Abstract
Aberrant rule- and reward-based processes underpin abnormalities of socio-emotional behaviour in major dementias. However, these processes remain poorly characterized. Here we used music to probe rule decoding and reward valuation in patients with frontotemporal dementia (FTD) syndromes and Alzheimer’s disease (AD) relative to healthy age-matched individuals. We created short melodies that were either harmonically resolved (‘finished’) or unresolved (‘unfinished’); the task was to classify each melody as finished or unfinished (rule processing) and rate its subjective pleasantness (reward valuation). Results were adjusted for elementary pitch and executive processing; neuroanatomical correlates were assessed using voxel-based morphometry. Relative to healthy older controls, patients with behavioural variant FTD showed impairments of both musical rule decoding and reward valuation, while patients with semantic dementia showed impaired reward valuation but intact rule decoding, patients with AD showed impaired rule decoding but intact reward valuation and patients with progressive non-fluent aphasia performed comparably to healthy controls. Grey matter associations with task performance were identified in anterior temporal, medial and lateral orbitofrontal cortices, previously implicated in computing diverse biological and non-biological rules and rewards. The processing of musical rules and reward distils cognitive and neuroanatomical mechanisms relevant to complex socio-emotional dysfunction in major dementias.
Keywords: music, reward, emotion, Alzheimer’s disease, frontotemporal dementia
Introduction
Disturbances of complex emotional and social behaviour are a defining hallmark of frontotemporal dementia (FTD) and may be prominent in other neurodegenerative diseases, notably Alzheimer’s disease (AD) (Rascovsky et al., 2011; Sturm et al., 2013; Warren et al., 2013; Sapey-Triomphe et al., 2015; Clark et al., 2016). The spectrum of socio-emotional dysfunction in these diseases is complex and poorly understood; however, certain core themes emerge. These include impaired understanding of social norms and their violation (potentially leading to phenomena such as faux pas, loss of decorum, insensitivity to others’ discomfiture and diffusion of social boundaries: Ibanez and Manes, 2012) and abnormal affective responses to homeostatic signals (leading, e.g. to apathy or abnormal hedonic investment in potentially rewarding, aversive or banal stimuli: Zhou and Seeley, 2014), as well as altered reactivity to emotional cues from complex environments (Sturm et al., 2015) or reduced ability to use semantic regularities in anticipating future events (Bertoux et al., 2014; Irish and Piolino, 2016). These processes might be collectively conceptualized as impaired ‘rule’ decoding and altered reward valuation from socio-emotional and other kinds of signals. Behaviourally, a major unifying goal of such processes is to respond appropriately to salient events in the world at large: ‘salience’ is a property often associated with stimuli that violate expectations (‘rules’) established by prior experience of related stimuli and salient stimuli frequently have reward or aversive potential, according to their intrinsic (biological or cognitive) value and behavioural context (Wallis, 2007; Lang et al., 2009; Dalton et al., 2012; Ibanez and Manes, 2012; Schultz, 2013; Zhou and Seeley, 2014).
Emerging evidence in neurodegenerative populations has underlined the clinical and neuroanatomical relevance of rule and reward processing. Large-scale cerebral networks coding salience (comprising insula, anterior cingulate and orbitofrontal cortex) and reward value (comprising ventral striatal and mesolimbic dopaminergic circuitry) have been implicated in the pathogenesis of socio-emotional phenotypes in FTD (Wallis, 2007; Lang et al., 2009; Pievani et al., 2011; Halabi et al., 2013; Perry et al., 2014, 2015; Zhou and Seeley, 2014; Bocchetta et al., 2016). Altered interactions between salience, reward and temporo-parietal ‘default-mode’ networks underpin socio-emotional dysfunction in AD (Ismail et al., 2008; O’Reilly et al., 2013; Zhou and Seeley, 2014; Fletcher et al., 2015b; Perry et al., 2015; Ahmed et al., 2016). Deficient integration of signal processing with central reward evaluation and autonomic regulatory mechanisms may constitute a common effector pathway in a range of diseases (Zhou and Seeley, 2014; Fletcher et al., 2015a,b). However, the study of rule processing, salience detection and associated reward processing in the dementias poses a number of challenges. These include the complexity of natural socio-emotional scenarios, the diversity of contingent phenomena (spanning the gamut of primary biological and secondary hedonic stimuli) and the difficulty of manipulating relevant stimulus parameters under experimental conditions.
As a candidate model system for analysing rule decoding and reward valuation in dementia, music has a number of attractive attributes. Music is universal, ubiquitous and pleasurable for most listeners; its effects are grounded in cerebral ciricuitry that mediates pattern resolution and hedonic processing, overlapping the distributed networks implicated in FTD and AD (Koelsch et al., 2000; Blood and Zatorre, 2001; Janata et al., 2002; Steinbeis et al., 2006; Pressnitzer et al., 2011; Salimpoor et al., 2011, 2013, 2015; Seger et al., 2013; Clark et al., 2014, 2015a). The socio-emotional resonance of music suggests it may be a fertile model for exploring the building blocks of more complex inter-personal and affective behaviours in daily life (Clark et al., 2015a). Music necessarily unfolds over time, mirroring the dynamic nature of socio-emotional interactions. On the other hand, despite its propensity to engage biological reward circuitry, music is an autonomous signalling system that does not depend on extra-musical associations. Moreover, the characteristics of music that convey salience and hedonic value are highly codified and relatively straightforward to vary experimentally. The ‘rules’ governing harmonic structures are acquired implicitly by normal listeners through exposure to the music of the dominant culture and are engaged rapidly and automatically even during non-attentive listening (Tillmann, 2005; Huron, 2006; Loui et al., 2009; Koelsch et al., 2013; Choi et al., 2014). Indeed, music may be an ideal probe of the interface between cognition and emotion, via the medium of psychological expectancy (Krumhansl, 1997; Huron, 2006).
The relations between rule-based musical expectancy, arousal and valence (perceived pleasantness) are complex and influenced by the specific musical context. The interplay of tension and resolution is a fundamental mechanism underlying a range of psychophysiological (arousal and valence) effects associated with the manipulation of musical structure (Krumhansl, 1997; Steinbeis et al., 2006; Gingras et al., 2016; Tsai and Chen, 2015). Musical pleasure and reward (though separable phenomena) are typically closely correlated (Salimpoor et al., 2013): although ambiguity or surprise in more complex music and musical ‘scenes’ can be intensely pleasurable (Huron, 2006; Pressnitzer et al., 2011), melodies with a harmonic structure that fulfils expectation or resolves ambiguity tend to reduce subjective tension and are usually perceived as subjectively pleasurable or rewarding, while lack of resolution or confounded expectation is associated with increased subjective tension and negative affect (Steinbeis et al., 2006; Gingras et al., 2016; Tsai and Chen, 2015; Tsai et al., 2015). This propensity of music to create psychological expectancy (‘pay-off’ vs disappointment) links it naturally to reward valuation, and indeed this is already recognized in the music neuroscience literature (Li et al., 2015; Tsai and Chen, 2015). Reward is a complex, multidimensional psychophysiological construct, but generally entails the potential for consummatory behaviour or active updating of stimulus value, completion or resolution even in the absence of overt goal-directed action (Schultz, 2013; Perry et al., 2015).
From a clinical perspective, altered behavioural responses to music are well documented in many patients with dementia and may stratify FTD and AD syndromes. Recent evidence suggests that behavioural variant (bv)FTD may impair the analysis of musical structure (in particular, anticipation of structure unfolding in time) as well as hedonic valuation of music, whereas semantic dementia (SD) may be associated with relatively preserved processing of musical structure and progressive non-fluent aphasia (PNFA) and AD with relatively preserved hedonic valuation of music (Omar et al., 2010; Weinstein et al., 2011; Hsieh et al., 2012; Downey et al., 2013; Fletcher et al., 2013, 2014, 2015b; Henley et al., 2014; Agustus et al., 2015).
Here we used music as a paradigm to assess rule decoding and reward valuation in the canonical syndromes of FTD and AD, referenced to healthy older individuals. We presented novel melodies that either resolved according to the rules of classical harmony or did not resolve: the behavioural task was to decide whether or not each melody resolved (rule decoding) and to rate its pleasantness (reward valuation). We adopted this tonal expectancy task (rather than simply assessing, e.g. detection of dissonant or scale-deviant notes) because we wished to model the dynamic scenarios of natural, extra-musical, socio-emotional behaviour in daily life: in more complex social scenarios, the potential for reward (here, modelled as harmonic resolution) rather than the delivery of ‘punishment’ (a dissonant note) tends to be the prime mover of behaviour (Milinski and Rockenbach, 2012). At the same time, we wished to avoid any requirement for explicit labelling of musical emotions, a somewhat artificial task that is known to be impaired in FTD syndromes (Omar et al., 2011). We assessed neural correlates of musical rule and reward processing using voxel-based morphometry of patients’ brain MR images. Based on previous evidence, we hypothesized that bvFTD would be associated with abnormalities of both musical rule decoding (categorization of tonally resolved vs unresolved melodies) and reward valuation (emotional rating of these melodies) (Omar et al., 2010; Downey et al., 2013; Henley et al., 2014; Agustus et al., 2015; Fletcher et al., 2015b) while SD would show abnormal musical reward valuation despite relatively preserved rule decoding (Omar et al., 2010; Weinstein et al., 2011; Hsieh et al., 2012; Fletcher et al., 2013, 2015b) and PNFA and AD would show abnormal musical rule decoding despite relatively preserved reward valuation (Rohrer et al., 2012; Fletcher et al., 2015b; Golden et al., 2017). Based on previous work in the healthy brain and in patients with focal brain damage, we further hypothesized that harmonic classification of melodies would have a neuroanatomical correlate in superior temporal and prefrontal areas that mediate the analysis of musical and other rule-based patterns (Koelsch et al., 2000, 2013; Janata et al., 2002; Salimpoor et al., 2013, 2015; Seger et al., 2013; Clark et al., 2015a); while the rating of harmonic pleasantness would have a correlate in inferior frontal areas (including orbitofrontal cortex and inferior frontal gyrus) implicated in the affective labelling of music and other stimuli (Blood and Zatorre, 2001; Salimpoor et al., 2011, 2013, 2015; Clark et al., 2014, 2015a) and the integration of semantic and emotional information (Koelsch et al., 2006; Belyk et al., 2017).
Materials and methods
Participants
In total 14 patients with typical AD (henceforth, ‘AD’) led by episodic memory decline, 11 patients with bvFTD, 6 patients with SD and 8 patients with PNFA were recruited. All patients fulfilled consensus clinical criteria for the relevant syndromic diagnosis (Gorno-Tempini et al., 2011; Rascovsky et al., 2011; Dubois et al., 2014). Twenty-two healthy older individuals with no history of neurological or psychiatric disorders also participated. Demographic details (including an assessment of past musical training and current listening habits) and clinical characteristics of the study cohort are summarized in Table 1. None of the participants had a history of clinically significant hearing loss or congenital amusia. One patient in the bvFTD group and five patients in the SD group had a history of abnormal craving for music or ‘musicophilia’ (Fletcher et al., 2013). All participants had a comprehensive general neuropsychological assessment (summarized in Table 1) and audiometric screening of peripheral hearing function (adapted from a commercial screening audiometry package; details in Supplementary Material). Brain imaging (MRI/CT) revealed a profile of atrophy compatible with the syndromic diagnosis in all patients; no brain images showed a significant cerebrovascular burden. In total 10 of 11 patients in the AD group for whom CSF was available had a protein marker profile suggesting underlying AD pathology (total CSF tau: beta-amyloid1-42 ratio >1, based on local laboratory reference ranges); CSF findings in 11 of 13 patients with other syndromes provided no evidence for underlying AD pathology. At the time of assessment, 13 patients in the AD group were receiving symptomatic treatment with donepezil and 2 with memantine; 1 other patient (in the PNFA group) was receiving donepezil.
Table 1.
General demographic, clinical and neuropsychological characteristics of participant groups
| Characteristic | Healthy controls | bvFTD | SDa | PNFA | AD |
|---|---|---|---|---|---|
| General | |||||
| No. (m:f) | 11: 11 | 9: 2 | 4: 2 | 2: 6 | 8: 6 |
| Age (years) | 68.5(5.1) | 65.8 (7.6) | 66.2 (5.2) | 71.5 (7.8) | 68.6 (6.7) |
| Musical training (years) | 4.5(3.4) | 4.8 (3.4) | 4.3 (4.4) | 3 (2.5) | 4.4 (2.9) |
| Musical listening (h/wk) | 9.7(10.1) | 5.5 (4.7)b | 8.8 (8.6)b | 5.5 (7.4) | 8.6 (11.0) |
| Education (years) | 16.7(2.0) | 15.3 (3.4) | 14.2 (3.1) | 16.9 (2.2) | 15 (2.4) |
| MMSE (/30) | N/A | 25 (3.8) | 27 (2.5) | 23 (9.5) | 21 (5.0) |
| Symptom duration (years) | N/A | 9.8 (5.5) | 6.3 (1.8) | 6.9 (3.7) | 6.3 (1.9) |
| Neuropsychological | |||||
| General intellect: IQ | |||||
| WASI verbal IQ | 119 (7.0) | 91 (16.5) | 87 (11.5) | 88 (16.1) | 98 (14.4) |
| WASI performance IQ | 121(10.6) | 104 (15.5) | 114 (19.1) | 103 (18.9) | 90 (21.5) |
| NART estimated premorbid IQ | 122 (4.7) | 108 (12.2) | 107 (12.1) | 104 (15.8) | 113 (9.0) |
| Executive skills | |||||
| WASI Block Design (/71) | 46 (13.0) | 33 (13.4) | 41 (19.8) | 21 (18.1) | 18 (13.8) |
| WASI Matrices (/32) | 25 (4.1) | 21 (5.6) | 24 (6.9) | 20 (6.7) | 13 (7.8) |
| WMS-R digit span forward (/12) | 9 (2.1) | 9 (2.5) | 11 (1.5) | 7 (2.0) | 6 (2.2) |
| WMS-R digit span reverse (/12) | 8 (2.0) | 7 (2.7) | 10 (2.2) | 3 (2.2) | 5 (1.7) |
| D-KEFS Stroop colour (s) | 29 (4.2) | 40 (10.3) | 37 (10.2) | 67 (20.9) | 54 (22.5) |
| D-KEFS Stroop word (s) | 21 (3.7) | 27 (8.0) | 22 (5.3) | 52 (24.6) | 37 (19.4) |
| D-KEFS Stroop interference (s) | 58 (17.1) | 81 (36.2) | 62 (27.5) | 149 (37.3) | 107 (53.2) |
| Letter fluency (F: total)c | 16 (5.5) | 9 (4.6) | 11 (4.9) | 4 (2.7) | 11 (5.0) |
| Category fluency (animals: total) | 24 (5.4) | 12 (3.8) | 6 (2.8) | 10 (3.4) | 12 (5.4) |
| Trails A (s)d | 32 (9.0) | 45 (17.3) | 35 (20.6) | 69 (37.2) | 73 (48.0) |
| Trails B (s) | 73 (20.4) | 148 (81.7) | 89 (48.0) | 233 (67.5) | 175 (62.9) |
| WAIS-R Digit Symbol (total) | 56 (10.7) | 34 (8.7) | 44 (11.2) | 27 (12.0) | 20 (15.6) |
| Episodic memory | |||||
| RMT words (/50) | 48 (1.8) | 37 (8.8) | 33 (6.6) | 47 (3.7) | 30 (5.8) |
| RMT faces (/50) | 44 (4.1) | 33 (6.1) | 32 (8.1) | 37 (5.7) | 33 (6.4) |
| Camden PAL (/24) | 20 (2.5) | 9 (6.6) | 5 (4.5) | 17 (4.5) | 4 (4.0) |
| Language skills | |||||
| WASI Vocabulary (/80) | 71 (3.2) | 50(18.7) | 42 (17.6) | 39 (17.9) | 56 (10.0) |
| WASI Similarities (/48) | 39 (4.8) | 26(8.4) | 22 (8.7) | 28 (7.2) | 26 (11.4) |
| GNT (/30) | 26 (2.2) | 8 (9.4) | 0 (0.8) | 17 (7.7) | 16 (6.7) |
| NART (/50) | 43 (3.6) | 31(11.5) | 28 (10.5) | 30 (12.8) | 36 (7.2) |
| Single word repetition (/45) | N/A | N/A | 45 (1.0) | 33 (15.4) | N/A |
| Sentence repetition (/10) | N/A | N/A | 10 (0.5) | 6 (4.4) | N/A |
| Semantic memory | |||||
| BPVS (/150) | 148 (1.9) | 128(21.2) | 120 (14.8) | 144 (4.8) | 145 (3.0) |
| Synonyms concrete(/25) | N/A | N/A | 18 (2.6) | 22 (2.9) | N/A |
| Synonyms abstract(/25) | N/A | N/A | 17 (3.2) | 21 (3.5) | N/A |
| Posterior cortical skills | |||||
| GDA (/24) | 16 (5.0) | 13(6.6) | 12(8. 4) | 5 (4.1) | 5 (6.3) |
| VOSP Object Decision (/20) | 19 (1.6) | 17(1.9) | 17 (2.7) | 16 (5.6) | 16 (3.4) |
Notes: Mean (SD) scores are shown unless otherwise indicated; maximum scores are shown after tests (in parentheses). Bold denotes significantly different (P < 0.05) to the healthy control group.
AD, patient group with typical Alzheimer’s disease; BPVS, British Picture Vocabulary Scale (Dunn LM et al., 1982); bvFTD, patient group with behavioural variant frontotemporal dementia; D-KEFS, Delis Kaplan Executive System (Delis et al., 2001); GDA, Graded Difficulty Arithmetic (Jackson and Warrington, 1986); GNT, Graded Naming Test (McKenna and Warrington, 1983); MMSE, Mini-Mental State Examination score; N/A, not assessed; NART, National Adult Reading Test (Nelson, 1982); PAL, Paired Associate Learning test (Warrington, 1996); PIQ, performance IQ; PNFA, patient group with progressive non-fluent aphasia; RMT, Recognition Memory Test (Warrington, 1984); SD, patient group with semantic dementia; Synonyms, Concrete and Abstract Word Synonyms Test (Warrington et al., 1998); SD, semantic dementia; VIQ, verbal IQ; VOSP, Visual Object and Spatial Perception Battery (Warrington and James, 1991); WAIS-R, Wechsler Adult Intelligence Scale – Revised (Wechsler, 1981); WASI, Wechsler Abbreviated Scale of Intelligence (Wechsler, 1997); WMS-R, Wechsler Memory Scale - Revised (Wechsler, 1987).
Three patients (two with musicophilia) had predominantly left-sided temporal lobe atrophy, one (with musicophilia) had predominantly right-sided temporal lobe atrophy and two (also with musicophilia) had more symmetrical, bilateral temporal lobe atrophy.
includes patients with musicophilia (five in SD group, one in bvFTD group);
Words generated in 1 min beginning with letter F (Gladsjo et al., 1999).
Time to complete Trails in seconds (maximum time achievable 2.5 min on task A, 5 min on task B) (Lezak et al., 2004).
The study was approved by the local institutional ethics committee and all participants gave informed consent in accordance with Declaration of Helsinki guidelines.
Musical assessment
Assessment of tonal expectancy
To assess processing of tonal expectancy (musical harmonic) rules and reward value, short monophonic melodies were composed by an experienced musician (O.M.). Melody endings were manipulated such that the melody sounded ‘finished’ (tonally resolved) or ‘unfinished’ (tonally unresolved). Melodies in the ‘finished’ and ‘unfinished’ conditions were closely matched for loudness, length, timbre, instrumentation, key, pitch velocity and tempo. All note sequences were synthesized with piano timbre as digital wavefiles using Logic Pro X. Tempo was fixed at 120 beats/min for all stimuli; melodies varied in length between three and five bars; however, the two conditions did not differ in mean length (number of bars or duration; see Supplementary Material). Examples of stimuli are presented in Figure 1; the complete set is notated in Supplementary Figure S1, together with further details of stimulus preparation and pilot testing.
Fig. 1.
Examples of stimuli used in the music experimental battery: ‘finished’ (tonally resolved) and ‘unfinished’ (tonally unresolved) melodies presented in the tonal (harmonic) expectancy test (see text for further details).
Melodies were presented in randomized order and the task on each trial was to decide first, if the melody sounded ‘finished’ or ‘unfinished’; and second, to rate how pleasing was the ending of the melody (‘How did the tune leave you feeling?’) on a 5-point Likert scale (1, not at all pleased; 5, very pleased; see Supplementary Figure S2).
Assessment of pitch pattern processing
To provide a measure of elementary pitch pattern processing, we adopted a procedure similar to other tests of basic serial pitch discrimination originating in the classic work of Seashore (1919). We assessed participants’ ability to detect pitch direction changes in sequentially presented note pairs; the notes comprising each pair differed in pitch by either a tone or a semitone. Notes had piano timbre and duration 1 s with an inter-note gap of 1 s. Twenty trials (pairs) were presented and the direction of the pitch shift between notes was varied randomly across trials (10 ascending, 10 descending). The task on each trial was to decide if the second note was ‘higher’ or ‘lower’ than the first note.
Experimental procedure
Stimuli were presented from a notebook computer running Matlab via headphones (Audio-Technica) at a comfortable listening level (at least 70 dB) in a quiet room. Participants were first familiarised with each test using practice examples to ensure they understood the task and were able to comply reliably. Participant responses were recorded for offline analysis. During the tests no feedback was given about performance and no time limits were imposed. All participants completed the experimental tests comfortably in <30 min.
Analysis of behavioural data
All behavioural data were analysed using Stata12. Demographic characteristics and neuropsychological data were compared between participant groups using Fisher’s exact test for categorical variables, and for continuous variables, either two sample t-tests or Wilcoxon rank-sum tests where t-test assumptions were materially violated (e.g. due to skewed data distribution).
Logistic regression was used to analyse accuracy of melody and pitch direction decisions (correct vs incorrect) and pleasantness ratings (dichotomized based on a rating ≥3 (‘pleasing’) or <3 (‘not pleasing’) to avoid over-estimating the effect of gradations of pleasantness). For each analysis a global test was first used to jointly compare all groups, followed by pairwise group comparisons where a significant overall group effect was found. An interaction term was included to examine whether there was differential performance between groups for ‘finished’ vs ‘unfinished’ melodies. To take account of potentially confounding factors, age, gender, reverse digit span (an index of executive function and specifically, auditory working memory) and (in the assessment of tonal expectancy) performance on the pitch direction task were included as covariates in the regression model.
In separate post hoc analyses, we used the non-parametric Spearman coefficient to assess the extent of any correlation between tonal expectancy and pitch direction processing performance and any correlation of musical task performance with years of prior musical training, auditory working memory (reverse digit span) or general executive capacity [Wechsler Abbreviated Scale of Intelligence (WASI) Matrices score, an index of overall disease severity] within the patient cohort.
A threshold P < 0.05 was accepted as the criterion for statistical significance in all analyses.
Brain image acquisition and voxel-based morphometry analysis
Brain MRI data for voxel-based morphometry were acquired for 34 patients (12 AD, 11 bvFTD, 5 SD and 6 PNFA) on a Siemens Trio 3Tesla MRI scanner using a 32-channel phased array head-coil and a sagittal 3D magnetization prepared rapid gradient echo T1-weighted volumetric sequence (echo time/repetition time/inversion time = 2.9/2200/900 ms, dimensions 256 × 256 × 208, voxel size 1.1 × 1.1 × 1.1 mm). Volumetric brain images were assessed visually in all planes to ensure adequate coverage and to exclude artefacts or significant motion. Pre-processing of patient brain MR images was performed using the Segment routine and the DARTEL toolbox of SPM12 (Ashburner, 2007). Further details of imaging analysis are available in Supplementary Material. A study-specific mean brain image template, for displaying results, was created by warping all bias-corrected native space whole-brain images to the final DARTEL template in MNI space and calculating the average of the warped brain.
Using the framework of the general linear model, multiple regression was used to examine associations between regional grey matter volume and accuracy of melody classification as ‘finished’ or ‘unfinished’ (raw score), pleasantness rating of melodies (relative likelihood of rating unfinished melodies as ‘not pleasing’) and pitch direction task performance over the combined patient cohort. In separate design matrices, voxel intensity (an index of grey matter volume) was modelled as a function of each relevant musical behavioural characteristic, including syndromic group membership, age, gender, total intracranial volume and reverse digit span as nuisance covariates in all matrices. For each model, separate contrasts (one-tailed t-tests) assessed positive linear associations between grey matter and the parameter of interest across the combined patient cohort. Statistical parametric maps (SPMs) were thresholded at P < 0.05 after family-wise error (FWE) correction for multiple voxel-wise comparisons over the whole brain.
Results
General participant characteristics
Patient and healthy control groups did not differ in age (P = 0.41), gender (P = 0.16), educational background (χ2 = 6.40, P = 0.20) or musical training (χ2 = 1.51, P = 0.80) and syndromic groups had similar mean symptom duration (χ2 = 2.59, p = 0.50). Patient groups showed the anticipated profiles of general neuropsychological impairment (Table 1). Peripheral hearing function varied between participant groups [combined audiometric tone detection score, see Supplementary Material; overall F(4, 53) = 7.32, P < 0.001, Supplementary Table S1); however, the absolute value of the difference was small and there was no significant correlation between peripheral hearing and accuracy on melody classification performance over the entire participant group (rho = −0.09, P = 0.50) nor within the combined patient cohort (rho = −0.08, P = 0.64).
Tonal expectancy processing
Group performance profiles on tonal expectancy tasks are summarized in Table 2 and in Supplementary Tables S2 and S3. Individual raw data are presented in Supplementary Figures S3–S5.
Table 2.
Summary of performance on music cognition tests for patient groups relative to healthy controls
| Test characteristic | bvFTD | SD | PNFA | AD |
|---|---|---|---|---|
| Tonal expectancy task: accuracy classifying melodies | ||||
| All | 0.47 (0.25–0.86) | 0.61 (0.30–1.25) | 0.66 (0.30–1.44) | 0.55 (0.33–0.91) |
| Finished | 0.30 (0.12–0.75) | 0.53 (0.17–1.71) | 1.07 (0.43–2.65) | 0.36 (0.18–0.74) |
| Unfinished | 0.95 (0.45–2.02) | 0.79 (0.29–2.14) | 0.32 (0.10–0.99) | 1.16 (0.44–3.01) |
| Interaction | 2.87 (0.91–9.01)a | 0.99 (0.20–4.81) | 0.50 (0.18–1.35) | 3.81 (1.17–12.4)a |
| Tonal expectancy task: rating of melodies as ‘not pleasing’ | ||||
| All | 0.46 (0.14–1.48) | 0.16 (0.04–0.60)a,b | 1.13 (0.48–2.69) | 0.79 (0.36–1.75) |
| Finished | 1.1 (0.34–3.30) | 0.52 (0.14–1.92) | 0.84 (0.31–2.26) | 1.07 (0.42–2.75) |
| Unfinished | 0.32 (0.09–1.10) | 0.10 (0.03–0.36) | 1.45 (0.42–4.97) | 0.72 (0.28–1.72) |
| Interaction | 0.30 (0.11–0.85)a | 0.19 (0.09–0.39)a,b | 1.72 (0.39–7.61) | 0.65 (0.25–1.71) |
| Pitch direction task | ||||
| Accuracy | 0.40 (0.18–0.91) | 0.34 (0.13–0.87) | 0.51 (0.12–2.10) | 0.57 (0.12–2.69) |
Notes: For tonal expectancy test data, odds ratios (95% confidence intervals) are shown for correctly classifying melodies as ‘finished’ vs ‘unfinished’ and for rating the endings of melodies as ‘not pleasing’ vs ‘pleasing’ (see text), relative to the healthy control group; ‘interaction’ here represents the odds of a score difference for ‘finished’ vs ‘unfinished’ melodies, expressed for each patient group relative to healthy controls. For the pitch direction task, the OR represents the relative accuracy of pitch direction labelling relative to healthy control performance. For all odds ratios, confidence intervals including 1 indicate no significant difference between that patient group and healthy controls for the parameter of interest. For all comparisons, patient group performance profiles that differed significantly (P < 0.05) from the healthy control group are shown in bold; asignificantly different (P < 0.05) from PNFA group; bsignificantly different (P < 0.05) from AD group; AD, patient group with typical Alzheimer’s disease, bvFTD, patient group with behavioural variant frontotemporal dementia; PNFA, patient group with progressive non-fluent aphasia; SD, patient group with semantic dementia.
There was evidence of an overall group performance difference in the odds of correctly classifying melodies as ‘finished’ or ‘unfinished’ (P = 0.003; Table 2 and Supplementary Figure S3). Relative to the healthy control group, the bvFTD and AD groups each showed overall significantly less accurate classification of melodies (P < 0.05), driven principally by incorrect classification of ‘finished’ melodies (Table 2). The SD and PNFA groups did not show a significant deficit of melody classification. There were no significant differences between syndromic groups. Whereas healthy controls were equivalently accurate in classifying ‘finished’ and ‘unfinished’ melodies [odds ratio (OR) = 1.4 (95% confidence interval (CI) 0.7–2.9), P = 0.38], the AD group was significantly less accurate classifying ‘finished’ than ‘unfinished’ melodies (P < 0.001). Across the patient cohort, accuracy of melody classification did not correlate with prior musical training (rho = 0.19, P = 0.25) or auditory working memory (reverse digit span, rho = 0.20, p = 0.23) but was significantly correlated with a general measure of non-verbal executive function (WASI Matrices score, rho = 0.46, P = 0.003).
There was further evidence of an overall group difference in pleasantness ratings of ‘unfinished’ vs ‘finished’ melodies (P < 0.0001; Table 2 and Supplementary Figure S4). The healthy control group was significantly more likely to rate ‘unfinished’ than ‘finished’ melodies as ‘not pleasing’ [OR = 7.7; CI 3.8–15.7]; the ranges of individual raw healthy control ratings for ‘finished’ and ‘unfinished’ melodies overlapped (Supplementary Figure S4 and Supplementary Table S2), suggesting that controls did not simply rate melodies to align explicitly with their melody classification. Patient groups showed a qualitatively similar profile, in that all groups tended to rate ‘finished’ melodies as more pleasant than ‘unfinished’ melodies (Supplementary Figure S4). However, both the bvFTD and SD groups showed significantly less discrepant pleasantness rating profiles than did the healthy control group (P < 0.05) and the SD group was also significantly less likely than healthy controls to rate ‘unfinished’ melodies as ‘not pleasing’ (P < 0.001), while the PNFA and AD groups showed a profile comparable to healthy controls. Comparing disease groups revealed significant differences between the pleasantness rating profiles of the bvFTD and SD groups vs the PNFA and AD groups (P < 0.05). Patient group pleasantness rating profiles were similar for the complete melody set and when restricted to those melodies correctly classified as ‘finished’ or ‘unfinished’ (compare Supplementary Figures S4 and S5, see Supplementary Table S3): both the bvFTD and SD groups were significantly less likely than healthy controls to rate correctly classified melodies as ‘not pleasing’ (P < 0.05) while the PNFA and AD groups rated correctly classified melodies similarly to healthy controls.
Pitch direction processing
Relative to healthy controls, a deficit of pitch direction processing was evident in the bvFTD group (P = 0.03) and SD group (P = 0.03), but not the PNFA group (P = 0.35) or AD group (P = 0.48) (Table 2 and Supplementary Figure S3). Within the patient cohort, performance on the pitch direction and tonal expectancy tasks showed a borderline significant correlation (P = 0.05, rho = 0.31; analyses of tonal expectancy performance were adjusted for pitch direction score). Performance on the pitch direction task was significantly correlated with prior musical training (rho = 0.58, P = 0.0002) but not with executive measures (reverse digit span, rho = 0.26, P = 0.11; WASI Matrices score, rho = 0.28, P = 0.08).
Neuroanatomical associations
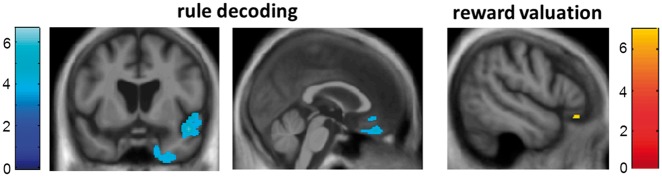
Significant neuroanatomical associations are summarized in Table 3 and SPMs are presented in Figure 2.
Table 3.
Summary of grey matter associations of tonal expectancy processing in patient cohort
| Region | Peak coordinate (mm) |
Z score | P value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Accuracy of classifying melodies | |||||
| Entorhinal cortex | 24 | 0 | –50 | 5.22 | 0.008 |
| Anterior superior temporal gyrus | 48 | 3 | –18 | 4.94 | 0.025 |
| Anterior superior temporal sulcus | 56 | –10 | –8 | 4.91 | 0.029 |
| Medial orbitofrontal cortex | 4 | 40 | −22 | 4.92 | 0.028 |
| Pleasantness rating of melodies | |||||
| Inferior frontal gyrus (pars orbitalis) | −51 | 33 | −15 | 5.69 | 0.001 |
Notes: The Table shows statistically significant positive associations between grey matter volume and accuracy of classifying melodies (‘finished’ vs ‘unfinished’) and pleasantness rating of melodies (likelihood of rating unfinished melodies as ‘not pleasing’), based on a voxel-based morphometric analysis of brain MR images for the combined patient cohort. Local maxima coordinates are presented with coordinates in MNI standard stereotactic space, thresholded at P < 0.05 after FWE correction for multiple voxel-wise comparisons over the whole brain. No significant associations were identified for the pitch direction task at the prescribed threshold.
Fig. 2.
Statistical parametric maps (SPMs) of regional grey matter volume positively associated with tonal expectancy parameters in the combined patient cohort are presented (see also Table 3); t scores are coded on the colour bars. Grey matter associations of accuracy of melody classification signify musical rule decoding, indicated in blue; grey matter associations of melody pleasantness rating signify musical reward valuation, indicated in red-orange (see text for details). SPMs are overlaid on coronal (left) and sagittal (middle, right) sections of the normalized study-specific T1-weighted mean brain MR image, selected to highlight right anterior superior temporal and entorhinal cortex (left), right medial orbitofrontal cortex (middle) and left inferior frontal gyrus pars orbitalis (right). SPMs are thresholded for display purposes at P < 0.001 uncorrected, however local maxima of areas shown were each significant at P < 0.05 after FWE correction for multiple voxel-wise comparisons over the whole brain (see Table 3).
Significant associations between grey matter atrophy and impaired melody classification accuracy over the combined patient cohort were identified in right entorhinal cortex, anterior superior temporal gyrus and sulcus and medial orbitofrontal cortex, thresholded at P < 0.05FWE after correction for multiple voxel-wise comparisons over the whole brain. A significant association between grey matter atrophy and abnormal pleasantness rating of melodies (a tendency to rate unresolved melodies as less unpleasant than healthy controls) was identified in left inferior frontal gyrus (pars orbitalis), thresholded at P < 0.05FWE after correction for multiple voxel-wise comparisons over the whole brain. No significant grey matter associations of pitch direction processing were identified for the combined patient cohort at the prescribed threshold.
Discussion
Here we have shown that music models impairments of rule and reward processing in cardinal syndromes of FTD and AD. When compared with healthy controls, patients with bvFTD had both less accurate classification of melodies based on harmonic structure (impaired rule decoding) and altered affective responses to harmonic completion (abnormal reward valuation); patients with SD had abnormal reward valuation despite preserved rule decoding; patients with AD had normal reward valuation despite impaired rule decoding; and patients with PNFA showed a relatively normal profile. Taken together, these profiles argue for dissociable signatures of musical rule decoding and reward valuation across dementia syndromes.
Melody classification in the bvFTD and AD groups was impaired after taking elementary pitch pattern perception (performance on the pitch direction task) into account and classification accuracy did not correlate with auditory working memory or prior musical training. It is therefore likely that the tonal expectancy test indexed a relatively specific impairment of musical rule processing. Such cognitive modularity may have contributed to the (on face value, somewhat surprising) preserved performance of the present PNFA group on melody classification, despite other evidence for impaired syntactical and pitch pattern deficits in this syndrome (Rohrer et al., 2012; Golden et al., 2017). On the other hand, tonal expectancy was aligned with general executive capacity, suggesting that this musical task may track disease severity. Abnormalities of musical reward valuation in bvFTD and SD were evident even in the context of correct rule decoding (for correctly classified melodies), further demonstrating that the processes of rule decoding and reward valuation are dissociable. The profiles of musical valuation exhibited by these syndromic groups were qualitatively similar to healthy controls despite quantitative differences, raising the possibility that more complex effects (such as biased ‘gain’ or altered precision of reward expectancy) might be revealed by larger cohort studies with scope to further refine the model of musical hedonic valuation in these diseases.
The musical profiles identified here resonate with behaviours exhibited by patients with FTD and AD in various other experimental and social contexts. Impaired rule-based processing on semantic categorization tasks has been demonstrated in bvFTD and AD (Grossman et al., 2001, 2003). More pertinently, these patients show impaired anticipation of future events based on previously experienced regularities (Irish et al., 2012; Irish and Piolino, 2016): a general mechanism for impaired decision-making. Patients with bvFTD show markedly impaired detection of rule violations in social scenarios (faux pas) and increased risk taking behaviour and reduced anticipation of future regret (aberrant reward prediction) (Rahman et al., 1999; Torralva et al., 2007; Bertoux et al., 2014; Bora et al., 2015). The present findings support previous evidence that detection of salience (rule violation) in socio-emotional contexts is generally impaired in bvFTD while perceptual and cognitive detection of salient events may be disengaged from affective reactivity in this syndrome (Sturm et al., 2006; Chiong et al., 2013; Clark et al., 2015b, 2016). In contrast, patients with AD have difficulty using rules to make decisions about future outcomes, but remain sensitive to affective outcomes (reward value) (Delazer et al., 2007, Dohnel et al., 2008; Sinz et al., 2008). AD and PNFA are substantially less likely than patients with bvFTD to exhibit strikingly aberrant responses to social rule violations (Bora et al., 2015; Clark et al., 2016), perhaps reflecting the relative extent to which affective awareness is preserved in these syndromes. In SD, decision-making based on modelling of future events depends critically on semantic function (Irish et al., 2011, 2012; Irish and Piolino, 2016): music (in contrast to most other rule-based systems) does not rely on extraneous semantic associations, perhaps accounting for the preserved ability to use musical rules exhibited by patients with SD here and in previous studies (Hailstone et al., 2009; Omar et al., 2010; Weinstein et al., 2011). Nevertheless (in line with the present profile), SD is frequently associated with abnormal valuation of biological and other rewards, including music (Fletcher et al., 2013, 2015a,b; Ahmed et al., 2014, 2015, 2016).
The neuroanatomical profiles identified here underline the involvement of brain networks mediating rule analysis, rule violation (salience) detection and reward evaluation in these diseases (Perry et al., 2014, 2015). Impaired tonal expectancy (classification of melodies) was associated with grey matter loss in right anterior superior and inferior temporal cortices and medial orbitofrontal cortex. In line with previous work (Peretz et al., 2001; Koelsch et al., 2006, 2013; Khalfa et al., 2008; Fujisawa and Cook, 2011; Hailstone et al., 2011; Omar et al., 2011; Hsieh et al., 2012; Salimpoor et al., 2013; Seger et al., 2013; Bonfiglio et al., 2015), this network may link cortical mechanisms mediating the structural analysis of melodies and harmonic hierarchies with paralimbic and orbitofrontal mechanisms mediating the cognitive representation and anticipation of musical emotion and reward. More generically, antero-medial temporal areas store previously learned knowledge and templates about sensory objects and regularities whereas prefrontal cortices implement and assess violations in rule-based algorithms (Michelon et al., 2003; Christensen et al., 2011; Nazimek et al., 2013; Cohen, 2014; Gauvin et al., 2016; Henderson et al., 2016). Altered valuation of musical reward (affective rating of melodies) was associated with grey matter loss in left inferior frontal gyrus pars orbitalis. Functionally, this region behaves as a subdivision of lateral orbitofrontal cortex and links cortical mechanisms analysing hierarchical and rule-based patterns [such as linguistic and musical ‘syntax’) with mechanisms representing reward value (Belyk et al., 2017); it has been implicated previously in representing musical tension associated with violation of harmonic expectancy and anticipation of musical rhythms (Lehne et al., 2014; Vuust et al., 2014)]. We did not find neuroanatomical correlates in striatal or other subcortical structures previously implicated in processing musical reward (Omar et al., 2011; Salimpoor et al., 2013; Li et al., 2015). Previous studies have generally employed music familiar to the participants or explicit (e.g. monetary) valuation of music, perhaps implying that other motivational, emotional or subjective factors engage these subcortical mechanisms.
Impairments of social and emotional functioning remain difficult to assess in cognitively impaired patients, limiting the identification of relevant biomarkers and the design of effective interventions. Taken together, the present findings suggest that music may provide a useful paradigm for deconstructing complex behavioural disturbances in FTD and AD. Beyond its universality and relative tractability, music (as a dynamic stimulus that unfolds in time) inherently entails predictive coding: the anticipation of musical structure based on internalized models that are continually updated (Huron, 2006). Aberrant predictive coding has emerged as a key organizing principle in computational accounts of major psychiatric disorders (Friston et al., 2014; Adams et al., 2016) and may play a similarly fundamental role in the phenomenology of neurodegenerative diseases characterized by deficient simulation of future events and consequences (Rahman et al., 1999; Torralva et al., 2007; Irish et al., 2012; Bertoux et al., 2014; Irish and Piolino, 2016). Music might generate novel dynamic biomarkers that can detect and track alterations in this core disease mechanism in the dementias, analogous to the next generation disease markers recently proposed for psychiatric illness (Friston et al., 2014; Adams et al., 2016).
This study suggests a number of directions for future work. Larger patient cohorts will be required to characterize the specificity of musical reward phenotypes for particular diseases while taking account of intrinsic individual variation in the cognitive decoding and hedonic valuation of music (Clark et al., 2014; Salimpoor et al., 2015; Sachs et al., 2016; Supplementary Figures S3–S5). Voxel-based morphometry is necessarily an associational technique and in clinical populations the associations observed are ‘windowed’ by the distribution of tissue damage imposed by the target disease. A complete picture of neurodegenerative musical phenotypes will entail functional neuroimaging and connectivity approaches that can delineate network-level effects directly (Salimpoor et al., 2013; Sachs et al., 2016), in addition to autonomic and other physiological metrics that may reveal disease-linked dissociations between implicit and explicit coding of musical reward (Balconi et al., 2015; Fletcher et al., 2015c; Sturm et al., 2015). The potential relevance of music as a biomarker will only be defined by longitudinal studies, including pre-symptomatic genetic cohorts. Assessment of hearing functions more generally (including the role of peripheral hearing) also warrants further study in common dementias; although peripheral hearing changes did not materially affect the key findings here, more elementary auditory processes may nevertheless be relevant to the broader characterization of these syndromes and as biomarkers in their own right (Hardy et al., 2016). Acknowledging these caveats, the present findings provide a case for music as a useful probe of aberrant rule and reward mechanisms in the dementias. We propose a reappraisal of music as a unique window on complex behaviours in neurodegenerative disease.
Supplementary data
Supplementary data are available at SCAN online.
Supplementary Material
Acknowledgements
We are grateful to all participants for their involvement and to Dr Jonathan Schott for giving us access to his research cohort for inclusion in the study. The Dementia Research Centre is supported by Alzheimer’s Research UK, the Brain Research Trust and the Wolfson Foundation.
Funding
This work was funded by the Alzheimer’s Society, the Wellcome Trust, the UK Medical Research Council, the NIHR Queen Square Dementia Biomedical Research Unit and NIHR University College London Hospitals Trust Biomedical Research Centre. C.N.C. was supported by The National Brain Appeal – Frontotemporal Dementia Research Fund. H.L.G. was supported by an Alzheimer Research UK PhD Fellowship. J.D.R. is funded by an MRC Clinician Scientist Fellowship (MR/M008525/1) and has received funding from the NIHR Rare Disease Translational Research Collaboration. S.J.C. is supported by an Alzheimer Research UK Senior Research Fellowship and an ESRC/NIHR (Grant no ES/K006711/1). J.D.W. was supported by a Wellcome Trust Senior Clinical Fellowship (Grant No 091673/Z/10/Z).
Conflict of interest. None declared.
References
- Adams R.A., Huys Q.J., Roiser J.P. (2016). Computational Psychiatry: towards a mathematically informed understanding of mental illness. Journal of Neurology, Neurosurgery, and Psychiatry, 87(1), 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agustus J.L., Mahoney C.J., Downey L.E., et al. (2015). Functional MRI of music emotion processing in frontotemporal dementia. Annals of the New York Academy of Sciences, 1337(1), 232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R.M., Iodice V., Daveson N., Kiernan M.C., Piguet O., Hodges J.R. (2015). Autonomic dysregulation in frontotemporal dementia. Journal of Neurology, Neurosurgery, and Psychiatry, 86(9), 1048–9. [DOI] [PubMed] [Google Scholar]
- Ahmed R.M., Irish M., Kam J., et al. (2014). Quantifying the eating abnormalities in frontotemporal dementia. JAMA Neurology, 71(12), 1540–6. [DOI] [PubMed] [Google Scholar]
- Ahmed R.M., Irish M., Piguet O., et al. (2016). Amyotrophic lateral sclerosis and frontotemporal dementia: distinct and overlapping changes in eating behaviour and metabolism. Lancet Neurology, 15(3), 332–42. [DOI] [PubMed] [Google Scholar]
- Ashburner J. (2007). A fast diffeomorphic image registration algorithm. Neuroimage, 38(1), 95–113. [DOI] [PubMed] [Google Scholar]
- Balconi M., Cotelli M., Brambilla M., et al. (2015). Understanding emotions in frontotemporal dementia: the explicit and implicit emotional cue mismatch. Journal of Alzheimer’s Diseases, 46(1), 211–25. [DOI] [PubMed] [Google Scholar]
- Belyk M., Brown S., Lim J., Kotz S.A. (2017). Convergence of semantics and emotional expression within the IFG pars orbitalis. Neuroimage, 156, 240–8. [DOI] [PubMed] [Google Scholar]
- Bertoux M., Cova F., Pessiglione M., Hsu M., Dubois B., Bourgeois-Gironde S. (2014). Behavioral variant frontotemporal dementia patients do not succumb to the Allais paradox. Frontiers in Neuroscience, 8, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood A.J., Zatorre R.J. (2001). Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proceedings of the National Academy of Sciences of the United States of America, 98(20), 11818–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchetta M., Gordon E., Marshall C.R., et al. (2016). The habenula: an under-recognised area of importance in frontotemporal dementia? Journal of Neurology, Neurosurgery, and Psychiatry, 87, 910–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfiglio L., Virgillito A., Magrini M., et al. (2015). N400-like responses to three-chord harmonic sequences with unexpected out of key endings: scalp topography, cortical sources, and perspectives for a clinical use. Archives Italiennes de Biologie, 153(1), 1–18. [DOI] [PubMed] [Google Scholar]
- Bora E., Walterfang M., Velakoulis D. (2015). Theory of mind in behavioural-variant frontotemporal dementia and Alzheimer’s disease: a meta-analysis. Journal of Neurology, Neurosurgery, and Psychiatry, 86(7), 714–9. [DOI] [PubMed] [Google Scholar]
- Chiong W., Wilson S.M., D'Esposito M., et al. (2013). The salience network causally influences default mode network activity during moral reasoning. Brain, 136(6), 1929–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi I., Bharadwaj H.M., Bressler S., Loui P., Lee K., Shinn-Cunningham B.G. (2014). Automatic processing of abstract musical tonality. Frontiers in Human Neuroscience, 8, 988.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen T.A., Lockwood J.L., Almryde K.R., Plante E. (2011). Neural substrates of attentive listening assessed with a novel auditory Stroop task. Frontiers in Human Neuroscience, 4, 236.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C.N., Downey L.E., Warren J.D. (2014). Music biology: all this useful beauty. Current Biology, 24(6), R234–7. [DOI] [PubMed] [Google Scholar]
- Clark C.N., Downey L.E., Warren J.D. (2015a). Brain disorders and the biological role of music. Social Cognitive and Affective Neuroscience, 10(3), 444–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C.N., Nicholas J.M., Gordon E., et al. (2016). Altered sense of humor in dementia. Journal of Alzheimer’s Diseases, 49(1), 111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C.N., Nicholas J.M., Henley S.M., et al. (2015b). Humour processing in frontotemporal lobar degeneration: a behavioural and neuroanatomical analysis. Cortex, 69, 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.X. (2014). A neural microcircuit for cognitive conflict detection and signaling. Trends in Neuroscience, 37(9), 480–90. [DOI] [PubMed] [Google Scholar]
- Dalton M.A., Weickert T.W., Hodges J.R., Piguet O., Hornberger M. (2012). Impaired acquisition rates of probabilistic associative learning in frontotemporal dementia is associated with fronto-striatal atrophy. Neuroimage Clinics, 2, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delazer M., Sinz H., Zamarian L., Benke T. (2007). Decision-making with explicit and stable rules in mild Alzheimer’s disease. Neuropsychologia, 45(8), 1632–41. [DOI] [PubMed] [Google Scholar]
- Delis D.C., Kaplan E., Kramer J. (2001) Delis–Kaplan Executive Function Scale, San Antonio TX: The Psychological Corporation. [Google Scholar]
- Dohnel K., Sommer M., Ibach B., Rothmayr C., Meinhardt J., Hajak G. (2008). Neural correlates of emotional working memory in patients with mild cognitive impairment. Neuropsychologia, 46(1), 37–48. [DOI] [PubMed] [Google Scholar]
- Downey L.E., Blezat A., Nicholas J., et al. (2013). Mentalising music in frontotemporal dementia. Cortex, 49(7), 1844–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B., Feldman H.H., Jacova C., et al. (2014). Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria'. Lancet Neurol, 13(6), 614–29. [DOI] [PubMed] [Google Scholar]
- Dunn L.M., Whetton C., Pintilie D. (1982) The British Picture Vocabulary Scale, Windsor: NFER- Nelson Publishing Co. [Google Scholar]
- fil.ion.ucl.ac.uk/spm/. (1994–2013). SPM by members & collaborators of the Wellcome Trust Centre for Neuroimaging. in T. F. M. Group (ed.), Internet.
- Fletcher P.D., Clark C.N., Warren J.D. (2014). Music, reward and frontotemporal dementia. Brain, 137(Pt 10), e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P.D., Downey L.E., Golden H.L., et al. (2015a). ain and temperature processing in dementia: a clinical and neuroanatomical analysis. Brain, 138(Pt 11),3360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P.D., Downey L.E., Golden H.L., et al. (2015b). Auditory hedonic phenotypes in dementia: A behavioural and neuroanatomical analysis. Cortex, 67, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P.D., Downey L.E., Witoonpanich P., Warren J.D. (2013). The brain basis of musicophilia: evidence from frontotemporal lobar degeneration. Frontiers in Psychology, 4, 347.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P.D., Nicholas J.M., Shakespeare T.J., et al. (2015c). P hysiological phenotyping of dementias using emotional sounds. Alzheimer’s and Dementia (Amsterdam), 1(2), 170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Stephan K.E., Montague R., Dolan R.J. (2014). Computational psychiatry: the brain as a phantastic organ. Lancet Psychiatry, 1(2), 148–58. [DOI] [PubMed] [Google Scholar]
- Fujisawa T.X., Cook N.D. (2011). The perception of harmonic triads: an fMRI study. Brain Imaging Behaviour, 5(2), 109–25. [DOI] [PubMed] [Google Scholar]
- Gauvin H.S., De Baene W., Brass M., Hartsuiker R.J. (2016). Conflict monitoring in speech processing: An fMRI study of error detection in speech production and perception. Neuroimage, 126, 96–105. [DOI] [PubMed] [Google Scholar]
- Gingras B., Pearce M.T., Goodchild M., Dean R.T., Wiggins G., McAdams S. (2016). Linking melodic expectation to expressive performance timing and perceived musical tension. Journal of Experimental Psychology: Human Perception and Performance, 42, 594–609. [DOI] [PubMed] [Google Scholar]
- Gladsjo J.A., Schuman C.C., Evans J.D., Peavy G.M., Miller S.W., Heaton R.K. (1999). Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment, 6(2), 147–78. [DOI] [PubMed] [Google Scholar]
- Golden H.L., Clark C.N., Nicholas J.M., et al. (2017). Music perception in dementia. Journal of Alzheimer’s Diseases, 55(3), 933–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Hillis A.E., Weintraub S., et al. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M., Robinson K., Bernhardt N., Koenig P. (2001). A rule-based categorization deficit in Alzheimer’s disease? Brain and Cognition, 45(2), 265–76. [DOI] [PubMed] [Google Scholar]
- Grossman M., Smith E.E., Koenig P.L., Glosser G., Rhee J., Dennis K. (2003). Categorization of object descriptions in Alzheimer’s disease and frontotemporal dementia: limitation in rule-based processing. Cognitve, Affective, and Behavioral Neuroscience, 3(2), 120–32. [DOI] [PubMed] [Google Scholar]
- Hailstone J.C., Omar R., Warren J.D. (2009). Relatively preserved knowledge of music in semantic dementia. Journal of Neurology, Neurosurgery, and Psychiatry, 80(7), 808–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailstone J.C., Ridgway G.R., Bartlett J.W., et al. (2011). Voice processing in dementia: a neuropsychological and neuroanatomical analysis. Brain, 134(9), 2535–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halabi C., Halabi A., Dean D.L., et al. (2013). Patterns of striatal degeneration in frontotemporal dementia. Alzheimer’s Disease and Associated Disorders, 27(1), 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy C., Marshall C., Golden H., et al. (2016). Hearing and dementia. Journal of Neurology, 263(11), 2339–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J.M., Choi W., Lowder M.W., Ferreira F. (2016). Language structure in the brain: a fixation-related fMRI study of syntactic surprisal in reading'. Neuroimage, 132, 293–300. [DOI] [PubMed] [Google Scholar]
- Henley S.M., Downey L.E., Nicholas J.M., et al. (2014). Degradation of cognitive timing mechanisms in behavioural variant frontotemporal dementia. Neuropsychologia, 65, 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh S., Hornberger M., Piguet O., Hodges J.R. (2012). Brain correlates of musical and facial emotion recognition: evidence from the dementias'. Neuropsychologia, 50(8), 1814–22. [DOI] [PubMed] [Google Scholar]
- Huron D. (2006) Sweet Anticipation: Music and the Psychology of Expectation, Cambridge: MIT Press. [Google Scholar]
- Ibanez A., Manes F. (2012). Contextual social cognition and the behavioral variant of frontotemporal dementia. Neurology, 78(17), 1354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish M., Addis D.R., Hodges J.R., Piguet O. (2012). Considering the role of semantic memory in episodic future thinking: evidence from semantic dementia. Brain, 135(7), 2178–91. [DOI] [PubMed] [Google Scholar]
- Irish M., Piguet O., Hodges J.R. (2012). Self-projection and the default network in frontotemporal dementia. Nature Reviews of Neurology, 8(3), 152–61. [DOI] [PubMed] [Google Scholar]
- Irish M., Piolino P. (2016). Impaired capacity for prospection in the dementias - theoretical and clinical implications. British Journal of Clinical Psychology, 55(1), 49–68. [DOI] [PubMed] [Google Scholar]
- Ismail Z., Herrmann N., Rothenburg L.S., et al. (2008). A functional neuroimaging study of appetite loss in Alzheimer’s disease. Journal of the Neurological Sciences, 271(1–2), 97–103. [DOI] [PubMed] [Google Scholar]
- Jackson M., Warrington E.K. (1986). Arithmetic skills in patients with unilateral cerebral lesions. Cortex, 22(22), 611–20. [DOI] [PubMed] [Google Scholar]
- Janata P., Birk J.L., Van Horn J.D., Leman M., Tillmann B., Bharucha J.J. (2002). The cortical topography of tonal structures underlying Western music. Science, 298(5601), 2167–70. [DOI] [PubMed] [Google Scholar]
- Khalfa S., Guye M., Peretz I., et al. (2008). Evidence of lateralized anteromedial temporal structures involvement in musical emotion processing. Neuropsychologia, 46(10), 2485–93. [DOI] [PubMed] [Google Scholar]
- Koelsch S., Fritz T., V Cramon D.Y., Müller K., Friederici A.D. (2006). Investigating emotion with music: an fMRI study. Human Brain Mapping, 27(3), 239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S., Gunter T., Friederici A.D., Schroger E. (2000). Brain indices of music processing: “nonmusicians” are musical. Journal of Cognitive Neuroscience, 12(3), 520–41. [DOI] [PubMed] [Google Scholar]
- Koelsch S., Rohrmeier M., Torrecuso R., Jentschke S. (2013). rocessing of hierarchical syntactic structure in music. Proceedings of the National Academy of Sciences of the United States of America, 110, 15443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumhansl C.L. (1997). An exploratory study of musical emotions and psychophysiology. Canadian Journal of Experimental Psychology, 51(4), 336–53. [DOI] [PubMed] [Google Scholar]
- Lang S., Kroll A., Lipinski S.J., et al. (2009). Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. European Journal of Neuroscience, 29(4), 823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehne M., Rohrmeier M., Koelsch S. (2014). Tension-related activity in the orbitofrontal cortex and amygdala: an fMRI study with music. Social Cognitive and Affective Neuroscience, 9(10), 1515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak M., Howieson D., Loring D. (2004) Neuropsychological Assessment, New York, Oxford University Press. [Google Scholar]
- Li C.W., Chen J.H., Tsai C.G. (2015). Listening to music in a risk-reward context: the roles of the temporoparietal junction and the orbitofrontal/insular cortices in reward-anticipation, reward-gain, and reward-loss’. Brain Research, 1629, 160–70. [DOI] [PubMed] [Google Scholar]
- Loui P., Wu E.H., Wessel D.L., Knight R.T. (2009). A generalized mechanism for perception of pitch patterns. Journal of Neuroscience, 29(2), 454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna P., Warrington E.K. (1983) Graded Naming Test Manual, Windsor: NFER-Nelson Publishing Company. [Google Scholar]
- Michelon P., Snyder A.Z., Buckner R.L., McAvoy M., Zacks J.M. (2003). Neural correlates of incongruous visual information. An event-related fMRI study. Neuroimage, 19(4), 1612–26. [DOI] [PubMed] [Google Scholar]
- Milinski M., Rockenbach B. (2012). On the interaction of the stick and the carrot in social dilemmas. Journal of Theoretical Biology, 299, 139–43. [DOI] [PubMed] [Google Scholar]
- Nazimek J.M., Hunter M.D., Hoskin R., Wilkinson I., Woodruff P.W. (2013). Neural basis of auditory expectation within temporal cortex. Neuropsychologia, 51(11), 2245–50. [DOI] [PubMed] [Google Scholar]
- Nelson H.E. (1982) Nelson Adult Reading Test Manual, London: The National Hospital for Nervous Diseases. [Google Scholar]
- O'Reilly J.X., Schuffelgen U., Cuell S.F., Behrens T.E., Mars R.B., Rushworth M.F. (2013). Dissociable effects of surprise and model update in parietal and anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America, 110(38), E3660–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar R., Hailstone J.C., Warren J.E., Crutch S.J., Warren J.D. (2010). The cognitive organization of music knowledge: a clinical analysis. Brain, 133(4), 1200–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar R., Henley S.M., Bartlett J.W., et al. (2011). ′The structural neuroanatomy of music emotion recognition: evidence from frontotemporal lobar degeneration′. Neuroimage, 56(3), 1814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz I., Blood A.J., Penhune V., Zatorre R. (2001). Cortical deafness to dissonance. Brain, 124(Pt 5), 928–40. [DOI] [PubMed] [Google Scholar]
- Perry D.C., Sturm V.E., Seeley W.W., Miller B.L., Kramer J.H., Rosen H.J. (2014). Anatomical correlates of reward-seeking behaviours in behavioural variant frontotemporal dementia. Brain, 137(6), 1621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D.C., Sturm V.E., Wood K.A., Miller B.L., Kramer J.H. (2015). Divergent processing of monetary and social reward in behavioral variant frontotemporal dementia and Alzheimer disease. Alzheimer’s Diseases and Associated Disorders, 29(2), 161–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pievani M., de Haan W., Wu T., Seeley W.W., Frisoni G.B. (2011). Functional network disruption in the degenerative dementias. Lancet Neurology, 10(9), 829–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressnitzer D., Suied C., Shamma S.A. (2011). Auditory scene analysis: the sweet music of ambiguity. Frontiers in Human Neuroscience, 5, 158.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S., Sahakian B.J., Hodges J.R., Rogers R.D., Robbins T.W. (1999). Specific cognitive deficits in mild frontal variant frontotemporal dementia. Brain, 122(8), 1469–93. [DOI] [PubMed] [Google Scholar]
- Rascovsky K., Hodges J.R., Knopman D., et al. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134(9), 2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J.D., Sauter D., Scott S., Rossor M.N., Warren J.D. (2012). Receptive prosody in nonfluent primary progressive aphasias. Cortex, 48(3), 308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M.E., Ellis R.J., Schlaug G., Loui P. (2016). Brain connectivity reflects human aesthetic responses to music. Social Cognitive and Affective Neuroscience, 11, 884–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimpoor V.N., Benovoy M., Larcher K., Dagher A., Zatorre R.J. (2011). Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nature of Neuroscience, 14(2), 257–62. [DOI] [PubMed] [Google Scholar]
- Salimpoor V.N., van den Bosch I., Kovacevic N., McIntosh A.R., Dagher A., Zatorre R.J. (2013). Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science, 340(6129), 216–9. [DOI] [PubMed] [Google Scholar]
- Salimpoor V.N., Zald D.H., Zatorre R.J., Dagher A., McIntosh A.R. (2015). Predictions and the brain: how musical sounds become rewarding. Trends in Cognitive Sciences, 19(2), 86–91. [DOI] [PubMed] [Google Scholar]
- Sapey-Triomphe L.A., Heckemann R.A., Boublay N., et al. (2015). Neuroanatomical correlates of recognizing face expressions in mild stages of Alzheimer’s disease. PLoS One, 10(12), e0143586.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. (2013). Updating dopamine reward signals. Current Opinion in Neurobiology, 23(2), 229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seashore C.E. (1919) The Psychology of Musical Talent. New York: Silver, Burdett and Company. [Google Scholar]
- Seger C.A., Spiering B.J., Sares A.G., et al. (2013). Corticostriatal contributions to musical expectancy perception. Journal of Cognitive Neuroscience, 25(7), 1062–77. [DOI] [PubMed] [Google Scholar]
- Sinz H., Zamarian L., Benke T., Wenning G.K., Delazer M. (2008). Impact of ambiguity and risk on decision making in mild Alzheimer’s disease. Neuropsychologia, 46(7), 2043–55. [DOI] [PubMed] [Google Scholar]
- Steinbeis N., Koelsch S., Sloboda J.A. (2006). The role of harmonic expectancy violations in musical emotions: evidence from subjective, physiological, and neural responses. Journal of Cognitive Neuroscience, 18(8), 1380–93. [DOI] [PubMed] [Google Scholar]
- Sturm V.E., Rosen H.J., Allison S., Miller B.L., Levenson R.W. (2006). Self-conscious emotion deficits in frontotemporal lobar degeneration. Brain, 129(9), 2508–16. [DOI] [PubMed] [Google Scholar]
- Sturm V.E., Yokoyama J.S., Eckart J.A., et al. (2015). Damage to left frontal regulatory circuits produces greater positive emotional reactivity in frontotemporal dementia. Cortex, 64, 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm V.E., Yokoyama J.S., Seeley W.W., Kramer J.H., Miller B.L., Rankin K.P. (2013). Heightened emotional contagion in mild cognitive impairment and Alzheimer's disease is associated with temporal lobe degeneration'. Proceedings of the National Academy of Sciences of the United States of America, 110(24), 9944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmann B. (2005). Implicit investigations of tonal knowledge in nonmusician listeners. Annals of the New York Academy of Sciences, 1060, 100–10. [DOI] [PubMed] [Google Scholar]
- Torralva T., Kipps C.M., Hodges J.R., et al. (2007). The relationship between affective decision-making and theory of mind in the frontal variant of fronto-temporal dementia. Neuropsychologia, 45(2), 342–9. [DOI] [PubMed] [Google Scholar]
- Tsai C.-G., Chen C.-P. (2015). Musical Tension over Time: Listeners’ Physiological Responses to the ‘Retransition’ in Classical Sonata Form'. Journal of New Music Research, 44(3), 271–86. [Google Scholar]
- Tsai C.G., Chen C.C., Wen Y.C., Chou T.L. (2015). Neuromagnetic brain activities associated with perceptual categorization and sound-content incongruency: a comparison between monosyllabic words and pitch names. Frontiers in Human Neuroscience, 9, 455.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuust P., Gebauer L.K., Witek M.A. (2014). Neural underpinnings of music: the polyrhythmic brain. Advances in Experimental Medicine and Biology, 829, 339–56. [DOI] [PubMed] [Google Scholar]
- Wallis J.D. (2007). Orbitofrontal cortex and its contribution to decision-making. Annual Review of Neuroscience, 30, 31–56. [DOI] [PubMed] [Google Scholar]
- Warren J.D., Rohrer J.D., Rossor M.N. (2013). Clinical review. Frontotemporal dementia. BMJ, 347, f4827.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington E.K. (1984) Recognition Memory Test: Manual. Berkshire, UK: NFER‐Nelson. [Google Scholar]
- Warrington E.K. (1996) The Camden Memory Test Battery. Hove: Psychology Press. [Google Scholar]
- Warrington E.K., James M. (1991) The Visual Object and Space Perception Battery. Bury St. Edmunds UK: Thames Valley Test Company. [Google Scholar]
- Warrington E.K., McKenna P., Orpwood L. (1998). Single word comprehension: a concrete and abstract word synonym test. Neuropsychological Rehabilitation, 8(2), 143–54. [Google Scholar]
- Wechsler D. (1981) Wechsler Adult Intelligence Scale‐-Revised, New York, Psychological Corporation. [Google Scholar]
- Wechsler D. (1987) WMS-R: Wechsler Memory Scale-Revised: Manual. Harcourt: Brace Jovanovich. [Google Scholar]
- Wechsler D. (1997) Wechsler Adult Intelligence Scale–Third Edition: Administration and Scoring Manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Weinstein J., Koenig P., Gunawardena D., McMillan C., Bonner M., Grossman M. (2011). Preserved musical semantic memory in semantic dementia. Archives in Neurology, 68(2), 248–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Seeley W.W. (2014). Network dysfunction in Alzheimer’s disease and frontotemporal dementia: implications for psychiatry. Biological Psychiatry, 75(7), 565–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.