Abstract
Theta-defensins (θ-defensins) are macrocyclic peptides expressed exclusively in granulocytes and selected epithelia of Old World monkeys. They contribute to anti-pathogen host defense responses by directly killing a diverse range of microbes. Of note, θ-defensins also modulate microbe-induced inflammation by affecting the production of soluble tumor necrosis factor (sTNF) and other proinflammatory cytokines. Here, we report that natural rhesus macaque θ-defensin (RTD) isoforms regulate sTNF cellular release by inhibiting TNF-α–converting enzyme (TACE; also known as a disintegrin and metalloprotease 17; ADAM17), the primary pro-TNF sheddase. Dose-dependent inhibition of cellular TACE activity by RTDs occurred when leukocytes were stimulated with live Escherichia coli cells as well as numerous Toll-like receptor agonists. Moreover, the relative inhibitory potencies of the RTD isoforms strongly correlated with their suppression of TNF release by stimulated blood leukocytes and THP-1 monocytes. RTD isoforms also inhibited ADAM10, a sheddase closely related to TACE. TACE inhibition was abrogated by introducing a single opening in the RTD-1 backbone, demonstrating that the intact macrocycle is required for enzyme inhibition. Enzymologic analyses showed that RTD-1 is a fast binding, reversible, non-competitive inhibitor of TACE. We conclude that θ-defensin–mediated inhibition of pro-TNF proteolysis by TACE represents a rapid mechanism for the regulation of sTNF and TNF-dependent inflammatory pathways. Molecules with structural and functional features mimicking those of θ-defensins may have clinical utility as TACE inhibitors for managing TNF-driven diseases.
Keywords: tumor necrosis factor (TNF), defensin, ADAM, shedding, enzyme inhibitor, proteolytic enzyme, inflammation, cytokine, ADAM17, sheddase inhibitors, TACE, theta defensin
Introduction
Mammalian defensins are host defense peptides composed of three structural families, designated as α-, β-, or θ-defensins. The three defensin families are genetically related, but are distinguished by amino acid chain length and distinctive tridisulfide motifs that are characteristic of each family (1–4). Isolation and characterization of each of the defensin families was the result of studies aimed at identifying antimicrobial substances in granulocytes or epithelial cells implicated in host defense (5, 6). Among defensins, θ-defensins are unique in that they are 18-amino acid macrocyclic peptides known to be expressed only in cells of Old World monkeys and are the only cyclic polypeptides known in animals (7).
Multiple θ-defensin isoforms are expressed in neutrophils and monocytes of rhesus monkeys (six) (8) and olive baboons (10) (9), each of which is a macrocyclic octadecapeptide that includes six disulfide-linked cysteines (Fig. 1). The sequence diversity of θ-defensins results from the binary combinations of gene-encoded nonapeptides that are ligated head-to-tail to form the θ-defensin backbone (Fig. 1) (10). Humans and great apes lack θ-defensins due to stop codon interruptions in the signal peptide coding regions of the respective θ-defensin precursors (11).
Figure 1.

θ-Defensin isoforms. The θ-defensin structural motif is defined by an 18-amino acid backbone stabilized by three conserved disulfide bonds. Other invariant residues are shaded and net charges of RTDs 1–5 are listed. θ-Defensin isoforms are produced by homo- or heterodimeric head-to-tail ligation of nonapeptides excised from one or two of three known propeptides. The ribbon structure of RTD-1 (top) shows the substituent nonapeptides (blue and gold) spliced to form the mature macrocycle. S7 is an acyclic version of RTD-1 with an opening between Cys-3 and Arg-4 (red arrow), one of the two peptide bonds formed during post-translational formation of the macrocyclic backbone.
Like α- and β-defensins, numerous θ-defensin isoforms have antimicrobial activities against bacteria, fungi, and viruses (2–4, 7–10, 12, 13). However, among the three peptide families, θ-defensins are distinguished by their unique immunomodulating properties. For example, the prototype θ-defensin RTD-12 reduced lethality in murine models of polymicrobial sepsis, Escherichia coli bacteremia, and severe acute respiratory syndrome coronavirus infection, and the therapeutic effects in each model were associated with significant reductions in tissue proinflammatory cytokine and chemokine levels (14, 15). Studies in the mouse severe acute respiratory syndrome coronavirus model strongly implicated host-directed anti-inflammatory effects of rhesus macaque θ-defensin (RTD-1) because the peptide had no direct antiviral activity (15). RTD-1 was also effective in reducing pulmonary pathology in murine models of endotoxic lung injury (16) and cystic fibrosis (17) by moderating inflammatory responses. Also, RTD-1 arrested joint inflammation in a rat model of rheumatoid arthritis (RA), pristane-induced arthritis, an autoimmune disease characterized by dysregulated proinflammatory cytokines and erosive joint changes similar to those associated with RA (18). Parenteral administration of RTD-1 to rats with established pristane-induced arthritis rapidly induced arrest of disease progression and resolution of arthritis that correlated with significant reductions in proinflammatory cytokines in joint tissues (78).
Soluble tumor necrosis factor (sTNF) is produced when pro-TNF, a type II transmembrane protein, is cleaved at the cell surface by TACE (a disintegrin and metalloprotease 17; ADAM17) (19–21). TACE is a membrane-anchored zinc metalloprotease and is responsible for “shedding” the ectodomain of TNF and many additional cytokines, growth factors, receptors, and adhesion molecules (22, 23). Dysregulated TACE activity has been associated with disruption of cytokine homeostasis, elevating levels of TNF in chronic and acute inflammatory diseases including RA, sepsis, and colitis (24–29) as well as cancer progression (22, 30). Inhibition of TACE activity with broad-spectrum metalloprotease inhibitors prevents TNF release from cell surfaces, suppressing levels of sTNF (31–33). In a previous study on the kinetics of RTD-1 inhibition of TNF release by E. coli-stimulated leukocytes we found that suppression of sTNF occurred rapidly upon peptide addition to the bacteria/blood incubation mixture (14). We hypothesized that the blockade of sTNF release is mediated by inhibition of pro-TNF ectodomain shedding. Thus, we performed studies to evaluate the effects of RTD isoforms on the TNFα convertase TACE and the related sheddase, ADAM10.
Results
RTD-1 suppresses TNF release by human blood leukocytes stimulated by diverse TLR agonists
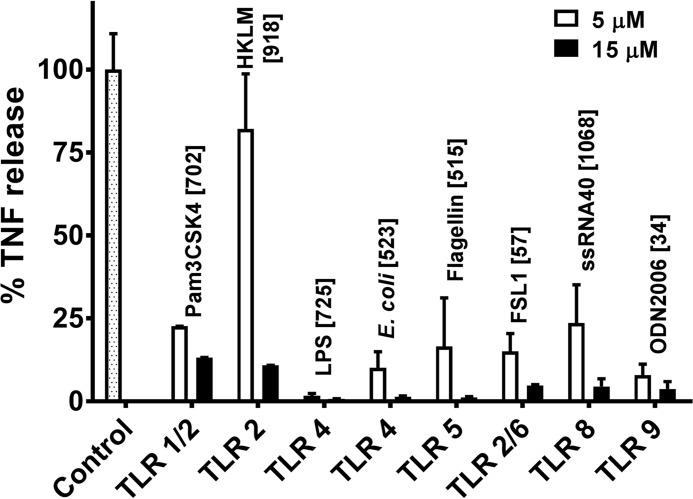
In a previous study we showed that RTD-1 suppressed the release of several pro-inflammatory cytokines, including TNF, IL-1α, IL-1β, IL-6, IL-8, CCL2, CCL3, and CCL4 by human buffy coat leukocytes stimulated with agonists of TLRs 2, 4, 5, and 8 (14). TNF release was markedly suppressed irrespective of the stimulus (14). In the current study we analyzed the effects of 5 or 15 μm RTD-1 on TNF secretion by human blood leukocytes stimulated with an expanded panel of TLR ligands including agonists of TLRs 1/2, 2, 4, 5, 2/6, 8, and 9. In the absence of peptide, stimulated cells secreted 30–1100 pg/ml of TNF (Fig. 2). Consistent with our previous report, RTD-1 dose-dependently reduced TNF release by agonists for TLRs 2, 4, 5, and 8 and also had similar effects on cells stimulated with ligands for TLRs 1/2, 2/6, and 9 (Fig. 2). These findings, and the rapid blockade of TNF release observed when blood leukocytes were stimulated with E. coli cells in the presence of RTD-1, suggested that the peptide regulates proteolytic release of TNF.
Figure 2.
RTD-1 suppresses TNF release from blood leukocytes. Human buffy coat leukocytes cells were stimulated with a panel of TLR agonists and treated with vehicle or 5 or 15 μm RTD-1. sTNF release is shown as a percent of TNF released compared with peptide-free controls for each agonist (pg/ml of sTNF). Bars represent mean ± S.D. of two independent experiments performed in duplicate.
RTD-1 inhibits TNF release by THP-1 cells but does not affect downstream signaling of sTNF in colonic epithelial cells
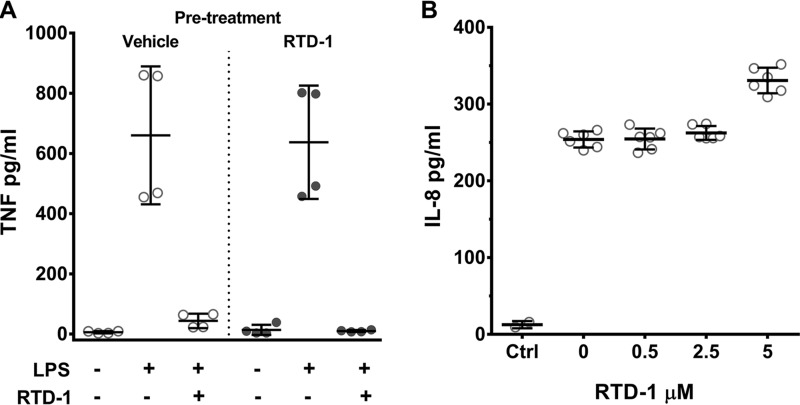
We previously showed that RTD-1 dose-dependently suppressed TNF release by lipopolysaccharide (LPS)-stimulated THP-1 monocytes (14). To determine whether RTD-1 pretreatment of THP-1 cells blocked LPS-induced TNF secretion, cells were incubated for 60 min with 5 μm RTD-1 or vehicle, washed, and stimulated with LPS in the presence or absence of 5 μm RTD-1. As shown in Fig. 3A, suppression of LPS-induced TNF secretion only occurred if RTD-1 was present when the cells were stimulated, as washout of the peptide prior to LPS stimulation had no effect on TNF release. This result was not due to neutralization of LPS as we showed previously that RTD-1 does not block the endotoxic properties of LPS (14).
Figure 3.
RTD-1 inhibition of TNF release is reversible and does not block signaling by sTNF. A, THP-1 macrophages were pre-treated with vehicle (open circles) or 5 μm RTD-1 (closed circles) for 60 min after which medium was removed and the cells washed. Cells were then treated with vehicle or RTD-1 (5 μm) with or without LPS, incubated for 4 h, and sTNF levels were determined. B, HT-29 cells were treated with 0–5 μm RTD-1, stimulated with 500 pg/ml of rTNF for 4 h, and supernatant IL-8 was quantified. Control incubations (Ctrl) lacked TNF and produced no IL-8 response, as did samples treated with RTD-1 alone (data not shown). Scatter plots represent mean ± S.D. of two independent experiments performed in duplicate (A) or triplicate (B).
Cellular responses to soluble TNF are mediated by paracrine and autocrine signaling, resulting in further TNF release and activation of other downstream inflammatory effects (34). To determine whether RTD-1 affects downstream TNF signaling, we tested whether the peptide alters the response of HT-29 cells to TNF. HT-29 cells express TNF receptor I (TNFRI) and release IL-8 in response to sTNF (35). Consistent with previous reports, TNF-stimulated HT-29 cells induced robust IL-8 release (Fig. 3B). This was unaffected by the co-incubation with RTD-1 (Fig. 3B), and RTD-1 alone had no effect on IL-8 release by HT-29 cells (data not shown). Thus, RTD-1 does not affect TNF signaling by direct neutralization of sTNF nor does the peptide appear to interfere with sTNF/TNFRI signaling in HT-29 cells.
Inhibition of TACE by θ-defensins
RTD-1 blockade of TNF release by human leukocytes stimulated with E. coli cells is extremely rapid (14), and the peptide down-regulates TNF release by leukocytes irrespective of the stimulating TLR ligand (Fig. 2). Based on these findings, we hypothesized that RTD-1 inhibits TNF release by inhibition of its mobilization from the cell surface by its principal convertase, TACE (ADAM17). RTD-1 dose-dependently inhibited recombinant human TACE (rhTACE) cleavage of its fluorogenic substrate (Fig. 4A) with an IC50 = 0.11 ± 0.04 μm. Of note, the inhibition by RTD-1 did not require preincubation of the peptide and the TACE enzyme. As discussed later, we found that RTD-1 rapidly bound to and inhibited the enzyme. The sigmoidal inhibitory response of RTD-1 was ∼10-fold less potent than that of marimastat, a small molecule inhibitor of zinc matrix metalloproteinases (36) (Fig. 4A). A single chain opening in the RTD-1 backbone, producing the S7 analog (Fig. 1), reduced inhibitory potency >99% (Fig. 4A). We also tested the TACE inhibitory activities of human α-defensins (human neutrophil peptides, HNPs 1–4) that have proinflammatory and/or immune activating properties (37–43). Neither HNP-2 nor HNP-4 inhibited TACE (Fig. 4A). We then evaluated the effects of RTD-1, S7, marimastat, and human α-defensins for their inhibition of TNF release by LPS-stimulated THP-1 cells. None of the HNPs, nor S7 were effective in blocking TNF release, whereas RTD-1 and marimastat dose-dependently inhibited sTNF release (Fig. 4B), consistent with the TACE inhibitory activity of each compound (Fig. 4A).
Figure 4.
θ-Defensins inhibit TACE and suppress TNF release by LPS-stimulated THP-1 monocytes. A and C, inhibition of recombinant TACE proteolytic activity is shown as percent change in rate of product formation relative to peptide-free control. Enzyme reactions were performed for 30 min at 22 °C with 2 nm TACE and 10 μm substrate (R&D Systems, ES003). Data represent mean ± S.E. of 3 independent experiments containing 2–3 technical repeats each. B and D, suppression of TNF release from THP-1 monocytes stimulated with LPS and peptides or marimastat (MRM). Results are expressed as mean ± S.D. of 2- 6 individual experiments containing 2 technical repeats each.
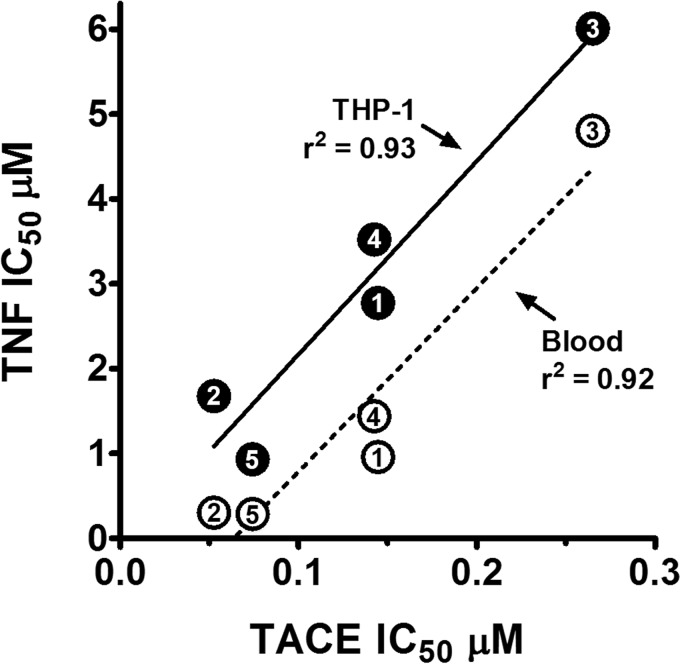
In a previous study, we showed that natural RTD isoforms (RTDs 1–5) varied markedly in their inhibition of TNF release by stimulated blood leukocytes or THP-1 monocytes (14). Therefore we evaluated RTDs 1–5 for their relative inhibition of rhTACE. Each peptide dose-dependently inhibited TACE, but with IC50 values that ranged from 50 to 265 nm, giving an inhibitory hierarchy of RTD-2 ≥ RTD-5 > RTD-4 ≥ RTD-1 > RTD-3 (Fig. 4C, Table 1). These peptides were then evaluated for their inhibition of sTNF release by LPS-stimulated THP-1 cells. The hierarchy of dose-dependent inhibition of sTNF release (Fig. 4D) was the same as that obtained for TACE inhibition by RTDs 1–5. Indeed, the TACE IC50 values of the RTDs were highly correlated with their TNF release IC50 values in assays using LPS-stimulated THP-1 cells or E. coli-stimulated blood leukocytes (Fig. 5).
Table 1.
Relative TACE inhibitory activities of RTDs (nm ± S.D.)
| Peptide | IC50 |
|---|---|
| RTD-1 | 141 ± 14 |
| RTD-2 | 52 ± 3 |
| RTD-3 | 285 ± 52 |
| RTD-4 | 132 ± 22 |
| RTD-5 | 55 ± 7 |
| S7 | >1000 |
| Marimastat | 7.6 ± 0.2 |
Figure 5.
TACE inhibition correlates with suppression of soluble TNF release from stimulated THP-1 monocytes and whole blood. IC50 values of RTDs 1–5 for sTNF release by LPS-stimulated THP-1 monocytes (●) and E. coli-stimulated whole blood (○) were plotted against IC50 values of each peptide for rTACE inhibition. Symbol numbers refer to RTDs 1–5 with correlation coefficients shown (THP-1, p = 0.0082; blood, p = 0.0107).
θ-Defensins inhibit TACE activities of THP-1 and HT-29 cells
To test whether the effects of θ-defensins on rhTACE are replicated with TACE-expressing cells, we incubated RTDs 1–5 with THP-1 macrophages and HT-29 epithelial cells in the presence of a fluorogenic substrate that mimics the cleavage site of membrane-bound TNF, allowing for continuous, real-time measurement of TACE activity in live cells (44, 45). After a brief lag, substrate conversion in RTD-free controls was linear for 60 min. RTDs dose-dependently inhibited substrate hydrolysis by both THP-1 macrophages and HT-29 cells (Fig. 6). Nearly maximum inhibition of THP-1 cell TACE was achieved with 5 μm RTDs 1, 3, and 5, whereas RTDs 2 and 4 were slightly less inhibitory at this concentration (Fig. 6A). The absence of complete inhibition at the highest RTD levels tested is similar to findings reported for the small molecule TACE inhibitors GM6001 and BB94 (45).
Figure 6.
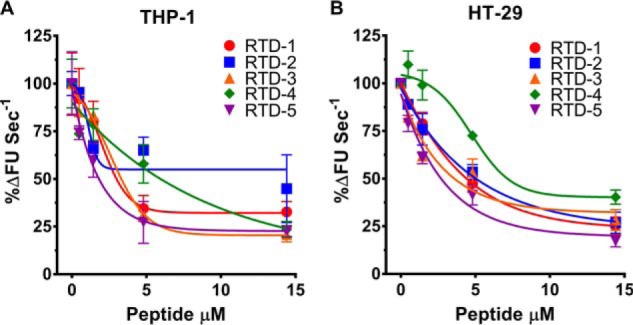
Real time measurement of RTD inhibition of cellular TACE. RTDs 1–5 were incubated with THP-1 macrophages or HT-29 cells in the presence of 10 μm fluorogenic substrate (R&D Systems, ES003). Substrate conversion was monitored continuously for 60 min at 37 °C and plotted as a function of percent product formation rate relative to peptide-free controls. Results are shown as mean ± S.D. of two independent experiments containing 2–3 technical repeats each.
θ-Defensins inhibit TACE and ADAM10 expressed on COS7 cells
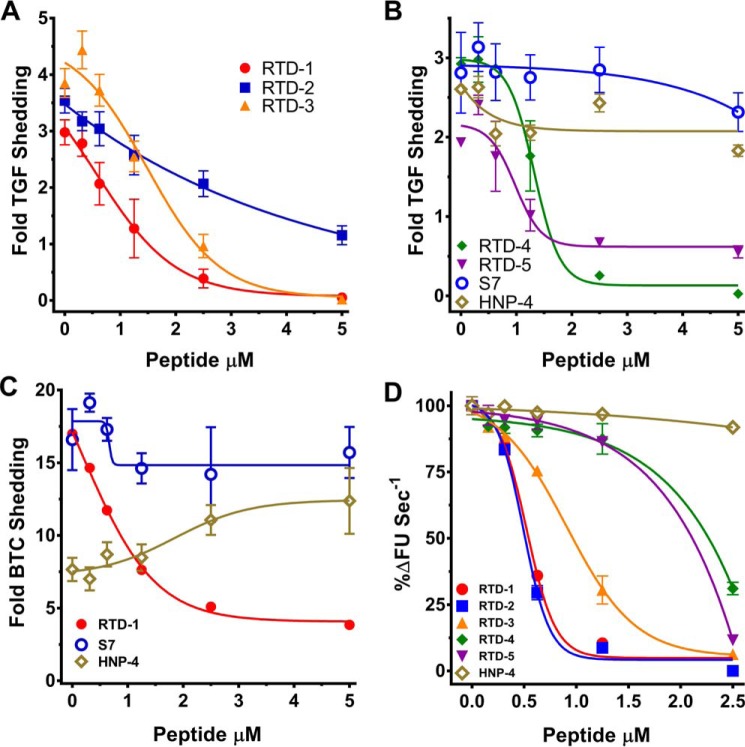
To further evaluate the effect of θ-defensins on sTNF mobilization, we analyzed the inhibitory activities of RTDs 1–5 on TACE and the related ADAM10 in COS7 cells (46, 47) co-expressing their respective AP-TGFα and AP-BTC reporter substrates (48, 49). Phorbol 12-myristate 13-acetate (PMA) stimulation increased TACE shedding of TGFα 2–4-fold compared with unstimulated cells and RTD 1–5 inhibited PMA-stimulated TACE activity in a dose-dependent manner (Fig. 7, A and B). Of note, as observed with THP-1 cells (Fig. 4, A and B), acyclic S7 and α-defensin HNP-4 were ineffective in inhibiting TACE activity (Fig. 7B), providing further evidence that macrocyclic RTDs uniquely interact with TACE to inhibit enzymatic activity.
Figure 7.
RTDs inhibit cellular TACE and ADAM10 sheddase activities. A and B, RTDs, but not acyclic S7 or α-defensin HNP-4, suppress TACE-mediated ectodomain shedding of TGFα in COS7 cells. Data are expressed as fold-TGFα shedding relative to constitutive TGFα release. C, RTD-1, but not S7 or HNP-4, inhibits ADAM10-dependent cleavage of BTC in COS7 cells. Data are expressed as fold BTC shedding relative to constitutive BTC release. Data in panels A--C represent mean ± S.D. of a representative experiment containing 3 technical repeats. D, RTDs inhibit rADAM10 proteolysis of its fluorogenic substrate. Enzyme reactions were performed for 30 min at 37 °C with 1 nm ADAM10 and 10 μm substrate (R&D Systems, ES010). Enzyme inhibition is expressed as percent change in substrate conversion rate relative to peptide-free controls. Data represent mean ± S.D. of 2 independent experiments containing 2 technical repeats each.
We assessed the effect of RTDs on ADAM10, a TACE-related metalloprotease, by monitoring processing of AP-BTC. Ionomycin stimulation of COS7 cells increased ADAM10 cleavage of AP-BTC by 10–20-fold more than unstimulated cells (Fig. 7C). RTD-1 dose-dependently reduced ionomycin-stimulated ADAM10 activity, but acyclic S7 had little inhibitory activity. HNP-4 enhanced ADAM10 activity by an unknown mechanism that was not investigated further. The effect of θ-defensins on ADAM10 was analyzed further by testing for inhibition rADAM10 by RTDs 1–5. Although each RTD inhibited ADAM10 as a function of peptide concentration (Fig. 7D), the inhibitory potency was lower, and the isoform-specific hierarchy differed, compared with inhibition of RTDs of rTACE and cellular TACE expressed by THP-1 cells (Figs. 4C and 5).
RTD-1 is a fast-binding non-competitive inhibitor of TACE
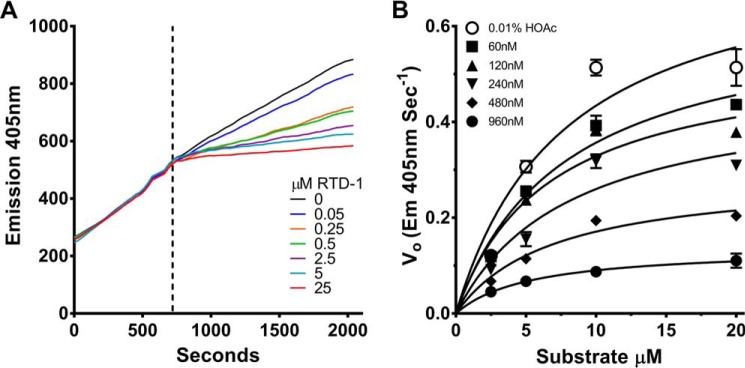
As noted above, the hypothesis that RTDs inhibit TACE was based in part on the exceedingly rapid blockade of sTNF release by E. coli-stimulated leukocytes exposed to RTD-1 (14). To further study the kinetics of inhibition, we analyzed the temporal effect of RTD-1 addition to a steady state reaction of fluorogenic substrate cleavage by rTACE. As shown in Fig. 8A, addition of RTD-1 very rapidly inhibited enzyme activity and the degree of blockade was dose-dependent. These findings are consistent with rapid binding of RTD-1 to TACE. Additionally, a large molar ratio of RTD-1 (0.05–25 μm) to TACE (2 nm) is required for proteolytic inhibition, indicating that RTD-1 is not a tight-binding inhibitor.
Figure 8.
RTD-1 is a fast binding, non-competitive inhibitor of TACE. A, kinetics of RTD-1 inhibition of TACE (2 nm) was evaluated in a steady-state TACE/FRET-substrate (10 μm substrate, R&D Systems, ES003) cleavage reaction at 22 °C. RTD-1, at the concentrations indicated was added to steady-state reaction mixtures at 10 min (dotted line) after reaction initiation. Results shown are from a single representative experiment performed twice containing 2 technical repeats each. B, Michaelis-Menten kinetics for RTD-1 inhibition of rTACE (2 nm) with multiple concentrations of substrate (ES003) at 22 °C. Data represent mean ± S.D. of a single representative experiment performed three times, each performed in triplicate.
Michaelis-Menten kinetics of RTD-1 inhibition of rhTACE was determined by measuring substrate conversion in reactions with varied substrate and peptide concentrations (Fig. 8B). RTD-1 inhibition of TACE could not be overcome by increasing concentrations of substrate, thus Vmax decreased with increasing RTD-1 concentrations, whereas Km remained unaffected, kinetics consistent with classical non-competitive inhibition. Best fit modeling of enzyme inhibition kinetics was performed (see “Experimental procedures”) disclosing that RTD-1 is indeed a non-competitive TACE inhibitor that likely binds to an exosite that modifies interaction of the enzyme with pro-TNF and related soluble substrates. As the Ki value for a non-competitive inhibitor is the same as its IC50 value, the values reported in Table 1 reflect both the measured IC50 and apparent Ki values for each peptide.
Discussion
ADAMs are key regulators of growth factor signaling, development, tumor progression, and inflammation (22, 50, 51), and among known ADAMs TACE plays a central role in both epidermal growth factor receptor signaling and proinflammatory pathways (52). TACE-mediated shedding of epidermal growth factor receptor ligands is required for normal organogenesis (53–57). TACE is also critical in generating sTNF (19–21), the ectodomain of pro-TNF, as well as the production of other proinflammatory cytokines and their receptors (58). Regulation of TACE activation is rapid and reversible and recent studies have demonstrated the important role of two membrane proteins, inactive Rhomboid 1 and 2, which selectively regulate activation of TACE in different mouse tissues (29, 59–61). The current study discloses a previously unknown mechanism of sTNF regulation wherein macrocyclic θ-defensins allosterically inhibit TACE. To our knowledge, θ-defensins are the only known endogenous inhibitors of TACE sheddase activity other than tissue inhibitor of matrix metalloproteinases 3 (TIMP3), which has been shown to play a critical role in regulating pro-TNF shedding (62–65).
Enzymology experiments demonstrate that θ-defensin inhibition of TACE is rapid, non-tight binding, reversible, and non-competitive. Inhibition of TACE by RTDs explains how RTD-1 is able to suppress sTNF release by blood leukocytes stimulated by an array of TLR agonists (Fig. 2). Moreover, the correlation between potency of θ-defensin isoforms in blocking TACE enzymatic activity and inhibition of sTNF release supports the conclusion that θ-defensins regulate sTNF production by TACE inhibition (Fig. 5). This was further validated using cell-based assays that showed that RTDs inhibit TACE and ADAM10 shedding of the ectodomains of their respective membrane-bound substrates.
θ-Defensins are expressed at high levels in neutrophils and monocytes of rhesus macaques (8), olive baboons (9), and African green monkeys.3 Despite the high degree of sequence conservation among RTDs 1–5 (identity in 11 of 18 residue positions), the TACE inhibitory potency of RTDs varied by more than 5-fold. Of note, the hierarchy of TACE inhibition of RTDs 1–5 strongly correlated with the suppression of TNF release by each isoform from stimulated THP-1 monocytes and whole blood leukocytes.
θ-Defensins are readily measurable in the plasma of baboons with experimental E. coli bacteremia, and are released by granulocytes challenged with E. coli in vitro (66). Moreover, RTDs are present in saliva of healthy macaques.3 In clinical sepsis, high levels of human neutrophil α-defensins are released into the circulation (67). We hypothesize that θ-defensins are endogenous regulators of TACE, ADAM10, and potentially other matrix metalloproteases in Old World monkeys. Because α-defensins stimulate proinflammatory pathways (38–43) and lack inhibitory activities against TACE or ADAM10, TACE inhibition by θ-defensins may underlie the differential susceptibility of humans and other great apes to endotoxin, compared with endotoxin resistance of Old World monkeys that express θ-defensins (68, 69).
RTD-1 markedly suppressed mRNA expression and release of TNF, IL-1β, and IL-8 by LPS-stimulated THP-1 macrophages (70). Suppression of transcriptional activation was associated with suppression of NF-κB and MAPK signaling with a concomitant increase in pAkt, which negatively regulates these pathways (70). Thus θ-defensins are pleiotropic effectors of host defense and inflammation. Like α- and β-defensins, they are potent, broad spectrum microbicides. However, unlike their acyclic counterparts, θ-defensins also function as anti-inflammatory molecules that moderate proinflammatory stimuli at both transcriptional and post-translational levels.
Given the importance of TACE as a regulator of sTNF, numerous laboratories have sought to identify selective TACE inhibitors for pharmacologic regulation of the enzyme's sheddase activity to antagonize TNF expression in diseases such as rheumatoid arthritis, inflammatory bowel diseases, diabetes, and sepsis. Small molecules evaluated for this purpose have included succinates, hydroxamates, sulfonamides, γ-lactams, β-benzamido compounds, benzothiadiazepine inhibitors, and zinc chelators (31, 71–74), each designed to competitively target the zinc-containing catalytic site (31). To date none of these programs has produced an approved TACE-targeted drug due to lack of efficacy and/or off target toxicities (27, 31, 75, 76). Perhaps molecules with structural and functional features embodied in θ-defensins may provide a new avenue for development of TACE inhibitors for treatment of TNF-driven diseases.
Experimental procedures
Human subjects
All studies involving human subjects were approved by an Institutional Review Board at the Keck School of Medicine, University of Southern California (IRB HS-09-00280) and were fully compliant with the Declaration of Helsinki principles.
Peptides
The hydrochloride salts of RTD 1–5 were produced by solid-phase synthesis and purified (≥98%) (4), and human neutrophil α-defensins 1–4 (≥98%) were purified from human buffy coat leukocytes as described previously (77). Peptides were dissolved in 0.01% (v/v) acetic acid or HPLC-grade water, and peptide concentrations were confirmed by coupled liquid chromatography-mass spectrometry (LC-MS) using previously quantified standards.
Bacteria and TLR agonists
E. coli number UCI 9021, a human blood isolate, was obtained from the clinical laboratory at the University of California Irvine Medical Center. Bacteria were cultured from single colonies, harvested by centrifugation, washed, and suspended in phosphate-buffered saline (PBS) (14). Bacterial density was determined by absorbance at 620 nm and correlated with colony forming units grown on tryptic soy agar. The following Toll-like receptor (TLR) agonists were from Invivogen (San Diego, CA): Pam3CSK4 synthetic triacylated lipopeptide (TLR 1/2), heat-killed Listeria monocytogenes (TLR 2), E. coli K12 LPS (TLR 4), Salmonella typhimurium flagellin (TLR 5), FSL1 synthetic (TLR2/6), ssRNA40 (TLR 8), and ODN2006 unmethylated CpG oligonucleotide (TLR 9).
Cell culture
THP-1 human monocytes (ATCC TIB-202) cells were cultured in RPMI 1640 containing 10% FBS and 100 units/ml of penicillin/streptomycin in 5% CO2. Cells (5 × 105/well in 24-well plates) were treated with 100 nm PMA (Sigma) for 48 h, washed with warm PBS, suspended in fresh medium, and incubated for 24 h prior to use in assays described below. Human colonic epithelial HT-29 cells (ATCC HTB-38) were grown to confluence in 24-well tissue culture dishes in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 100 units/ml of penicillin/streptomycin, washed, and cultured in RPMI 1640, 100 units/ml of penicillin/streptomycin plus 5% human EDTA plasma. HT-29 cells were incubated with 0–5 μm RTD-1 plus 500 pg/ml of recombinant human TNF (Invivogen). After a 4-h incubation at 37 °C in 5% CO2 culture, supernatants were analyzed for soluble IL-8 by ELISA (Life Technologies).
TNF release assays
Kinetics of TNF release by stimulated leukocytes was determined as described previously (14). Briefly, THP-1 cells (5 × 105 cells/ml) were stimulated for 4 h with the indicated concentrations of TLR agonist in the presence of 0–5 μm of RTDs 1–5 at 37 °C in 5% CO2. TNF in cell-free supernatants was determined by ELISA (Life Technologies). For studies using peripheral blood leukocytes, EDTA-anticoagulated blood was obtained from healthy adult volunteers, and buffy coat leukocytes were harvested by centrifugation at 200 × g. Cells were washed twice with 3–5 ml of RPMI, counted with a hemocytometer, and suspended at 5 × 105 cells/ml in RPMI + 5% human EDTA plasma. Leukocytes were incubated for 4 h with the indicated concentrations of TLR ligands or 100 colony forming units/ml of E. coli number UCI 9021, and TNF in clarified supernatants was quantified by ELISA.
TACE and ADAM10 inhibition assays
Proteolytic activities of recombinant human TACE (R&D Systems, 930-ADB, 52 kDa, full ectodomain) and ADAM10 (R&D Systems, 936-AD, 52 kDa, full ectodomain) were measured using fluorogenic substrates Mca-PLAQAV-Dpa-RSSSR-NH2 (R&D Systems, ES003) and Mca-KPLGL-Dpa-AR-NH2 (R&D Systems, ES010), respectively. Assays were performed in F16 black Maxisorp 96-well plates in 25 mm Tris, 2.5 μm ZnCl2, 0.005% Brij-35 (v/v) (pH 9.0). Individual RTDs were added to TACE (0.1 μg/ml, 2 nm) or ADAM10 (0.05 μg/ml, 1 nm) at final concentrations of 0–1.5 μm, followed by addition of 10 μm fluorogenic substrates. Marimastat (Sigma, number 154036-60-8) was used as the control metalloproteinase inhibitor. Substrate conversion was measured every 30 s for 30 min at 22 (TACE) or 37 °C (ADAM10) in a SpectraMax M5e fluorometer (Molecular Devices; 320ex/405em). Vmax was calculated for each sample and transformed into percent change in product formation rate (mean ± S.E., n = 6). Inhibition curves were fitted with a non-linear variable slope curve using GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego, CA). Half-maximal inhibition concentrations (IC50 values) were obtained from fitted curves.
Cellular sheddase assays
PMA-differentiated THP-1 cells and HT-29 cells were grown in Black μClear CELLSTAR 96-well plates (Greiner Bio-one, Monroe, NC), washed, and suspended in assay buffer (20 mm Tris, 154 mm NaCl, 1% human serum, pH 7.4). At t = 0, 0–15 μm of individual θ-defensins (RTDs 1–5) and 10 μm fluorogenic TNF substrate (Mca-PLAQAV-Dpa-RSSSR-NH2) were added to cells and incubated at 37 °C for 60 min. Fluorescence (320ex/405em) was measured at 30-s intervals. Vmax was calculated for each sample and transformed into percent sheddase activity relative to solvent controls.
Effects of RTDs on cellular TACE and ADAM10 activities were also analyzed using transfected COS7 (ATCC) cells expressing alkaline phosphatase (AP)-tagged substrates of the respective enzymes (48, 49). Briefly, COS7 cells transiently transfected with AP-tagged transforming growth factor-α (AP-TGFα; TACE substrate) or AP-betacellulin (AP-BTC; ADAM10 substrate) were cultured overnight, washed, starved 2–4 h in Opti-MEM (Gibco), and incubated for 10 min with 0–5 μm RTDs 1–5 in fresh media. TACE activity was induced by addition of 25 ng/ml of PMA for 30–45 min, after which culture aliquots (supernatants) were collected and cells were lysed. Samples of cell supernatants and lysates were added to AP assay buffer (100 mm Tris, 100 mm NaCl, 20 mm MgCl2, pH 9.5). After addition of 2% (v/v) of 50 mg/ml aqueous p-nitrophenylphosphate substrate (Thermo Scientific 34045), TACE activity was quantified as AP conversion of p-nitrophenylphosphate. Cellular ADAM10 activity was determined similarly using COS7 cells transfected with AP-tagged betacellulin. ADAM10 activity was induced with 2.5 μm ionomycin. All assays were performed in triplicate.
Enzyme kinetics
RTD-1 (0–960 nm) was incubated with 2.5, 5, 10, and 20 μm Mca-PLAQAV-Dpa-RSSSR-NH2 (320ex/405em) in the presence of 25 ng/ml of rTACE and enzyme activity was measured every 30 s for 90 min. Initial velocity (Vo) was plotted as a function of substrate concentration (mean ± S.D., n = 3), subjected to Michaelis-Menten kinetics analyses, which were compared with best-fit models for competitive, non-competitive, uncompetitive, and mixed modeling inhibition using GraphPad Prism. Inhibition of rTACE by RTD-1 was also determined by addition of 0–25 μm RTD-1 to steady-state (Vs) TACE-Mca-PLAQAV-Dpa-RSSSR-NH2 reactions in which substrate conversion was monitored for 10 min prior to addition of peptide, and then for 25 min after the addition of the indicated concentration of RTD-1.
Author contributions
J. B. S., D. Q. T., and M. E. S. conceptualization; J. B. S., T. M., and P. A. T. data curation; J. B. S., T. M., D. Q. T., P. A. T., P. T., C. P. B., A. J. O., and M. E. S. formal analysis; J. B. S., T. M., and C .P. B. validation; J. B. S., T. M., D. Q. T., P. A. T., P. T., and C. P. B. investigation; J. B. S. visualization; J. B. S., T. M., D. Q. T., P. T., C. P. B., and M. E. S. methodology; J. B. S. and M. E. S. writing-original draft; J. B. S., T. M., C. P. B., A. J. O., and M. E. S. project administration; J. B. S., T. M., D. Q. T., P. A. T., P. T., C. P. B., A. J. O., and M. E. S. writing-review and editing; D. Q. T., P. A. T., A. J. O., and M. E. S. supervision; D. Q. T., C. P. B., A. J. O., and M. E. S. funding acquisition; P. A. T. and M. E. S. resources.
Acknowledgments
The Southern California Clinical and Translational Science Institute was supported by Grant UL1TR000130, and the University of Southern California Norris Cancer Center was supported by Grant P30CA014089 from the NCI, National Institutes of Health.
This work was supported by National Institutes of Health Grants AR068833 (to D. Q. T.), Grants AI22931 and DE021341 (to M. E. S.), and GM64750 (to C. P. B.) and Arthritis Foundation Grant 5997 (to M. E. S.). The following authors declare competing interests in relationship to their affiliations with Oryn Therapeutics: D. Q. T. is Scientific Director, M. E. S. is Chief Scientific Officer, A. J. O. is a minority investor. Oryn Therapeutics has licensed technologies disclosed in US patents 6,335,318; 6,514,727; 6,890,537; 7,119,070; 7,399,823 B1, 7,462,598, and 9,346,866 B2. The affiliation of the authors with Oryn Therapeutics does not alter author adherence to JBC policies on sharing data and materials. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
V. Basso, A. Garcia, D. Q. Tran, J. B. Schaal, P. Tran, D. Ngole, Y. Aqeel, P. Tongaonkar, A. J. Ouellette, and M. E. Selsted, unpublished data.
- RTD
- rhesus macaque θ-defensin
- RA
- rheumatoid arthritis
- sTNF
- soluble tumor necrosis factor
- TACE
- tumor necrosis factor α-converting enzyme
- ADAM
- a disintegrin and metalloprotease 17
- LPS
- lipopolysaccharide
- rhTACE
- recombinant human TACE
- HNP
- human neutrophil peptides
- PMA
- phorbol 12-myristate 13-acetate
- TLR
- Toll-like receptor
- AP
- alkaline phosphatase
- BTC
- betacellulin
- Mca
- 7-methoxycoumarin-4-yl-acetyl
- Dpa
- N-3-(2,4-dinitrophenyl)-l-2,3-diaminopropionyl.
References
- 1. Selsted M. E. (2007) A pocket guide to explorations of the defensin field. Curr. Pharm. Des. 13, 3061–3064 10.2174/138161207782110363 [DOI] [PubMed] [Google Scholar]
- 2. Selsted M. E. (2004) θ-Defensins: cyclic antimicrobial peptides produced by binary ligation of truncated alpha-defensins. Curr. Protein Pept. Sci. 5, 365–371 10.2174/1389203043379459 [DOI] [PubMed] [Google Scholar]
- 3. Tran D., Tran P., Roberts K., Osapay G., Schaal J., Ouellette A., and Selsted M. E. (2008) Microbicidal properties and cytocidal selectivity of rhesus macaque θ-defensins. Antimicrob. Agents Chemother. 52, 944–953 10.1128/AAC.01090-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tran D., Tran P. A., Tang Y. Q., Yuan J., Cole T., and Selsted M. E. (2002) Homodimeric theta-defensins from rhesus macaque leukocytes: isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. J. Biol. Chem. 277, 3079–3084 10.1074/jbc.M109117200 [DOI] [PubMed] [Google Scholar]
- 5. Ganz T. (2003) Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3, 710–720 10.1038/nri1180 [DOI] [PubMed] [Google Scholar]
- 6. Selsted M. E., and Ouellette A. J. (2005) Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6, 551–557 10.1038/ni1206 [DOI] [PubMed] [Google Scholar]
- 7. Lehrer R. I., Cole A. M., and Selsted M. E. (2012) θ-Defensins: cyclic peptides with endless potential. J. Biol. Chem. 287, 27014–27019 10.1074/jbc.R112.346098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tongaonkar P., Tran P., Roberts K., Schaal J., Osapay G., Tran D., Ouellette A. J., and Selsted M. E. (2011) Rhesus macaque θ-defensin isoforms: expression, antimicrobial activities, and demonstration of a prominent role in neutrophil granule microbicidal activities. J. Leukoc. Biol. 89, 283–290 10.1189/jlb.0910535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garcia A. E., Osapay G., Tran P. A., Yuan J., and Selsted M. E. (2008) Isolation, synthesis, and antimicrobial activities of naturally occurring θ-defensin isoforms from baboon leukocytes. Infect. Immun. 76, 5883–5891 10.1128/IAI.01100-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tang Y. Q., Yuan J., Osapay G., Osapay K., Tran D., Miller C. J., Ouellette A. J., and Selsted M. E. (1999) A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated α-defensins. Science 286, 498–502 10.1126/science.286.5439.498 [DOI] [PubMed] [Google Scholar]
- 11. Nguyen T. X., Cole A. M., and Lehrer R. I. (2003) Evolution of primate θ-defensins: a serpentine path to a sweet tooth. Peptides 24, 1647–1654 10.1016/j.peptides.2003.07.023 [DOI] [PubMed] [Google Scholar]
- 12. Gallo S. A., Wang W., Rawat S. S., Jung G., Waring A. J., Cole A. M., Lu H., Yan X., Daly N. L., Craik D. J., Jiang S., Lehrer R. I., and Blumenthal R. (2006) θ-Defensins prevent HIV-1 Env-mediated fusion by binding gp41 and blocking 6-helix bundle formation. J. Biol. Chem. 281, 18787–18792 10.1074/jbc.M602422200 [DOI] [PubMed] [Google Scholar]
- 13. Tai K. P., Kamdar K., Yamaki J., Le V. V., Tran D., Tran P., Selsted M. E., Ouellette A. J., and Wong-Beringer A. (2015) Microbicidal effects of α- and θ-defensins against antibiotic-resistant Staphylococcus aureus and Pseudomonas aeruginosa. Innate Immun. 21, 17–29 10.1177/1753425913514784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schaal J. B., Tran D., Tran P., Ösapay G., Trinh K., Roberts K. D., Brasky K. M., Tongaonkar P., Ouellette A. J., and Selsted M. E. (2012) Rhesus macaque θ-defensins suppress inflammatory cytokines and enhance survival in mouse models of bacteremic sepsis. PLoS ONE 7, e51337 10.1371/journal.pone.0051337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wohlford-Lenane C. L., Meyerholz D. K., Perlman S., Zhou H., Tran D., Selsted M. E., and McCray P. B. Jr. (2009) Rhesus θ-defensin prevents death in a mouse model of severe acute respiratory syndrome coronavirus pulmonary disease. J. Virol. 83, 11385–11390 10.1128/JVI.01363-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jayne J. G., Bensman T. J., Schaal J. B., Park A. Y. J., Kimura E., Tran D., Selsted M. E., and Beringer P. M. (2017) Rhesus θ-defensin-1 attenuates endotoxin-induced acute lung injury by inhibiting proinflammatory cytokines and neutrophil recruitment. Am. J. Respir. Cell Mol. Biol. 10.1165/rcmb.2016-0428OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bensman T. J., Jayne J. G., Sun M., Kimura E., Meinert J., Wang J. C., Schaal J. B., Tran D., Rao A. P., Akbari O., Selsted M. E., and Beringer P. M. (2017) Efficacy of Rhesus θ-defensin-1 in experimental models of Pseudomonas aeruginosa lung infection and inflammation. Antimicrob. Agents Chemother. 61, e00154–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patten C., Bush K., Rioja I., Morgan R., Wooley P., Trill J., and Life P. (2004) Characterization of pristane-induced arthritis, a murine model of chronic disease: response to antirheumatic agents, expression of jointcytokines, and immunopathology. Arthritis Rheum. 50, 3334–3345 10.1002/art.20507 [DOI] [PubMed] [Google Scholar]
- 19. Black R. A., Rauch C. T., Kozlosky C. J., Peschon J. J., Slack J. L., Wolfson M. F., Castner B. J., Stocking K. L., Reddy P., Srinivasan S., Nelson N., Boiani N., Schooley K. A., Gerhart M., Davis R., et al. (1997) A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature 385, 729–733 10.1038/385729a0 [DOI] [PubMed] [Google Scholar]
- 20. Horiuchi K. (2013) A brief history of tumor necrosis factor α-converting enzyme: an overview of ectodomain shedding. Keio J. Med. 62, 29–36 10.2302/kjm.2012-0003-RE [DOI] [PubMed] [Google Scholar]
- 21. Moss M. L., Jin S. L., Milla M. E., Bickett D. M., Burkhart W., Carter H. L., Chen W. J., Clay W. C., Didsbury J. R., Hassler D., Hoffman C. R., Kost T. A., Lambert M. H., Leesnitzer M. A., McCauley P., et al. (1997) Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-α. Nature 385, 733–736 10.1038/385733a0 [DOI] [PubMed] [Google Scholar]
- 22. Murphy G. (2008) The ADAMs: signalling scissors in the tumour microenvironment. Nat. Rev. Cancer 8, 929–941 10.1038/nrc2459 [DOI] [PubMed] [Google Scholar]
- 23. Gooz M. (2010) ADAM-17: the enzyme that does it all. Crit. Rev. Biochem. Mol. Biol. 45, 146–169 10.3109/10409231003628015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chalaris A., Adam N., Sina C., Rosenstiel P., Lehmann-Koch J., Schirmacher P., Hartmann D., Cichy J., Gavrilova O., Schreiber S., Jostock T., Matthews V., Hasler R., Becker C., Neurath M. F., et al. (2010) Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J. Exp. Med. 207, 1617–1624 10.1084/jem.20092366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohta S., Harigai M., Tanaka M., Kawaguchi Y., Sugiura T., Takagi K., Fukasawa C., Hara M., and Kamatani N. (2001) Tumor necrosis factor-α (TNF-α) converting enzyme contributes to production of TNF-α in synovial tissues from patients with rheumatoid arthritis. J. Rheumatol. 28, 1756–1763 [PubMed] [Google Scholar]
- 26. Rose-John S. (2013) ADAM17, shedding, TACE as therapeutic targets. Pharmacol. Res. 71, 19–22 10.1016/j.phrs.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 27. Saftig P., and Reiss K. (2011) The “A Disintegrin And Metalloproteases” ADAM10 and ADAM17: novel drug targets with therapeutic potential? Eur. J. Cell Biol. 90, 527–535 10.1016/j.ejcb.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 28. Horiuchi K., Kimura T., Miyamoto T., Takaishi H., Okada Y., Toyama Y., and Blobel C. P. (2007) Cutting edge: TNF-α-converting enzyme (TACE/ADAM17) inactivation in mouse myeloid cells prevents lethality from endotoxin shock. J. Immunol. 179, 2686–2689 10.4049/jimmunol.179.5.2686 [DOI] [PubMed] [Google Scholar]
- 29. Issuree P. D., Maretzky T., McIlwain D. R., Monette S., Qing X., Lang P. A., Swendeman S. L., Park-Min K. H., Binder N., Kalliolias G. D., Yarilina A., Horiuchi K., Ivashkiv L. B., Mak T. W., Salmon J. E., and Blobel C. P. (2013) iRHOM2 is a critical pathogenic mediator of inflammatory arthritis. J. Clin. Invest. 123, 928–932 10.1172/JCI66168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Richards F. M., Tape C. J., Jodrell D. I., and Murphy G. (2012) Anti-tumour effects of a specific anti-ADAM17 antibody in an ovarian cancer model in vivo. PLoS ONE 7, e40597 10.1371/journal.pone.0040597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DasGupta S., Murumkar P. R., Giridhar R., and Yadav M. R. (2009) Current perspective of TACE inhibitors: a review. Bioorg. Med. Chem. 17, 444–459 10.1016/j.bmc.2008.11.067 [DOI] [PubMed] [Google Scholar]
- 32. Mohler K. M., Sleath P. R., Fitzner J. N., Cerretti D. P., Alderson M., Kerwar S. S., Torrance D. S., Otten-Evans C., Greenstreet T., and Weerawarna K. (1994) Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature 370, 218–220 10.1038/370218a0 [DOI] [PubMed] [Google Scholar]
- 33. McGeehan G. M., Becherer J. D., Bast R. C. Jr, Boyer C. M., Champion B., Connolly K. M., Conway J. G., Furdon P., Karp S., and Kidao S. (1994) Regulation of tumour necrosis factor-α processing by a metalloproteinase inhibitor. Nature 370, 558–561 10.1038/370558a0 [DOI] [PubMed] [Google Scholar]
- 34. Hayden M. S., and Ghosh S. (2014) Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 26, 253–266 10.1016/j.smim.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Y., Wang Y., Zhang F., Wang K., Liu G., Yang M., Luan Y., Zhao Z., Zhang J., Cao X., and Zhang D. (2015) Allyl methyl disulfide inhibits IL-8 and IP-10 secretion in intestinal epithelial cells via the NF-κB signaling pathway. Int. Immunopharmacol. 27, 156–163 10.1016/j.intimp.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 36. de Meijer V. E., Sverdlov D. Y., Popov Y., Le H. D., Meisel J. A., Nosé V., Schuppan D., and Puder M. (2010) Broad-spectrum matrix metalloproteinase inhibition curbs inflammation and liver injury but aggravates experimental liver fibrosis in mice. PLoS ONE 5, e11256 10.1371/journal.pone.0011256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Armstrong-James D., Meintjes G., and Brown G. D. (2014) A neglected epidemic: fungal infections in HIV/AIDS. Trends Microbiol. 22, 120–127 10.1016/j.tim.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 38. Chaly Y. V., Paleolog E. M., Kolesnikova T. S., Tikhonov I. I., Petratchenko E. V., and Voitenok N. N. (2000) Neutrophil α-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur. Cytokine Netw. 11, 257–266 [PubMed] [Google Scholar]
- 39. Khine A. A., Del Sorbo L., Vaschetto R., Voglis S., Tullis E., Slutsky A. S., Downey G. P., and Zhang H. (2006) Human neutrophil peptides induce interleukin-8 production through the P2Y6 signaling pathway. Blood 107, 2936–2942 10.1182/blood-2005-06-2314 [DOI] [PubMed] [Google Scholar]
- 40. Lillard J. W. Jr, Boyaka P. N., Chertov O., Oppenheim J. J., and McGhee J. R. (1999) Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc. Natl. Acad. Sci. U.S.A. 96, 651–656 10.1073/pnas.96.2.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Presicce P., Giannelli S., Taddeo A., Villa M. L., and Della Bella S. (2009) Human defensins activate monocyte-derived dendritic cells, promote the production of proinflammatory cytokines, and up-regulate the surface expression of CD91. J. Leukoc. Biol. 86, 941–948 10.1189/jlb.0708412 [DOI] [PubMed] [Google Scholar]
- 42. Syeda F., Liu H. Y., Tullis E., Liu M., Slutsky A. S., and Zhang H. (2008) Differential signaling mechanisms of HNP-induced IL-8 production in human lung epithelial cells and monocytes. J. Cell Physiol. 214, 820–827 10.1002/jcp.21279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van Wetering S., Mannesse-Lazeroms S. P., Van Sterkenburg M. A., Daha M. R., Dijkman J. H., and Hiemstra P. S. (1997) Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am. J. Physiol. 272, L888–L896 [DOI] [PubMed] [Google Scholar]
- 44. Kirkegaard T., Pedersen G., Saermark T., and Brynskov J. (2004) Tumour necrosis factor-α converting enzyme (TACE) activity in human colonic epithelial cells. Clin. Exp. Immunol. 135, 146–153 10.1111/j.1365-2249.2004.02348.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alvarez-Iglesias M., Wayne G., O'Dea K. P., Amour A., and Takata M. (2005) Continuous real-time measurement of tumor necrosis factor-α converting enzyme activity on live cells. Lab. Invest. 85, 1440–1448 10.1038/labinvest.3700340 [DOI] [PubMed] [Google Scholar]
- 46. Le Gall S. M., Maretzky T., Issuree P. D., Niu X. D., Reiss K., Saftig P., Khokha R., Lundell D., and Blobel C. P. (2010) ADAM17 is regulated by a rapid and reversible mechanism that controls access to its catalytic site. J. Cell Sci. 123, 3913–3922 10.1242/jcs.069997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Le Gall S. M., Bobé P., Reiss K., Horiuchi K., Niu X. D., Lundell D., Gibb D. R., Conrad D., Saftig P., and Blobel C. P. (2009) ADAMs 10 and 17 represent differentially regulated components of a general shedding machinery for membrane proteins such as transforming growth factor α, L-selectin, and tumor necrosis factor alpha. Mol. Biol. Cell 20, 1785–1794 10.1091/mbc.E08-11-1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maretzky T., Yang G., Ouerfelli O., Overall C. M., Worpenberg S., Hassiepen U., Eder J., and Blobel C. P. (2009) Characterization of the catalytic activity of the membrane-anchored metalloproteinase ADAM15 in cell-based assays. Biochem. J. 420, 105–113 10.1042/BJ20082127 [DOI] [PubMed] [Google Scholar]
- 49. Sahin U., Weskamp G., Kelly K., Zhou H. M., Higashiyama S., Peschon J., Hartmann D., Saftig P., and Blobel C. P. (2004) Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol. 164, 769–779 10.1083/jcb.200307137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Blobel C. P. (2005) ADAMs: key components in EGFR signalling and development. Nat. Rev. Mol. Cell Biol. 6, 32–43 10.1038/nrm1548 [DOI] [PubMed] [Google Scholar]
- 51. Huovila A. P., Turner A. J., Pelto-Huikko M., Kärkkäinen I., and Ortiz R. M. (2005) Shedding light on ADAM metalloproteinases. Trends Biochem. Sci. 30, 413–422 10.1016/j.tibs.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 52. Zunke F., and Rose-John S. (2017) The shedding protease ADAM17: physiology and pathophysiology. Biochim. Biophys. Acta 1864, 2059–2070 10.1016/j.bbamcr.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 53. Scheller J., Chalaris A., Garbers C., and Rose-John S. (2011) ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 32, 380–387 10.1016/j.it.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 54. Peschon J. J., Slack J. L., Reddy P., Stocking K. L., Sunnarborg S. W., Lee D. C., Russell W. E., Castner B. J., Johnson R. S., Fitzner J. N., Boyce R. W., Nelson N., Kozlosky C. J., Wolfson M. F., Rauch C. T., et al. (1998) An essential role for ectodomain shedding in mammalian development. Science 282, 1281–1284 10.1126/science.282.5392.1281 [DOI] [PubMed] [Google Scholar]
- 55. Franzke C. W., Cobzaru C., Triantafyllopoulou A., Löffek S., Horiuchi K., Threadgill D. W., Kurz T., van Rooijen N., Bruckner-Tuderman L., and Blobel C. P. (2012) Epidermal ADAM17 maintains the skin barrier by regulating EGFR ligand-dependent terminal keratinocyte differentiation. J. Exp. Med. 209, 1105–1119 10.1084/jem.20112258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jackson L. F., Qiu T. H., Sunnarborg S. W., Chang A., Zhang C., Patterson C., and Lee D. C. (2003) Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 22, 2704–2716 10.1093/emboj/cdg264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sternlicht M. D., Sunnarborg S. W., Kouros-Mehr H., Yu Y., Lee D. C., and Werb Z. (2005) Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development 132, 3923–3933 10.1242/dev.01966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pruessmeyer J., and Ludwig A. (2009) The good, the bad and the ugly substrates for ADAM10 and ADAM17 in brain pathology, inflammation and cancer. Semin. Cell Dev. Biol. 20, 164–174 10.1016/j.semcdb.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 59. Li X., Maretzky T., Weskamp G., Monette S., Qing X., Issuree P. D., Crawford H. C., McIlwain D. R., Mak T. W., Salmon J. E., and Blobel C. P. (2015) iRhoms 1 and 2 are essential upstream regulators of ADAM17-dependent EGFR signaling. Proc. Natl. Acad. Sci. U.S.A. 112, 6080–6085 10.1073/pnas.1505649112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McIlwain D. R., Lang P. A., Maretzky T., Hamada K., Ohishi K., Maney S. K., Berger T., Murthy A., Duncan G., Xu H. C., Lang K. S., Häussinger D., Wakeham A., Itie-Youten A., Khokha R., et al. (2012) iRhom2 regulation of TACE controls TNF-mediated protection against Listeria and responses to LPS. Science 335, 229–232 10.1126/science.1214448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Adrain C., Strisovsky K., Zettl M., Hu L., Lemberg M. K., and Freeman M. (2011) Mammalian EGF receptor activation by the rhomboid protease RHBDL2. EMBO Rep. 12, 421–427 10.1038/embor.2011.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Amour A., Slocombe P. M., Webster A., Butler M., Knight C. G., Smith B. J., Stephens P. E., Shelley C., Hutton M., Knäuper V., Docherty A. J., and Murphy G. (1998) TNF-α converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 435, 39–44 10.1016/S0014-5793(98)01031-X [DOI] [PubMed] [Google Scholar]
- 63. Lee M. H., Verma V., Maskos K., Becherer J. D., Knäuper V., Dodds P., Amour A., and Murphy G. (2002) The C-terminal domains of TACE weaken the inhibitory action of N-TIMP-3. FEBS Lett. 520, 102–106 10.1016/S0014-5793(02)02776-X [DOI] [PubMed] [Google Scholar]
- 64. Wisniewska M., Goettig P., Maskos K., Belouski E., Winters D., Hecht R., Black R., and Bode W. (2008) Structural determinants of the ADAM inhibition by TIMP-3: crystal structure of the TACE-N-TIMP-3 complex. J. Mol. Biol. 381, 1307–1319 10.1016/j.jmb.2008.06.088 [DOI] [PubMed] [Google Scholar]
- 65. Mahmoodi M., Sahebjam S., Smookler D., Khokha R., and Mort J. S. (2005) Lack of tissue inhibitor of metalloproteinases-3 results in an enhanced inflammatory response in antigen-induced arthritis. Am. J. Pathol. 166, 1733–1740 10.1016/S0002-9440(10)62483-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Garcia A. E. (2011) Theta-defensins from olive baboon leukocytes: isolation, synthesis, and host defense properties. Ph.D. thesis, University of California, Irvine [Google Scholar]
- 67. Panyutich A. V., Panyutich E. A., Krapivin V. A., Baturevich E. A., and Ganz T. (1993) Plasma defensin concentrations are elevated in patients with septicemia or bacterial meningitis. J. Lab. Clin. Med. 122, 202–207 [PubMed] [Google Scholar]
- 68. Haudek S. B., Natmessnig B. E., Fürst W., Bahrami S., Schlag G., and Redl H. (2003) Lipopolysaccharide dose response in baboons. Shock 20, 431–436 10.1097/01.shk.0000090843.66556.74 [DOI] [PubMed] [Google Scholar]
- 69. Redl H., Bahrami S., Schlag G., and Traber D. L. (1993) Clinical detection of LPS and animal models of endotoxemia. Immunobiology 187, 330–345 10.1016/S0171-2985(11)80348-7 [DOI] [PubMed] [Google Scholar]
- 70. Tongaonkar P., Trinh K. K., Schaal J. B., Tran D., Gulko P. S., Ouellette A. J., and Selsted M. E. (2015) Rhesus macaque θ-defensin RTD-1 inhibits proinflammatory cytokine secretion and gene expression by inhibiting the activation of NF-κB and MAPK pathways. J. Leukoc. Biol. 98, 1061–1070 10.1189/jlb.3A0315-102R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Niu X., Umland S., Ingram R., Beyer B. M., Liu Y. H., Sun J., Lundell D., and Orth P. (2006) IK682, a tight binding inhibitor of TACE. Arch. Biochem. Biophys. 451, 43–50 10.1016/j.abb.2006.03.034 [DOI] [PubMed] [Google Scholar]
- 72. Sengupta P., Puri C. S., Chokshi H. A., Sheth C. K., Midha A. S., Chitturi T. R., Thennati R., Murumkar P. R., and Yadav M. R. (2011) Synthesis, preliminary biological evaluation and molecular modeling of some new heterocyclic inhibitors of TACE. Eur. J. Med. Chem. 46, 5549–5555 10.1016/j.ejmech.2011.09.018 [DOI] [PubMed] [Google Scholar]
- 73. Wang Z., Wang L., Fan R., Zhou J., and Zhong J. (2016) Molecular design and structural optimization of potent peptide hydroxamate inhibitors to selectively target human ADAM metallopeptidase domain 17. Comput. Biol. Chem. 61, 15–22 10.1016/j.compbiolchem.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 74. Xue C. B., He X., Corbett R. L., Roderick J., Wasserman Z. R., Liu R. Q., Jaffee B. D., Covington M. B., Qian M., Trzaskos J. M., Newton R. C., Magolda R. L., Wexler R. R., and Decicco C. P. (2001) Discovery of macrocyclic hydroxamic acids containing biphenylmethyl derivatives at P1′, a series of selective TNF-α converting enzyme inhibitors with potent cellular activity in the inhibition of TNF-α release. J. Med. Chem. 44, 3351–3354 10.1021/jm0155502 [DOI] [PubMed] [Google Scholar]
- 75. Fingleton B. (2008) MMPs as therapeutic targets: still a viable option? Semin. Cell Dev. Biol. 19, 61–68 10.1016/j.semcdb.2007.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fingleton B. (2007) Matrix metalloproteinases as valid clinical targets. Curr. Pharm. Des. 13, 333–346 10.2174/138161207779313551 [DOI] [PubMed] [Google Scholar]
- 77. Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., and Lehrer R. I. (1985) Defensins: natural peptide antibiotics of human neutrophils. J. Clin. Invest. 76, 1427–1435 10.1172/JCI112120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schaal J. B., Tran D. Q., Subramanian A., Patel R., Laragione T., Roberts K. D., Trinh K., Tongaonkar P., Tran P. A., Minond D., Fields G. B., Beringer P., Ouellette A. J., Gulko P. S., and Selsted M. E. (2017) Suppression and resolution of autoimmune arthritis by rhesus theta-defensin-1, an immunomodulatory macrocyclic peptide. PLoS One 12, e0187868 10.1371/journal.pone.0187868 [DOI] [PMC free article] [PubMed] [Google Scholar]