglobulin (IVIG) 400 mg/kg for 5 consecutive days, her tetraparesis started to improve, and she became able to walk by the 10th hospital day. However, on the 4th hospital day, she developed left facial palsy. Although she was treated with valaciclovir, mild left facial weakness remained. Aside from this, her weakness was fully recovered by the 30th hospital day. The follow-up CSF study showed a decreased cell count, and the NCS showed the improvement of the CMAP amplitudes. She was discharged on the 41st hospital day.
In 2016, at 79 years of age, she was transferred to our hospital for progressive tetraparesis. Seven days before transfer, she had been admitted to another hospital due to persistent fever and severe cough, which lasted for approximately one week. A chest roentgenogram showed consolidation of the middle lobe of the right lung, and she was diagnosed with pneumonia. Her fever immediately subsided after treatment with ceftriaxone (2 g/day). However, 3 days later, she again had a fever of 38°C, and developed hypertension with a systolic blood pressure exceeding 200 mmHg. She was treated with roxoprophen and nifedipine. On the same day, she had mild numbness of both hands. On the next day, she developed a headache and weakness of the right extremities. Two days later, the weakness of her extremities became generalized, and urination became difficult. She was then transferred to our hospital.
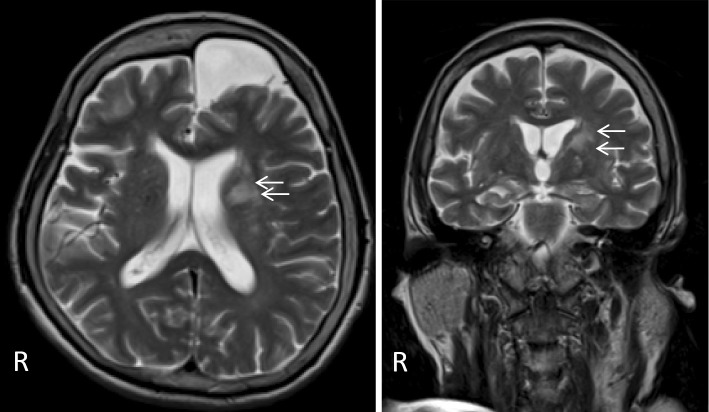
In the emergency room, her temperature was 35.3°C, her blood pressure was 136/84 mmHg, and her heart rate was 80 beats/min. She had mild somnolence, E3V5M6 (Glasgow Coma Scale). Nuchal stiffness and radicular pains in the four limbs were noted. Abducent ocular movement was bilaterally incomplete. Left facial palsy and mild dysarthria were noted. Her muscle strength, as assessed by the Medical Research Council (MRC) scale, was 2/5 for all four limbs. Weakness was prominent in the distal portion of the upper limbs, and her grip strength was 0 kg/0 kg. A sensory examination showed the disturbed position sense of her right hand and leg. While DTRs of the four limbs were abolished, the number of jaw jerks increased, and the plantar responses were extensor bilaterally. Blood tests showed mild leukocytosis (WBC 10,900 /μL) and a normal C-reactive protein (CRP) level (0.30 mg/dL). The sedimentation rate was 43 mm/h. The levels of vitamins B1 (24 ng/mL) and B12 (912 pg/mL) were normal. Her angiotensin-converting enzyme (ACE) level was not elevated (6.4 mIU/mL). The patient was negative for tumor markers, including carcinoembryonic antigen (CEA), CA19-9, soluble interleukin-2 receptor, CYFRA21-1, pro-gastrin-releasing peptide, and sialyl Lewis X-1. Serum virological tests for herpes simplex virus (HSV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), HIV, and human T-lymphotropic virus type 1 (HTLV-1) were negative, and an interferon gamma release assay for tuberculosis (T-SPOTⓇ) was also negative. Her M. pneumoniae antibody titers were 40-fold the normal limit. Serum autoantibodies, including antinuclear antibody (ANA), anti-cyclic citrullinated peptide (CCP) antibody, proteinase-anti-neutrophil cytoplasmic antibody (PR3-ANCA), myeloperoxidase (MPO)-anti-neutrophil cytoplasmic antibody (ANCA), and anti-SS-A/Ro and anti-SS-B/Ro antibodies were negative. Tests for antiglycolipid antibodies were performed, and the patient was found to be positive for anti-Gal-C IgG antibodies. She was negative for anti-Gal-C IgM and anti-ganglioside antibodies. A urinalysis revealed no remarkable findings. A CSF study on the 1st hospital day showed an opening pressure of 12 cmH2O, 40 leukocytes/μL (97% mononuclear cells and 3% polymorphs), protein 65 mg/dL, glucose 73 mg/dL, and IgG index 0.73. Virological tests for HSV and CMV in the CSF were negative, and adenosine deaminase was not elevated (2.5 IU/mL). An electroencephalogram (EEG) showed diffuse slowing. An NCS was performed on the 2nd hospital day (Table). The motor NCS showed severely decreased CMAP amplitudes in each nerve. While nerve conduction velocities (NCVs) were normal, the distal latency (DL) of the CMAPs was prolonged in the right tibial nerve. F waves were not induced in any nerves. The sensory NCS showed that the SNAP amplitude in the right median nerve was mildly decreased. Chest and abdominal CT revealed no remarkable findings, with the exception of mild infiltration in the right lung. Head MRI showed a focal lesion at the left corona radiata on T2WI and fluid-attenuated inversion recovery (FLAIR) imaging (Figure); no abnormalities were detected on whole spine MRI.
Table.
Results of the Motor and Sensory Nerve Conduction Studies before and after Treatment.
| Motor nerve conduction studies | |||
|---|---|---|---|
| Rt. median nerve | Rt. ulnar nerve | Rt. tibial nerve | |
| Distal latency (ms) | 4.6 (6.1) | 2.9 (4.1) | 6.1 (5.7) |
| CMAP (mV) | 1.8 (7.5) | 2.7 (6.7) | 4.4 (5.4) |
| MCV (m/s) | 64.9 (49.3) | 71.0 (47.4) | 50.4 (53.4) |
| F-wave occurrence (%) | 0 (56) | 0 (75) | 0 (75) |
| Sensory nerve conduction studies | |||
| Rt. median nerve | Rt. ulnar nerve | Rt. sural nerve | |
| SNAP (μV) | 9.5 (3.2) | 10.2 (0.8) | 14.9 (14.1) |
| SCV (m/s) | 53.0 (58.5) | 57.2 (54.1) | 66.2 (59.5) |
CMAP: compound muscle action potential, MCV: motor conduction velocity, SNAP: sensory nerve action potential, SCV: sensory conduction velocity
Data are before treatment (after treatment).
Figure.
Head MRI. Head MRI shows a high-intensity lesion at the left corona radiata on T2WI (arrows). An arachnoid cyst is also seen at the front of the left frontal lobe.
Based on these findings, the patient was diagnosed with GBS associated with acute disseminated encephalomyelitis (ADEM). Considering the success of the previous treatment, she was treated with IVIG (400 mg/kg/day) for 5 consecutive days from the 2nd hospital day. The treatment was also successful this time, and her consciousness became clear and her weakness improved to MRC grade 4/5 by the 10th hospital day. She became able to use a spoon and chopsticks, and could walk with a cane by the 20th hospital day. A follow-up NCS was performed on the 15th hospital day. A motor NCS showed improvement of the CMAP amplitudes in all nerves, and F waves were induced. On the other hand, the motor conduction velocities of the right median and ulnar nerves were mildly decreased. A sensory NCS showed decreased SNAP amplitudes of the right median and ulnar nerves. These results were consistent with the criteria for acute inflammatory demyelinating polyneuropathy (AIDP) proposed by Ho et al. (9). A follow-up CSF study on the 22nd hospital day showed a decreased cell count (15/μL), while the protein level was not reduced (73 mg/dL). She was transferred to the previous hospital on the 37th hospital day. At the time, she was able to walk without assistance.
Discussion
We described the case of an elderly woman with recurrent GBS. Recurrent GBS has been reported to be characterized by development at a younger age (<30 years of age), relatively mild symptoms, and presentation as Miller Fisher syndrome (10). The present case, however, did not seem to be consistent with these characteristics. On the other hand, it has also been suggested that patients with recurrent GBS show stereotypic manifestations with each episode (10, 11). In this regard, there were several characteristic similarities between the two episodes of the present case: prominent weakness in the upper limbs, CSF pleocytosis, and a good response to IVIG. Furthermore, Notturno et al. reported that patients with recurrent GBS are likely to develop demyelinating polyneuropathy (12), and the patient in the present case also showed a demyelinating pattern on the NCSs that were performed in each episode. As for the preceding events, the patient developed respiratory infections caused by undetermined agents in both episodes. On the other hand, several reports have described stereotypic manifestations in recurrent GBS with different preceding events (10, 11). Based on these, Kuitwaard et al. suggested that intrinsic host factors rather than the preceding agents might play a more important role in recurrent GBS (10).
In the literature, several types of anti-glycolipid antibodies have been detected in recurrent GBS patients: GM1 IgM/IgG, GM1b IgG, GM2 IgM/IgG, GD1a IgG, GD1b IgG, GalNac-GD1a IgM/IgG, GT1a IgG, and GQ1b IgG (11-15). In contrast, anti-Gal-C IgG antibodies were detected in our patient's serum on the second episode. To the best of our knowledge, this is the first case report describing recurrent GBS in a patient with this antibody. Samukawa et al. reported the clinical manifestations of anti-Gal-C antibody-seropositive GBS: demyelinating polyneuropathy occurred four times more frequently than axonal polyneuropathy, and sensory disturbance and autonomic failure were frequently observed (7). Actually, the second episode of the present case was consistent with Ho's criteria for AIDP, and sensory disturbance, dysuria, and abnormal hypertension were observed. Thus, the antibody was likely to have played an important role in the pathogenesis of the present case.
Gal-C is distributed not only in the PNS but also in the CNS (6). In the second episode of the present case, the patient experienced fever, headache, mild disturbance of consciousness, meningeal signs, and CSF pleocytosis, indicating meningoencephalitis. On head MRI, a white matter lesion was detected in the left corona radiata. Although follow-up MRI was not performed, this likely represented CNS demyelination. Based on this finding, the second episode was diagnosed as a complication of GBS and ADEM. An association between the two conditions has already been reported, and similar conditions have been described as “encephalomyeloradiculoneuropathy” or “acute severe combined demyelination” (2-4). In these cases, it was hypothesized that the autoantibody for the specific epitope shared by the PNS and CNS was the causative agent (2). Thus, the anti-Gal-C antibody might have been related to ADEM in the present case. In support of this, several clinical studies have reported that anti-Gal-C antibodies were sometimes detected in the serum of patients with immune-mediated encephalitis or ADEM (8, 16). Samukawa et al. reported that 4 of 25 ADEM patients were positive for anti-Gal-C antibodies, and that 3 of the 4 positive patients showed tetraparesis and motor-dominant axonal neuropathy (16). While these patients were resistant to steroid therapy, IVIG was successful. There seemed to be similarities between these reported cases and the present case. Anti-Gal-C antibodies may play an important role in the involvement of both the PNS and the CNS.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors would like to thank Prof. Susumu Kusunoki (Department of Neurology, Kinki University Medical School) for conducting the analysis of the anti-glycolipid antibodies.
References
- 1. Winer JB. Guillain-Barré syndrome. BMJ 337: 227-231, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Bernard G, Riou E, Rosenblatt B, et al. Simultaneous Guillain-Barré syndrome and acute disseminated encephalomyelitis in the pediatric population. J Child Neurol 23: 752-757, 2008. [DOI] [PubMed] [Google Scholar]
- 3. Blennow G, Gamstrop I, Rosenberg R. Encephalo-myelo-radiculo-neuropathy. Dev Med Child Neurol 10: 485-490, 1968. [DOI] [PubMed] [Google Scholar]
- 4. Amit R, Glick B, Itzchak Y, et al. Acute severe combined demyelination. Childs Nerv Syst 8: 354-356, 1992. [DOI] [PubMed] [Google Scholar]
- 5. Saida T, Saida K, Dorfman SH, et al. Experimental allergic neuritis induced by sensitization with galactocerebroside. Science 204: 1103-1106, 1979. [DOI] [PubMed] [Google Scholar]
- 6. Griot-Wenk M, Griot C, Pfister H, et al. Antibody-dependent cellular cytotoxicity in antimyelin antibody-induced oligodendrocyte damgage in vitro. J Neuroimmunol 33: 145-155, 1991. [DOI] [PubMed] [Google Scholar]
- 7. Samukawa M, Hamada Y, Kuwahara M, Japanese GBS Study Group, et al. Clinical features in Guillain-Barré syndrome with anti-Gal-C antibody. J Neurol Sci 337: 55-60, 2014. [DOI] [PubMed] [Google Scholar]
- 8. Hanzawa F, Fuchigami T, Ishii W, et al. A 3-year-old boy with Guillain-Barré syndrome and encephalitis associated with Mycoplasma pneumoniae infection. J Infect Chemother 20: 134-138, 2014. [DOI] [PubMed] [Google Scholar]
- 9. Ho TW, Mishu B, Li CY, et al. Guillain-Barré syndrome in northern China. Relationship to Campylobacter jejuni infection and anti-glycolipid antibodies. Brain 118: 597-605, 1995. [DOI] [PubMed] [Google Scholar]
- 10. Kuitwaard K, van Koningsveld R, Ruts L, Jacobs BC, van Doorn PA. Recurrent Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry 80: 56-59, 2009. [DOI] [PubMed] [Google Scholar]
- 11. Pyun SY, Jeong JH, Bae JS. Recurrent Guillin-Barré syndrome presenting stereotypic manifestations, positive antiganglioside antibodies, and raid recovery. Clin Neurol Neruosurg 139: 230-233, 2015. [DOI] [PubMed] [Google Scholar]
- 12. Notturno F, Kokubun N, Sekiguki Y, et al. Demyelinating Guillain-Barré syndrome recurs more frequently than axonal subtypes. J Neurol Sci 365: 132-136, 2015. [DOI] [PubMed] [Google Scholar]
- 13. Ishii J, Yuki N, Kawamoto M, Yoshimura H, Kusunoki S, Kohara N. Recurrent Guillain-Barré syndrome, Miller Fisher syndrome and Bickerstaff brainstem encephalitis. J Neurol Sci 364: 59-64, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Togawa J, Nakaoku Y, Hagiwara M, et al. Similarities of serum anti-ganglioside antibodies in first and third episodes of recurrent Guillain-Barré syndrome: case report. J Neurol 262: 1360-1362, 2015. [DOI] [PubMed] [Google Scholar]
- 15. Tagawa Y, Yuki N, Hirata K. Antiganglioside antibodies in various episodes in a patient with recurrent Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry 65: 952, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Samukawa M, Hirano M, Tsugawa J, et al. Refractory acute disseminated encephalomyelitis with anti-galactocerebroside antibody. Neurosci Res 74: 284-289, 2012. [DOI] [PubMed] [Google Scholar]