ABSTRACT
Although it has been shown that some mannose-binding lectins (MBLs) exhibit significant activity against HIV infection, little is known about whether N-acetylgalactosamine (GalNAc)-binding lectins have the ability to inhibit HIV infection. Here, we demonstrate that a soybean-derived lectin (SBL) with GalNAc-binding affinity could potently suppress HIV infection of macrophages in a dose-dependent fashion. Unlike the MBLs, which block HIV only through binding to the glycosylated envelope proteins (gp120 and gp41) of the virus, SBL inhibited HIV at multiple steps of the virus infection/replication cycle. SBL could activate the beta interferon (IFN-β)–STAT signaling pathway, resulting in the upregulation of a number of antiviral interferon-stimulated genes (ISGs) in macrophages. In addition, SBL treatment of macrophages induced the production of C-C chemokines, which bind to HIV entry coreceptor CCR5. Deglycosylation of cell surface galactosyl moieties or presaturation of GalNAc-binding capacity could compromise SBL-mediated induction of the antiviral factors. Furthermore, SBL exerted its anti-HIV activity in the low nanomolar range with no mitogenic effect on CD4+ T cells, a major advantage in the development of SBL as a potential anti-HIV agent compared with MBLs. These data indicate a necessity to further investigate SBL as an alternative and cost-effective anti-HIV natural product.
IMPORTANCE Mannose-binding lectins (MBLs) can block the attachment of HIV to target cells and have been suggested as anti-HIV microbicides. However, the mitogenic effect of MBLs on CD4+ T cells limits this potential in clinical settings. Lectins with galactose (Gal)- or N-acetylgalactosamine (GalNAc)-binding specificity are another important category of carbohydrate-binding proteins (CBP). Compared to high-mannose N-linked glycans, GalNAc-type glycans present much less in HIV gp120 or gp41 glycosylation. Here, we demonstrate that GalNAc-specific soybean lectin (SBL) triggers antiviral signaling via recognition of the cell surface galactosyl group of macrophages, which results in the suppression of HIV at multiple steps. More importantly, SBL has no mitogenic effect on the activation of CD4+ T cells, a major advantage in the development of Gal/GalNAc-specific lectins as naturopathic anti-HIV agents.
KEYWORDS: soybean lectin, IFN-β, HIV, GalNAc, mitogenicity
INTRODUCTION
Lectins are a heterogeneous group of specific carbohydrate-binding proteins (CBP) that are distributed across a variety of species and are particularly prevalent in legumes and grains (1). These nonimmunoglobulin proteins are characterized by their binding affinity for complex carbohydrates, which are abundant on cell surfaces or associated with virulence factors. The N-linked glycans account for almost 50% of the molecular weight of the heavily glycosylated HIV envelope glycoproteins (gp120 and gp41), and about 33% of these N-linked glycans are high-mannose type (2–5). The lectins with mannose-binding capacity (mannose-binding lectins [MBLs]), such as the lectins from banana (BanLec) (6, 7), from snowdrop (GNA) (8), from the cyanobacterium Microcystis aeruginosa (microvirin [MVN]) (9), and from the red alga Griffithsia sp. (Griffithsin [GRFT]) (10), have been reported to have significant anti-HIV activity via interaction with the mannose moieties on viral envelope proteins. Therefore, MBLs have been suggested for use as antiviral microbicides to prevent HIV sexual transmission (11).
Although many studies have been focused on the binding of the MBLs to HIV envelope glycoproteins, there is little information about the impact of lectins binding to the target cell glycans upon HIV infection. It is important to note that the mammalian cell surface exhibits a variety of both N-linked and O-linked glycans, including galactosyl groups affiliated with membrane-bound proteins or glycolipids (http://www.functionalglycomics.org), which are involved in essential biological functions (12). It has been reported that human recombinant macrophage galactose-type C-type lectin could interfere with the function of regulatory T cells through binding to cell surface N-acetylgalactosamine (GalNAc) (13). Recombinant human galectin 9 (rhGal-9) with galactose (Gal) binding specificity could potently reverse HIV latency through oligosaccharides on the T cell surface and induce robust expression of host antiviral factors (14). RhGal-9 has also been shown to interact with Tim-3 on activated CD4+ T cells, resulting in the downregulation of HIV coreceptors and rendering cells less susceptible to HIV infection and replication (15). Soybean lectin (SBL) is a glycoprotein isolated from the seeds of soybeans that has binding affinity to GalNAc. It has been documented that SBL could inhibit HIV reverse transcriptase (RT) in a non-cell-culture system (16). Here, we investigate the antiviral activity of SBL on HIV infection of macrophages and explore the underlying mechanism(s) of SBL-mediated viral inhibition. Our data demonstrate that GalNAc-specific SBL can induce multiple antiviral factors via recognition of macrophage surface galactosyl moieties and has potential to be further developed as an anti-HIV-1 naturopathic agent.
RESULTS
SBL inhibits HIV infection of macrophages.
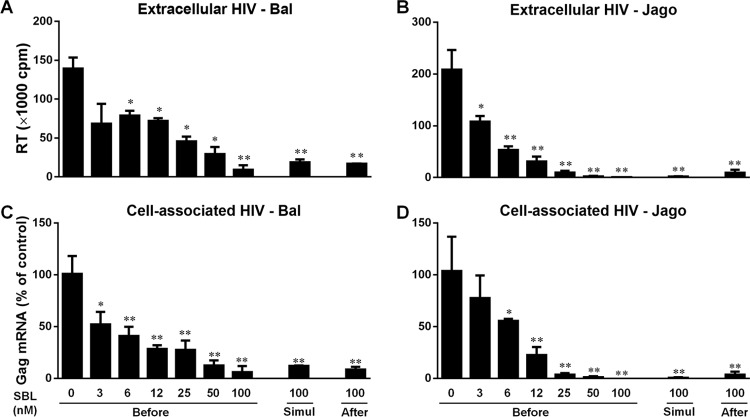
We first determined the anti-HIV potency of SBL under several treatment conditions. As shown in Fig. 1, SBL treatment of macrophages prior to HIV infection for 24 h dose-dependently inhibited HIV infections (50% inhibitory concentration [IC50], 3.4 nM for strain Bal and 6.0 nM for strain Jago). At a concentration of 100 nM, SBL inhibited nearly 90% of HIV infection at both the extracellular and intracellular levels. In addition, SBL treatment of macrophages during HIV infection (simultaneously) or 24 h postinfection also significantly suppressed viral infection (Fig. 1). The inhibitory activity of SBL against HIV should not be attributed to the cytotoxic effect of SBL, as treatment with up to 1,000 nM SBL for 72 h showed no cytotoxicity to macrophages (data not shown).
FIG 1.
SBL inhibits HIV infection of macrophages. Macrophages were cultured in the presence or absence of the indicated concentrations of SBL either 24 h prior to HIV infection (Before), simultaneously (Simul), or 24 h postinfection (After). Three hours postinfection, unbound viruses were washed away and fresh medium containing the indicated concentrations of SBL was added to the cell cultures. (A and B) Cell-free supernatant was collected on day 12 postinfection for HIV Bal or HIV Jago RT assay. (C and D) The expression of cell-associated HIV gag mRNA was measured by qRT-PCR. The data are expressed as the means and SD of the results of three independent experiments with cells from three donors (*, P < 0.05; **, P < 0.01).
SBL induces rapid IFN-β induction and IRF3 phosphorylation.
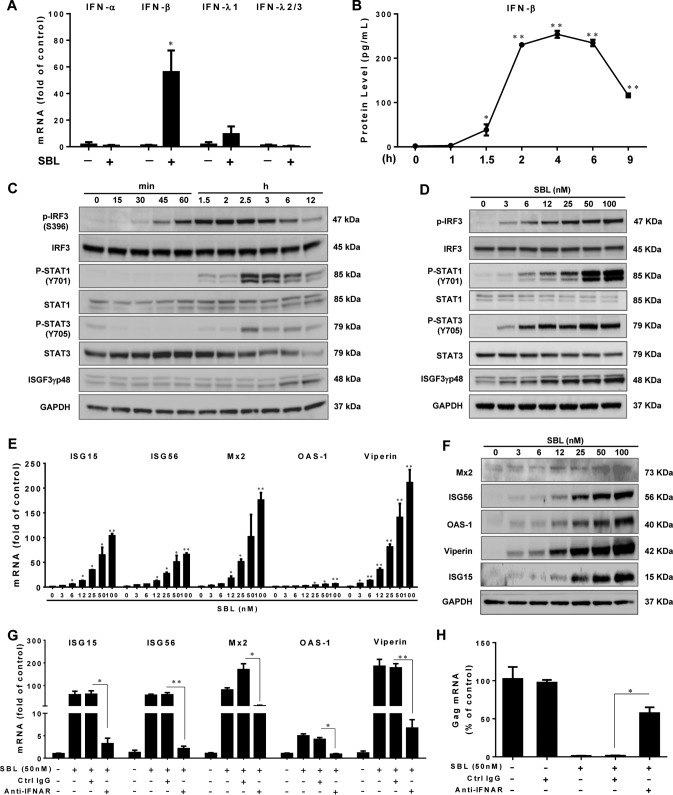
Type I interferons (IFNs) play a critical role in the innate immunity against viral infections. Interestingly, SBL specifically induced the expression of IFN-β (Fig. 2A). The secretion of IFN-β was induced as early as 1.5 h, lasted for the next 4 h, and then diminished afterward (Fig. 2B). Because IRF3 is a master regulator of IFN-β, we examined the impact of SBL on IRF3 expression. There was no change in the expression of total IRF3 in SBL-treated macrophages. In contrast, SBL rapidly induced IRF3 phosphorylation (Ser396) as early as 30 min posttreatment, with maximal induction reached at 2 h (Fig. 2C). The effect of SBL on IRF3 phosphorylation was also dose dependent (Fig. 2D).
FIG 2.
SBL induces the IRF3–IFN-β–STAT axis and ISG expression. (A) Macrophages were treated or not with SBL (50 nM) for 2 h, and IFN genes were examined by qPCR. (B) Macrophages were treated with 50 nM SBL for the indicated times, and IFN-β levels in cell-free medium were measured by ELISA. (C and D) Macrophages were treated with SBL (50 nM) for the indicated times (C) or with the indicated concentrations of SBL for 2 h (D). The expression of IRF3, STAT1/3, and ISGF3γp48 was determined by immunoblotting. (E and F) Macrophages were treated with the indicated concentrations of SBL for 6 h (E) or 24 h (F), and the expression of different ISGs was measured. (G and H) Macrophages were treated with the neutralization antibody to IFN-α/β receptor (anti-IFNAR; 10 μg/ml) or control IgG for 1 h and then incubated with SBL for an additional 6 h (G) or anti-IFNAR- or control IgG-treated macrophages were incubated with SBL for 24 h prior to HIV (strain Bal) infection (H). The expression of HIV gag was measured at 72 h postinfection. The data are expressed as means and SD of the results of three independent experiments (*, P < 0.05; **, P < 0.01).
SBL activates STAT kinetics and induces antiviral ISGs.
We next investigated the effect of SBL on STAT activation, which is essential for IFN-mediated antiviral signaling. Figure 2C shows that SBL increased total STAT1/3 expression and induced the phosphorylation of STAT1 and STAT3. STAT1/3 phosphorylation was observed as early as 1.5 h posttreatment and reached the maximal level at 2.5 h. In addition, SBL triggered the formation of interferon-stimulated transcription factor 3 gamma 48 kDa (ISGF3-γp48) starting from a later time point, 6 h posttreatment. SBL-induced STAT phosphorylation and ISGF3-γp48 expression was also dose dependent (Fig. 2D). The expression of a pattern of interferon-stimulated genes (ISGs) (ISG15, ISG56, OAS-1, Viperin, and Mx2) was upregulated by SBL at both the mRNA (Fig. 2E) and protein (Fig. 2F) levels. Preincubation of macrophages with anti-IFN-α/β receptor chain 2 (IFNAR) neutralization antibody significantly attenuated the induction of ISGs by SBL (Fig. 2G), as well as compromising SBL-mediated anti-HIV activity (Fig. 2H), suggesting that IFN-β was responsible for SBL-mediated ISG induction and anti-HIV activity.
SBL inhibits HIV infection of macrophages at the entry level.
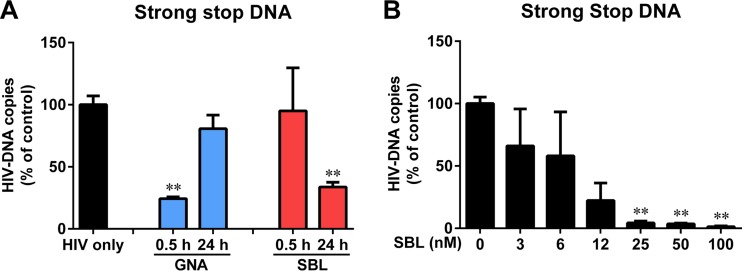
To determine whether SBL can function like MBLs that inhibit HIV infection through binding to the viral envelope protein, we compared SBL with GNA, one of the MBLs, with regard to HIV inhibition at the entry level. A short incubation of macrophages with GNA (0.5 h, without washing it off) inhibited the expression of HIV strong-stop DNA, an indicator of HIV entry. However, 24-h pretreatment with GNA (washing it off) of macrophages had no effect on HIV entry (Fig. 3A), implying that inhibition depends on the interaction of GNA with HIV. In contrast, we found that a short treatment of macrophages with SBL (0.5 h, without washing it off) could not suppress the HIV strong-stop DNA levels, while 24-h pretreatment of macrophages with SBL (washing it off) inhibited HIV entry (Fig. 3A). The extended SBL pretreatment inhibited strong-stop DNA expression in a dose-dependent manner (Fig. 3B).
FIG 3.
SBL inhibits HIV infection of macrophages at the entry level. (A) Macrophages were pretreated with GNA or SBL (50 nM) for 0.5 h (without washing it off) or 24 h (washing it off) prior to infection with DNase I-treated HIV (strain Bal). Three hours postinfection, the cellular DNA of the macrophages was harvested, and strong-stop DNA was quantified by real-time PCR. The number of copies was normalized to the control (100%). (B) Macrophages were pretreated with the indicated concentrations of SBL for 24 h prior to infection with DNase I-treated HIV (Bal). Three hours postinfection, the cellular DNA of the macrophages was harvested, and strong-stop DNA was quantified by qPCR. The data are expressed as the means and SD of the results of three independent experiments (**, P < 0.01).
SBL subdues macrophage susceptibility to HIV by inducing C-C chemokine expression.
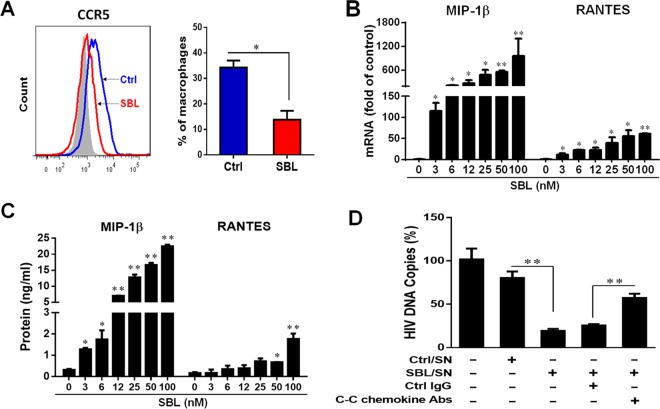
We also determined the impact of SBL on HIV coreceptor CCR5 expression. Although SBL had no effect on suppressing CCR5 expression at the mRNA level (data not shown), SBL treatment of macrophages significantly inhibited the surface expression of CCR5 as examined by flow cytometry (Fig. 4A). We then examined the effect of SBL on C-C chemokines, the natural ligands of CCR5. As shown in Fig. 4B and C, SBL pretreatment of macrophages dose-dependently induced C-C chemokine (MIP-1β and RANTES) expression at both the mRNA and protein levels. These C-C chemokines subdued the macrophages' susceptibility to HIV, as the SBL-conditioned medium (SBL-SN) exerted a suppressive effect on HIV strong-stop DNA expression, and neutralization antibodies to C-C chemokines could partially reverse the suppression (Fig. 4D).
FIG 4.
SBL subdues macrophage susceptibility to HIV by enhancing C-C chemokine expression. (A) Macrophages were incubated with or without SBL (50 nM) for 24 h. The CCR5 expression was measured by flow cytometry. (B and C) Macrophages were treated with the indicated concentrations of SBL for 6 h (B) or 24 h (C), and the expression of the C-C chemokines MIP-1β and RANTES at the mRNA (B) and protein (C) levels was measured. (D) Macrophages were incubated with or without SBL (50 nM) for 24 h, and the culture supernatants without SBL (Ctrl/SN) or with SBL (SBL/SN) were collected for treatment of autologous macrophages. In addition, SBL/SN was incubated with 20 μg/ml control IgG (Ctrl/IgG) or a mixture of neutralization antibodies to MIP-1α, MIP-1β, and RANTES for 1 h and then added to the macrophages for an additional 1 h prior to infection with DNase I-treated HIV (Bal). HIV strong-stop DNA was quantified at 3 h postinfection. The number of copies was normalized to the control (100%). The data are expressed as the means and SD of the results of three independent experiments (*, P < 0.05; **, P < 0.01).
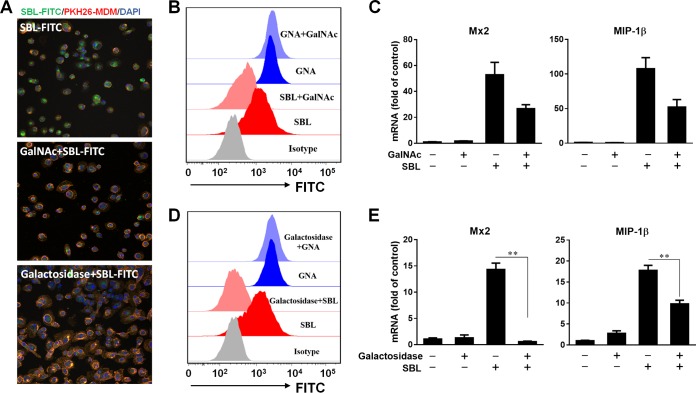
SBL triggers the antiviral response of macrophages via the recognition of surface galactosyl moieties.
To characterize the role of GalNAc-binding specificity in the SBL-induced antiviral response, we presaturated the sugar binding capacity of SBL by competitive inhibition assay. Incubation of fluorescein isothiocyanate (FITC)-conjugated SBL (SBL-FITC) with macrophages rapidly delivered the complex into the cells, as evidenced by the dispersed green fluorescence inside the PKH26-labeled plasma membrane (Fig. 5A). Preincubation of SBL-FITC with GalNAc decreased the entry of SBL into macrophages, as observed by confocal imaging (Fig. 5A). In addition, the binding of SBL-FITC to macrophages was also decreased by GalNAc competition (Fig. 5B). Consequently, GalNAc preincubation suppressed SBL-induced upregulation of Mx2 and MIP-1β (Fig. 5C). In contrast, GalNAc had no effect on the binding of GNA-FITC to macrophages (Fig. 5B), suggesting the inhibition was glycan specific. There was no induction of IFN-β, Mx2, and MIP-1β by GNA (see Fig. 7B). We next deglycosylated the macrophage surface glycans to examine whether cell surface galactosyl moieties are involved in SBL's action. Figure 5A and D show that galactosidase treatment almost abolished the entry of SBL-FITC into macrophages, as well as cell surface binding of SBL-FITC, but not the binding of GNA-FITC, confirming that the binding of SBL is surface galactosyl specific. Moreover, SBL-mediated induction of MIP-1β and Mx2 expression could be suppressed by galactosidase treatment of macrophages (Fig. 5E).
FIG 5.
SBL triggers the antiviral response of macrophages via the recognition of surface galactosyl moieties. SBL-FITC (50 nM) was incubated with or without GalNAc (200 mM) for 24 h and the mixtures were added to treat macrophages, or macrophages were pretreated with galactosidase for 24 h and then treated with SBL-FITC. (A) Cells were stained with PKH26 and Hoechst 33342 and visualized by confocal imaging after 1 h of SBL-FITC treatment. (B and D) Cells were washed with ice-cold PBS to halt binding, and the bound FITC-conjugated lectins were determined using flow cytometry. (C and E) Macrophages were treated for 6 h, and the expression of Mx2 and MIP-1β was measured by real-time RT-PCR. The data are expressed as the means and SD of the results of three independent experiments (**, P < 0.01).
FIG 7.
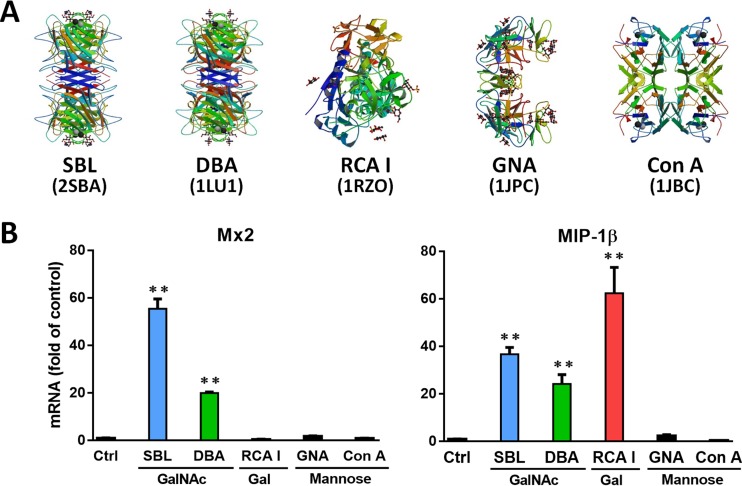
Effects of different sugar affinity lectins on ISG and C-C chemokine expression. (A) Three-dimensional (3D) structures of the indicated lectins with the respective accession codes from the Research Collaboratory for Structural Bioinformatics: Protein Data Bank (RCSB PDB). (B) Macrophages were treated with the indicated lectins (50 nM) for 6 h, and then cellular RNA was extracted and the expression of Mx2 and MIP-1β was measured by qPCR. The data are expressed as the means and SD of the results of three independent experiments (**, P < 0.01).
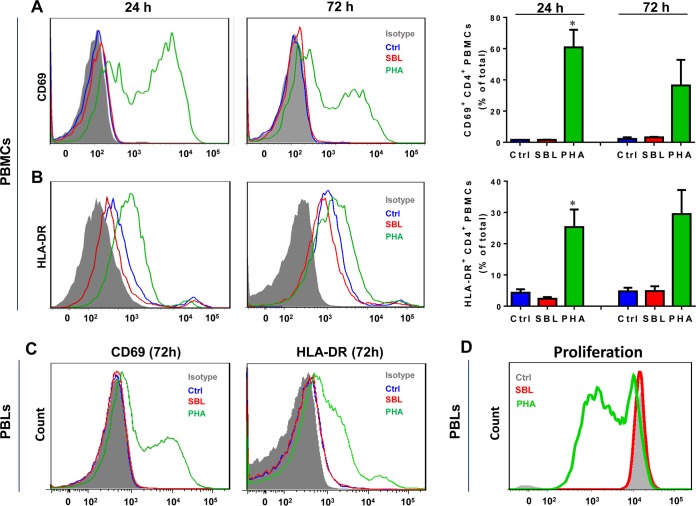
SBL has no mitogenic effect on CD4+ T cells.
The mitogenic activity of HIV candidate drugs may compromise their possible clinical application, as the activation of T cells would exacerbate HIV replication (17). We next investigated whether SBL affected peripheral blood mononuclear cell (PBMC)/peripheral blood lymphocyte (PBL) activation and used phytohemagglutinin (PHA) as a positive control. Not surprisingly, the well-known mitogenic lectin PHA significantly increased the cell surface expression of CD69 and HLA-DR on CD3+ CD4+ T cells in PBMCs (Fig. 6A and B). In contrast, SBL treatment did not increase the activation markers CD69 and HLA-DR on CD3+ CD4+ T cells up to 72 h in PBMCs (Fig. 6A and B). SBL also exhibited no stimulatory effect on the activation and proliferation of CD3+ CD4+ T cells in PBLs (Fig. 6C and D).
FIG 6.
Effect of SBL on activation or proliferation of CD4+ T cells. (A and B) PBMCs were treated with 50 nM SBL or PHA for 24 h or 72 h, respectively. The activation markers CD69 (A) and HLA-DR (B) on CD3+ CD4+ PBMCs were measured by flow cytometry. PHA was used as a positive control for CD3+ CD4+ PBMC activation. (Left and middle) Representative data from PBMCs obtained from one donor. (Right) Statistical data, expressed as means and SD, obtained from three independent experiments using PBMCs from three donors (*, P < 0.05). (C) PBLs were treated with 50 nM SBL or PHA for 72 h. The activation markers CD69 and HLA-DR on CD3+ CD4+ PBLs were measured by flow cytometry. PHA was used as a positive control for CD4+ PBL activation. (D) PBLs were labeled with carboxyfluorescein succinimidyl ester (CFSE) (5 μM) and incubated with 50 nM SBL or PHA for 72 h. The CFSE expression of CD3+ CD4+ T cells was measured by flow cytometry.
Gal/GalNAc lectins induce antiviral factors in macrophages.
Based on the finding that Gal/GalNAc-specific SBL could inhibit HIV infection of macrophages through induction of antiviral ISGs and C-C chemokines (Fig. 2 and 4), we further examined whether other Gal/GalNAc-specific lectins have effects on these antiviral factors. As shown in Fig. 7B, similar to SBL, Dolichos biflorus agglutinin (DBA) induced both Mx2 and MIP-1β expression, and Ricinus communis agglutinin I (RCA I) could induce the expression MIP-1β only. In contrast, the two MBLs GNA and concanavalin A (ConA) were not able to induce the expression of these antiviral factors in macrophages (Fig. 7B).
DISCUSSION
In the present study, we showed that the lectin isolated from soybeans (SBL) has potent inhibitory effect against HIV infection of macrophages at the nanomolar level (Fig. 1). SBL inhibits HIV, not only at the viral entry level, but also through inducing multiple cellular antiviral factors. Importantly, with a carbohydrate-binding affinity for GalNAc, SBL inhibited HIV infection with no mitogenic effect on the activation of CD4+ T cells (Fig. 6), as well as CD8+ T cells (data not shown). These results support the previous finding that SBL in its native form, which is divalent with respect to sugar binding, was nonmitogenic in stimulating lymphocytes (18). Therefore, this nonmitogenic property provides a superior advantage for the potential use of SBL in HIV treatment. Studies have shown that lectins with mitogenic activity can activate CD4+ T cells, which results in systemic inflammation and enhancement of HIV transmission (9, 19). The lectin from bananas (BanLec) suffers from potential side effects mediated by its mitogenicity, although it has previously been identified as a potent inhibitor of HIV infection (6). Increased cell surface expression of CD69 was observed in BanLec-treated CD4+ T cells, and this mitogenicity has been uncoupled from antiviral activity via bioengineering. The modified BanLec without mitogenicity has been suggested as a potential broad-spectrum antiviral agent (17). We revealed that two other Gal/GalNAc-specific plant lectins (DBA and RCA I) can also induce antiviral Mx2 and MIP-1β expression in macrophages (Fig. 7B). These two Gal/GalNAc-specific lectins are also known to have very little mitogenic effect on lymphocyte activation (20, 21). In this regard, the ability of natural SBL to suppress HIV infection or of other Gal/GalNAc-specific lectins to induce antiviral factors without inducing systemic activation of CD4+ T cells is an important property that fits the clinical setting and warrants further study.
Pathogen-associated or cell surface N-linked and O-linked glycans may constitute key targets for new therapeutics to treat HIV and possibly a variety of other infectious diseases. To date, extensive studies have been focused on MBLs that can block the binding of glycosylated HIV envelope proteins (high-mannose-type N-linked glycans) to target cells. Unlike the reported anti-HIV MBLs (e.g., BanLec and GNA) that target primarily high-mannose structures of HIV gp120, SBL has glycan binding specificity for the GalNAc moiety and to a lesser extent for galactose. Compared to high-mannose N-linked glycans, GalNAc-type glycans present much less in HIV gp120 or gp41 glycosylation. Therefore, a short treatment of macrophages with SBL had no effect on HIV infection at the entry level (Fig. 3A). In contrast, the pretreatment of macrophages with nanomolar levels of SBL for 24 h exerted HIV inhibition activity at multiple steps. Besides the inhibition of HIV entry by inducing C-C chemokine expression, SBL enhanced the expression of antiviral factors in macrophages. These findings have not been reported for other viricidal lectins, such as BanLec, GNA, or MVN. The GalNAc specificity was confirmed to be responsible for activation of the antiviral response in macrophages by SBL, as sugar competition or degalactosidase markedly reduced SBL's binding to cells and subsequent ISG and C-C chemokine expression (Fig. 5B).
The innate immune system is crucial as a first line of host defense against pathogens (22). A major aspect of the antiviral innate immune response is the induction and secretion of IFNs, which perform antiviral and immunomodulatory functions (23). We demonstrated that SBL could rapidly induce IRF3 phosphorylation and selectively induce IFN-β expression in macrophages. Canonical IFN-β signaling activates the JAK-STAT pathway, leading to a pattern of ISG transcription (24). We found that SBL treated macrophages produce several antiviral ISGs (ISG15, ISG56, OAS-1, Viperin, and Mx2). These ISGs are known to inhibit HIV by blocking several stages of the viral life cycle, including assembling, releasing, and inhibiting the capsid-dependent nuclear import of subviral complexes (25–27). In terms of antiviral ISG induction, SBL is similar to Gal-9 of human origin, which has been shown to potently reverse HIV latency through oligosaccharides on the T cell surface and to induce robust expression of host antiviral factors (such as APOBEC3G) (14). The mammalian Gal-9 also has a binding preference for glycans featuring galactose and its derivatives (28). Soluble Gal-9 has been reported to interact with Tim-3 on activated CD4+ T cells, resulting in the downregulation of HIV coreceptors and rendering cells less susceptible to HIV infection and replication (15). Thus, lectins with Gal/GalNAc-binding determinants may represent an important category of lectins that can trigger host cell innate antiviral responses to subdue their susceptibility to viral infection. Given that SBL is not mitogenic to T cells while it is capable of inducing the antiviral response of macrophages, the effect of SBL on the immune system warrants further study in animal models.
In summary, our findings reveal that GalNAc-specific SBL can initiate antiviral signaling via recognition of host cell surface glycan moieties. The ability of SBL to suppress HIV infection without inducing systemic activation implies the potential to explore naturopathic lectins as novel anti-HIV therapeutics targeting the cell surface glycan diversity.
MATERIALS AND METHODS
Reagents.
SBL (Glycine max) was purchased from Sigma-Aldrich (St. Louis, MO). Fluorescein-conjugated SBL (SBL-FITC), unconjugated and fluorescein-conjugated GNA (GNA-FITC), and unconjugated D. biflorus agglutinin (DBA), R. communis agglutinin I (RCA I), and ConA were purchased from Vector Laboratories (Burlingame, CA). The primary antibodies against phospho-IRF-3, phospho-STAT1, STAT1, phospho-STAT3, STAT3, ISG56, OAS-1, Viperin, ISG15, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Cell Signaling Technology (Danvers, MA). Rabbit aniti-OAS-1 antibody was purchased from Sigma-Aldrich. Mouse anti-IRF3 (SL-12) and goat anti-Mx2 antibodies were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). Anti-IFNAR was purchased from PBL Assay Science (Piscataway, NJ). Pacific Blue (PB) anti-CD4 monoclonal antibody (MAb), FITC anti-CD69 MAb, and phycoerythrin (PE) anti-HLA-DR MAb were from BD Biosciences (San Jose, CA).
Human primary monocyte-derived macrophages and PBMC cultures.
Purified human peripheral blood monocytes, PBMCs, and PBLs were obtained from the Human Immunology Core at the University of Pennsylvania (Philadelphia, PA). The monocytes were cultured in Corning CellBind multiwell plates at 37°C with 5% CO2 in Dulbecco's modified Eagle's culture medium containing 10% heat-inactivated fetal bovine serum (FBS) (HyClone, Logan, UT), 2 mM l-glutamine, 50 units/ml penicillin, 50 μg/ml streptomycin, and 25 mM HEPES (Invitrogen). The monocytes were differentiated in complete culture medium for 7 days, and the macrophages were characterized. For mitogenic activity assays or proliferation assays, PBMCs or PBLs were cultured in 48-well plates at 37°C with 5% CO2 in RPMI 1640 supplemented with 10% FBS for up to 72 h. The viability of the cells was determined by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS), assay (Promega, Madison, WI).
HIV infection of macrophages.
Human primary monocyte-derived macrophages were incubated with 20 ng of p24-gag antigen equivalent of Bal (AIDS Research and Reference Program, NIH) or Jago (Center for AIDS Research at the University of Pennsylvania) for 3 h. Unbound viruses were then removed by three washes with basal medium, and the cultures were replenished with fresh complete medium supplemented with corresponding concentrations of SBL. The culture media were collected every 3 days postinfection for HIV RT assay (29, 30). At day 12 postinfection, cellular RNA was also harvested for determination of the number of intracellular copies of HIV gag (31, 32).
Detection of strong-stop DNA of HIV.
To assess the viral entry of the laboratory-adapted HIV Bal infection, we examined the expression of HIV long terminal repeat (LTR) R/U5 DNA (strong-stop DNA), the first product of HIV reverse transcription, which is synthesized immediately after viral entry and uncoating (33, 34). Briefly, the viral stock was treated with 10 units/μl of RNase-free DNase I (Roche, Indianapolis, IN) for 30 min prior to its addition to macrophage cultures. The macrophages were then washed, and cellular DNA was extracted using Tri-Reagent (MRC, Cincinnati, OH) 3 h postinfection. Strong-stop DNA was quantified by real-time PCR. The primers were as follows: forward, 5′-TCTCTCTGGTTAGACCAGATCTG-3′, and reverse, 5′-ACTGCTAGAGATTTTCCACACTG-3′ (35).
Reverse transcription and qRT-PCR.
Total RNA was isolated as described previously (36) and then subjected to reverse transcription according to the manufacturer's protocol. Quantitative real-time PCR (qRT-PCR) was performed by using SYBR green master mix (Bio-Rad, CA) as described previously (29).
Reverse transcriptase assay.
Extracellular HIV RT activity in the macrophage culture supernatant was determined as described previously (29, 37). Briefly, 10 μl of supernatant was incubated with 50 μl of a cocktail containing poly(A), oligo(dT), MgCl2, and [32P]dTTP overnight at 37°C. The mixture (30 μl) was then spotted onto DE81 paper (Whatman International Ltd., United Kingdom), dried, and washed 4 times with 2× saline-sodium citrate buffer and once with 95% ethanol. The radioactivity was measured in a liquid scintillation counter (PerkinElmer, Waltham, MA).
Flow cytometric analysis.
PBMCs or PBLs were washed with phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and then stained with PE-Cy5-CD3 MAb, PB-CD4 MAb, FITC-CD69 MAb, and PE-HLA-DR MAb. For cell proliferation assays, PBLs were stained with 5 μM of cell-permeable amine-reactive fluorescent dye (CFSE; Invitrogen) and then treated with 50 nM SBL for 72 h. The stained cells were measured on a FACSCanto II (BD Bioscience) and analyzed using FlowJo software (Tree Star Inc., Ashland, OR). PHA (50 nM) was used as a positive control to activate the CD4+ T cells, whereas isotype IgG-stained cells were used as background controls.
Cell deglycosylation assay and sugar competitive-inhibition assay.
For surface deglycosylation, macrophages were treated with β-galactosidase (Sigma) for 24 h, washed with PBS, and then incubated with SBL-FITC for 1 h or with SBL for 6 h. For the sugar competitive-inhibition assay, SBL-FITC or SBL was incubated with 200 mM GalNAc at 37°C for 24 h, and the mixture was added to treat macrophages for an additional 1 h or 6 h. The binding of SBL-FITC to macrophages was examined by flow cytometry, and the expression of ISGs and C-C chemokines was examined by qRT-PCR.
ELISA.
The protein levels of IFN-β, MIP-1β, and RANTES in the culture supernatants of macrophages were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (IFN-β, PBL Assay Science; MIP-1β and RANTES, RayBiotech, Norcross, GA).
Immunoblotting.
Macrophages were lysed with RIPA buffer supplemented with protease/phosphatase inhibitors (Sigma). The protein concentration was determined by the bicinchoninic acid (BCA) method (ChemCruz, Dallas, TX). Equal amounts of lysates (20 μg) were separated on 4 to 12% Bis-Tris gels (Invitrogen). Following transfer to a nitrocellulose membrane, the proteins were analyzed by immunoblotting and visualized using enhanced chemiluminescence (Amersham, Bucks, United Kingdom) in a Fuji Film LAS-4000 imaging analyzer (GE Life Sciences, Piscataway, NJ).
Statistical analysis.
Data were expressed as means and standard deviations (SD) from at least 3 independent experiments, and statistical significance was measured by the Student t test or one-way analysis of variance, followed by the Newman-Keuls test where appropriate.
ACKNOWLEDGMENTS
We thank Servio H. Ramirez (Department of Pathology and Laboratory Medicine, Temple University) for providing technical support for immunofluorescence and flow cytometric analysis. Technical support from Xinji Guo (Cardiovascular Research Center at Temple University) for immunoblotting is also acknowledged.
This work was supported by the National Science and Technology Major Projects of Infectious Disease (2014ZX10001003-005 to W.H.), the National Natural Sciences Foundation of China (81571962 to W.H.), and the National Institutes of Health (DA040329 and MH109385 to J.L.; DA022177, DA042373, and DA041302 to W.H.).
REFERENCES
- 1.Akkouh O, Ng TB, Singh SS, Yin C, Dan X, Chan YS, Pan W, Cheung RC. 2015. Lectins with anti-HIV activity: a review. Molecules 20:648–668. doi: 10.3390/molecules20010648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizuochi T, Spellman MW, Larkin M, Solomon J, Basa LJ, Feizi T. 1988. Carbohydrate structures of the human-immunodeficiency-virus (HIV) recombinant envelope glycoprotein gp120 produced in Chinese-hamster ovary cells. Biochem J 254:599–603. doi: 10.1042/bj2540599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, Sodroski JG. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Bartesaghi A, Borgnia MJ, Sapiro G, Subramaniam S. 2008. Molecular architecture of native HIV-1 gp120 trimers. Nature 455:109–113. doi: 10.1038/nature07159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu P, Liu J, Bess J Jr, Chertova E, Lifson JD, Grise H, Ofek GA, Taylor KA, Roux KH. 2006. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature 441:847–852. doi: 10.1038/nature04817. [DOI] [PubMed] [Google Scholar]
- 6.Swanson MD, Winter HC, Goldstein IJ, Markovitz DM. 2010. A lectin isolated from bananas is a potent inhibitor of HIV replication. J Biol Chem 285:8646–8655. doi: 10.1074/jbc.M109.034926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferir G, Vermeire K, Huskens D, Balzarini J, Van Damme EJ, Kehr JC, Dittmann E, Swanson MD, Markovitz DM, Schols D. 2011. Synergistic in vitro anti-HIV type 1 activity of tenofovir with carbohydrate-binding agents (CBAs). Antiviral Res 90:200–204. doi: 10.1016/j.antiviral.2011.03.188. [DOI] [PubMed] [Google Scholar]
- 8.Hammar L, Hirsch I, Machado AA, De Mareuil J, Baillon JG, Bolmont C, Chermann JC. 1995. Lectin-mediated effects on HIV type 1 infection in vitro. AIDS Res Hum Retrovir 11:87–95. doi: 10.1089/aid.1995.11.87. [DOI] [PubMed] [Google Scholar]
- 9.Huskens D, Ferir G, Vermeire K, Kehr JC, Balzarini J, Dittmann E, Schols D. 2010. Microvirin, a novel alpha(1,2)-mannose-specific lectin isolated from Microcystis aeruginosa, has anti-HIV-1 activity comparable with that of cyanovirin-N but a much higher safety profile. J Biol Chem 285:24845–24854. doi: 10.1074/jbc.M110.128546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mori T, O'Keefe BR, Sowder RC II, Bringans S, Gardella R, Berg S, Cochran P, Turpin JA, Buckheit RW Jr, McMahon JB, Boyd MR. 2005. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem 280:9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- 11.Koharudin LM, Gronenborn AM. 2014. Antiviral lectins as potential HIV microbicides. Curr Opin Virol 7:95–100. doi: 10.1016/j.coviro.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varki A. 1993. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology 3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zizzari IG, Martufi P, Battisti F, Rahimi H, Caponnetto S, Bellati F, Nuti M, Rughetti A, Napoletano C. 2015. The macrophage galactose-type C-type lectin (MGL) modulates regulatory T cell Functions. PLoS One 10:e0132617. doi: 10.1371/journal.pone.0132617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdel-Mohsen M, Chavez L, Tandon R, Chew GM, Deng X, Danesh A, Keating S, Lanteri M, Samuels ML, Hoh R, Sacha JB, Norris PJ, Niki T, Shikuma CM, Hirashima M, Deeks SG, Ndhlovu LC, Pillai SK. 2016. Human galectin-9 is a potent mediator of HIV transcription and reactivation. PLoS Pathog 12:e1005677. doi: 10.1371/journal.ppat.1005677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elahi S, Niki T, Hirashima M, Horton H. 2012. Galectin-9 binding to Tim-3 renders activated human CD4+ T cells less susceptible to HIV-1 infection. Blood 119:4192–4204. doi: 10.1182/blood-2011-11-389585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin P, Ye X, Ng T. 2008. Purification of melibiose-binding lectins from two cultivars of Chinese black soybeans. Acta Biochim Biophys Sin 40:1029–1038. doi: 10.1111/j.1745-7270.2008.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swanson MD, Boudreaux DM, Salmon L, Chugh J, Winter HC, Meagher JL, Andre S, Murphy PV, Oscarson S, Roy R, King S, Kaplan MH, Goldstein IJ, Tarbet EB, Hurst BL, Smee DF, de la Fuente C, Hoffmann HH, Xue Y, Rice CM, Schols D, Garcia JV, Stuckey JA, Gabius HJ, Al-Hashimi HM, Markovitz DM. 2015. Engineering a therapeutic lectin by uncoupling mitogenicity from antiviral activity. Cell 163:746–758. doi: 10.1016/j.cell.2015.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schechter B, Lis H, Lotan R, Novogrodsky A, Sharon N. 1976. The requirement for tetravalency of soybean agglutinin for induction of mitogenic stimulation of lymphocytes. Eur J Immunol 6:145–149. doi: 10.1002/eji.1830060302. [DOI] [PubMed] [Google Scholar]
- 19.Gavrovic-Jankulovic M, Poulsen K, Brckalo T, Bobic S, Lindner B, Petersen A. 2008. A novel recombinantly produced banana lectin isoform is a valuable tool for glycoproteomics and a potent modulator of the proliferation response in CD3+, CD4+, and CD8+ populations of human PBMCs. Int J Biochem Cell Biol 40:929–941. doi: 10.1016/j.biocel.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 20.Closs O, Saltvedt E, Olsnes S. 1975. Stimulation of human lymphocytes by galactose-specific Abrus and Ricinus lectins. J Immunol 115:1045–1048. [PubMed] [Google Scholar]
- 21.Borrebaeck CAK, Etzler ME. 1982. Mitogenic properties of 2 carbohydrate binding-proteins from the Dolichos-Biflorus Plant. FEBS Lett 145:8–10. doi: 10.1016/0014-5793(82)81195-2. [DOI] [Google Scholar]
- 22.Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Seth RB, Sun L, Chen ZJ. 2006. Antiviral innate immunity pathways. Cell Res 16:141–147. doi: 10.1038/sj.cr.7310019. [DOI] [PubMed] [Google Scholar]
- 24.Ivashkiv LB, Donlin LT. 2014. Regulation of type I interferon responses. Nat Rev Immunol 14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okumura A, Lu G, Pitha-Rowe I, Pitha PM. 2006. Innate antiviral response targets HIV-1 release by the induction of ubiquitin-like protein ISG15. Proc Natl Acad Sci U S A 103:1440–1445. doi: 10.1073/pnas.0510518103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goujon C, Moncorge O, Bauby H, Doyle T, Ward CC, Schaller T, Hue S, Barclay WS, Schulz R, Malim MH. 2013. Human MX2 is an interferon-induced post-entry inhibitor of HIV-1 infection. Nature 502:559–562. doi: 10.1038/nature12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kane M, Yadav SS, Bitzegeio J, Kutluay SB, Zang T, Wilson SJ, Schoggins JW, Rice CM, Yamashita M, Hatziioannou T, Bieniasz PD. 2013. MX2 is an interferon-induced inhibitor of HIV-1 infection. Nature 502:563–566. doi: 10.1038/nature12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tandon R, Chew GM, Byron MM, Borrow P, Niki T, Hirashima M, Barbour JD, Norris PJ, Lanteri MC, Martin JN, Deeks SG, Ndhlovu LC. 2014. Galectin-9 is rapidly released during acute HIV-1 infection and remains sustained at high levels despite viral suppression even in elite controllers. AIDS Res Hum Retrovir 30:654–664. doi: 10.1089/aid.2014.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Wang Y, Wang X, Ye L, Zhou Y, Persidsky Y, Ho W. 2013. Immune activation of human brain microvascular endothelial cells inhibits HIV replication in macrophages. Blood 121:2934–2942. doi: 10.1182/blood-2012-08-450353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Ye L, Hou W, Zhou Y, Wang YJ, Metzger DS, Ho WZ. 2009. Cellular microRNA expression correlates with susceptibility of monocytes/macrophages to HIV-1 infection. Blood 113:671–674. doi: 10.1182/blood-2008-09-175000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bao R, Zhuang K, Liu J, Wu J, Li J, Wang X, Ho WZ. 2014. Lipopolysaccharide induces immune activation and SIV replication in rhesus macaques of Chinese origin. PLoS One 9:e98636. doi: 10.1371/journal.pone.0098636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Xiao Q, Zhou R, Wang Y, Xian Q, Ma T, Zhuang K, Zhou L, Guo D, Wang X, Ho WZ, Li J. 2016. Comparative analysis of immune activation markers of CD8+ T cells in lymph nodes of different origins in SIV-infected Chinese rhesus macaques. Front Immunol 7:371. doi: 10.3389/fimmu.2016.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balzarini J, Hatse S, Vermeire K, Princen K, Aquaro S, Perno CF, De Clercq E, Egberink H, Vanden Mooter G, Peumans W, Van Damme E, Schols D. 2004. Mannose-specific plant lectins from the Amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob Agents Chemother 48:3858–3870. doi: 10.1128/AAC.48.10.3858-3870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidtmayerova H, Alfano M, Nuovo G, Bukrinsky M. 1998. Human immunodeficiency virus type 1 T-lymphotropic strains enter macrophages via a CD4- and CXCR4-mediated pathway: replication is restricted at a postentry level. J Virol 72:4633–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Secchiero P, Zella D, Capitani S, Gallo RC, Zauli G. 1999. Extracellular HIV-1 tat protein up-regulates the expression of surface CXC-chemokine receptor 4 in resting CD4+ T cells. J Immunol 162:2427–2431. [PubMed] [Google Scholar]
- 36.Krogmann AO, Lusebrink E, Steinmetz M, Asdonk T, Lahrmann C, Lutjohann D, Nickenig G, Zimmer S. 2016. Proinflammatory stimulation of Toll-like receptor 9 with high dose CpG ODN 1826 impairs endothelial regeneration and promotes atherosclerosis in mice. PLoS One 11:e0146326. doi: 10.1371/journal.pone.0146326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willey RL, Smith DH, Lasky LA, Theodore TS, Earl PL, Moss B, Capon DJ, Martin MA. 1988. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol 62:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]