Abstract
Context:
Dentinal hypersensitivity (DH) is a chronic disorder in which patients report sharp and acute pain to a variety of stimuli. Till date, a standardized procedure to treat DH is missing, though several alternative treatment strategies have been designed, including laser therapies.
Aim:
The aim of the study was to treat DH with minimum chemical concentration and least laser energy level with longer follow-up period.
Materials and Methods:
One hundred and twenty patients were randomly divided into four groups: (i) Group 1-5% potassium nitrate (KNO3); (ii) Group 2 - gallium-aluminum-arsenide diode laser (62.2 J/cm2, wavelength - 980 nm, noncontact pulse mode, and power wattage - 0.5 W); (iii) Group 3 - combined 5% KNO3 and the diode laser; and (iv) Group 4 - placebo (control). The visual analog scale (VAS) scores were recorded, analyzed, and compared to tactile stimuli, cold water, and air blast tests at different intervals for 6 weeks.
Results:
Synergistic use of 5% KNO3 and diode laser (Group 3) significantly reduced the DH pain, which was almost negligible after 6th week (97%–99% of the pain was reported to be relieved) and showed promising results than any other studied groups. Further, the diode laser (Group 2) showed better results than 5% KNO3 (Group 1). One-way ANOVA and Bonferroni correction post hoc test revealed the combination of groups with significant differences in the mean VAS scores at the different interval of time (P < 0.01).
Conclusions:
Convincingly, the combined application of 5% KNO3 with the diode laser can be recommended for treating DH patients.
Key words: Dentinal hypersensitive patients, diode and chemical agent synergism, randomized clinical study, visual analog scale
INTRODUCTION
Dentinal hypersensitivity (DH), a painful chronic dentinal disorder, is characterized by short but sharp pain arising from exposed dentin to stimuli-thermal, evaporative, tactile, osmotic, or chemical.[1,2] The condition usually affects the surface of teeth – bicuspids and canines – near the cervical area. Brannstrom's hydrodynamic theory recognized DH as the change in fluid dynamics inside dentinal tubules.[3] Many periodontal therapies exposed the dentin to DH through attrition and abrasion.[4,5] Some other factors include the faulty brush technique, root denudation, aging, other periodontal diseases, and poor lifestyle.[6,7]
DH pain can range from mild discomfort to extreme severity. Often patients have a hard time in describing the pain levels. Therefore, a detailed history of the problem, its management, and diagnosis is in demand.[3] The treatment depends on the invasiveness standards including dentist's applied in-office procedures or patient-applied over-the-counter dentifrices. The practice of desensitizing agents is common in traditional therapy, such as the use of protein precipitates,[8] tubule-occluding agents,[9] and tubule sealants.[10] On the contrary, lasers proved to be more efficient than chemical agents/sealants.[11,12,13] Numerous studies observed that the application of lasers relieved the patient's discomfort for a longer period compared to any desensitizing agents.[14,15,16] Nevertheless, the clinical studies still debate for the best professional treatment, and the race is “on” to find the best cure for DH. In the search for a better treatment to DH, the synergistic effect of diode laser and potassium nitrate (KNO3) (a commonly used antihypersensitivity agent) was compared to the sole application of diode laser or KNO3 on DH patients.
MATERIALS AND METHODS
The research protocol was reviewed and approved by the Ethical Committee of the Institution. All patients were educated about the study, and then, written consent was acquired before enrollment in the study. The study was conducted for 6 weeks.
A flow diagram for complete methodology is presented in Figure 1. One hundred and twenty patients were diagnosed with the problem of DH with at least two hypersensitive teeth. The statistics of the studied patients include the mean age of 36.5 years for the age group range of 18–55 years and with a gender distribution of 69 men and 51 women [Table 1]. The evaluation was in response to air, cold water, and tactile stimuli recorded through visual analog scale (VAS). The values of VAS varied between 0–10; “0"– the absence of pain and “10"– intolerable pain. The inclusion criteria for patients enrollments in this study are based on any or combination of these: (a) Absence of undergoing desensitizing therapy, (b) absence of gestation or lactation, (c) nonallergic to the medicament employed in the study, (d) do not have systemic conditions causing or predisposing to DH (e.g., chronic acid regurgitation), (e) do not have excessive dietary or environmental exposure to the acids, (f) absence of any teeth or supportive structures with any other painful pathological defects, and (g) have baseline pain of above 5.0 VAS score.
Figure 1.
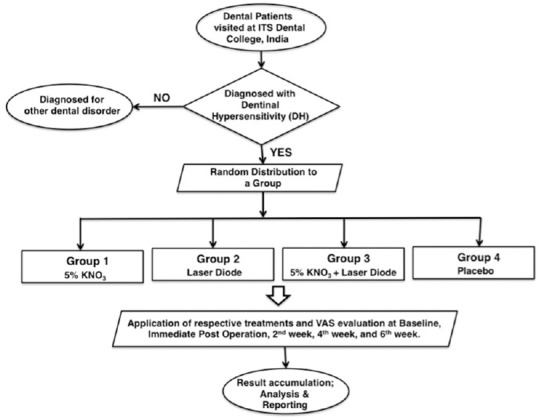
Flowchart indicating the methodology implemented in the present clinical study
Table 1.
Sample characteristics of included in the study

Only when a patient was diagnosed with DH, with their respective written consent, the patients were assigned equally but randomly to one of the four treatment groups (n = 30). Envelopes containing identifications for treatment groups were enclosed, mixed, and then numbered. Each participant was randomly selected to one of the following groups.
Group 1: 5% KNO3 was applied for 60 s on the tooth surface. In each visit of a patient, KNO3 was applied using a new disposable brush. Further, patients were restrained to rinse the teeth until 3 min
Group 2: Lased by a gallium-aluminum-arsenide (GaAlA) laser Photon Plus, Zolar co., 980 nm with 62.2 J/cm2 energy in noncontact mode and using a fiber of 320 μ diameter. Each site underwent three applications of 60 s
Group 3: Treated with both 5% KNO3 and GaAlA diode laser (980 nm with 62.2 J/cm2). The KNO3 gel was left on the tooth surface for 60 s before laser treatment
Group 4: Commercial toothpaste was applied (placebo–control) for 60 s.
Scaling and root planning were performed before the sensitivity treatment to all the patients. In addition, teeth vitality was assessed. The vitality was checked using an electric Pulp Tester (Gentle-Pulse, Parkell Electronics Division). All selected teeth were vital, which was the inclusion criteria. Oral hygiene instructions were provided to the patients and asked to perform tooth brushing twice daily (toothpaste was standardized after confirming the absence of any sensitivity check agents and handed over to the patients with strict instructions), employing modified bass technique.[17] VAS scores were measured at the baseline, immediate postoperative application, 2nd, 4th, and 6th week.
Patients were evaluated by employing three test stimuli. The examination sites were isolated with the help of cotton rolls and subjected to the following tests.[18] A total of three readings were recorded with an interval of 1 min. The mean and standard errors for the tests performed on each group were recorded.
Tactile test
To examine the affected area, a mechanical stimulus was applied carefully to the cervical area with the aid of a sharp dental explorer (17/23). The explorer was extended gently across the affected area, perpendicular to the long axis of the tooth. The examination was recapitulated thrice before scoring VAS scale, and then, the interpretation was noted.
Air blast test
The dental syringe was used to generate a blast of air of 60 lb/inch pressure implemented on the affected region of the tooth for 1 s from a distance of 10 mm. The discomfort scale score was then recorded.
Cold-water test
After isolating the particular tooth, 0.2 ml of ice-cold water in a pre-cooled 1 ml disposable syringe along to the suspected tooth surface, and the score was registered using the discomfort scale.
A time interval of 5 min was given between measures on a given tooth. Tooth sensitivity was recorded by marking the degree of discomfort on VAS. At the end of the baseline, immediate postoperative application, 2nd, 4th, and 6th week, VAS scores were collected. Any drop-outs were excluded from the study. Each patient included in the study was present at every follow-up period. In the recall period of study, no adverse effects for any subjective signs (like an allergic reaction, ulceration, or with objective signs such as redness of mucosa and staining of teeth) were observed. For all the follow-ups, the same examiner was assigned every time.
The statistical analysis was performed for each parameter. The mean and standard deviation were calculated using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA version 16.0 for Windows). One-way ANOVA and Bonferroni correction post hoc test were performed to analyze the differences in the various treatment groups at the respective intervals. P < 0.01 was considered to be statistically significant.
RESULTS
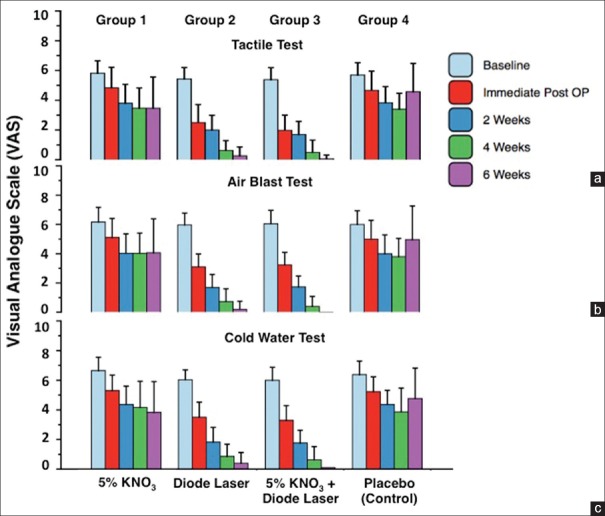
The VAS was scored for all groups (n = 30 in each group). There was negligible difference in VAS scores between the groups at baseline. A significant reduction in DH was observed for Group 3 (GaAlA laser diode and 5% KNO3) and Group 2 (GaAlA laser diode) than Group 1 (5% KNO3). For Group 1 (5% KNO3), the VAS value decreased by 40.3% for the tactile test [Figure 2a] whereas the same treatment decreased 34.1% [Figure 2b] and 42.4% [Figure 2c] for the air blast and cold-water tests. For Group 2 (diode laser), the tactile, air blast, and cold-water test observed for the pain reduction by 95.1%, 96.6%, and 93.4%, respectively, [Figure 2]. The synergistic application of 5% KNO3 and laser (Group 3) was the best treatment and lead to a reduction by 98.8% for the tactile test, 96.6% for air blast, and 98.3% for the cold-water test [Figure 3a]. A negligible change in VAS for the placebo (Group 4) was observed. The pain reduction for Group 4 patients was only by ~ 10%–15% from baseline to the 6-week period. The effectiveness of the treatment was highest for synergistic 5% KNO3 and diode laser application, followed by diode laser application than 5% KNO3 and placebo [Figure 3b].
Figure 2.
Variation in visual analog scale with time for different groups incorporated in the study. The results are summarized for (a) tactile test, (b) air blast test, and (c) cold water test
Figure 3.
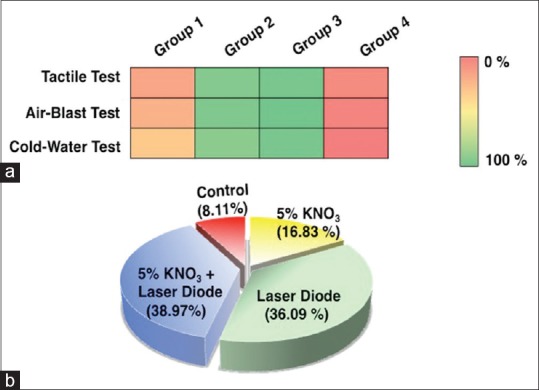
Statistical analysis indicating the variation in the results (a) heat plot showing variation in four different groups with three diagnostic tests, and (b) pie chart presenting the effectiveness of applications on treating dentinal hypersensitivity
To investigate the effect of a nontoxic oxidizing chemical on DH, KNO3 in diluted form (5%) was applied to the patients in Group 1. The tactile, air blast and cold-water tests were identical [Figure 2]. Patients reported a stable, moderate pain level by the end of the 4th week, which continued to be constant until the 6th week.
To evaluate the impact of noninvasive high energy on DH, the GaAlA laser treatment was implemented to the Group 2. All tests (tactile, air blast, and cold water) showed similar changes in the mean values of VAS at different intervals, where patients observed a gradual decrease in the pain over the time [Figure 2]. The VAS scores were almost half after the first application. Further, the VAS values were drastically reduced from 2nd week to the 4th week, and the negligible pain was recorded at the end of the 6th week.
To evaluate the combined effect of chemical reagent and laser, Group 3 patients were observed for changes in pain while implementing the synergistic application of 5% KNO3 and GaAlA laser diode. This group of patients showed a rapid decrease in pain compared to the rest group patients [Figure 2]. The tactile, air-blast, and cold-water tests were observed for a similar pattern in pain reduction. The baseline score reduced approximately by 2.7 times after the first treatment, which was almost negligible by the 6th week.
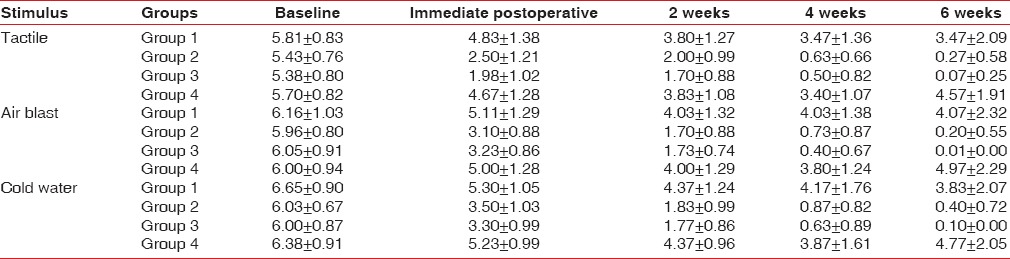
Group 4 was maintained as the placebo (control group). The variations from the baseline to 6th-week VAS values for the tactile test is from 5.700 ± 0.8263 to 4.567 ± 1.9061 [Figure 2a], for the air blast test is from 6.000 ± 0.9377 to 4.967 ± 2.2967 [Figure 2b], and for the cold-water test is from 6.383 ± 0.9067 to 4.767 ± 2.0542 [Figure 2c]. Therefore, Group 4 patients reported negligible changes in the VAS from baseline to 6 weeks indicating no reduction in pain for this group [Table 2].
Table 2.
Mean comparative values of visual analog scale scores over time according to the treatment groups, n=30, mean±standard deviation (Group 1-5% potassium nitrate; Group 2 - diode laser; Group 3-5% potassium nitrate + diode laser; Group 4 - Placebo)
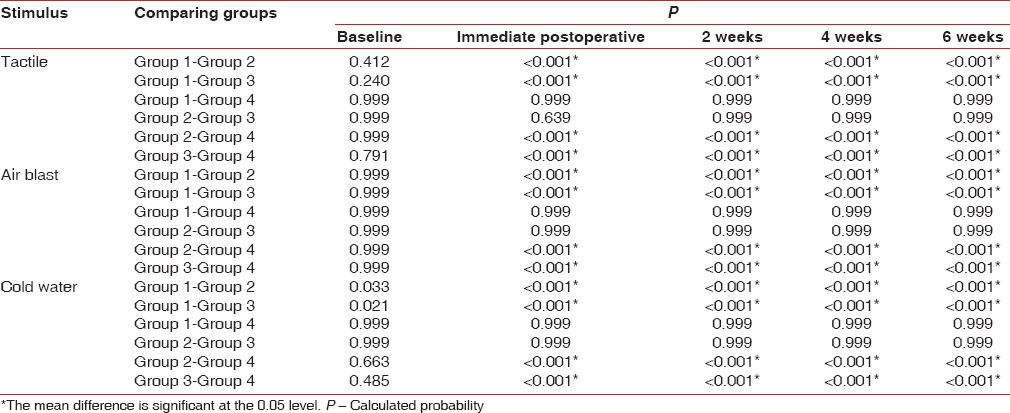
The comparison with one-way ANOVA analysis shows that the comparison of mean between and within the groups was statistically significant (P < 0.001) except at the baseline (tactile test P = 0.118; air-blast test P = 0.845; and cold-water test P = 0.009) [Table 3]. The Bonferroni Correction post hoc tests revealed the groups with significant mean differences [Table 4]. Comparing any two groups at the baseline was insignificant for any of the tests as the P values varied from 0.240–0.999. Interestingly, the mean differences for (a) Group 1 (5% KNO3)–Group 4 (placebo) and (b) Group 2 (diode laser)–Group 3 (synergistic application of 5% KNO3 and diode laser) were insignificant for any of the tests (P > 0.001). The comparison of mean VAS values for any other combinations of the groups, however, were significant from the immediate postoperative until the 6th week of the follow-up period (P < 0.001) [Table 4].
Table 3.
One-way ANOVA analysis for the differences in the various treatment groups at the respective intervals
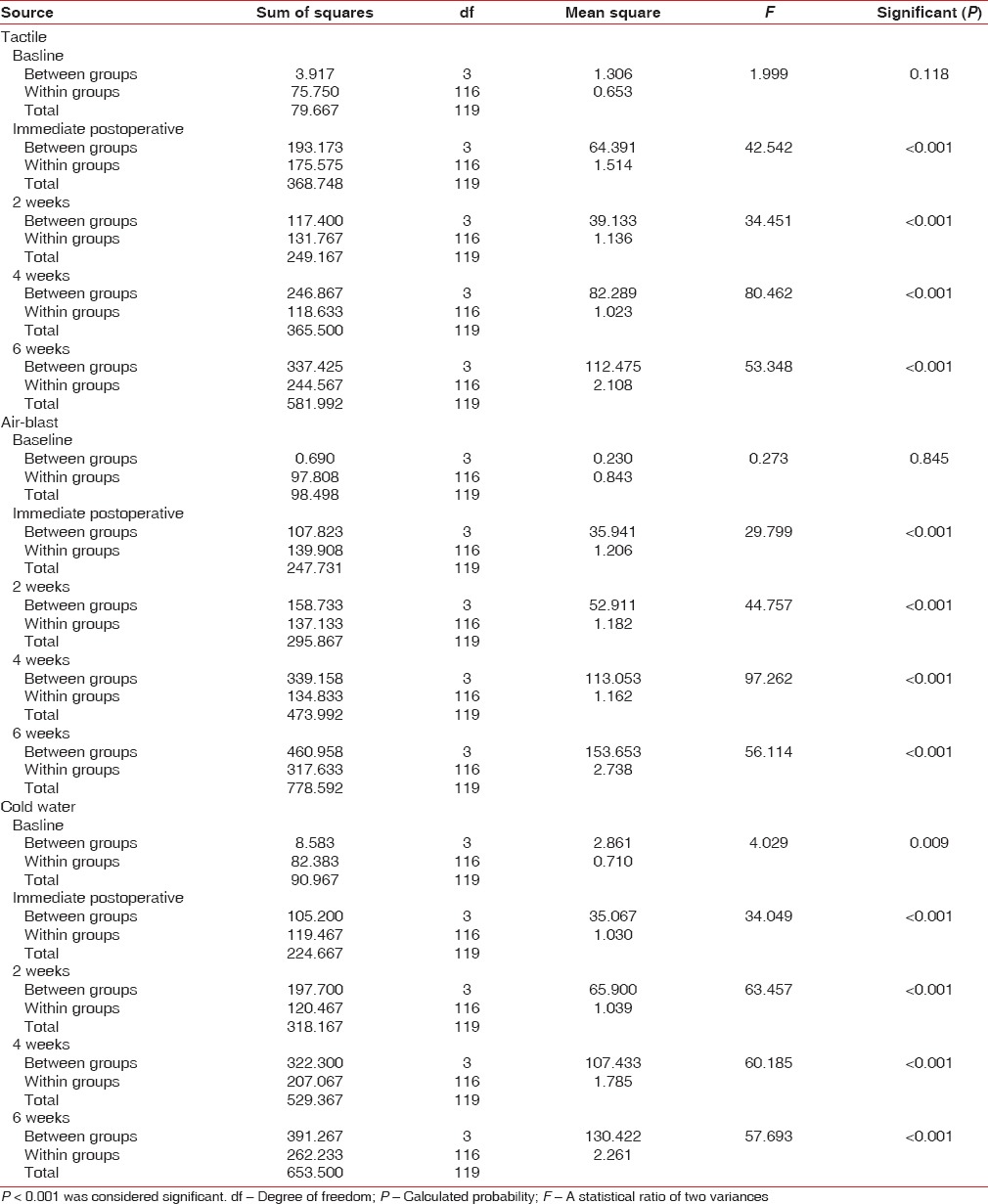
Table 4.
Intergroup multiple comparison using Bonferroni post hoc test at different period interval (Group 1-5% potassium nitrate, Group 2 - diode laser, Group 3-5% potassium nitrate + diode laser, and Group 4 - placebo)
DISCUSSION
DH is one of the most common complaints prevalent as high as 57% among adult dental patients.[19] The pain is localized caused by sudden movement of dentinal fluid excite the pulp's mechanoreceptors. Thus, the best possible solution so far is to remove the stimulus.[3,19] Although exact and the most appropriate method for effectively treating DH is still missing in the dental literature, the continuous efforts with various permutation and combinations are reported since the last decade. The primary focus of this study was on optimizing the limited chemical usage and laser diode energy without compromising the treatment outcome. Table 5 lists and compares our work with previously cited studies. It appears that our synergistic approach is so far the best solution to comfort DH patients.
Table 5.
Comparison of the present study with reported previous clinical reports on dentinal hypersensitivity
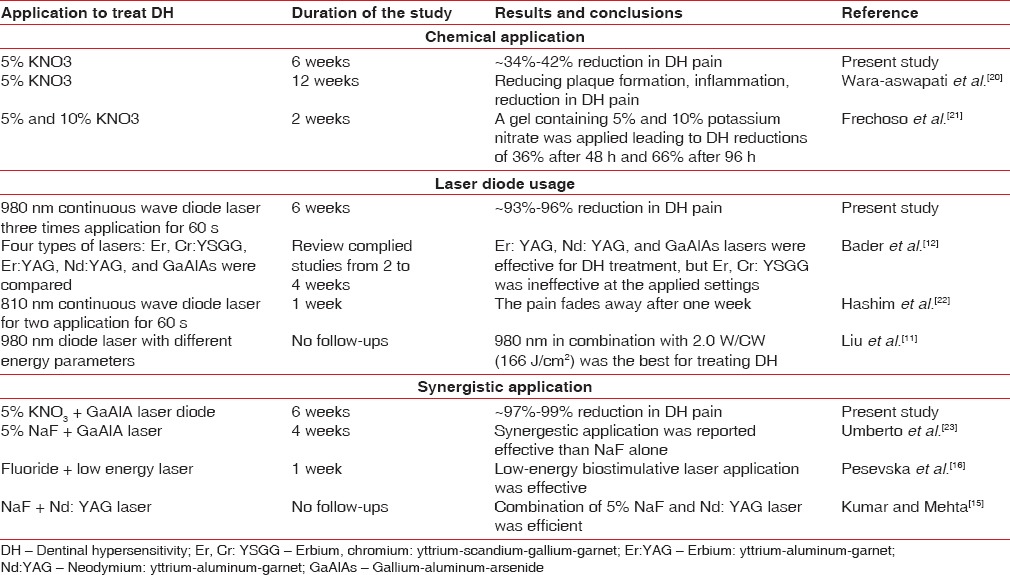
KNO3 is one of the most commonly used desensitizing agents.[20,21,24] Many researchers have reported unsteady responses to the application of KNO3 [Table 5] that may be attributed to the altered geographical and environmental factors. Frechoso et al. reported that 10% KNO3 eased the pain by 35.8% whereas 5% KNO3 by 11.8% after 48 h.[21] The increased concentration of KNO3 reduced the pain, but it may have unseen side effects. Hence, the concentration of KNO3 was kept constant to 5% for the study. In another study, Wara-aswapati et al. investigated the combined effect of triclosan (antiplaque and anti-inflammatory's agent), KNO3 (desensitizing agent), and sodium mono-fluoro-phosphate (anticaries agent).[20] This 4-week study suggested that the synergistic effect of these chemicals was much efficient in reducing the DH than the individual application. Similarly, the combined application of 2% strontium chloride and 5% potassium chloride had shown 54% reduction in 3 days.[24] The sole application of 5% KNO3 reduced pain by ~40.0% in 6 weeks. Since time to ease the patients is the key, 5% KNO3 alone may not be the best for treating DH.
Another method extensively applied for the treatment of DH is the use of laser diodes.[12,13,22] Liu et al. did an extensive study to determine safety parameters for the diode laser irradiating dentin with three different energy levels of 980 nm diode laser (166, 250, and 333 J/cm2).[11] They concluded that 2.0 W/CW (166 J/cm2) was the best setting for 980 nm diode laser, which effectively sealed the dentin without any morphological damage to the pulp tissue and odontoblasts. Since 166 J/cm2 was the starting energy level, the uncertainty of effectiveness of this energy was high over the different geographically location.[11] Hence, in this study, the energy level of diode laser was reduced by almost 2.5 times (62.2 J/cm2 with 980 nm) making the laser application much safer. The laser diode was applied for 60 s-in-line with previous studies.[22] A meta-analysis study was summarized about the type of lasers found effective for treating DH.[12] The analysis indicated that erbium: yttrium-aluminum-garnet, neodymium: yttrium-aluminum-garnet (Nd:YAG), and gallium-aluminum-arsenide (GaAlAs) garnet were efficacious in reducing DH. However, the high degree of heterogeneity required more clinical trials. In our study, GaAlA laser was used, which confirms the effectiveness of GaAlAs laser analysis done by Bader et al.[12]
Kumar and Mehta observed that 5% sodium fluoride combined with (Nd:YAG) laser diode application was considerably efficient in the treatment of DH than sole respective applications.[15] Similarly, Pesevska et al. concluded that the low-energy biostimulation laser application was proficient than traditional topical fluoride treatment.[16] In another study, Umberto et al. reported the efficacy of the laser diode was best when applied while using 5% sodium fluoride for DH treatment.[22] In line with literature, the synergistic application was more effective than the individual application of KNO3 or GaAlA laser diode. The laser might induce a slight melting of the dentin surface and rapidly seal the exposed dentin tubules whereas the KNO3 gel emphasized the reduction in the pain. The speculations on the synergistic mechanism are based on the literature survey and require future directions. Indeed, the synergistic mechanism study must stand out a complete area of research.
The present study is geographically limited to a particular region. The results presented in this study may vary with the locations; however, the conclusion drawn from this study and during the literature review are accounting for a generic advantage of the synergistic application of diode laser and chemical sealants/additives for the long-term treatment of DH. Nonetheless, the mechanism is relatively unknown. Therefore, in vitro studies using either scanning electron microscopy or histological analysis may provide an in-depth knowledge on the synergism.
CONCLUSIONS
The goal was to provide significant and immediate reduced discomfort to DH patients. Based on the literature and this study, the application of KNO3 and GaAlA laser diode (980 nm) setting was optimized to 5% and 66.2 J/cm2, respectively. When applied together, diode laser and KNO3 synergism showed the best results than the individual application for long-term DH pain relief and may be the recommended the treatment for DH.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Orchardson R, Gillam DG. Managing dentin hypersensitivity. J Am Dent Assoc. 2006;137:990–8. doi: 10.14219/jada.archive.2006.0321. [DOI] [PubMed] [Google Scholar]
- 2.Vieira AH, Santiago SL. Management of dentinal hypersensitivity. Gen Dent. 2009;57:120–6. [PubMed] [Google Scholar]
- 3.Braennstroem M, Astroem A. A study on the mechanism of pain elicited from the dentin. J Dent Res. 1964;43:619–25. doi: 10.1177/00220345640430041601. [DOI] [PubMed] [Google Scholar]
- 4.Birang R, Poursamimi J, Gutknecht N, Lampert F, Mir M. Comparative evaluation of the effects of Nd:YAG and Er:YAG laser in dentin hypersensitivity treatment. Lasers Med Sci. 2007;22:21–4. doi: 10.1007/s10103-006-0412-z. [DOI] [PubMed] [Google Scholar]
- 5.Addy M. Tooth brushing, tooth wear and dentine hypersensitivity – Are they associated? Int Dent J. 2005;55:261–7. doi: 10.1111/j.1875-595x.2005.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee WC, Eakle WS. Stress-induced cervical lesions: Review of advances in the past 10 years. J Prosthet Dent. 1996;75:487–94. doi: 10.1016/s0022-3913(96)90451-5. [DOI] [PubMed] [Google Scholar]
- 7.Marini MG, Greghi SL, Passanezi E, Sant'ana AC. Gingival recession: Prevalence, extension and severity in adults. J Appl Oral Sci. 2004;12:250–5. doi: 10.1590/s1678-77572004000300017. [DOI] [PubMed] [Google Scholar]
- 8.Gangarosa LP Sr. Current strategies for dentist-applied treatment in the management of hypersensitive dentine. Arch Oral Biol. 1994;39:101S–6S. doi: 10.1016/0003-9969(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 9.Kerns DG, Scheidt MJ, Pashley DH, Horner JA, Strong SL, Van Dyke TE, et al. Dentinal tubule occlusion and root hypersensitivity. J Periodontol. 1991;62:421–8. doi: 10.1902/jop.1991.62.7.421. [DOI] [PubMed] [Google Scholar]
- 10.Ianzano JA, Gwinnett AJ, Westbay G. Polymeric sealing of dentinal tubules to control sensitivity: Preliminary observations. Periodontal Clin Investig. 1993;15:13–6. [PubMed] [Google Scholar]
- 11.Liu Y, Gao J, Gao Y, Xu S, Zhan X, Wu B, et al. In vitro study of dentin hypersensitivity treated by 980-nm diode laser. J Lasers Med Sci. 2013;4:111–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Bader J, Balevi B, Farsai P, Flores-Mir C, Gunsolley J, Matthews D, et al. Lasers may reduce pain arising from dentin hypersensitivity. J Am Dent Assoc. 2014;145:e1–2. doi: 10.14219/jada.2013.56. [DOI] [PubMed] [Google Scholar]
- 13.Yaghini J, Mogharehabed A, Safavi N, Mohamadi M, Ashtiju F. Evaluation of the effect of low level laser therapy toothbrush in treatment of dentin hypersensitivity. J Lasers Med Sci. 2015;6:85–91. [PMC free article] [PubMed] [Google Scholar]
- 14.Lan WH, Liu HC, Lin CP. The combined occluding effect of sodium fluoride varnish and Nd:YAG laser irradiation on human dentinal tubules. J Endod. 1999;25:424–6. doi: 10.1016/S0099-2399(99)80271-4. [DOI] [PubMed] [Google Scholar]
- 15.Kumar NG, Mehta DS. Short-term assessment of the Nd:YAG laser with and without sodium fluoride varnish in the treatment of dentin hypersensitivity – A clinical and scanning electron microscopy study. J Periodontol. 2005;76:1140–7. doi: 10.1902/jop.2005.76.7.1140. [DOI] [PubMed] [Google Scholar]
- 16.Pesevska S, Nakova M, Ivanovski K, Angelov N, Kesic L, Obradovic R, et al. Dentinal hypersensitivity following scaling and root planing: Comparison of low-level laser and topical fluoride treatment. Lasers Med Sci. 2010;25:647–50. doi: 10.1007/s10103-009-0685-0. [DOI] [PubMed] [Google Scholar]
- 17.Poyato-Ferrera M, Segura-Egea JJ, Bullón-Fernández P. Comparison of modified bass technique with normal toothbrushing practices for efficacy in supragingival plaque removal. Int J Dent Hyg. 2003;1:110–4. doi: 10.1034/j.1601-5037.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 18.Clark GE, Troullos ES. Designing hypersensitivity clinical studies. Dent Clin North Am. 1990;34:531–44. [PubMed] [Google Scholar]
- 19.Terry DA. Cervical dentin hypersensitivity: Etiology, diagnosis, and management. Dent Today. 2011;30:61–2, 64, 68. [PubMed] [Google Scholar]
- 20.Frechoso SC, Menéndez M, Guisasola C, Arregui I, Tejerina JM, Sicilia A, et al. Evaluation of the efficacy of two potassium nitrate bioadhesive gels (5% and 10%) in the treatment of dentine hypersensitivity. A randomised clinical trial. J Clin Periodontol. 2003;30:315–20. doi: 10.1034/j.1600-051x.2003.20077.x. [DOI] [PubMed] [Google Scholar]
- 21.Wara-aswapati N, Krongnawakul D, Jiraviboon D, Adulyanon S, Karimbux N, Pitiphat W, et al. The effect of a new toothpaste containing potassium nitrate and triclosan on gingival health, plaque formation and dentine hypersensitivity. J Clin Periodontol. 2005;32:53–8. doi: 10.1111/j.1600-051X.2004.00631.x. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Hu D. Efficacy of a commercial dentifrice containing 2% strontium chloride and 5% potassium nitrate for dentin hypersensitivity: A 3-day clinical study in adults in china. Clin Ther. 2012;34:614–22. doi: 10.1016/j.clinthera.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 23.Hashim NT, Gasmalla BG, Sabahelkheir AH, Awooda AM. Effect of the clinical application of the diode laser (810 nm) in the treatment of dentine hypersensitivity. BMC Res Notes. 2014;7:31. doi: 10.1186/1756-0500-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umberto R, Claudia R, Gaspare P, Gianluca T, Alessandro del V. Treatment of dentine hypersensitivity by diode laser: A clinical study. Int J Dent. 2012;2012:858950. doi: 10.1155/2012/858950. [DOI] [PMC free article] [PubMed] [Google Scholar]