Abstract
Direct eye gaze is a powerful stimulus in social interactions, yet people vary considerably in the range of gaze lines that they accept as being direct (cone of direct gaze, CoDG). Here, we searched for a possible neural trait marker of these individual differences. We measured the width of the CoDG in 137 healthy participants and related their individual CoDG to their neural baseline activation as measured with resting electroencephalogram. Using a source-localization technique, we found that resting theta current density in the left temporo-parietal junction (TPJ) and adjacent posterior superior temporal sulcus (pSTS) was associated with the width of CoDG. Our findings suggest that the higher the baseline cortical activation in the left TPJ/pSTS, the wider the CoDG and thus the more liberal the individuals’ judgments were in deciding whether a looker stimulus was making eye contact or not. This is a first demonstration of the neural signatures underlying individual differences in the feeling of being looked at.
Keywords: resting EEG, neural trait marker, eye gaze, cone of direct gaze, mentalizing network
Introduction
Imagine getting on a crowded train. As you sit down on one of the remaining empty seats, you notice the stare of a person in the next compartment. Is this person looking at you? Do you know this person? Does she want to start a conversation? Or might she not be looking at you at all, but instead at the person behind you? If you assume that the woman on the train is looking at you and you decide to approach her, this might lead to an enjoyable social interaction. If on the other hand you assume that she is making eye contact with the person behind you, you may miss a pleasant encounter. Knowing where another person is looking is of central importance for social interactions (Argyle and Cook, 1976; Kleinke, 1986; Baron-Cohen, 1995). In human beings, eye contact is associated with approach orientation and affiliation motivation while averted gaze might signalize avoidance orientation and disinterest. Hence, if someone looks us in the eye we often interpret this as something positive, especially if this person shows a friendly expression (c.f. Lobmaier et al., 2008; Lobmaier and Perrett, 2011). In turn, the assumption that another person is making eye contact may invite us to reciprocate the affiliative orientation which can result in a positive social encounter.
There is a considerable range of gaze directions that are accepted as making eye contact (e.g. Gibson and Pick, 1963; Gamer and Hecht, 2007; Lobmaier et al., 2008; Lobmaier and Perrett, 2011; Harbort et al., 2017). This lead Gamer and Hecht (2007) to suggest that gaze direction should be thought of as a cone rather than of a ray as assumed in earlier studies (e.g. Gale and Monk, 2000; Symons et al., 2004). The cone of direct gaze (CoDG) refers to the range of gaze directions that an observer judges as being directed towards them: the wider the range, the more liberal the observer’s judgement. Interestingly, most people show a CoDG of considerable width (c.f. Gamer and Hecht, 2007; Gamer et al., 2011) but there seem to be remarkable individual differences in the range of gaze angles that are accepted as being direct (e.g. Ewbank et al., 2009; Gamer et al., 2011; Schulze et al., 2013a; Harbort et al., 2017). This means that observers are prone to assume mutual gaze even when the looker is actually looking away, but that some people show this bias more than others.
Attempts to explain individual variability in the width of the CoDG primarily focused on differences in social anxiety (Gamer et al., 2011; Jun et al., 2013; Schulze et al., 2013a; Schulze et al., 2013b; Harbort et al., 2017) and autistic traits (e.g. Matsuyoshi et al., 2014). These studies have found that individuals with higher social anxiety interpret a wider range of gaze lines as making eye contact whereas autism has been associated with narrower CoDG. Healthy participants lie somewhere in between. Hence there might be an optimal width of CoDG which does not reflect faultless discrimination of direct and averted gaze. Rather there seems to be an area of ambiguity which might in fact be beneficial in our daily social interactions, as has been illustrated in the crowded train scenario described above.
The present study aimed at using task-independent neural baseline activation measured by resting electroencephalogram (EEG) to reveal sources of individual differences in the width of CoDG. Measuring resting EEG involves recording electrical activity on the scalp when the participant is at rest to index patterns of baseline neural activation that are not related to any particular task. These patterns of baseline neural activation are ideal neural trait markers because they have been demonstrated to be highly specific (i.e. the extent to which an EEG pattern uniquely belongs to a given person; Dunki et al., 2000; Napflin et al., 2007) and highly stable over time (e.g. Dunki et al., 2000; Napflin et al., 2007; Cannon et al., 2012). Resting EEG is thus much like a neural ‘fingerprint’ and has been used to reveal sources of individual differences in time preferences (Gianotti et al., 2012), risk preferences (Jäncke et al., 2008; Gianotti et al., 2009; Studer et al., 2013), and social preferences (Knoch et al., 2010; Baumgartner et al., 2013; for a review, see Nash et al., 2015). Hence, this measure provides a promising neural trait marker to investigate possible sources for individual differences in the width of the CoDG.
To date, studies on the neural underpinnings of gaze processing primarily focused on brain activity during gaze perception tasks (i.e. task-evoked activity) and found an association between processing of gaze direction and the fusiform gyrus, the superior temporal sulcus (STS), the temporo-parietal junction (TPJ), medial prefrontal (mPFC) and orbitofrontal cortices (Conty et al., 2007; Itier and Batty, 2009; Senju and Johnson, 2009; for a review, see Hamilton, 2016). Although these findings do not identify neural traits responsible for people’s ability to process direct gaze, they can inform possible hypotheses about which brain structures may be involved when searching for neural dispositional determinants of the inter-individual variability in the CoDG.
Recent research has offered compelling evidence that the width of the CoDG is modulated by the emotional expression on the looker's face. Especially for happy faces, a wider range of gaze directions is accepted as being direct than for example for neutral or angry faces (Lobmaier et al., 2008; Lobmaier and Perrett, 2011), suggesting that gaze direction and facial expression are meaningfully combined in the processing of socially relevant facial information (c.f. Adams and Kleck, 2003, 2005). In the light of these findings, we included faces with happy and neutral expressions in the present research, to further investigate whether potential resting EEG predictions on the CoDG are modulated by the facial expression on the looker’s face.
We measured neural baseline activation using resting EEG in healthy individuals and examined whether these are related to the width of the CoDG in a subsequent gaze discrimination task which required participants to indicate whether a briefly presented face was making eye contact or not (c.f. Lobmaier et al., 2008; Lobmaier and Perrett, 2011). The presented face either showed a happy or neutral expression. In light of the above-mentioned findings, we might expect baseline activation in the TPJ, STS, mPFC and fusiform gyrus to be related to CoDG, however, we intentionally conducted whole-brain analyses without any a-priori hypotheses.
Materials and methods
Participants
One-hundred-thirty-seven right-handed students recruited at the University of Bern participated in the study. Informed consent was obtained from all participants. Participants indicated neither current nor previous history of neurological and psychiatric disorders or alcohol and drug abuse. All participants had normal or corrected to normal vision. Four male participants were excluded from the analysis due to inconsistent responses recorded during the behavioral task, which precluded the estimation of the behavioral indices used in the current study. One additional female participant was excluded because of an excessive amount of EEG artifacts, leaving a sample of 132 participants (104 women and 28 men). Mean age was 22.6 years (s.d. = 3.2, range: 19–47). The study was approved by the local Ethics Committee. All participants gave written informed consent and were informed of their right to discontinue participation at any time. Participants received 35 Swiss francs (CHF 1 = USD 1.05) for participation. We recruited for one academic year and collected as much data as possible during that time. Data were collected in a single wave and then analyzed (no analyses were calculated before all participants were tested).
Procedure
After obtaining written informed consent, participants were seated comfortably in a dimly lit, sound- and electrically shielded recording chamber with intercom connection to the experimenters. Participants were instructed that EEG recording was to be done while they rested with their eyes alternately open or closed, and that they would later participate in a gaze discrimination task. The resting EEG protocol consisted of the participants resting for 20 s with their eyes open, followed by 40 s with their eyes closed; this was repeated five times. Such a protocol guarantees minimal fluctuations in participants’ vigilance state. The instructions about eye opening/closing were given via intercom. Data analysis is based on the 200-s eyes-closed condition. After the recording of the resting EEG, the electrodes were removed and participants received written instructions for the gaze discrimination task.
Stimuli and gaze discrimination task
Three-dimensional face stimuli were created using the software package FaceGen Modeller 3.5.2 (Singular Inversions Inc., 2010) which enables the generation of face stimuli with a high level of realism. Faces of 5 Caucasian gender-neutral avatars were generated while expressing neutral and happy emotion (Figure 1A). To ensure that the perceptual features of different face stimuli did not affect the results, the 5 avatars were generated by using the ‘genetic’ tool. This tool allows to create highly similar faces with a predefined level of randomness (30%). The gaze direction of the faces was aligned with the head direction, so that nose, gaze fixation point and virtual camera lay on the same axis. The avatar faces obtained with this procedure were then rotated in one-degrees steps, producing 17 different viewing angles (from 1° to 8° to the left and right, and 0°). All stimuli [N = 180; 18 angles (0° angle was shown twice) x 2 emotional expressions x 5 avatars] were presented pseudo-randomly across three experimental blocks (60 trials each), with the constraint that each angle, emotional expression and face was equally distributed across the blocks.
Fig. 1.

(A) Stimuli samples of neutral and happy faces in three different views (–8°, 0°, +8°). (B) Timeline of the Gaze Discrimination Task: Faces were presented for 300 ms, followed by a response window of 1700 ms. Each trial is separated by a variable ITI interval (750–900 ms).
Participants were seated at a distance of 60 cm from a PC screen. Lighting was kept constant for all participants and the screen was manually adapted so that the eyes of the avatars were vertically aligned with the eyes of the participants. Each face was presented for 300 ms in the center of the screen, followed by a response window of 1700 ms, followed by an inter-trial interval (ITI) of variable duration (between 750 ms and 900 ms). During both the response window and the ITI period a fixation cross was shown (Figure 1B). Participants were asked to decide as fast as possible whether the presented face was gazing directly at them (‘yes response’ pressing the letter ‘A’ on the keyboard) or not (‘no response’ pressing the letter ‘L’ on the keyboard). The correspondence between yes/no responses and the response keys (i.e. ‘A’ vs ‘L’) was counterbalanced across participants.
Psychometric analysis of behavioral data
The proportion of yes and no responses across emotions and visual angles were used to compute the CoDG. As a first step, we calculated the percentage of times the participant decided that the face stimulus was gazing directly at him/her as a function of the gaze angle. We then fitted the data to a logistic function using an in-house algorithm to calculate the point of subjective equivalence (pse). The pse is defined as the angle at which a participant would be predicted to choose the yes and no responses with equal frequency (i.e. the percentage of yes and no responses each equals 50%). Such analysis was conducted separately for each emotion, and for left and right side gaze angles. Then, the CoDG of each emotion was calculated as the sum of the absolute values of the left and right side pse.
Resting EEG recording and pre-processing
Resting EEG was continuously recorded with a BrainAmp DC amplifier system using 60 Ag-AgCl electrodes mounted in an elastic cap and placed according to the international 10-10 system (Nuwer et al., 1998). The electrode at the position FCz was the recording reference, while the electrode at the position CPz served as ground. Data were recorded with a sampling rate of 500 Hz (bandwidth: 0.1–250 Hz). Horizontal and vertical eye movements were recorded with electrodes at the left and right outer canthi and at the left infraorbital area. Impedances were kept below 10 kΩ. Eye movement artifacts were corrected by independent component analysis. EEG signals from channels with corrupted signals were interpolated. A computerized artifact rejection was applied to the EEG collected at rest (maximal allowed voltage step: 15 μV/ms; minimal allowed activity in intervals of 100-ms length: 0.5 μV; maximal allowed amplitude: ±100 μV). Data were additionally examined visually to eliminate residual artifacts (e.g. large movement-related artifacts). All available artifact-free 2000-ms EEG epochs were extracted and recomputed against the average reference. On average, there were 87.2 epochs (s.d. = 16.8) available per participant. A Fast Fourier Transformation (using a square window) was applied to each epoch and channel to compute the power spectra with 0.5-Hz resolution. The spectra for each channel were averaged over all epochs for each participant. Absolute power spectra were integrated for the following seven independent frequency bands (Kubicki et al., 1979): Delta (1.5–6 Hz), theta (6.5–8 Hz), alpha1 (8.5–10 Hz), alpha2 (10.5–12 Hz), beta1 (12.5–18 Hz), beta2 (18.5–21 Hz) and beta3 (21.5–30 Hz).
Standardized low-resolution brain electromagnetic tomography (sLORETA; Pascual-Marqui et al., 2002) was used to estimate the intracerebral electrical sources that generated the scalp-recorded activity. sLORETA computes electrical neural activity as current density (A/m2) without assuming a predefined number of active sources. The sLORETA solution space consists of 6239 voxels (voxel size: 5 × 5 × 5 mm) and is restricted to cortical gray matter and hippocampi, as defined by the digitized Montreal Neurological Institute probability atlas. The sLORETA method has received considerable validation from studies combining EEG/MEG source localizations performed in conjunction with other localization methods, such as functional Magnetic Resonance Imaging (fMRI, Mobascher et al., 2009; Olbrich et al., 2009) and Positron Emission Tomography (Laxton et al., 2010). Further, the method has been validated with experimental data for which the true generators are known from invasive, implanted depth electrodes (Zumsteg et al., 2006a; Zumsteg et al., 2006b) and has been demonstrated to be able to correctly localize deep structures such as the anterior cingulate cortex (e.g. Pizzagalli et al., 2001) and mesial temporal lobes (e.g. Zumsteg et al., 2006a). Using the automatic regularization method in the sLORETA software, we chose the transformation matrix with the signal-to-noise ratio set to 10. To reduce confounds that have no regional specificity, for each participant, sLORETA images were normalized to a total power of one and then log-transformed before statistical analyses. Due to a relatively large range of age of our participants, we first regressed the putatitve age-influence out of sLORETA images. The standardized sLORETA residuals were then used for further analyses.
Statistical analysis
In order to explore the effect of the emotional expression of the faces on the CoDG, we conducted a paired t-test.
The main goal of this study was to examine whether subject-specific CoDG can be explained based on a task-independent neural trait marker. For that purpose, we conducted whole-brain regression analyses (separately for each frequency band and emotion) using the subject-specific CoDG as dependent variables. The non-parametric randomization approach (Nichols and Holmes, 2002) was used for estimating empirical probability distributions (number of randomizations used: 5000) and the corresponding critical probability thresholds (corrected for multiple comparisons). In a next step, for regions that displayed significant, whole-brain corrected correlations, the voxels with the strongest correlations (peak voxels) were used for the constructions of spherical regions of interest (ROIs; radius: 10 mm around the peak voxel). Mean current density within the ROIs were calculated and used for visualization.
Results
Behavioral results
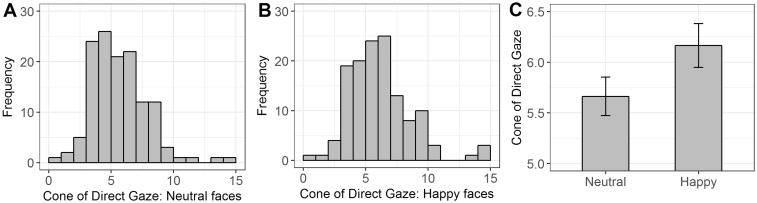
Large inter-individual differences in the CoDG were observed in both emotional expressions (Figure 2A and B). For neutral expressions the CoDG varied from 0.83° to 14.36° (M = 5.66°; s.d. = 2.18°), while for happy expressions the CoDG varied from 0.52° to 14.92° (M = 6.16°; s.d. = 2.47°). Paired t-test revealed a significant larger CoDG for happy expressions compared to neutral expressions [t(131) = 4.49, P < 0.001; Figure 2C].
Fig. 2.
Frequency plots of the CoDG for neutral (A), and happy (B) faces. Width of the CoDG across the two emotional expressions (C). Error bars depict standard errors.
Brain results
As demonstrated above, participants strongly differed in their subjective feeling of being looking at. Accordingly, we conducted whole-brain regression analyses using the CoDG (for neutral and happy faces, separately) as dependent variables. Using sLORETA as a source localization technique to estimate intra-cerebral sources underlying scalp-recorded resting EEG, we found that in the theta frequency band a cluster of voxels in the left TPJ and extending to the left posterior superior temporal sulcus (pSTS) showed significant negative correlations between current density and the two CoDG. In particular, baseline theta current density negatively correlated with the CoDG for neutral faces in the left TPJ (Figure 3A; cluster size = 500 mm3; MNI coordinates peak voxel: x = −60, y = –55, z = 30, Brodmann area 40). Baseline current density in the theta band was also negatively correlated with the CoDG for happy faces in the left TPJ and in the left pSTS (Figure 3B; cluster size = 1500 mm3; MNI coordinates peak voxel: x = −65, y = –50, z = 20, Brodmann area 22). Regression analyses conducted with the two ROIs (spheres of 10 mm radius around the peak voxels) revealed negative correlation coefficients of –0.24 for the CoDG for neutral faces (P = 0.007), and of –0.23 for the CoDG for happy faces (P = 0.008). Removing four participants who showed CoDG values larger than 2.5 s.d. or more from the mean did not affect the results, CoDG for neutral faces: r(125) = –0.21, P = 0.02; CoDG for happy faces: r(125) = –0.20, P = 0.03. Also, partialing out the covariation for participant sex did not affect the results, CoDG for neutral faces: r(129) = –0.23, P = 0.007; CoDG for happy faces: r(129) = –0.23, P = 0.009.
Fig. 3.
Relationship between the CoDG for the neutral (A) and happy (B) faces and the baseline theta current density. In each panel, on the left side, locations of the voxels that showed significant correlations (whole-brain corrected) are indicated in red (P < 0.05) or in yellow (0.05 < P < 0.10) and, on the right side, scatter plots (based on a 10 mm spherical ROI around the peaks of the negative correlations; including regression lines and confidence intervals 95%) are shown, demonstrating the relationship between the CoDG and baseline theta current density. Please note that the numbers reported in the upper right part of the scatter plots represent the coefficients of the correlations between theta current density in the ROIs and the CoDG. Voxels that showed correlations in both emotional expressions (conjunction: Theta current density vs CoDG for neutral faces ∩ Theta current density vs CoDG for happy faces) are shown in (C).
Conjunction analysis (Figure 3C) clearly indicated that the width of both CoDG (i.e. for neutral and for happy faces) was correlated with the baseline current density in the theta band in the same neural region, the left TPJ/pSTS. Our findings were specific to the left TPJ/pSTS; in no other brain region was baseline theta current density correlated with CoDG at the corrected significance threshold. Interestingly, lowering the threshold, very similar results regarding the left TPJ/pSTS were found in the delta band (P = 0.11; Supplementary Figure S1). No significant correlations were found in any other EEG frequency bands.
Discussion
Direct eye gaze is a powerful stimulus in social interactions and is often associated with interest and approach orientation. If we feel looked at, we may be invited to reciprocate the affiliative orientation which again may lead to positive social encounters. Accepting an extremely narrow range of gaze lines as making eye contact might result in missing out on potentially positive encounters, a too liberal sense of being looked at may instead be associated with either exaggerated feelings of self-importance or paranoia. Interestingly, most individuals’ range of gaze lines that are interpreted as being direct includes gaze angles that are averted to the left or right and people vary considerably in the range of gaze lines that they accept as being direct (e.g. Gamer and Hecht, 2007; Harbort et al., 2017). Recent research has linked such individual variability to social anxiety or autistic traits (e.g. Gamer et al., 2011; Jun et al., 2013; Schulze et al., 2013a; Schulze et al., 2013b; Matsuyoshi et al., 2014; von dem Hagen et al., 2014; Harbort et al., 2017). We provide first evidence that a specific neural trait marker-task-independent baseline theta current density in the left TPJ and in the left pSTS-is negatively correlated with the width of the CoDG. It is important to note that these findings are based on whole-brain corrected analyses. Thus, our results reveal a significant relation between baseline activation in the TPJ/pSTS and width of CoDG even without testing any a-priori hypotheses. As baseline slow wave oscillations (in the delta and theta band) likely reflect decreased cortical activation, these findings suggest that the higher the baseline cortical activation in the left TPJ/pSTS, the wider the CoDG and thus the more liberal the individuals’ judgments were in deciding whether a looker stimulus was making eye contact or not. Our interpretation of the functional significance of slow wave oscillations (in particular delta and theta current density) during rest (that is, not during task execution) is based on the observation that an increase in slow wave oscillations is typically observed during lower vigilance stages and increased subjective drowsiness (e.g. Strijkstra et al., 2003). Moreover, resting EEG-fMRI studies found negative correlations between theta power and the BOLD signal in regions close to the TPJ (Scheeringa et al., 2008; Luchinger et al., 2011; Feige et al., 2017). We acknowledge that some caution is warranted here as recent literature suggests a complex interpretation of the functional role of EEG slow waves at rest (see e.g. O’Gorman et al., 2013). Also, it is important to note that the functional significance of slow wave oscillations critically depends on whether participants are measured at rest or during task execution (e.g. Billeke et al., 2014).
The left TPJ is an important node of the Default Mode Network that is activated during social cognition tasks [e.g. Schilbach et al., 2008; for a meta-analysis see (Eickhoff et al., 2009; for a review see Li et al., 2014)]. In particular, it has been argued that TPJ as well as the adjacent pSTS play a fundamental role in the mentalizing system (c.f. Frith and Frith, 2006; Saxe, 2006). Given that gaze direction is an important stimulus for the attribution of mental states (e.g. Baron-Cohen, 1995; Khalid et al., 2016) it is particularly interesting that task-independent baseline activation in the TPJ/pSTS is related to individual differences in the feeling of being looked at. Online studies have shown increased activity in the TPJ/pSTS when participants encoded self-directed communicative intentions (c.f. Ciaramidaro et al., 2014). This leads us to the speculation that people with higher baseline activation in TPJ/sSTS, that is, people with higher perspective-taking abilities, are more likely to feel looked at than their counterparts with lower perspective-taking abilities. This would mean that people with higher perspective-taking abilities, rather than being highly accurate in detecting direct gaze, accept a wider range of gaze lines as looking at them, leading them to be more likely to approach the person who is supposedly making eye contact. Indeed, it might be more beneficial for our everyday social interactions to wrongly assume that someone is looking at us than missing a gaze that is directed at us (c.f. Mareschal et al., 2013). Obviously, this erroneous assumption that someone is looking at us when she is actually not becomes maladaptive if too extreme. Support for this may be found in clinical populations. For example, social anxiety disorder is associated with hyperactive TPJ in online studies (e.g. Gaebler et al., 2014; Boehme et al., 2015; for a review see Bruhl et al., 2014) as well as wide CoDG (Jun et al., 2013; Schulze et al., 2013a; Harbort et al., 2017). Clinical evidence shows that also the other extreme is maladaptive: People with autism spectrum disorder showed an abnormal increase in EEG delta and theta power at rest (for a review see Wang et al., 2013), have a hypoactive TPJ in online studies (e.g. Lombardo et al., 2011; Pantelis et al., 2015) and show a narrower CoDG (c.f. Matsuyoshi et al., 2014; von dem Hagen et al., 2014). Healthy individuals lie somewhere midway the continuum between individuals with autism spectrum disorder and individuals with social anxiety, both in respect to TPJ/pSTS activation and CoDG. Please note that in the present study, we recruited men and women who self-reported as having no current or previous history of neurological or psychiatric disorders. We did not specifically screen our participants for social anxiety or autistic traits, so it is possible that one or the other may have had elevated traits of either disorders.
An alternative interpretation would be that individual differences in the feeling of being looked at reflect differences in perceptual processes. The STS plays an important role in perception of gaze directions (e.g. Carlin and Calder, 2013), in particular, activity in this region has been found to be associated with processing of direct gaze in online studies (for reviews see Conty et al., 2007; Senju and Johnson, 2009). One could therefore speculate that higher baseline activation in the pSTS might increase the probability to falsely perceive an indirect gaze as being directed towards oneself.
We note that even though the baseline activation in the TPJ and pSTS represents a significant neural marker associated with the individual differences in CoDG, it explained approximately 5% of the variance only. It will have to be the aim of future studies to identify further factors explaining more variability in the width of the CoDG.
Paralleling earlier findings, we found wider CoDG for faces expressing positive emotions (i.e. happy) compared to neutral expressions. This has been interpreted as a self-referential positivity bias in gaze perception (e.g. Lobmaier et al., 2008; Lobmaier and Perrett, 2011), suggesting that we prefer to think that a friendly face is looking at us than a face showing a neutral or negative expression. We thus replicated this well documented positivity bias in gaze perception using highly controlled artificial avatar faces instead of 3D head models of real men and women as in earlier studies (e.g. Lobmaier et al., 2008; Lobmaier and Perrett, 2011). Interestingly, the strength of the correlations between baseline activation in the TPJ/pSTS and CoDG was not differentially related to the emotions expressed in the faces. However, the number of voxels that were significantly correlated with the CoDG was three times as large for happy faces than for faces showing a neutral expression. This might explain the larger CoDG in happy faces.
In conclusion, we herewith have identified a promising neural trait marker (i.e. task-independent baseline activation in the TPJ and pSTS) which helps to explain the individual variability in the feeling of being looked at.
Supplementary Material
Acknowledgement
The authors thank Alexander von Bergen, Peggy Kübler, Flavio Schmidig and Gina Spizzi for their help in data collection and processing.
Funding
This work was supported by the Swiss National Science Foundation [100019_166006 to D.K and L.G.]; and the Mens Sana Foundation [to D.K.].
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Adams R.B. Jr, Kleck R.E. (2003). Perceived gaze direction and the processing of facial displays of emotion. Psychological Science, 14(6), 644–7. [DOI] [PubMed] [Google Scholar]
- Adams R.B. Jr, Kleck R.E. (2005). Effects of direct and averted gaze on the perception of facially communicated emotion. Emotion, 5(1), 3–11. [DOI] [PubMed] [Google Scholar]
- Argyle M., Cook M. (1976). Gaze and Mutual Gaze. New York: Cambridge University Press. [Google Scholar]
- Baron-Cohen S. (1995) Mindblindness: An Essay on Autism and Theory of Mind. Cambridge, MA: The MIT Press. [Google Scholar]
- Baumgartner T., Gianotti L.R.R., Knoch D. (2013). Who is honest and why: Baseline activation in anterior insula predicts inter-individual differences in deceptive behavior. Biological Psychology, 94(1), 192–7. [DOI] [PubMed] [Google Scholar]
- Billeke P., Zamorano F., Lopez T., Rodriguez C., Cosmelli D., Aboitiz F. (2014). Someone has to give in: theta oscillations correlate with adaptive behavior in social bargaining. Social Cognitive and Affective Neuroscience, 9(12), 2041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme S., Miltner W.H., Straube T. (2015). Neural correlates of self-focused attention in social anxiety. Social Cognitive and Affective Neuroscience, 10(6), 856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhl A.B., Delsignore A., Komossa K., Weidt S. (2014). Neuroimaging in social anxiety disorder-a meta-analytic review resulting in a new neurofunctional model. Neuroscience and Biobehavioral Reviews, 47, 260–80. [DOI] [PubMed] [Google Scholar]
- Cannon R.L., Baldwin D.R., Shaw T.L., et al. (2012). Reliability of quantitative EEG (qEEG) measures and LORETA current source density at 30 days. Neuroscience Letters, 518(1), 27–31. [DOI] [PubMed] [Google Scholar]
- Carlin J.D., Calder A.J. (2013). The neural basis of eye gaze processing. Current Opinion in Neurobiology, 23(3), 450–5. [DOI] [PubMed] [Google Scholar]
- Ciaramidaro A., Becchio C., Colle L., Bara B.G., Walter H. (2014). Do you mean me? Communicative intentions recruit the mirror and the mentalizing system. Social Cognitive and Affective Neuroscience, 9(7), 909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conty L., N’Diaye K., Tijus C., George N. (2007). When eye creates the contact! ERP evidence for early dissociation between direct and averted gaze motion processing. Neuropsychologia, 45(13), 3024–37. [DOI] [PubMed] [Google Scholar]
- Dunki R.M., Schmid G.B., Stassen H.H. (2000). Intraindividual specificity and stability of human EEG: comparing a linear vs a nonlinear approach. Methods of Information in Medicine, 39, 78–82. [PubMed] [Google Scholar]
- Eickhoff S.B., Laird A.R., Grefkes C., Wang L.E., Zilles K., Fox P.T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewbank M.P., Jennings C., Calder A.J. (2009). Why are you angry with me? Facial expressions of threat influence perception of gaze direction. Journal of Vision, 9(16), 1–7. [DOI] [PubMed] [Google Scholar]
- Feige B., Spiegelhalder K., Kiemen A., et al. (2017). Distinctive time-lagged resting-state networks revealed by simultaneous EEG-fMRI. Neuroimage, 145(Pt A), 1–10. [DOI] [PubMed] [Google Scholar]
- Frith C.D., Frith U. (2006). The neural basis of mentalizing. Neuron, 50(4), 531–4. [DOI] [PubMed] [Google Scholar]
- Gaebler M., Daniels J.K., Lamke J.P., Fydrich T., Walter H. (2014). Behavioural and neural correlates of self-focused emotion regulation in social anxiety disorder. Journal of Psychiatry Neuroscience, 39(4), 249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale C., Monk A.F. (2000). Where am I looking? The accuracy of video-mediated gaze awareness. Perception and Psychophysics, 62(3), 586–95. [DOI] [PubMed] [Google Scholar]
- Gamer M., Hecht H. (2007). Are you looking at me? Measuring the cone of gaze. Journal of Experimental Psychology: Human Perception and Performance, 33(3), 705–15. [DOI] [PubMed] [Google Scholar]
- Gamer M., Hecht H., Seipp N., Hiller W. (2011). Who is looking at me? The cone of gaze widens in social phobia. Cognition and Emotion, 25(4), 756–64. [DOI] [PubMed] [Google Scholar]
- Gianotti L.R., Knoch D., Faber P.L., et al. (2009). Tonic activity level in the right prefrontal cortex predicts individuals’ risk taking. Psychological Science, 20(1), 33–8. [DOI] [PubMed] [Google Scholar]
- Gianotti L.R.R., Figner B., Ebstein R.P., Knoch D. (2012). Why some people discount more than others: baseline activation in the dorsal PFC mediates the link between COMT genotype and impatient choice. Frontiers in Neuroscience, 6, Article no: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J.J., Pick A.D. (1963). Perception of another person’s looking behavior. American Journal of Psychology, 76, 386–94. [PubMed] [Google Scholar]
- Hamilton A.F.D. (2016). Gazing at me: the importance of social meaning in understanding direct-gaze cues. Philosophical Transactions of the Royal Society B-Biological Sciences, 371(1686), 20150080.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbort J., Spiegel J., Witthöft M., Hecht H. (2017). The effects of social pressure and emotional expression on the cone of gaze in patients with social anxiety disorder. Journal of Behavior Therapy and Experimental Psychiatry, 55, 16–24. [DOI] [PubMed] [Google Scholar]
- Itier R.J., Batty M. (2009). Neural bases of eye and gaze processing: the core of social cognition. Neuroscience and Biobehavioral Reviews, 33(6), 843–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäncke L., Brunner B., Esslen M. (2008). Brain activation during fast driving in a driving simulator: the role of the lateral prefrontal cortex. NeuroReport, 19, 1127–30. [DOI] [PubMed] [Google Scholar]
- Jun Y.Y., Mareschal I., Clifford C.W.G., Dadds M.R. (2013). Cone of direct gaze as a marker of social anxiety in males. Psychiatry Research, 210(1), 193–8. [DOI] [PubMed] [Google Scholar]
- Khalid S., Deska J.C., Hugenberg K. (2016). The eyes are the windows to the mind: direct eye gaze triggers the ascription of others’ minds. Personality and Social Psychology Bulletin, 42(12), 1666–77. [DOI] [PubMed] [Google Scholar]
- Kleinke C.L. (1986). Gaze and eye contact: a research review. Psychological Bulletin, 100(1), 78–100. [PubMed] [Google Scholar]
- Knoch D., Gianotti L.R., Baumgartner T., Fehr E. (2010). A neural marker of costly punishment behavior. Psychological Science, 21(3), 337–42. [DOI] [PubMed] [Google Scholar]
- Kubicki S., Herrmann W.M., Fichte K., Freund G. (1979). Reflections on the topics: EEG frequency bands and regulation of vigilance. Pharmakopsychiatrie Und Neuropsychopharmakologie, 12(2), 237–45. [DOI] [PubMed] [Google Scholar]
- Laxton A.W., Tang-Wai D.F., McAndrews M.P., et al. (2010). A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Annals of Neurology, 68(4), 521–34. [DOI] [PubMed] [Google Scholar]
- Li W., Mai X., Liu C. (2014). The default mode network and social understanding of others: what do brain connectivity studies tell us. Frontiers in Human Neuroscience, 8, 74.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobmaier J.S., Perrett D.I. (2011). The world smiles at me: self-referential positivity bias when interpreting direction of attention. Cognition and Emotion, 25(2), 334–41. [DOI] [PubMed] [Google Scholar]
- Lobmaier J.S., Tiddeman B., Perrett D.I. (2008). Emotional expression modulates perceived gaze direction. Emotion, 8(4), 573–7. [DOI] [PubMed] [Google Scholar]
- Lombardo M.V., Chakrabarti B., Bullmore E.T., Consortium M.A., Baron-Cohen S. (2011). Specialization of right temporo-parietal junction for mentalizing and its relation to social impairments in autism. Neuroimage, 56(3), 1832–8. [DOI] [PubMed] [Google Scholar]
- Luchinger R., Michels L., Martin E., Brandeis D. (2011). EEG-BOLD correlations during (post-) adolescent brain maturation. Neuroimage, 56(3), 1493–505. [DOI] [PubMed] [Google Scholar]
- Mareschal I., Calder A.J., Clifford C.W. (2013). Humans have an expectation that gaze is directed toward them. Current Biology, 23(8), 717–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyoshi D., Kuraguchi K., Tanaka Y., Uchida S., Ashida H., Watanabe K. (2014). Individual differences in autistic traits predict the perception of direct gaze for males, but not for females. Molecular Autism, 5(1), 12.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobascher A., Brinkmeyer J., Warbrick T., et al. (2009). Fluctuations in electrodermal activity reveal variations in single trial brain responses to painful laser stimuli–a fMRI/EEG study. Neuroimage, 44(3), 1081–92. [DOI] [PubMed] [Google Scholar]
- Napflin M., Wildi M., Sarnthein J. (2007). Test-retest reliability of resting EEG spectra validates a statistical signature of persons. Clinical Neurophysiology, 118, 2519–24. [DOI] [PubMed] [Google Scholar]
- Nash K., Gianotti L.R., Knoch D. (2015). A neural trait approach to exploring individual differences in social preferences. Frontiers in Behavioral Neuroscience, 8, 458.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping, 15(1), 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuwer M.R., Comi G., Emerson R., et al. (1998). IFCN standards for digital recording of clinical EEG. International Federation of Clinical Neurophysiology. Electroencephalography and Clinical Neurophysiology, 106(3), 259–61. [DOI] [PubMed] [Google Scholar]
- O’Gorman R.L., Poil S.S., Brandeis D., et al. (2013). Coupling between resting cerebral perfusion and EEG. Brain Topography, 26, 442–57. [DOI] [PubMed] [Google Scholar]
- Olbrich S., Mulert C., Karch S., et al. (2009). EEG-vigilance and BOLD effect during simultaneous EEG/fMRI measurement. Neuroimage, 45(2), 319–32. [DOI] [PubMed] [Google Scholar]
- Pantelis P.C., Byrge L., Tyszka J.M., Adolphs R., Kennedy D.P. (2015). A specific hypoactivation of right temporo-parietal junction/posterior superior temporal sulcus in response to socially awkward situations in autism. Social Cognitive and Affective Neuroscience, 10(10), 1348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui R.D., Esslen M., Kochi K., Lehmann D. (2002). Functional imaging with low-resolution brain electromagnetic tomography (LORETA): a review. Methods and Findings in Experimental and Clinical Pharmacology, 24(Suppl C), 91–5. [PubMed] [Google Scholar]
- Pizzagalli D., Pascual-Marqui R.D., Nitschke J.B., et al. (2001). Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. American Journal of Psychiatry, 158, 405–15. [DOI] [PubMed] [Google Scholar]
- Saxe R. (2006). Uniquely human social cognition. Current Opinion in Neurobiology, 16(2), 235–9. [DOI] [PubMed] [Google Scholar]
- Scheeringa R., Bastiaansen M.C., Petersson K.M., Oostenveld R., Norris D.G., Hagoort P. (2008). Frontal theta EEG activity correlates negatively with the default mode network in resting state. International Journal of Psychophysiology, 67(3), 242–51. [DOI] [PubMed] [Google Scholar]
- Schilbach L., Eickhoff S.B., Rotarska-Jagiela A., Fink G.R., Vogeley K. (2008). Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the ‘default system’ of the brain. Consciousness and Cognition, 17(2), 457–67. [DOI] [PubMed] [Google Scholar]
- Schulze L., Lobmaier J.S., Arnold M., Renneberg B. (2013a). All eyes on me?! Social anxiety and self-directed perception of eye gaze. Cognition and Emotion, 27, 1305–13. [DOI] [PubMed] [Google Scholar]
- Schulze L., Renneberg B., Lobmaier J.S. (2013b). Gaze perception in social anxiety and social anxiety disorder. Frontiers in Human Neuroscience, 7, Article no: 872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A., Johnson M.H. (2009). The eye contact effect: mechanisms and development. Trends in Cognitive Sciences, 13(3), 127–34. [DOI] [PubMed] [Google Scholar]
- Strijkstra A.M., Beersma D.G., Drayer B., Halbesma N., Daan S. (2003). Subjective sleepiness correlates negatively with global alpha (8-12 Hz) and positively with central frontal theta (4-8 Hz) frequencies in the human resting awake electroencephalogram. Neuroscience Letters, 340(1), 17–20. [DOI] [PubMed] [Google Scholar]
- Studer B., Pedroni A., Rieskamp J. (2013). Predicting risk-taking behavior from prefrontal resting-state activity and personality. PLoS ONE, 8(10), e76861.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons L.A., Lee K., Cedrone C.C., Nishimura M. (2004). What are you looking at? Acuity for triadic eye gaze. Journal of General Psychology, 131(4), 451–69. [PMC free article] [PubMed] [Google Scholar]
- von dem Hagen E.A., Stoyanova R.S., Rowe J.B., Baron-Cohen S., Calder A.J. (2014). Direct gaze elicits atypical activation of the theory-of-mind network in autism spectrum conditions. Cerebral Cortex, 24(6), 1485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Barstein J., Ethridge L.E., Mosconi M.W., Takarae Y., Sweeney J.A. (2013). Resting state EEG abnormalities in autism spectrum disorders. Journal of Neurodevelopmental Disorders, 5(1), 24.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumsteg D., Friedman A., Wieser H.G., Wennberg R.A. (2006a). Propagation of interictal discharges in temporal lobe epilepsy: correlation of spatiotemporal mapping with intracranial foramen ovale electrode recordings. Clinical Neurophysiology, 117, 2615–26. [DOI] [PubMed] [Google Scholar]
- Zumsteg D., Lozano A.M., Wieser H.G., Wennberg R.A. (2006b). Cortical activation with deep brain stimulation of the anterior thalamus for epilepsy. Clinical Neurophysiology, 117, 192–207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.