Abstract
Individuals react differently to social experiences; for example, people who are more sensitive to negative social experiences, such as being excluded, may be more likely to adapt their behavior to fit in with others. We examined whether functional brain connectivity during social exclusion in the fMRI scanner can be used to predict subsequent conformity to peer norms. Adolescent males (n = 57) completed a two-part study on teen driving risk: a social exclusion task (Cyberball) during an fMRI session and a subsequent driving simulator session in which they drove alone and in the presence of a peer who expressed risk-averse or risk-accepting driving norms. We computed the difference in functional connectivity between social exclusion and social inclusion from each node in the brain to nodes in two brain networks, one previously associated with mentalizing (medial prefrontal cortex, temporoparietal junction, precuneus, temporal poles) and another with social pain (dorsal anterior cingulate cortex, anterior insula). Using predictive modeling, this measure of global connectivity during exclusion predicted the extent of conformity to peer pressure during driving in the subsequent experimental session. These findings extend our understanding of how global neural dynamics guide social behavior, revealing functional network activity that captures individual differences.
Keywords: fMRI, functional connectivity, mentalizing, network, social pain
Introduction
Social connection is fundamental to human well-being (Pinquart and Sörensen, 2000; Kawachi and Berkman, 2001; Helliwell and Putnam, 2004) and survival (Berkman and Syme, 1978; House et al., 1982; Kawachi et al., 1996), whereas disconnection from social ties negatively impacts emotional and physical health (Holt-Lunstad et al., 2010; Eisenberger and Cole, 2012; Cacioppo and Cacioppo, 2014). Consequently, people work to remain connected to others and avoid social exclusion. When these connections are disrupted, such as when a member of a group is excluded, the individual may feel ‘social pain’ (Eisenberger et al., 2003; Eisenberger and Lieberman, 2004; MacDonald and Leary, 2005) and attempt to understand others’ thoughts and feelings (i.e. ‘mentalizing’; Frith & Frith, 2003) in service of reconnecting with others (Maner et al., 2007). One way that people develop and maintain social connections with those around them is by conforming to others’ attitudes and behavior (Cialdini and Goldstein, 2004). Importantly, however, individuals respond differently to social exclusion, even within the same peer groups (Fenigstein, 1979; Nezlek et al., 1997; Zadro et al., 2006; Waldrip, 2007; DeWall et al., 2012; Cascio et al., 2015a), and therefore may be differentially disposed to conform in service of maintaining social harmony. Critically, the extent to which a broad range of brain systems become synchronized with responses in the social pain and mentalizing systems during social exclusion may index the influence of these systems in responding to social threat. In turn, people who show higher synchrony between social pain and mentalizing systems and a broader range of other processing may also be more disposed to conformity. To test this possibility, we directly examine whether individual differences in functional connectivity between social pain and mentalizing regions, with the adolescent brain as a whole in response to exclusion, can capture and account for individual differences in conformity behavior.
Research into adolescent social decision-making motivates our proposed link between neural responses to social exclusion and conformity in adolescents (for reviews, see Blakemore, 2008, and Blakemore and Robbins, 2012). Compared to adults, adolescents show high sensitivity to rejection (Somerville, 2013) and willingness to conform to peer influence, including risk taking in the presence of a peer (Gardner and Steinberg, 2005). Health-risk behaviors are associated with the leading causes of morbidity and mortality among adolescents in the United States (Kann et al., 2016), emphasizing the value of understanding the causes of risky behaviors during this developmental period. Previous research has identified regions of the brain that continue to develop during adolescence (Giedd, 2004) and their effect on behavior (Spear, 2000; Steinberg, 2007); recently, more attention has been given to functional networks in the adolescent brain (Supekar et al., 2009; Whelan et al., 2012). In this work, we expand upon the theory of developing brains by exploring how global connectivity in the brain, with particular focus on key systems of interest, can predict behavior.
The connectivity methods used here offer important insights into the neural responses to social exclusion. First, the mental processes that take place during periods of exclusion, such as feelings of dejection and thoughts about others’ reasons for rejection, may fluctuate over the course of an exclusion episode; connectivity is sensitive to the synchronicity of these fluctuations throughout the brain. Second, high-level processing, including social processing, involves large networks of brain regions rather than isolated areas (Blakemore, 2008). On a broader scope, a growing body of research demonstrates that large-scale interactions between key regions of interest and the rest of the brain may provide additional information, complementing regional activity in capturing current mental states (Van den Heuvel and Hulshoff Pol, 2010; Bassett et al., 2015; Medaglia et al., 2015; Brooks et al., 2016; Muraskin et al., 2016, 2017; Garcia et al., 2017; Passaro et al., 2017).
Our connectivity analysis is focused on brain networks implicated in social pain (Eisenberger et al., 2003) and mentalizing (Frith and Frith, 2003) processes, which might take on more importance globally in the brain during exclusion, relative to inclusion, for individuals who are more reactive to the exclusion experience (Eisenberger et al., 2003; Masten et al., 2009). Previous research demonstrates the high cost of social exclusion (for a review, see Williams, 2007) and the fact that neural reactivity to exclusion varies across individuals (Falk et al., 2014). If social pain and mentalizing have more global importance in interpreting experiences during exclusion for some individuals, we would expect not only univariate changes (for a review, see Eisenberger, 2015) but also changes in global connectivity between regions associated with social pain and mentalizing and the rest of the brain. Past research demonstrates that conformity is one way that participants try to regain acceptance (DeWall, 2010) and potentially preempt further exclusion. Therefore, people whose global brain response to exclusion is coordinated more strongly with social pain and mentalizing systems may be more predisposed to conform. Yet, despite recent studies that have begun to consider functional connectivity among single regions during social tasks (Bolling et al., 2011; Meyer, 2012; Puetz et al., 2014), little is known about larger-scale network dynamics during social experiences.
We hypothesized that the extent to which people conform to their peers can be predicted using changes in brain dynamics linked to social pain and mentalizing when people are faced with social exclusion (cf., Falk et al., 2014). Although there is promise of predicting behavior from network dynamics (Bassett et al., 2011; Baldassarre et al., 2012), research has not yet linked brain network dynamics during social tasks in the neuroimaging environment to objectively logged social behaviors measured outside of the scanner. To this end, we examined the relationship between global connectivity of regions implicated in social pain and mentalizing during social exclusion and inclusion as predictors of conformity to peer influence on simulated driving outside of the scanner a week later. We focused on brain dynamics during exclusion given past research demonstrating that conformity is one way that participants try to regain acceptance (DeWall, 2010) and potentially preempt further exclusion. Conformity to driving risk attitudes in teens served as our outcome because teens’ driving risk is socially influenced (Simons-Morton et al., 2005, 2014; Bingham et al., 2016) and has important real-world consequences (Ouimet et al., 2010).
Materials and methods
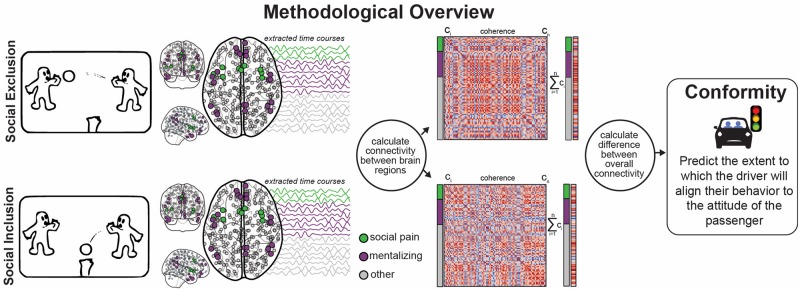
This research was part of a five-study investigation of the effects of peer influence on teen driving (Falk et al., 2014; Simons-Morton et al., 2014; Cascio et al., 2015b; Bingham et al., 2016; Schmälzle et al., 2017). A previous study in this research program found an association between neural activation during exclusion and risk taking (Falk et al., 2014), and prior reports on the driving simulator data reported here noted substantial individual variability in susceptibility to influence (Bingham et al., 2016). In this analysis, we extend these results by examining a measure of global functional connectivity during exclusion vs inclusion and investigate its predictive relationship with individual differences in conformity (Figure 1).
Fig. 1.
Study overview. fMRI BOLD data were collected during Cyberball, a virtual ball-tossing game that simulates social exclusion and social inclusion. Functional brain activity was extracted from regions in a whole-brain parcellation (gray), including regions previously associated with social pain (green) and mentalizing (purple). Connectivity was then computed between all region pairs, and the difference in connectivity during social exclusion and social inclusion was used to predict subsequent conformity to the attitude of a peer passenger during a driving simulator.
Participants
Fifty-seven right-handed, neurotypical, male participants aged 16 or 17 completed both portions of the study. Each participant had received a Level 2 Michigan driver’s license (unsupervised driving allowed with several restrictions) at least 4 months prior to the study, drove at least twice per week, had normal or corrected-to-normal vision and was insensitive to simulator sickness. Participants were told that the purpose of the study was to examine the physiology of driving. The University of Michigan Institutional Review Board approved the study procedures; participants provided written assent, and their legal guardians provided written informed consent.
Neuroimaging data collection: Cyberball (fMRI)
Participants were told that they would play a variety of computer games while in a functional magnetic resonance imaging (fMRI) scanner, some alone and one, called Cyberball, with two other participants; the other ‘participants’ were in reality controlled by a computer (Williams et al., 2000; Williams and Jarvis, 2006). Participants played two rounds of Cyberball. The first condition simulated social inclusion by having each player (the actual participant and the two simulated players) receive the ball equally often; in the second condition, the game started the same as the first but the two computer-controlled players soon began throwing the ball only between each other, excluding the participant. Both the inclusion and exclusion conditions lasted approximately 2.5 min (74 brain volumes for each condition).
Neuroimaging data were acquired using a 3 Tesla GE Signa MRI scanner. Functional images were recorded using a reverse spiral sequence (TR = 2000 ms, echo time = 30 ms, flip angle = 90°, 43 axial slices, field of view = 220 mm, slice thickness = 3 mm, voxel size = 3.44 × 3.44 × 3.0 mm).
To enhance coregistration and normalization, in-plane T1-weighted images (43 slices, slice thickness = 3 mm, voxel size = 0.86 × 0.86 × 3.0 mm) and high-resolution T1-weighted images (SPGR, 124 slices, slice thickness = 1.02 × 1.02 × 1.2 mm) were also acquired. The first four volumes were not acquired. The functional data were preprocessed and analyzed using Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK), and images despiked using the 3dDespike program as implemented in the AFNI toolbox (Cox, 1996; Cox and Hyde, 1997; Gold et al., 1998). The volumes were then corrected for slice time acquisition differences and spatially realigned to the first functional image. Functional and structural images were coregistered by aligning the in-plane T1 images to the mean functional image, and then the in-plane image was registered to the high-resolution T1 images. Structural images were then segmented into gray matter, white matter and cerebal spinal fluid (using SPM8) to create a mask for data extraction and then normalized to the skull-stripped MNI template provided by FSL.
Need-Threat Scale: measuring the effects of ostracism
Following the fMRI scan, participants completed several questionnaires, including the Need-Threat Scale (Van Beest and Williams, 2006). This assessment quantifies the perceived threat to participants’ social needs experienced during Cyberball on a scale of 1–7, with lower scores indicating higher threat. Scores ranged from 1.7 to 6.1, with a mean of 3.48 and a standard deviation of 0.96.
Neuroimaging data analysis: global connectivity
We identified a set of brain areas associated with social pain, including the dorsal anterior cingulate cortex (dACC) and anterior insula (AI) (Eisenberger, 2003; Eisenberger and Lieberman, 2004; Lamm and Singer, 2010; Cacioppo et al., 2013; Rotge et al., 2015), and a separate set involved in mentalizing, including the temporoparietal junction (TPJ), temporal pole (TP), precuneus (PC), and dorsomedial and ventromedial prefrontal cortex (dmPFC and vmPFC, respectively) (Frith and Frith, 2003; Frith and Frith, 2006; D’Argembeau et al., 2007; Van Overwalle and Baetens, 2009). Following a standard preprocessing stream (see above), we used a previously published whole-brain parcellation (Power et al., 2011) to define 264 regions.
Since the regions of the social pain and mentalizing networks are subdivided in the whole-brain atlas used (Power et al., 2011), we calculated the distance between each centroid in the networks (D’Argembeau et al., 2007; Schmälzle et al., 2017; coordinates in Supplementary Figure S1) and the center of every atlas region and chose the three atlas regions of minimal distance from it. This resulted in 30 nodes to represent the two theory-driven networks from this atlas (depicted by green and purple nodes for social pain and mentalizing, respectively, in Figure 1).
We estimated functional connectivity between brain regions during the social inclusion and exclusion conditions of the Cyberball game by calculating the coherence (Rosenberg et al., 1989) between every pair of regions (see Supplementary data for details). Previous research in task-based fMRI has demonstrated the importance of considering frequencies of up to 0.15 Hz in order to account for a broad range of potential hemodynamic response function shapes (Sun et al., 2004, 2005; but see Cordes et al., 2001). To this end, we analyzed coherence for nine frequency bands between 0.0625 and 0.1458 Hz (Lauritzen et al., 2009) and used a data-driven approach to select the one that was most predictive (0.1146 Hz).
For each region, we derived a measure of ‘global connectivity’ for the social inclusion condition and one for the social exclusion condition. We created a graph with 264 nodes, one for each region in the whole-brain atlas (Power et al., 2011), and the edge weight between nodes was determined by their coherence. The weighted degree of a region is the sum of its coherence to every other region in the brain and represents the extent to which the region is connected to the rest of the brain. We used this weighted degree summation as the global connectivity metric for each region, computing it separately for the two Cyberball conditions. We then subtracted the weighted degree during the inclusion condition from the weighted degree during the exclusion condition. The difference in a node’s weighted degree encapsulates the effect that social exclusion has on the region’s global connectivity (Figure 1). We then use the global connectivity metric for each region as the feature set in our predictive model to predict a participant’s behavioral conformity in a subsequent driving simulator session. Finally, we calculated the Pearson correlation between the global connectivity measure and the Need-Threat Scale of ostracism for each of the regions selected in the final model.
Behavioral data collection: driving simulator
Approximately one week after the fMRI session, participants returned for the driving simulator session. After a short practice drive, participants completed a solo practice drive in the simulator and then two drives, one solo and one with a confederate as a passenger. Order of the drive conditions was counterbalanced between participants. Each drive lasted 10–15 min, and the participant approached either 9 or 10 traffic lights that were timed to turn red before the participant cleared the intersection. The timing of the yellow lights was fine tuned after the first 8 participants and was held constant for the final 49 participants. To validate that the timing changes did not influence the results, we performed a two-sided t-test to compare conformity between the first 8 participants and the final participants and determined that the two samples were not significantly different; t(55) = 1.33, P = 0.190. Additional information about the driving simulator can be found in the Supplementary data.
Each participant was randomly assigned (between participants) to one of two conditions for the passenger drive: risk-averse or risk-accepting passenger. In both conditions, participants completed a pre-drive survey after which a similar-aged, male confederate arrived late. In the risk-averse condition, he explained, ‘Sorry I was a little late getting here. I tend to drive slower, plus I hit every yellow light’, whereas the risk-accepting confederate said, ‘Sorry I was a little late getting here. Normally I drive way faster, but I hit like every red light’.
In addition, before the passenger drive, the participant and confederate watched two videos together, one showing high-risk driving from the passenger seat and one showing low-risk driving from the same perspective; the order in which the videos were shown was random. After each video, the participant and confederate were asked to rate on a scale of 1–10 how similar their driving was to the driving shown in the video and how likely they would be to ride with the driver in the video. The confederate responded second and gave responses that were less risky than the participant’s in the risk-averse condition or riskier than the participant’s in the risk-accepting condition.
Finally, during the passenger drive, the confederate was told to navigate using a map, which provided an excuse to make comments about the participant’s driving behavior during the drive. In the risk-accepting condition, the confederate stated the speed limit when the participant was driving below it, while in the risk-averse condition, he noted reduced-speed zones.
Behavioral data analysis: driving and conformity
For each drive, we derived a measure of conformity by calculating the percentage of intersections where the driver failed to stop in each drive and subtracting them, such that a positive difference in either condition indicates that the participant conformed to the confederate’s attitude by driving in a riskier manner in the risk-accepting condition and more safely in the risk-averse condition. This estimation of conformity serves as the dependent variable for our analysis.
To confirm that passenger type influenced driving behavior, we computed the incidence rate ratio (IRR) of failure to stop at red lights during the passenger drives between the participants with risk-accepting passengers and those with risk-averse passengers, treating each intersection as a separate event.
Cross-validated analysis: connectivity predicts conformity
We used a predictive modeling pipeline to examine whether brain connectivity predicts behavioral conformity in an out-of-scanner driving task a week later, using global connectivity of regions as features. The primary analysis used 30 regions from the theory-driven brain areas as features; a second (see Supplementary data) used all 264 from the whole-brain parcellation. To prevent overfitting, we created 57 splits into training and test sets, each one leaving out one participant from the training set, and performed feature selection within each split, resulting in a model with seven features (see Supplementary data for details on feature selection).
Results
We investigated whether functional connectivity during exclusion relative to inclusion in the Cyberball task in the scanner predicted conformity to a confederate passenger in a simulated drive one week later. Our primary analysis examined connectivity of theory-driven regions involved in mentalizing and social pain. Functional connectivity was computed between each region and the rest of the brain, and these metrics of global connectivity were used as features in a cross-validated predictive model to predict conformity in the driving simulator.
Behavioral conformity responses in the driving simulator
We first examined drivers’ conformity to the attitude of their confederates defined as the amount to which they moved their driving behavior in the direction of the confederates during the passenger drive relative to the solo drive, i.e. increased risk in the presence of a passenger in the risk-accepting condition and decreased risk in the presence of a passenger in the risk-averse condition. Twenty-six drivers were randomized to the risk-accepting condition, and 31 were in the risk-averse condition. Those with risk-accepting passengers drove through more red lights than those with risk-averse passengers during their passenger drive (IRR = 1.31, 95% CI = [1.03, 1.66], P = 0.03). The distribution of conformity in the risk-accepting (M = 1.90% change in risk toward the passenger’s attitude, s.d. = 24.65%) and risk-averse conditions (M = 1.92%, s.d. = 25.14%) was nearly identical. The combined sample had a mean of 1.91% change toward the confederate’s attitude and a standard deviation of 24.65%. Consistent with behavioral reports using these data (Bingham et al., 2016), these behavioral results indicate a bias toward conformity to the confederate’s attitude, but the large amount of variance confirms its value for understanding individual differences in susceptibility to social influence for risky behaviors.
Global connectivity from theory-driven regions to the rest of the brain predicts subsequent conformity
We then investigated whether individual differences in the global connectivity of social pain and mentalizing regions (Figure 1, green and purple regions, respectively) during Cyberball could account for the substantial amount of variability in conformity found in the subsequent driving session. We used global connectivity from the 30 theory-driven regions as features in a leave-one-out cross-validation to predict a participant’s conformity score in the driving simulator one week later. A parallel analysis conducted considering all 264 regions in the whole-brain parcellation (Power et al., 2011) confirmed the importance of these networks (see Supplementary data).
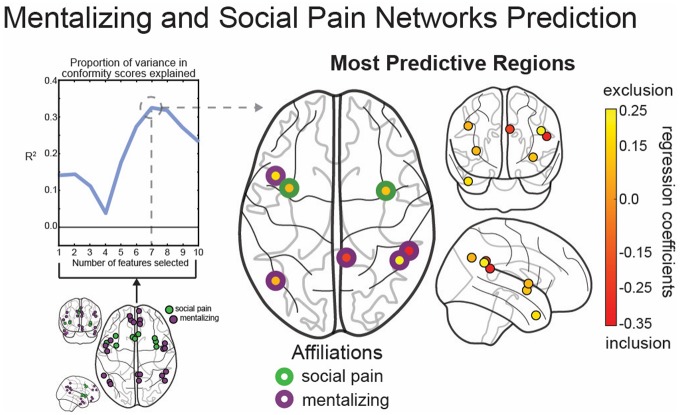
To assess global connectivity, we first computed the difference in a region’s global connectivity with the rest of the brain between the two social conditions of Cyberball (social exclusion−social inclusion). We then used these global connectivity measures for each person within our theory-driven regions of interest to predict individual differences in conformity during the driving session. As shown in Figure 2 (top left), the best prediction was achieved from 7 of the 30 theory-driven regions; these 7 regions’ global connectivity predicted individual differences in conformity with an out-of-sample R2 of 0.325 (root mean-squared error of 20.07 in cross-validation). Two of the three regions in the social pain network were selected (left and right AI), along with four of the seven regions in the mentalizing network (two regions in the right TPJ and one each in the left TPJ, left TP, and PC). In Figure 2, the regions in the social pain network are outlined in green, and the regions in the mentalizing network are outlined in purple; the center color for each region reflects the regression coefficient to further characterize the predictive relationship between global connectivity from each region and subsequent conformity. For five of the seven regions selected, more connectivity during social exclusion than inclusion was associated with behavioral conformity.
Fig. 2.
The regions in the social pain and mentalizing networks whose connectivity to the rest of the brain is most predictive of conformity; regions pictured in green and purple, respectively, in bottom left. Top left: The out-of-sample R2 values obtained when selecting between 1 and 10 regions as features in our model, with seven features being most predictive of conformity. Right: The seven regions selected by our feature-selection algorithm. The regions with a green outline are in the social pain network, while the regions with a purple outline are in the mentalizing network. The color of the center of each region indicates its regression coefficient. For yellow regions, more connectivity during social exclusion than social inclusion is predictive of conformity to the confederate’s attitude; for red regions, the opposite is true.
To assess convergence between the features selected by our model and self-reports of distress during exclusion, we examined correlations between global connectivity in our key regions of interest and scores on the Need-Threat Scale. We found a significant correlation between the global connectivity of one region of the right TPJ and need threat (R = 0.387, P = 0.003) and a marginally significant correlation between global connectivity of the right AI and need threat (R = −0.260, P = 0.050), indicating that greater global connectivity of the right TPJ in response to social exclusion is associated with more needs being met, whereas greater global connectivity of the right AI in response to social exclusion is associated with fewer needs being met.
The correlations between actual conformity and conformity predicted by our model were similar for participants with a risk-accepting passenger (R = 0.538) and those with a risk-averse passenger (R = 0.608), indicating that connectivity is predictive of conformity regardless of passenger type (see also Supplementary Figure S3).
To verify that our predictive accuracy does not arise from chance or overfitting, we performed a permutation test on the data, training our model on 10 000 permutations of the dependent variable and using it to predict the shuffled dependent variables in leave-one-out cross-validation. The true model achieved a better R2 than 99.99% of the shuffled models (P = 0.0002; Ojala and Garriga, 2010). As a final test of significance, we generated 1000 random subsets of 30 regions from the entire 264-region whole-brain atlas (Power et al., 2011) and ran our model starting with these regions instead of our 30 theory-driven regions. For each random subset of 30 regions, we tested models using between 1 and 10 features and empirically selected the optimal number of features for this subset of regions. This led to 1000 optimized models. Only 0.7% of these optimized models outperformed our original model, with more than two thirds of the total features selected in those models coming from among the features selected from our networks-of-interest or whole-brain models (see Supplementary data). The median out-of-sample R2 score among all 1000 optimized models was 0.055.
Finally, we examined whether global connectivity during either of the two Cyberball conditions could predict conformity as well as did their difference. Individual differences in connectivity during social exclusion were substantially predictive of conformity (R2 = 0.274), although not as strongly as the difference in connectivity between social exclusion and social inclusion (R2 = 0.325); individual differences in connectivity during social inclusion had little predictive power (R2 = 0.096).
Collectively, these results indicate that global connectivity during social exclusion, either alone or in comparison to connectivity during social inclusion, can predict individual differences in subsequent conformity behavior 1 week later. This highlights the value of examining global connectivity to understand individual variability in real-world social situations.
Discussion
Social connection is fundamental to well-being, and a motivating force for a wide range of behaviors, including conformity (Cialdini and Goldstein, 2004; Maner et al., 2007). Previous research has characterized a neural alarm system that responds to social pain (Eisenberger, 2003; Eisenberger and Lieberman, 2004; Lamm and Singer, 2010; Rotge et al., 2015), as well as a broader set of brain regions that allow people to understand others’ mental states (Frith and Frith, 2003, 2006; D’Argembeau et al., 2007; Van Overwalle and Baetens, 2009). Using a Cyberball game, we show that individual differences in the degree to which key brain regions implicated in social pain and mentalizing change their connectivity with the rest of the brain in response to social exclusion predict conformity to peer attitudes in a driving simulator a week later. Thus, the current research uses a novel application of a network neuroscience measure to highlight how individual differences in network connectivity in response to a social experience, such as exclusion, predict sensitivity to social influence in a real-world setting.
Although we observed nonsignificant trends in group-averaged neural responses to social exclusion, not all participants showed equal levels of differentiation in their global connectivity between exclusion and inclusion. Similarly, in the initial report of the driving simulator data used here, the authors noted substantial individual variability in tendency to conform (Bingham et al., 2016). Previous research suggested that sensitivity to social pain might prime individuals to preempt exclusion in other social contexts by conforming (Maner et al., 2007; Peake et al., 2013; Falk et al., 2014). For example, Falk et al. (2014) found that univariate increases in brain activity within social pain and mentalizing regions of interest were associated with greater driving risk taking in the presence of a peer, compared to driving alone. No prior research, however, has examined how social pain and mentalizing regions might change their global connectivity with the rest of the brain in response to social threats, nor how this relates to real-world relevant decision making. In this study, we hypothesized that individual differences in connectivity between social pain and mentalizing systems with the rest of the brain would relate to behavioral conformity responses in an unrelated driving context, if social pain and mentalizing have more global importance in interpreting experiences during exclusion for some individuals. Consistent with this hypothesis, coherence in brain networks involved in responding to social cues (i.e. social pain and mentalizing networks) during social exclusion compared to social inclusion predicted approximately one-third of the variance in the degree to which participants conformed to peers’ driving preferences a week later. This result substantially extends past research on social behavior and the brain by demonstrating that the global connectivity of social pain and mentalizing systems in response to exclusion maps onto the inclination to conform to peer attitudes. These data are consistent with the idea that conformity is a means to preserve one’s position in a group and that a person who experiences a greater reaction to exclusion may take greater actions to prevent such an experience in other contexts.
The current findings also extend past research by revealing information about how the brain helps navigate the social world. In line with past research suggesting that specific control points in the brain, and particularly within the default mode network, help transition the brain to execute different tasks (Gu et al., 2015; Betzel et al., 2016; Muldoon et al., 2016; Medaglia et al., 2016), we find that global connectivity between key social pain and mentalizing regions predicts individual differences in susceptibility to peer influence. In other words, greater changes in global brain connectivity may be associated with flexibly altering behaviors to adjust to social situations. Specifically, our method uses a novel application of a functional connectivity measure that allowed us to identify the regions whose global brain dynamics were the most predictive of behavior change. During our main analysis, these regions were selected from two hypothesized networks of interest, namely networks previously associated with social pain and mentalizing. Prior work has shown that activity in the social pain and mentalizing networks can be used to predict subsequent behavior change (Hein et al., 2010; Carter et al., 2012). Here, we show that functional connections between both regions in the social pain (e.g. bilateral AI) and mentalizing networks (bilateral TPJ), and the rest of the brain are associated with later individual differences in tendency to change behavior. Both the right TPJ and right AI also appeared in our whole-brain analysis (see Supplementary data), suggesting the robustness of these results. Moreover, the global connectivity of the right TPJ and right AI was associated with self-reported need threat after the exclusion task; this indicates that these two regions’ brain dynamics are not only predictors of future conformity but also correlates of a subjective response to social exclusion.
The key roles of the AI and TPJ in our models and in relation to the Need-Threat Scale may elucidate the psychological significance of our method. The right AI has been identified as a ‘causal outflow hub’ (Sridharan et al., 2008; Menon and Uddin, 2010; Uddin et al., 2011), meaning that its activity is predictive of that of a large number of other regions in the brain. Similarly, the TPJ also functions as a hub of connectivity, integrating activity in different regions into a single coherent social context and affecting processing throughout the brain (Carter and Huettel, 2013). As the features in our model are the cumulative (i.e. ‘global’) functional connectivity of each region, it is to be expected that the regions that are most predictive of behavior change are those that serve as focal points, integrating and influencing other regions. This influence is also seen in relation to self-reported need threat: of the seven regions in our predictive model, only the global connectivity of the right TPJ and right AI were related to the Need-Threat Scale of ostracism.
Taken together, these results highlight the importance of considering not only how individual brain regions are modulated by social experiences but also how those regions communicate with the rest of the brain more globally. We find that social context (i.e. exclusion vs inclusion) causes different changes across individuals in the extent to which key regions implicated in social pain and mentalizing become more globally connected to the rest of the brain. Further, individual differences in the extent of this shift were significantly predictive of later conformity to driving norms expressed by a peer.
Future directions
Our results show a predictive relationship between brain activity and social influence in our sample of 16- and 17-year-old, primarily Caucasian, males from Southeast Michigan. Future research could examine possible changes in this relationship across developmental periods, including whether the brain regions involved in responding to social exclusion fluctuate over time or play a differential role in the brain’s global connectivity dependent on developmental stage (see Vijayakumar et al., 2017 for a univariate perspective on this question). It would also be interesting to examine the extent to which this relationship generalizes across other socio-demographic populations since cultural variation has been shown to influence social processing, including social orientation (individualism vs collectivism; Kitayama and Markus, 1991), decision making (Iyengar and Lepper, 1999), and team performance (Wagner et al., 2012).
Conclusion
This work shows that the functional connectivity of brain regions associated with social pain and mentalizing in response to social exclusion is able to predict subsequent conformity. This result highlights the power of considering global connectivity as predictor and is a first step toward understanding how neural connectivity informs our interaction with the social world. The technique that we developed in the process, using overall connectivity of regions as predictors, addresses common limitations of other connectivity techniques while capturing processes that are averaged away in models based on mean activation. Our method is likely to have applications for developing predictive models based on network dynamics, which in turn provide parsimonious explanations relating brain activity, social context and behavior.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the Communication Neuroscience laboratory for research assistance and the staff of the University of Michigan fMRI Center as well as Jean T. Shope, Marie Claude Ouimet, Anuj K. Pradhan, Kristin Shumaker, Jennifer LaRose, Farideh Almani and Johanna Dolle for collaboration on a larger study from which these data were drawn and assistance with data collection. The authors gratefully acknowledge Andrew Suzuki, Robin Liu, Ryan Bondy, Matthew Sweet, Cary Welsh, Andrea I. Barretto, Jennifer LaRose, Farideh Almani, Alyssa Templar and Kristin Shumaker for research assistance. They thank Ralf Schmälzle for provision of ROIs.
Funding
The research was supported by (i) the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development contract # HHSN275201000007C (PI: C.R.B.); (ii) the University of Michigan Injury Center Pilot Grant (PI: E.B.F.); (iii) #NIH/NICHD IR21HD073549-01A1 (PI: E.B.F.); (iv) an NIH Director’s New Innovator Award #1DP2DA03515601 (PI: E.B.F.); and (v) the U.S. Army Research Laboratory, including work under Cooperative Agreement W911NF-10-2-0022 and W911NF-16-2-0165.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Baldassarre A., Lewis C.M., Committeri G., Snyder A.Z., Romani G.L., Corbetta M. (2012). Individual variability in functional connectivity predicts performance of a perceptual task. Proceedings of the National Academy of Sciences of the United States of America, 109(9), 3516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D.S., Wymbs N.F., Porter M.A., Mucha P.J., Carlson J.M., Grafton S.T. (2011). Dynamic reconfiguration of human brain networks during learning. Proceedings of the National Academy of Sciences of the United States of America, 108, 7641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D.S., Yang M., Wymbs N.F., Grafton S.T. (2015). Learning-induced autonomy of sensorimotor systems. Nature Neuroscience, 18(5), 744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman L.F., Syme S.L. (1978). Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. American Journal of Epidemiology, 109, 186–204. [DOI] [PubMed] [Google Scholar]
- Betzel R.F., Gu S., Medaglia J.D., Pasqualetti F., Bassett D.S. (2016). Optimally controlling the human connectome: the role of network topology. Scientific Reports, 6, 30770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham C.R., Simons-Morton B.G., Pradhan A.K., et al. (2016). Peer passenger norms and pressure: experimental effects on simulated driving among teenage males. Transportation Research Part F: Traffic Psychology and Behaviour, 41, 124–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J. (2008). The social brain in adolescence. Nature Reviews Neuroscience, 9(4), 267–77. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Robbins T.W. (2012). Decision-making in the adolescent brain. Nature Neuroscience, 15(9), 1184–91. [DOI] [PubMed] [Google Scholar]
- Bolling D.Z., Pitskel N.B., Deen B., Crowley M.J., Mayes L.C., Pelphrey K.A. (2011). Development of neural systems for processing social exclusion from childhood to adolescence: neural systems for processing social exclusion. Developmental Sciebce, 14(6), 1431–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J.R., Garcia J.O., Kerick S.E., Vettel J.M. (2016). Differential functionality of right and left parietal activity in controlling a motor vehicle. Frontiers in Systems Neuroscience, 10, article 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo J.T., Cacioppo S. (2014). Social relationships and health: the toxic effects of perceived social isolation: social relationships and health. Social and Personality Psychology Compass, 8, 58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo S., Frum C., Asp E., Weiss R.M., Lewis J.W., Cacioppo J.T. (2013). A Quantitative meta-analysis of functional imaging studies of social rejection. Scientific Reports, 3, article 2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R.M., Bowling D.L., Reeck C., Huettel S.A. (2012). A distinct role of the temporal-parietal junction in predicting socially guided decisions. Science, 337, 109–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R.M., Huettel S.A. (2013). A nexus model of the temporal–parietal junction. Trends in Cognitive Sciences, 17(7), 328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio C.N., Carp J., O'Donnell M.B., et al. (2015b). Buffering social influence: neural correlates of response inhibition predict driving safety in the presence of a peer. Journal of Cognitive Neuroscience, 27(1), 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio C.N., Konrath S.H., Falk E.B. (2015a). Narcissists’ social pain seen only in the brain. Social Cognitive and Affective Neuroscience, 10, 335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cialdini R.B., Goldstein N.J. (2004). Social influence: compliance and conformity. Annual Review of Psychology, 55, 591–621. [DOI] [PubMed] [Google Scholar]
- Cordes D., Haughton V.M., Arfanakis K., et al. (2001). Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. American Journal of Neuroradiology, 22, 1326–33. [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–73. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Hyde J.S. (1997). Software tools for analysis and visualization of fMRI data. NMR in Biomedicine, 10, 171–8. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A., Ruby P., Collette F., et al. (2007). Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of Cognitive Neuroscience, 19, 935–44. [DOI] [PubMed] [Google Scholar]
- DeWall C.N. (2010). Forming a basis for acceptance: excluded people form attitudes to agree with potential affiliates. Social Influence, 5(4), 245–60. [Google Scholar]
- DeWall C.N., Masten C.L., Powell C., Combs D., Schurtz D.R., Eisenberger N.I. (2012). Do neural responses to rejection depend on attachment style? An fMRI study. Social Cognitive and Affective Neuroscience, 7, 184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I. (2003). Does rejection hurt? An fMRI study of social exclusion. Science, 302(5643), 290–2. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I. (2015). Social pain and the brain: controversies, questions, and where to go from here. Annual Review of Psychology, 66(1), 601–29. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Cole S.W. (2012). Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nature Neuroscience, 15(5), 669–74. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D. (2004). Why rejection hurts: a common neural alarm system for physical and social pain. Trends in Cognitive Sciences, 8, 294–300. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D., Williams K.D. (2003). Does rejection hurt? An fMRI study of social exclusion. Science, 302(5643), 290–2. [DOI] [PubMed] [Google Scholar]
- Falk E.B., Cascio C.N., Brook O'Donnell M., et al. (2014). Neural responses to exclusion predict susceptibility to social influence. Journal of Adolescent Health, 54(5), S22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenigstein A. (1979). Self-consciousness, self-attention, and social interaction. Journal of Personality and Social Psychology, 37(1), 75. [Google Scholar]
- Frith C.D., Frith U. (2006). The neural basis of mentalizing. Neuron, 50(4), 531–4. [DOI] [PubMed] [Google Scholar]
- Frith U., Frith C.D. (2003). Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London Series B Biological Sciences, 358, 459–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J.O., Brooks J., Kerick S., Johnson T., Mullen T.R., Vettel J.M. (2017). Estimating direction in brain-behavior interactions: proactive and reactive brain states in driving. NeuroImage, 150, 239–49. [DOI] [PubMed] [Google Scholar]
- Gardner M., Steinberg L. (2005). Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental Psychology, 41, 625–35. [DOI] [PubMed] [Google Scholar]
- Giedd J.N. (2004). Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences, 1021(1), 77–85. [DOI] [PubMed] [Google Scholar]
- Gold S., Christian B., Arndt S., et al. (1998). Functional MRI statistical software packages: a comparative analysis. Human Brain Mapping, 6(2), 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S., Pasqualetti F., Cieslak M., et al. (2015). Controllability of structural brain networks. Nature Communications, 6, 8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G., Silani G., Preuschoff K., Batson C.D., Singer T. (2010). Neural responses to ingroup and outgroup members’ suffering predict individual differences in costly helping. Neuron, 68, 149–60. [DOI] [PubMed] [Google Scholar]
- Helliwell J.F., Putnam R.D. (2004). The social context of well-being. Philosophical Transactions of the Royal Society of London Series B Biological Sciences, 359, 1435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J., Smith T.B., Layton J.B. (2010). Social relationships and mortality risk: a meta-analytic review. PLOS Medicine, 7(7), e1000316.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House J.S., Robbins C., Metzner H.L. (1982). The association of social relationships and activities with mortality: prospective evidence from the Tecumseh Community Health Study. American Journal of Epidemiology, 116, 123–40. [DOI] [PubMed] [Google Scholar]
- Iyengar S.S., Lepper M.R. (1999). Rethinking the value of choice: a cultural perspective on intrinsic motivation. Journal of Personality and Social Psychology, 76, 349–66. [DOI] [PubMed] [Google Scholar]
- Kann L., McManus T., Harris W.A., et al. (2016). Youth risk behavior surveillance – United States, 2015. Surveillance Summaries, 65(6), 1–174. [DOI] [PubMed] [Google Scholar]
- Kawachi I., Berkman L.F. (2001). Social ties and mental health. Journal of Urban Health, 78(3), 458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi I., Colditz G.A., Ascherio A., et al. (1996). A prospective study of social networks in relation to total mortality and cardiovascular disease in men in the USA. Journal of Epidemiology and Community Health, 50(3), 245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama S., Markus H.R. (1991). Culture and the self: implications for cognition, emotion, and motivation. Psychological Review, 98, 224–53. [Google Scholar]
- Lamm C., Singer T. (2010). The role of anterior insular cortex in social emotions. Brain Structure and Function, 214(5–6), 579–91. [DOI] [PubMed] [Google Scholar]
- Lauritzen T.Z., D'Esposito M., Heeger D.J. (2009). Top-down flow of visual spatial attention signals from parietal to occipital cortex. Journal of Vision, 9, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald G., Leary M.R. (2005). Why does social exclusion hurt? The relationship between social and physical pain. Psychological Bulletin, 131, 202–23. [DOI] [PubMed] [Google Scholar]
- Maner J.K., DeWall C.N., Baumeister R.F., Schaller M. (2007). Does social exclusion motivate interpersonal reconnection? Resolving the “porcupine problem.” Journal of Personality and Social Psychology, 92, 42–55. [DOI] [PubMed] [Google Scholar]
- Masten C.L., Eisenberger N.I., Borofsky L.A., et al. (2009). Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience, 4(2), 143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medaglia J.D., Gu S., Pasqualetti F., et al. (2016) Cognitive control in the controllable connectome. arXiv Preprint. arXiv: 1606.09185.
- Medaglia J.D., Lynall M.E., Bassett D.S. (2015). Cognitive network neuroscience. Journal of Cognitive Neuroscience, 27(8), 1471–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function, 214( 5–6: ), 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M.L., Masten C.L., Ma Y., et al. (2012). Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Social Cognitive and Affective Neuroscience, 8(4), 446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon S.F., Pasqualetti F., Gu S., et al. (2016). Stimulation-Based Control of Dynamic Brain Networks. PLOS Computational Biology, 12(9), e1005076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraskin J., Dodhia S., Lieberman G., et al. (2016). Brain dynamics of post‐task resting state are influenced by expertise: insights from baseball players. Human Brain Mapping, 37(12), 4454–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraskin J., Sherwin J., Lieberman G., et al. (2017). Fusing multiple neuroimaging modalities to assess group differences in perception–action coupling. Proceedings of the IEEE, 105(1), 83–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezlek J.B., Kowalski R.M., Leary M.R., Blevins T., Holgate S. (1997). Personality moderators of reactions to interpersonal rejection: depression and trait self-esteem. Personality and Social Psychology Bulletin, 23, 1235–44. [Google Scholar]
- Ojala M., Garriga G.C. (2010). Permutation tests for studying classifier performance. Journal of Machine Learning Research, 11, 1833–63. [Google Scholar]
- Ouimet M.C., Simons-Morton B.G., Zador P.L., et al. (2010). Using the U.S. National Household Travel Survey to estimate the impact of passenger characteristics on young drivers’ relative risk of fatal crash involvement. Accident Analysis and Prevention, 42(2), 689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaro A.D., Vettel J.M., McDaniel J., Lawhern V., Franaszczuk P.J., Gordon S.M. (2017). A novel method linking neural connectivity to behavioral fluctuations: behavior-regressed connectivity. Journal of Neuroscience Methods, 279, 60–71. [DOI] [PubMed] [Google Scholar]
- Peake S.J., Dishion T.J., Stormshak E.A., Moore W.E., Pfeifer J.H. (2013). Risk-taking and social exclusion in adolescence: neural mechanisms underlying peer influences on decision-making. Neuroimage, 82, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinquart M., Sörensen S. (2000). Influences of socioeconomic status, social network, and competence on subjective well-being in later life: a meta-analysis. Psychology and Aging, 15(2), 187–224. [DOI] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., et al. (2011). Functional network organization of the human brain. Neuron, 72(4), 665–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puetz V.B., Kohn N., Dahmen B., et al. (2014). Neural response to social rejection in children with early separation experiences. Journal of the American Academy of Child and Adolescent Psychiatry, 53(12), 1328–37. [DOI] [PubMed] [Google Scholar]
- Rosenberg J.R., Amjad A.M., Breeze P., Brillinger D.R., Halliday D.M. (1989). The Fourier approach to the identification of functional coupling between neuronal spike trains. Progress in Biophysics and Molecular Biology, 53, 1–31. [DOI] [PubMed] [Google Scholar]
- Rotge J.Y., Lemogne C., Hinfray S., et al. (2015). A meta-analysis of the anterior cingulate contribution to social pain. Social Cognitive and Affective Neuroscience, 10(1), 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmälzle R., O’Donnell M.B., Garcia J.O., et al. (2017). Brain connectivity dynamics during social exclusion reflect social network structure. Proceedings of the National Academy of Sciences of the United States of America, 114(20), 5153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons-Morton B., Lerner N., Singer J. (2005). The observed effects of teenage passengers on the risky driving behavior of teenage drivers. Accident Analysis and Prevention, 37, 973–82. [DOI] [PubMed] [Google Scholar]
- Simons-Morton B.G., Bingham C.R., Falk E.B., et al. (2014). Experimental effects of injunctive norms on simulated risky driving among teenage males. Health Psychology, 33(7), 616–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H. (2013). The teenage brain: sensitivity to social evaluation. Current Directions in Psychological Science, 22(2), 121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. (2000). The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews, 24, 417–63. [DOI] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America, 105, 12569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. (2007). Risk taking in adolescence: new perspectives from brain and behavioral science. Current Directions in Psychological Science, 16(2), 55–9. [Google Scholar]
- Sun F.T., Miller L.M., D’Esposito M. (2004). Measuring interregional functional connectivity using coherence and partial coherence analyses of fMRI data. Neuroimage, 21, 647–58. [DOI] [PubMed] [Google Scholar]
- Sun F.T., Miller L.M., D’Esposito M. (2005). Measuring temporal dynamics of functional networks using phase spectrum of fMRI data. Neuroimage, 28, 647–58. [DOI] [PubMed] [Google Scholar]
- Supekar K., Musen M., Menon V. (2009). Development of large-scale functional brain networks in children. PLoS Biology, 7(7), e1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K.S., Ryali S., Menon V. (2011). Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. Journal of Neuroscience, 31(50), 18578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beest I., Williams K.D. (2006). When inclusion costs and ostracism pays, ostracism still hurts. Journal of Personality and Social Psychology, 91(5), 918–28. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel M.P., Hulshoff Pol H.E. (2010). Exploring the brain network: a review on resting-state fMRI functional connectivity. European Neurospychoparmacology, 20, 519–34. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F., Baetens K. (2009). Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage, 48(3), 564–84. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N., Cheng T.W., Pfeifer J.H. (2017). Neural correlates of social exclusion across ages: a coordinate-based meta-analysis of functional MRI studies. Neuroimage, 153, 359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J.A., Humphrey S.E., Meyer C.J., Hollenbeck J.R. (2012). Individualism-collectivism and team member performance: another look. Journal of Organizational Behavior, 33, 946–63. [Google Scholar]
- Waldrip A.M. (2007). The power of ostracism: can personality influence reactions to social exclusion? Dissertation, University of Texas-Arlington, 194 pp.
- Whelan R., Conrod P.J., Poline J.B., et al. (2012). Adolescent impulsivity phenotypes characterized by distinct brain networks. Nature Neuroscience, 15(6), 920–5. [DOI] [PubMed] [Google Scholar]
- Williams K.D. (2007). Ostracism. Annual Review of Psychology, 58, 425–52. [DOI] [PubMed] [Google Scholar]
- Williams K.D., Cheung C.K.T., Choi W. (2000). Cyberostracism: effects of being ignored over the Internet. Journal of Personality and Social Psychology, 79, 748–62. [DOI] [PubMed] [Google Scholar]
- Williams K.D., Jarvis B. (2006). Cyberball: a program for use in research on interpersonal ostracism and acceptance. Behavioral Research Methods, 38, 174–80. [DOI] [PubMed] [Google Scholar]
- Zadro L., Boland C., Richardson R. (2006). How long does it last? The persistence of the effects of ostracism in the socially anxious. Journal of Experimental Social Psychology, 42, 692–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.