Abstract
The personality dimensions of neuroticism and extraversion are strongly associated with emotional experience and affective disorders. Previous studies reported functional magnetic resonance imaging (fMRI) activity correlates of these traits, but no study has used brain-based measures to predict them. Here, using a fully cross-validated approach, we predict novel individuals’ neuroticism and extraversion from functional connectivity (FC) data observed as they simply rested during fMRI scanning. We applied a data-driven technique, connectome-based predictive modeling (CPM), to resting-state FC data and neuroticism and extraversion scores (self-reported NEO Five Factor Inventory) from 114 participants of the Nathan Kline Institute Rockland sample. After dividing the whole brain into 268 nodes using a predefined functional atlas, we defined each individual’s FC matrix as the set of correlations between the activity timecourses of every pair of nodes. CPM identified networks consisting of functional connections correlated with neuroticism and extraversion scores, and used strength in these networks to predict a left-out individual’s scores. CPM predicted neuroticism and extraversion in novel individuals, demonstrating that patterns in resting-state FC reveal trait-level measures of personality. CPM also revealed predictive networks that exhibit some anatomical patterns consistent with past studies and potential new brain areas of interest in personality.
Keywords: neuroticism, extraversion, functional connectivity, predictive models, resting-state fMRI
Introduction
Attempts to predict individuals’ personality traits have permeated through the ages, from Babylonian horoscopic astrology in second millennium B.C. (Holden, 2013) to modern questionnaires such as the Myers-Briggs personality scale (Carlyn, 1977). Although machines that read out personality traits from brain activity have so far only existed in science fiction, such as the novel Divergent, functional magnetic resonance imaging (fMRI) may begin to make this a reality. This study demonstrates that two Big Five personality traits (Eysenck, 1967; Costa and McCrae, 1990), neuroticism and extraversion, can be predicted from functional brain connectivity (FC)—the degree to which fMRI activity in anatomically distinct brain regions are related through time. Specifically, FC is based on synchronous fluctuations in blood oxygenation level-dependent (BOLD) signals, which are thought to reflect engagement of these brain areas in similar or common processes. FC can be calculated from fMRI data obtained while the subject is at rest or performing a task; this study specifically generates predictive models of neuroticism and extraversion based on resting-state FC.
Neuroticism is generally associated with negative affect (Larsen and Ketelaar, 1989; Robinson et al., 2007) and tendency to worry and be anxious (Canli et al., 2001), whereas extraversion is characterized as sensitivity to positive and pleasurable social and environmental cues and generally associated with positive affect (Larsen and Ketelaar, 1989; Watson and Clark, 1997). Of the five personality dimensions, neuroticism and extraversion have garnered special interest because of their connection to emotional experience and development of affective disorders. These two traits have been found to be the most predictive of happiness (Hayes and Joseph, 2003) and anxiety and depressive disorders (Kotov et al., 2010). Furthermore, in a study involving pharmacological treatment of unipolar depression, neuroticism was most strongly associated with depressed mood out of the Big Five traits, whereas extraversion was most predictive of treatment outcome (Bagby et al., 1995).
Early studies on neural correlates of neuroticism and extraversion identified localized brain regions whose reactivity to emotional stimuli was correlated with these traits. The amygdala was especially ubiquitous; neuroticism was correlated with amygdala activation in response to emotional conflict and negative stimuli, and extraversion to happy expressions (Canli et al., 2001, 2002; Haas et al., 2007). Work identifying relationships between these traits and functional brain connectivity emerged more recently; while participants viewed angry and fearful faces, connectivity between amygdala and dorsomedial prefrontal cortex was positively correlated with neuroticism, whereas connectivity between amygdala and anterior cingulate cortex (ACC) was negatively correlated with neuroticism, suggesting self-referential processing of negative emotions and lowered ACC control over amygdala in highly neurotic individuals (Cremers et al., 2010).
In addition to neural correlates identified from task-based FC, recent studies have examined resting-state FC patterns associated with neuroticism and extraversion, but mostly in relation to specific regions. Neuroticism and extraversion seem to be associated with resting-state connectivity of regions consistent with their psychological qualities—neuroticism with areas associated with fear and self-evaluation, and extraversion with areas associated with social tendencies, reward-seeking and motivation (Adelstein et al., 2011). Individuals high on extraversion exhibited increased amygdala connectivity with temporal pole and insula, suggesting adaptive socioemotional functioning (Aghajani et al., 2013). Conversely, higher neuroticism was associated with decreased amygdala connectivity with these areas (Aghajani et al., 2013), suggesting impairments in awareness and recognition of socioemotional cues in highly neurotic individuals, as found in previous literature (McCrae and Costa, 1991). Decreased amygdala and insula connectivity was also associated with anxiety and depression (Etkin et al., 2009). Furthermore, neuroticism was associated with increased influence of amygdala on areas associated with cognitive regulation, such as the middle frontal gyrus, suggesting modulation on cognitive regulation in neurotic individuals (Pang et al., 2016). In addition to FC of specific areas, highly neurotic individuals also exhibited weaker whole-brain FC, with connections that more resemble those of random networks and functional subnetworks that were more difficult to cleanly delineate (Servaas et al., 2015).
Activity and FC patterns have been studied and identified with regards to known levels of neuroticism and extraversion; however, this study shows that we can use resting-state FC to predict the level of these traits in novel individuals based on a whole-brain, data-driven, cross-validated approach. A new technique, connectome-based predictive modeling (CPM) (Shen et al., 2017), has been shown to predict fluid intelligence (Finn et al., 2015), attention (Rosenberg et al., 2016a,b, 2017, 2018; Yoo et al., 2018), and reading comprehension (Jangraw et al., 2018) using whole-brain FC. CPM examines functional connections between every possible pair of pre-defined nodes in the entire brain (including cortex, subcortex and cerebellum), identifies networks consisting of the connections most correlated with observed trait scores, and uses these networks to generate models that predict scores. Rather than constraining to specific areas of interest, CPM considers nodes across the whole brain and treats each connection independently and equally, assessing the correlation of each connection with observed scores across subjects. This then gives this method the power to elucidate potential new brain areas that might be related to the constructs of interest and worthy of further study.
In this study, we obtained resting-state imaging and psychological assessment data from 114 individuals in the NKI-Rockland Sample. Neuroticism and extraversion scores were measured from the NEO-Five Factor Inventory, a self-reported questionnaire that assesses the individual’s level of each Big Five trait. To apply CPM, we first divided the brain into 244 nodes using a pre-defined functional atlas. We then generated 244-by-244 connectivity matrices, which consisted of the functional time-course correlation (‘edge’) between every pair of nodes. Using leave-one-out cross validation, CPM generated predictive networks consisted of edges most correlated with neuroticism and extraversion scores and used these networks on the left-out individual to predict his/her personality scores. We then correlated the observed and predicted scores of all individuals to assess the predictive power of these networks. Using CPM, we show that resting-state whole-brain FC is not only related to neuroticism and extraversion levels, but can predict these levels in novel individuals.
Materials and methods
Participants
We used the Nathan Kline Institute Rockland Sample (NKI-RS), an ongoing study that aims to create and openly share a large-scale community sample including physiological, psychological assessment and neuroimaging data (Nooner et al., 2012). Written consent was obtained from all participants according to the Institutional Review Boards of NKI and Montclair State University. We obtained 162 individuals with matched imaging and psychological assessment data. After excluding individuals due to excessive motion and poor registrations of anatomical to functional scans, 114 individuals [age 18–85 (mean = 38), 68 females] remained. Three individuals had a diagnosis of attention deficit hyperactivity disorder (ADHD), Tourette’s syndrome or a past eating disorder.
Neuroticism and extraversion assessment
As part of the Rockland Sample, individuals completed the NEO Five Factor Inventory, a 60-item psychological personality inventory that assesses the five personality domains: openness to experience, conscientiousness, extraversion, agreeableness and neuroticism (McCrae and Costa, 2010). It is a shortened version of the NEO Personality Inventory, intended to measure variability in individuals without psychopathology, and has been shown to be reliable and consistent (McCrae and Costa, 2004). Each item is a short description that assesses the traits and tendencies associated with the five domains (e.g. ‘I laugh easily’; ‘At times I have felt bitter and resentful’), and participants were asked to rate their opinion to each item on a Likert scale of 0–4 (0—strongly agree; 2—neutral; 4—strongly disagree). Each individual was scored on each of the five domains, with higher scores representing higher levels. Since we were most interested in neuroticism and extraversion because of their associations with emotional experience and affective disorders, we only looked at scores for these two domains. Both raw total scores and T-scores normalized for the individuals’ age range were available for each domain, but we used raw scores for analysis since raw and T-scores were highly correlated (neuroticism r = 0.98, extraversion r = 0.99 for the 114 individuals). Both neuroticism and extraversion scores were normally distributed (neuroticism: mean = 19.11, SD = 8.05, range 2–40; extraversion: mean = 29.90, SD = 6.18, range 11–44).
Imaging protocols and preprocessing
We obtained the high-resolution T1-weighted anatomical magnetization prepared rapid gradient echo (MPRAGE) scan and two sets of 10-min resting-state functional scans at different temporal resolutions from NKI-RS. Detailed scanning parameters are detailed on the NKI-RS site (http://fcon_1000.projects.nitrc.org/indi/enhanced/mri_protocol.html). In brief, the data were collected on Siemens Magnetom TrioTim syngo MR B17 with the following parameters: MPRAGE repetition time (TR) = 2900 ms, echo time (TE) = 2.52 ms, slice thickness =1 mm; low temporal resolution functional: TR = 1400 ms, TE = 30 ms, slice thickness = 2.0 mm; high temporal resolution functional: TR = 645 ms, TE = 30 ms, slice thickness = 3.0 mm.
The majority of fMRI data processing and analysis was performed using Bioimage Suite (Joshi et al., 2011) and custom Matlab scripts. Motion correction was performed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Linear and quadratic shift, mean signal from cerebrospinal fluid, global signal and a 24-parameter motion model including six motion parameters and temporal derivatives along with their squares were regressed from the data. Images were temporally smoothed with a zero mean unit variance Gaussian filter and iteratively smoothed to a smoothness of 4 mm (Friedman et al., 2006; Scheinost et al., 2014). Regression of the 24-parameter motion model and iterative smoothing have been shown to minimize motion confounds associated with FC (Satterthwaite et al., 2013; Yan et al., 2013; Scheinost et al., 2014).
Construction of predictive networks
To test the ability of resting-state FC to predict a novel individual’s personality scores, we applied CPM (Finn et al., 2015; Rosenberg et al., 2016a; Shen et al., 2017) to the resting-state fMRI data and neuroticism and extraversion scores (obtained from the self-reported NEO Five Factor Inventory) of 114 subjects from the NKI-RS. We obtained both sets of functional scans (TR = 645 and TR = 1400 ms).
We computed FC matrices from each set of scans for each individual. To do so, we parcellated the brain into 268 nodes using a predefined functional atlas that maximized the similarity of voxel-wise time courses within each node (Shen et al., 2013) and included the whole brain (cortex, subcortex, cerebellum and brain stem). The 268-node atlas was warped from MNI space into single-subject space via concatenation of a linear registration computed using FSL (Jenkinson et al., 2002) and a non-linear registration (Scheinost et al., 2017) between the functional images, MPRAGE scans, and the MNI brain. All transformation pairs were calculated independently, combined into a single transform, and inverted, warping the functional atlas into single participant space. This single transformation reduces interpolation error because the functional atlas is warped to an individual with only one transformation.
However, because some scans did not include full coverage, we excluded nodes that were completely missing (0% of voxels comprising the node were present) in at least one individual. Twenty-four nodes and their associated edges were excluded from analysis. Nodes were excluded from the prefrontal, parietal, temporal, limbic and brainstem macroscale regions, with the most exclusion proportion-wise from the motor (7 out of 21 nodes) and brainstem (2 out of 9 nodes) regions (see Supplementary Material Section S1 for number of nodes excluded per region). All subsequent analyses were based on the remaining 244 nodes.
The time course was calculated for each node by averaging the BOLD signal time courses of each voxel within the node. The 244-by-244 connectivity matrices, then, consisted of the Fisher-normalized Pairwise Pearson correlation (‘edge’) of every node with every other node. Because of the relatively short run-time (10 min per run) and the high similarity between the connectivity matrices obtained from the two scans (reported in the ‘Combining resting-states scans’ section), we used the average of the two connectivity matrices in CPM. Each edge in the final connectivity matrix for each individual used in analysis was the mean of that edge in the individual’s TR645 and TR1400 matrices.
To obtain networks used in prediction, we computed the Pearson correlation between every edge in the connectivity matrices and observed scores across individuals, obtaining an r-value for each edge with an associated P-value. As in prior studies, with a P-value threshold of 0.01, edges that were positively correlated with observed scores made up the positive prediction network, and edges that were negatively correlated with observed scores made up the negative prediction network. For each subject, a single summary statistic, ‘network strength’, was calculated for each network by summing the edges in the network (Finn et al., 2015, Rosenberg et al., 2016a).
Predicted scores were generated in a leave-one-out basis. For each set of n−1 individuals, positive and negative networks were generated and their strengths were calculated. For neuroticism, positive network ranged from 388 to 524 edges, and negative network ranged from 294 to 449 edges; for extraversion, positive network ranged from 235 to 312 edges, and negative network ranged from 276 to 353 edges. Linear models were constructed to relate positive and negative network strengths to observed scores. The models were then applied to the novel individual’s positive and negative network strengths to generate predicted scores for the individual. A GLM was also constructed to combine the positive and negative network strengths to generate predicted scores.
Combining resting-state scans
Each individual had two connectivity matrices, one calculated from the TR = 645 ms (TR645) scans and the other from the TR = 1400 ms (TR1400) scans. Using FC fingerprinting (Finn et al., 2015), we found that the TR645 and TR1400 matrices were very similar. FC fingerprinting allows us to identify the matrix from a set of matrices that is most similar to another matrix of interest. To summarize, we assessed the similarity of each individual’s connectivity matrix from one set of scans to every individual’s connectivity matrices from the other set of scans, where similarity was defined as the Pearson correlation of the vectorized forms of the two matrices. Then, for every matrix in each set of scans (target matrix), we found the most similar matrix from the other set of scans (the matrix with the highest correlation coefficient with the target matrix). We counted as success if the target matrix and the most similar matrix were from the same individual—i.e. we successfully identified the individual’s matrix from the other set of scans based on the individual’s target matrix (Finn et al., 2015). Success rate identifying individuals from the TR1400 set using targets from the TR645 set was 91%, and success rate identifying individuals in the other direction was 96%. Furthermore, pairs of matrices from the same individual had an average correlation coefficient of 0.64, whereas pairs of matrices from different individuals had an average correlation coefficient of only 0.38.
Registration and motion controls
Registrations of anatomical to functional scans were visually inspected using Bioimage Suite, and individuals with poor registrations were excluded from further analyses. Individuals with excessive head motion, defined a priori as >2 mm translation, >3° rotation or >0.15 mm mean frame-to-frame displacement during the run, were also excluded from analysis (Rosenberg et al., 2016b). A total of 162 individuals had imaging data with matching psychological assessment data. Out of the 162 individuals, 9 were excluded due to excessive head motion in the high TR scans, another 11 due to excessive head motion in the low TR scans, another 26 due to poor registrations in either or both runs, and a final two subjects were excluded due to anomalous distribution of edges in their final connectivity matrices. Data exclusion was performed prior to all analyses. After exclusions, 114 individuals were left with good registrations and acceptable levels of head motion for both runs for further analyses.
To further control for potential motion confounds, we also ran partial Pearson correlation between observed scores with predicted scores while controlling for head motion (defined as the average of the mean frame-to-frame motion of both runs).
Permutation testing
To assess the significance of the r-values between observed scores and predicted scores generated from leave-one-out cross validation, we conducted permutation testing by running the leave-one-out pipeline 1000 times, each time using randomly shuffled observed scores to generate predictive networks and predicted scores. The 1000 Pearson correlations between observed and predicted scores composed null distributions of r-values against which we assessed the r-values presented in ‘Results’ section. P-values were calculated as 1 + (the number of permutations that generated r-values equal to or larger than our presented r-values)/1001.
We used this permutation method to calculate the P-values for all r-values between observed and predicted scores, i.e. for our main prediction results and all results from our age-, motion- and gender-control.
Age-control methods
Our data included participants in a wide age range (18–85 years); thus, to control for age effects in prediction, we applied three age-control methods in addition to our main, non-age-controlled results.
Instead of running Pearson correlation at the final correlation between predicted and observed scores, we ran partial Pearson correlation of predicted and observed scores while controlling for age (Table 1a in ‘Results’ section).
We excluded any edge that was significantly correlated with age (P < 0.01) from predictive networks. That is, we first generated predictive networks by selecting edges that were correlated with trait scores without regards to age (as per our method from main results), but before building a predictive model, we removed any edge significantly correlated with age from these networks. This conservative method might also filter out edges that covary with trait scores, potentially decreasing predictive power; however, we found that this method still produced significant predictions for both neuroticism and extraversion (Table 1b).
Last, we generated predictive networks that uniquely predicted personality scores when controlling for age. Instead of filtering out age-correlated edges from our original predictive networks as in method (ii), we used new predictive networks composed of edges whose partial Pearson correlation with personality scores while controlling for age passed the P < 0.01 threshold. This correction should, ideally, reveal edges that predict personality rather than age (Table 1c).
Table 1.
The results of different methods for correcting for age
| Neuroticism | Extraversion | |
|---|---|---|
| Main results | r = 0.22, P = 0.033 | r = 0.22, P = 0.045 |
| a. controlling for age at final correlation | r = 0.11, P = 0.21 | r = 0.21, P = 0.042 |
| b. filtering out age correlates at edge selection | r = 0.22, P = 0.003 | r = 0.23, P = 0.008 |
| c. controlling for age at edge selection | r = 0.19, P = 0.063 | r = 0.23, P = 0.033 |
Predictions were assessed for three age-control methods and compared with our main, non-age-controlled results. We first controlled for age at the final correlation between predicted and observed scores (a). Then, we introduced age control in the predictive networks by excluding from prediction edges that were significantly correlated with age (b). Finally, we generated predictive networks that uniquely predicted trait scores by using partial correlation to control for age at the edge selection step (c). GLM predictions are presented for simplicity. P values were determined with permutation testing.
Gender-control methods
Potential gender differences in FC could confound our analyses. We controlled for gender in our predictions by a method similar to age-control method (ii); i.e. we removed from our predictive networks any edge that significantly differed between males and females with a two-tailed two-sample t-test (P < 0.05).
Results
Neuroticism and extraversion prediction
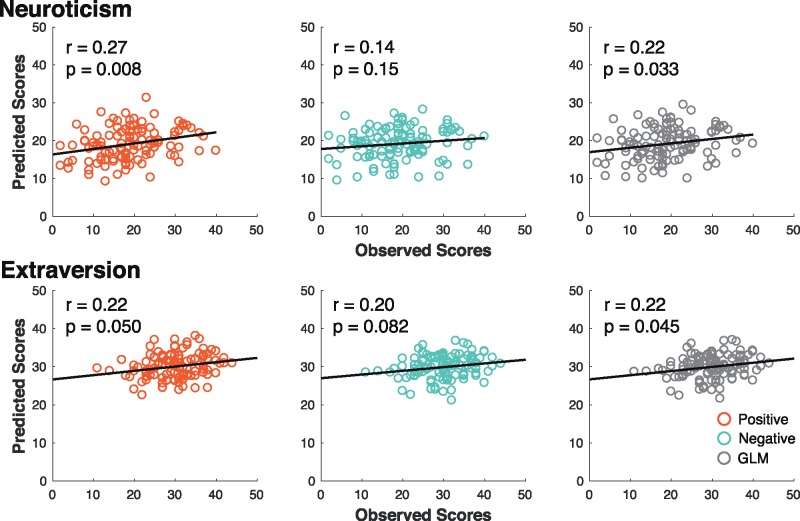
Correlating predicted and observed scores (Pearson), we found that CPM significantly predicted neuroticism [positive network r = 0.27, P = 0.008; negative network r = 0.14, P = 0.15; general linear model (GLM) r = 0.22, P = 0.033] and extraversion (positive network r = 0.22, P = 0.050; negative network r = 0.20, P = 0.082; GLM r = 0.22, P = 0.045) in novel individuals (Figure 1). The P-values for these results and all subsequent cross-validation results were calculated through permutation testing (detailed method described in ‘Materials and methods’ section). For neuroticism, the common positive network (consisted of edges included in positive prediction in every iteration of leave-one-out) contained 206 edges (0.69% of the 29 646 total possible edges), and the common negative network contained 128 edges (0.43% of all possible edges). For extraversion, the common positive network contained 101 edges and the common negative network contained 106 edges (∼0.35% of all possible edges).
Fig. 1.
CPM predicts neuroticism and extraversion scores in novel individuals. Scatterplot of predicted scores vs observed scores for neuroticism and extraversion. Predicted scores were generated using edges positively correlated with prediction (positive network) and negatively correlated with prediction (negative network). A GLM was also constructed to combine positive and negative networks to generate predicted scores. P values were determined with permutation testing.
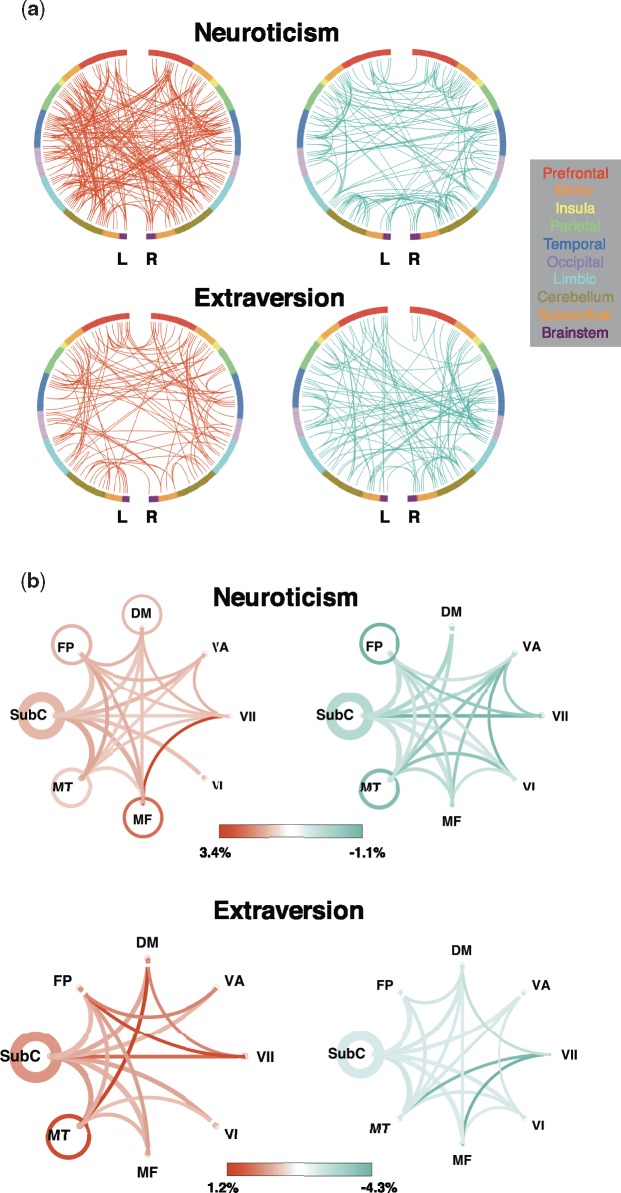
CPM also reveals the functional anatomy of predictive networks. To this end, we divided the 244 nodes into ten macroscale regions per hemisphere and applied the parcellation to the common positive and negative networks to look at the number of connections between regions involved in prediction (Figure 2a). To further investigate the role of functional sub-networks, we used a parcellation scheme that divides the 244 nodes into eight canonical networks: subcortical-cerebellum (SubC), motor, medial frontal (MF), visual I (VI), visual II (VII), visual association, default mode (DM) and frontoparietal (FP) (Finn et al., 2015). We applied the parcellation to the common positive and negative networks and looked at the proportion of edges in each network pair that was utilized in prediction (Figure 2b).
Fig. 2.
Canonical network pairs in positive and negative prediction. (a) Edges between macroscale regions. Each semicircle represents a hemisphere of the brain, and nodes are organized around the circle by anatomical location. Edges are represented by lines; red edges are stronger in individuals with higher scores, and blue edges are stronger in individuals with lower scores. (b) Proportion of total edges of each canonical functional network pair utilized in prediction, calculated by dividing the number of edges in each network pair included in prediction by the total number of possible edges between that pair of networks. The higher the proportion, the more ‘utilized’ the network pair is in prediction. Orange represents higher utilization in positive prediction and green represents higher utilization in negative prediction. Line saturation indicates the proportion of edges of the particular network pair involved in prediction: the darker the line, the higher proportion. Line width indicates the total number of edges possible in the pair: the thicker the line, the more possible edges. Curves show between-network connections and circles show within-network connections.
Positive and negative predictive networks involve areas across the whole brain. However, not all anatomical regions or subnetworks were involved equally, and some trends emerge: e.g. the motor region, motor functional network and different sets of network connections involving the VII functional network were highly involved in all four predictions; the cerebellum was highly involved in prediction, especially negative prediction of neuroticism. The significance of the involvement of these networks is further discussed in ‘Discussion’ section.
Age control
Our data included participants in a wide age range (18–85 years), and neuroticism scores were significantly negatively correlated with age (r = −0.27, P = 0.0032), while extraversion scores were not correlated with age (r = 0.071, P = 0.45), as expected based on previous work (Srivastava et al., 2003). Since FC has been shown to change with age (Hampson et al., 2012), we wanted to control for the possibility of CPM predictions being driven by age rather than personality traits, especially neuroticism. To control for age effects, we applied three age-control methods, as described in detail in ‘Materials and methods’ section, in addition to our main, non-age-controlled results. The results for each are presented in Table 1.
All predictions remained significant (most with P < 0.05, one with P = 0.063), except for neuroticism prediction when age-control method (i) was applied. This loss in predictive power when age was controlled in the final correlation could be explained by the possibility that predictions were driven by age when age was not controlled in generating predictive networks. However, attempts to generate predictive networks that more uniquely predict trait scores instead of age still yielded significant predictions (methods (ii) and (iii)), suggesting brain networks still predict neuroticism and extraversion levels even after controlling for age.
It is important to note that though controlling for age is important for predicting personality trait levels beyond age, our goal was to build a model and identify brain networks that could predict neuroticism and extraversion levels. Age seems to be an inherent factor in neuroticism level and thus could be treated as an aid in prediction, giving merit to our non-age-controlled main method despite the drop in predictive power after factoring in age.
Motion control
Head motion, defined as the average of the mean frame-to-frame motion of both runs, was negatively correlated with observed neuroticism scores (r = −0.18, P = 0.050) and positively correlated with observed extraversion scores (r = 0.13, P = 0.17). Predicted neuroticism scores were also negatively correlated with head motion (GLM r = −0.26, P = 0.0054). Predicted extraversion scores, however, did not correlate with head motion (GLM r = 0.093, P = 0.33).
To further control for effects of motion, we ran partial Pearson correlation between observed and predicted scores while controlling for head motion. We found that prediction achieved trend-level significance for neuroticism (GLM r = 0.18, P = 0.075) and significance for extraversion (GLM r = 0.21, P = 0.048) after controlling for motion.
Gender control
Previous studies have found that resting-state FC differs between genders. For example, resting-state FC of amygdala sub-regions with various brain regions differs between adolescent boys and girls during normal development (Alarcón et al., 2015). Gender differences in resting-state FC were also found in various disease states such as multiple sclerosis (Koenig et al., 2013) and PTSD (Helpman et al., 2017). Although our sample revealed no statistically significant gender difference in neuroticism level (mean male = 18.48; mean female = 19.54; two-tailed two-sample t-test: P = 0.49) or extraversion level (mean male = 28.93; mean female = 30.56; two-tailed two-sample t-test: P = 0.17), we nonetheless wanted to minimize potential gender confounds in prediction. We did so by excluding from our predictive networks all edges that significantly differed between males and females with a two-tailed two-sample t-test (P < 0.05). We found that predictions were still significant for neuroticism (GLM r = 0.25, P = 0.004) and extraversion (GLM r = 0.18, P = 0.014).
Discussion
This study shows that resting-state FC predicts neuroticism and extraversion in novel individuals using CPM. Previous studies have identified neural correlates of neuroticism and extraversion using task-based or resting-state functional imaging. However, to our knowledge, this is the first study to predict a novel individual’s neuroticism and extraversion scores from an fMRI scan. Importantly, significant predictions were obtained using imaging data collected when individuals were not engaged in any explicit task. Furthermore, on a broader scale, this study extends the utility of CPM from predicting cognitive abilities such as fluid intelligence and attention (Finn et al., 2015; Rosenberg et al., 2016a,b) to predicting personality traits.
Past studies suggested that neural reactivity might provide a more direct and objective measure of personality and prediction of behavioral outcomes than self-reported measures. For example, a functional imaging study found that dorsal ACC activity explained nearly five times the variance of a behavioral outcome associated with neuroticism than self-reported neuroticism scores did (Eisenberger et al., 2005). These results raise the possibility that functional predictive networks might be less influenced by subjective factors and capture more individual variability than self-report measures do, potentially implicating this method as a more objective identification of neuroticism and extraversion levels in individuals. Furthermore, it is worth emphasizing that this study predicted neuroticism and extraversion scores using resting-state instead of task-based fMRI data. Resting-state fMRI could alleviate burdens associated with performing tasks in the scanner, thus allowing prediction on individuals who might have difficulty doing so.
Personality network characteristics
Some brain regions and networks involved in predicting neuroticism and extraversion levels correspond to brain areas associated with these traits in past studies. Previous literature on the FC correlates of these traits mostly concentrated on the amygdala, such as increased amygdala connectivity with middle frontal gyrus (Pang et al., 2016) and decreased amygdala connectivity with temporal pole and insula (Aghajani et al., 2013) in high neurotic individuals. Although the relatively large size of nodes used in this study (average size 4.8 cm3) prevents us from inferring specific involvement of the amygdala (average size 1.24 cm3; Brabec et al., 2010), we did see trends that match these previous results—a connection between temporal region and the node that contains left amygdala negatively predicted neuroticism; on a broader scale, more limbic-prefrontal cortex edges were included in positive than negative prediction of neuroticism (16 vs 1 edges), and more limbic-insula edges were involved in negative than positive prediction of neuroticism (6 vs 0).
Additionally, because CPM identifies predictive network edges in a data-driven manner, not limited to a priori regions of interest, it can reveal potential new areas of interest related to neuroticism and extraversion. Besides the amygdala, a previous seed-based study found that resting-state FC in primary motor and sensory regions (such as the occipital cortex) correlated with personality traits (Adelstein et al., 2011). We also found that motor region connections with limbic and temporal regions positively predicted extraversion. Motor functional network connections with the DM and SubC networks were highly utilized in positive and negative prediction of extraversion, respectively. However, it remains unclear how to interpret the associations of the motor network with personality traits (Adelstein et al., 2011). Similarly, connections involving the VII network were highly utilized in prediction of both traits—e.g. VII-MF connections were positively predictive of neuroticism, while VII-SubC and FP connections were positively predictive of extraversion. Little research has been done on associations between visual brain networks and personality, but some studies have shown that individuals high on extraversion and neuroticism exhibit differences in visual attention and fixation (Kaspar and Konig, 2012). Thus, VII network might also be worthwhile to investigate in future studies.
Furthermore, the cerebellum was particularly important for prediction, especially parietal-cerebellar connections and intra-cerebellar connections in positive and negative prediction of neuroticism, respectively. Little work has addressed the relationship between the cerebellum and personality traits, but past studies have observed personality changes following cerebellar lesions (Mariën et al., 2009; Stoodley and Schmahmann, 2010), and found that cerebellar volume is associated with higher neuroticism and other personality traits in healthy individuals (Schutter et al., 2012; Picerni et al., 2013). Similarly, the cerebellum also played a prominent role in predicting sustained attention using CPM (Rosenberg et al., 2016a,b), which provided further evidence of involvement of brain areas other than executive control areas in ADHD. Therefore, it could be worthwhile to investigate the role intra-cerebellar and cerebellar-cortical FC in personality traits.
Limitations, implications and future directions
Because each individual node in our study involved a large group of voxels, the role of specific structures—e.g. the amygdala—is difficult to isolate with precision. A node might include many other voxels besides the structure of interest, and a structure might cross multiple nodes; thus, comparisons to FC of small, specific regions in the brain would be difficult to draw.
Our exclusion of 10% of the nodes from analyses due to signal dropout or a lack of whole-brain coverage could entail losses of crucial information both in prediction and identifying regions and networks involved in neuroticism and extraversion, so future studies including these missing nodes could be valuable.
Finally, though we did control for potential major confounds such as age and motion, other constructs might interact with our predictions as well. For one, since mood states and personality traits could interact, mood might be a confound in our predictions. Mood states were not collected at the time of scan; however, our measure of personality, the NEO Five-Factor Inventory, has high test-retest reliability and thus is unlikely to reflect transient mood states (McCrae and Costa, 2004). Second, since neuroticism and extraversion were correlated, one trait might influence predictions of the other; however, controlling for one trait while predicting the other did not change our central results (see Supplementary Material Section S2 for prediction results).
Summary
In sum, connectome-based predicting modeling based on resting-state data is able to predict the level of neuroticism and extraversion in novel individuals. Although we only assessed predictions of neuroticism and extraversion using planned comparisons based on the strong connections of these traits to emotional experience and affective disorders, CPM is a general method that can be extended to predict other traits and clinical constructs.
Supplementary data
Supplementary data are available at SCAN online.
Funding
This work was supported by National Institutes of Health MH108591 to M.M.C., EB009666 to R.T.C., T32 DA022975 to D.S., and National Science Foundation BCS 1558497 to M.M.C. M.D.R. was supported by a National Science Foundation Graduate Research Fellowship.
Conflict of interest. None declared.
Supplementary Material
References
- Adelstein J.S., Shehzad Z., Mennes M., et al. (2011). Personality is reflected in the brain’s intrinsic functional architecture. PLoS One, 6(11), e27633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajani M., Veer I.M., van Tol M.-J., et al. (2014). Neuroticism and extraversion are associated with amygdala resting-state functional connectivity. Cognitive, Affective, and Behavioral Neuroscience, 14(2), 836–48. [DOI] [PubMed] [Google Scholar]
- Alarcón F., Cservenka A., Rudolph M.D., Fair D.A., Nagel B.J. (2015). Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage, 115, 235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby R.M., Joffe R.T., Parker J.D.A., Kalemba V., Harkness K.L. (1995). Major Depression and the Five-Factor Model of Personality. Journal of Personality Disorders, 9(3), 224–34. [Google Scholar]
- Brabec J., Rulseh A., Hoyt B., et al. (2010). Volumetry of the human amygdala - An anatomical study. Psychiatry Research – Neuroimaging, 182(1), 67–72. [DOI] [PubMed] [Google Scholar]
- Canli T., Sivers H., Whitfield S.L., Gotlib I.H., Gabrieli J.D.E. (2002). Amygdala response to happy faces as a function of extraversion. Science, 296(5576), 2191. [DOI] [PubMed] [Google Scholar]
- Canli T., Zhao Z., Desmond J.E., Kang E., Gross J., Gabrieli J.D. (2001). An fMRI study of personality influences on brain reactivity to emotional stimuli. Behavioral Neuroscience, 115(1), 33–42. [DOI] [PubMed] [Google Scholar]
- Carlyn M. (1977). An assessment of the Myers-Briggs Type Indicator. Journal of Personality Assessment, 41(5), 461–73. [DOI] [PubMed] [Google Scholar]
- Costa P.T., McCrae R.R. (1990). Personality disorders and The Five-Factor model of personality. Journal of Personality Disorders, 4(4), 362–71. [Google Scholar]
- Cremers H.R., Demenescu L.R., Aleman A., et al. (2010). Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. NeuroImage, 49(1), 963–70. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D., Satpute A.B. (2005). Personality from a controlled processing perspective: an fMRI study of neuroticism, extraversion, and self-consciousness. Cognitive, Affective and Behavioral Neuroscience, 5(2), 169–81. [DOI] [PubMed] [Google Scholar]
- Etkin A., Prater K.E., Schatzberg A.F., Menon V., Greicius M.D. (2009). Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of General Psychiatry, 66(12), 1361–72. [DOI] [PubMed] [Google Scholar]
- Eysenck H.J. (1967). The biological basis of personality. American Lecture Series, Publication No 689 A Monograph in the Bannerstone Division of American Lectures in Living Chemistry, xvii, 399. https://doi.org/10.1038/1991031a0.
- Finn E.S., Shen X., Scheinost D., et al. (2015). Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nature Neuroscience, 18(11), 1664–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L., Glover G.H. & The FBIRN Consortium. (2006). Reducing interscanner variability of activation in a multicenter fMRI study: controlling for signal-to-fluctuation-noise-ratio (SFNR) differences. NeuroImage, 33(2), 471–81. [DOI] [PubMed] [Google Scholar]
- Haas B.W., Omura K., Constable R.T., Canli T. (2007). Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behavioral Neuroscience, 121(2), 249–56. [DOI] [PubMed] [Google Scholar]
- Hampson M., Tokoglu F., Shen X., Scheinost D., Papademetris X., Constable R.T. (2012). Intrinsic Brain Connectivity Related to Age in Young and Middle Aged Adults. PLoS One, 7(9), e44067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes N., Joseph S. (2003). Big 5 correlates of three measures of subjective well-being. Personality and Individual Differences, 34(4), 723–7. [Google Scholar]
- Helpman L., Xi Z., Suarez-Jimenez B., Lazarov A., Neria Y. (2017). Sex differences in resting state functional connectivity (rs-FC) of limbic circuits in PTSD. Biological Psychiatry, 81(10), Supplement, S38–9. [Google Scholar]
- Holden J.M. (2013). A History of Horoscopic Astrology. Tempe, AZ: American Federation of Astrologers. [Google Scholar]
- Jangraw D.C., Gonzalez-Castillo J., Handwerker D.A., et al. (2018). A functional connectivity-based neuromarker of sustained attention generalizes to predict recall in a reading task. NeuroImage, 166, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. (2002). Improved methods for the registration and motion correction of brain images. NeuroImage, 17(2), 825–41. [DOI] [PubMed] [Google Scholar]
- Joshi A., Scheinost D., Okuda H., et al. (2011). Unified framework for development, deployment and robust testing of neuroimaging algorithms. Neuroinformatics, 9(1), 69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar K., König P. (2012). Emotions and personality traits as high-level factors in visual attention: a review. Frontiers in Human Neuroscience, 6, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig K.A., Lowe M.J., Lin J., et al. (2013). Sex differences in resting-state functional connectivity in multiple sclerosis. American Journal of Neuroradiology, 34(12), 2304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R., Gamez W., Schmidt F., Watson D. (2010). Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychological Bulletin, 136(5), 768–821. [DOI] [PubMed] [Google Scholar]
- Larsen R.J., Ketelaar T. (1989). Extraversion, neuroticism and susceptibility to positive and negative mood induction procedures. Personality and Individual Differences, 10(12), 1221–8. [Google Scholar]
- Mariën P., Baillieux H., De Smet H.J., et al. (2009). Cognitive, linguistic and affective disturbances following a right superior cerebellar artery infarction: a case study. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior, 45(4), 527–36. [DOI] [PubMed] [Google Scholar]
- McCrae R.R., Costa P.T. (1991). Adding Liebe und Arbeit: the full Five-Factor Model and Well-Being. Personality and Social Psychology Bulletin, 17(2), 227–32. [Google Scholar]
- McCrae R.R., Costa P.T. (2004). A contemplated revision of the NEO Five-Factor Inventory. Personality and Individual Differences, 36(3), 587–96. [Google Scholar]
- McCrae R.R., Costa P.T., Jr. (2010). NEO Inventories professional manual for the NEO Personality Inventory-3, NEO Five-Factor Inventory-3, and NEO Personality Inventory-Revised. Lutz, FL: PAR. [Google Scholar]
- Nooner K.B., Colcombe S.J., Tobe R.H., et al. (2012). The NKI-Rockland Sample: A Model for Accelerating the Pace of Discovery Science in Psychiatry. Frontiers in Neuroscience, 6, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y., Cui Q., Wang Y., et al. (2016). Extraversion and neuroticism related to the resting-state effective connectivity of amygdala. Scientific Reports, 6(1), 35484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picerni E., Petrosini L., Piras F., et al. (2013). New evidence for the cerebellar involvement in personality traits. Frontiers in Behavioral Neuroscience, 7, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.D., Ode S., Moeller S.K., Goetz P.W. (2007). Neuroticism and affective priming: evidence for a neuroticism-linked negative schema. Personality and Individual Differences, 42(7), 1221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M.D., Finn E.S., Scheinost D., Constable R.T., Chun M.M. (2017). Characterizing Attention with Predcitve Network Models. Trends in Cognitive Sciences, 21(4), 290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M.D., Finn E.S., Scheinost D., et al. (2016a). A neuromarker of sustained attention from whole-brain functional connectivity. Nature Neuroscience, 19(1), 165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M.D., Zhang S., Hsu W.-T., et al. (2016b). Methylphenidate modulates functional network connectivity to enhance attention. Journal of Neuroscience, 36(37), 9547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M.D., Hsu W.-T., Scheinost D., Constable R.T., Chun M.M. (2018). Connectome-based models predict separable components of attention in novel individuals. Journal of Cognitive Neuroscience, 30(2), 160–73. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Elliott M.A., Gerraty R.T., et al. (2013). An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage, 64, 240–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D., Papademetris X., Constable R.T. (2014). The impact of image smoothness on intrinsic functional connectivity and head motion confounds. NeuroImage, 95, 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D., Kwon S.H., Lacadie C., et al. (2017). Alterations in anatomical covariance in the prematurely born. Cereb Cortex, 27, 534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter D.J.L.G., Koolschijn P.C.M.P., Peper J.S., Crone E.A., Fontenelle L. (2012). The cerebellum link to neuroticism: a volumetric MRI association study in healthy volunteers. PLoS One, 7(5), e37252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaas M.N., Geerligs L., Renken R.J., et al. (2015). Connectomics and neuroticism: an altered functional network organization. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 40(2), 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Finn E.S., Scheinost D., et al. (2017). Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nature of Protocols, 12(3), 506–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Tokoglu F., Papademetris X., Constable R.T. (2013). Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. NeuroImage, 82, 403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., John O.P., Gosling S.D., Potter J. (2003). Development of personality in early and middle adulthood: set like plaster or persistent change? Journal of Personality and Social Psychology, 84(5), 1041–53. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. (2010). Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex: A Journal Devoted to the Study of the Nervous System & Behavior, 46(7), 831–44. 14 p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L.A. (1997). Extraversion and its positive emotional core. In: R. Hogan, J. Johnson, S. Briggs (Eds.), Handbook of personality psychology, San Diego: Academic Press, pp. 767–93. https://doi.org/10.1016/B978-012134645-4/50030-5. [Google Scholar]
- Yan C.G., Cheung B., Kelly C., et al. (2013). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage, 76, 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo K., Rosenberg M.D., Hsu W.-T., et al. (2018). Connectome-based predictive modeling of attention: Comparing different functional connectivity measures and prediction methods across datasets. NeuroImage, 167, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.