Abstract
Drought and heat are major abiotic stresses that reduce crop productivity and weaken global food security, especially given the current and growing impacts of climate change and increases in the occurrence and severity of both stress factors. Plants have developed dynamic responses at the morphological, physiological and biochemical levels allowing them to escape and/or adapt to unfavorable environmental conditions. Nevertheless, even the mildest heat and drought stress negatively affects crop yield. Further, several independent studies have shown that increased temperature and drought can reduce crop yields by as much as 50%. Response to stress is complex and involves several factors including signaling, transcription factors, hormones, and secondary metabolites. The reproductive phase of development, leading to the grain production is shown to be more sensitive to heat stress in several crops. Advances coming from biotechnology including progress in genomics and information technology may mitigate the detrimental effects of heat and drought through the use of agronomic management practices and the development of crop varieties with increased productivity under stress. This review presents recent progress in key areas relevant to plant drought and heat tolerance. Furthermore, an overview and implications of physiological, biochemical and genetic aspects in the context of heat and drought are presented. Potential strategies to improve crop productivity are discussed.
Keywords: drought, heat, stress, crop, productivity, genomics, biotechnology, agronomy
Introduction
Drought and heat can reduce crop productivity and yields leading to lower income for farmers. Reduction in yield by as much as 40% was observed for maize and 21% for wheat at approximately a 40% water reduction (Daryanto et al., 2016). In the case of cowpea, an important crop in Africa, yield reduction can vary between 34 and 68% depending on the developmental timing of the drought stress (Farooq et al., 2017). Reductions in crop productivity will be further exacerbated by the impacts of climate change. The Intergovernmental Panel on Climate Change (IPCC) report concludes with unequivocal evidence that the air and ocean temperatures have warmed, and the concentrations of greenhouse gases have increased (IPCC, 2014). Both of these factors have direct influences on plant growth and crop yields (Bita and Gerats, 2013; Stocker et al., 2013). Between 1880 and 2012, land and ocean temperature data show an average global warming of 0.85°C based on multiple independently produced datasets (Stocker et al., 2013). The atmospheric concentrations of the greenhouse gases carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O) have also risen, with net emissions approaching 300 ppm in the recent years (Stocker et al., 2013). Climate change is impacting the earth's crust resulting in infrequent and erratic precipitations, elevated temperatures, and expansion of affected land areas under flood or water deficit. These adverse conditions are contributing to development of drought-prone areas and consequently on the plant growth and crop productivity. Though the impact of increased CO2 on crop yield is a debated topic with some researchers arguing that elevated CO2 will increase photosynthesis. However, Gray et al. (2016) showed that rising CO2 did not counteract the effect of severe drought on photosynthesis and yield.
Several independent studies have demonstrated the effects of increased temperature and water stress on crop yields. For example in Canada, the extreme events that occurred during 2001 and 2002 and the droughts and floods during 2010 and 2011 had a devastating impact on crop yield reducing by as much as 50% (Wheaton et al., 2008). Between 1980 and 2016, major USA disasters, exceeding a billion dollars each per year, indicates that when drought and heat are combined, they cause expanded agricultural losses of more than $220 billion (Oceanic Atmospheric Administration website, 2015).
In developing countries, the impact of heat and drought is equally significant. For example in Morocco, the economy is largely dependent on agriculture with approximately 40% of the labor force involved. In years of drought, crop productivity is significantly reduced and thus directly affects the livelihood of farmers and consequently the economy. Wheat is a staple crop where it is used for bread (haxaploid wheat) and for couscous (Durum wheat). The effect of drought is significant on wheat productivity. For example, cereal productivity was reduced in 2015–16 by approx. 50% from to 7.5M tons production on an average year to 3.4M tons in 2015–16. Sub-Saharan countries are also negatively affected in years with significant drought. Crop failure due to drought was reported in West Africa, with droughts between 1975 and 1985 resulting in a per capita food production decline of 25% (Epule et al., 2013).
We propose that despite the complexity of plant responses to heat and drought and the limited improvements so far achieved in mitigating the effects of these stresses, there are opportunities, highlighted in this review, to improve productivity of plants under these stresses.
Drought vs. heat stress
At the whole plant level, all abiotic stresses induce a cascade of physiological and molecular events resulting, in some cases, in similar responses. Drought, high salinity and cold can all be exhibited as a physiological dehydration at the cellular level (Vinocur and Altman, 2005).
Drought stress takes place when soil and atmospheric humidity is low and the ambient air temperature is high. This condition is the result of an imbalance between the evapotranspiration flux and water intake from the soil (Lipiec et al., 2013). Heat stress is defined as the rise in soil and air temperature beyond a threshold level for a minimum amount of time such that permanent harm to plant growth and development occur. A detailed multi-location study highlights the impact of temperature effects on the yields. These adverse conditions are contributing to development of drought-prone areas and consequently on the plant growth and crop productivity of major crops (Zhao et al., 2017).
It is important that both drought and heat have to be considered together because their combined effect is higher than when taken individually (Dreesen et al., 2012). In general, abiotic stress including heat and drought are controlled by multiple genes and the underpinning mechanisms are more complex than other traits such as biotic stresses that are generally characterized by monogenic resistance. In addition, other abiotic and/or biotic stresses often have additive influence to heat and drought response making the study additionally challenging.
Water shortage and soil salinity undeniably represent major challenges facing productivity as they trigger oxidative, osmotic and temperature stresses (Reynolds and Tuberosa, 2008; Landi et al., 2017). Moreover, heat and water stress were also reported to be linked, as the reduced stomatal conductance and transpiration under these conditions may induce heat stress as leaf temperature rises (Król, 2013). Under field conditions, when water deficit and high temperature occurs simultaneously, growth and performance of the plants decline rapidly especially in tropical and sub-tropical environments (Wahid et al., 2007; De Boeck et al., 2015; Niinemets, 2015; Zandalinas et al., 2016, 2017).
Leaf structure is affected by higher temperature often causing development of thinner leaves with higher leaf area (Loveys et al., 2002; Luomala et al., 2005; Poorter et al., 2009). These gross morphological changes are underpinned with changes in leaf anatomy. Leaves which develop under water deficit generally have smaller cells and higher stomatal density (Tisné et al., 2010; Shahinnia et al., 2016). Wahid et al. (2007) reported comparable effects of high temperature and water deficit on cell density, but limited data are available with respect to leaf anatomy changes in response to high temperature.
Physiological responses to heat and drought stresses
Plants have adapted dynamic responses to handle abiotic stresses at the morphological, physiological, and biochemical levels, allowing them to survive under variable environmental conditions (Huber and Bauerle, 2016). Plant physiological responses to drought and heat stresses can be classified into two distinct mechanisms. Avoidance mechanisms are mainly morphological and physiological adjustments that provide an escape to the water or heat stress, including increased root system, reduced stomatal number and conductance, decreased leaf area, increased leaf thickness, and leaf rolling or folding to lessen evapotranspiration (Sicher et al., 2012; Goufo et al., 2017). Cuticular wax biosynthesis, on the surfaces of the aerial plant parts, is also strictly associated with an adaptive response (Lee and Suh, 2013). Tolerance traits maintain tissue hydrostatic pressure, by cellular and biochemical modifications, mainly through osmotic adjustments (Khan et al., 2015; Blum, 2017).
Under these water challenging conditions, plants perceive stresses through various sensors involved in response signaling. These are transduced by various pathways in which many signaling and transcriptional factors play important and specific functions (Hirayama and Shinozaki, 2010). Moreover, some plants have built-in mechanisms to trigger hydraulic, chemical, or electrical long-distance signals to launch systemic stress responses. Under drought, a decrease in root hydraulic conductivity manifests to prevent water losses from the plant to the dry soil. High temperature can also increase root moisture loss to harmful levels (Parent et al., 2010).
Water transport within a plant occurs under tension as determined by soil water availability and the atmospheric vapor pressure deficit, creating turgor pressure within cells. Physiological adjustments that maintain turgor pressure are important under changing environmental conditions. Water transport in roots is affected by various components such as root anatomy, water availability and salts in the soil (Boursiac et al., 2005). All of these factors are influenced by the activity of aquaporins, which are integral membrane proteins that function as channels to transfer select small solutes and water (Maurel et al., 2008; Vandeleur et al., 2014).
Photosynthesis comprises various components, including the photosystems and photosynthetic pigments, the electron transport system, and CO2 reduction pathways. A stress-induced negative effect on any component in these systems may lead to a reduction in the overall photosynthetic performance. Studies have shown that photosynthetic efficiency and transpiration rates decrease under water, salt, and heat stress when applied individually or in combination (Arbona et al., 2013; Zandalinas et al., 2016). This is mainly a consequence of stress-induced stomatal closure but also can occur by other nonstomatal limitations such as decreased leaf expansion, leaf senescence and inappropriate functioning of the photosynthetic machinery (Wahid et al., 2007; Saibo et al., 2009; Rahnama et al., 2010). This latter effect situation is frequently attributed to the lower internal availability of CO2, along with the inhibition of key photosynthetic enzymes and ATP synthases (Zlatev and Lidon, 2012; Zandalinas et al., 2016). Both heat and water stresses are reported to decrease electron transport, degrade proteins, and release magnesium and calcium ions from their protein-binding partners (Wahid et al., 2007; Rexroth et al., 2011; Zlatev and Lidon, 2012; Zandalinas et al., 2016). Extended exposure to high temperature also triggers a decrease in chlorophyll content, increased amylolytic activity, thylakoid grana disintegration and disruption of assimilates' transport (Kozłowska et al., 2007).
Decreases in photosynthetic rate are directly linked to Water Use Efficiency (WUE), which is one of the most important parameters in crop response to osmotic imbalances. A number of reports have shown that decreases in net photosynthetic rate are associated with stomatal closure resulting in increased WUE (net CO2 assimilation rate/transpiration) (Ruggiero et al., 2017). Stomatal closure is thought to have a more inhibitory effect on transpiration of water than on CO2 diffusion into the leaf tissues (Sikuku et al., 2010). A widely employed tool for screening physiological traits associated with WUE or “Transpiration Efficiency” is the use of carbon isotope discrimination, which reflects both CO2 exchange and water economy. This type of tool is critical to assess phenotypic variation within a large breeding population (Ellsworth and Cousins, 2016). Recent advances in photosynthesis research may open new opportunities to address the relevant issues when crop plants face adverse drought and heat stress (Ort et al., 2015; Kromdijk et al., 2016).
The reproductive phase of development is the most sensitive stage to high temperature stress in several crops including wheat (Farooq et al., 2011; Shanmugam et al., 2013; Dwivedi et al., 2017), chickpea (Kalra et al., 2008; Devasirvatham et al., 2012; Kaushal et al., 2013), maize (Cairns et al., 2012), and Sorghum (Djanaguiraman et al., 2014; Singh et al., 2016). The reproductive processes involving pollen and stigma viability, pollination anthesis, pollen tube growth, and early embryo development are particularly vulnerable to heat stress (Giorno et al., 2013). However, in general, male reproductive tissues are much more sensitive at all stages of development to high temperature stress than female reproductive tissues (Hedhly, 2011).
Biochemical responses to heat and drought stresses
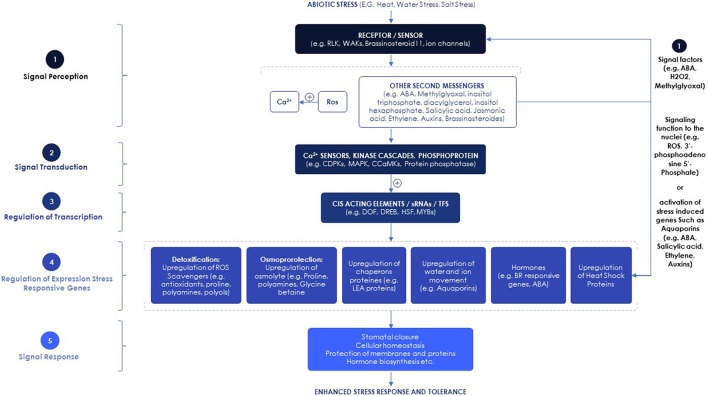
Signal perception through cell-surface receptors is a common feature of all living organisms. During stress conditions, reception of signals from the environment activates signaling cascades as a first step in a response (Figure 1) (Shulaev et al., 2008). Different types of receptors perceive various signals and stimuli from the environment. In the 1990s, the first receptor kinase protein, the Receptor-Like Kinase (RLK), was described in plants (Walker and Zhang, 1990; He et al., 1996). In vascular plants, a subfamily of RLKs known as WAKs (Wall-Associated Kinases) receives signals from the environment and other adjacent cells as a necessary step to activate appropriate signaling cascades (Shulaev et al., 2008). Since the finding of the first RLK, considerable efforts have been dedicated to characterizing additional specific receptor-kinase genes see in the Supplementary Data Table S1 for additional molecular factors involved in plant response to heat and drought stresses.
Figure 1.
Signaling pathways involved in plant abiotic stress responses.
Stress perception is followed by the activation of systemic signaling cascades. Studies show that aquaporin proteins, key factors contributing to hydraulic conductivity, are regulated at the biochemical level by environmental stimuli with changes such as phosphorylation (Johansson et al., 1998), cytoplasmic pH and calcium (Gerbeau et al., 2002; Alleva et al., 2006) or re-localization to intracellular compartments (Boursiac et al., 2008).
Plant hormones, have also been shown to be implicated in root and shoot long-distance signaling and the control of hydraulic conductivity. Abscisic acid (ABA) is the most critical hormone involved in regulating tolerance to abiotic stresses such as drought, salinity, cold, heat and wounding (Zhang et al., 2006; Lata and Prasad, 2011). ABA has long been acknowledged as a major chemical root-to-shoot stress signal (Schachtman and Goodger, 2008), inducing inhibition of leaf expansion and short-term responses like stomatal closure. ABA is involved in the regulation of systemic responses to abiotic stress before there are any detectable changes in leaf water or nutrient status (Bauer et al., 2013; Suzuki et al., 2013). Osmotic stress results in the synthesis or catabolism of several other growth regulators, including auxin, cytokinins, ethylene, gibberellins, brassinosteroids, jasmonic acid and other factors (eg. nitrogen, pH) that have been shown to be involved in the regulation of physiological processes through their action as signal molecules in signaling networks (Nakashima and Yamaguchi-Shinozaki, 2013; Mittler and Blumwald, 2015; Verma et al., 2016).
Increased intracellular Ca2+ levels are also induced under stress conditions by several signal molecules such as: inositol trisphosphate, inositol hexaphosphate, diacylglycerol and Reactive Oxygen Species (ROS) (Hirayama and Shinozaki, 2010). Calcium binding proteins, that function as Ca2+ sensors, perceive the elevated Ca2+ levels (Kudla et al., 2010), which can lead to the activation of calcium dependent protein kinases. The activated kinases or phosphatases can phosphorylate or dephosphorylate specific transcription factors (TFs), thus regulating the expression levels of stress-responsive genes (Reddy et al., 2011). The activated Ca2+ sensors can also bind to cis-elements of major stress-responsive gene promoters or can interact with DNA-binding proteins regulating these genes, resulting in their activation or suppression (Kudla et al., 2010; Reddy et al., 2011).
An early heat stress response was reported to induce Ca2+ influx and cytoskeletal restructuring, which causes the upregulation of mitogen activated protein kinases and calcium dependent protein kinase signaling cascades (Wahid et al., 2007; Ashraf and Harris, 2013). This signaling cascade leads to production of antioxidants and compatible osmolytes (for osmotic adjustment) and the expression of heat shock proteins. The main impacts of heat stress are protein denaturation, instabilities in nucleic acids and cytoskeletal structure, increased membrane fluidity, inactivation of the synthesis and degradation of proteins, and loss of membrane integrity (Howarth, 2005; Wahid et al., 2007). Severe cellular injuries or loss can occur at moderately high temperatures, after long-term exposure, or after extremely short-term exposure to very high temperatures (Wahid et al., 2007). This may reduce ion flux and lead to production of ROS and other toxic compounds which severely affect plant growth (Howarth, 2005). Expression of heat shock proteins as well as other types of protective proteins is an effective adaptive strategy under conditions of high temperatures exposure—with the expression of said protective proteins often correlating with stress tolerance (Wahid et al., 2007), photosynthesis and WUE (Camejo et al., 2005), membrane stability (Ahn and Zimmerman, 2006), and maintenance of cellular hydration (Wahid and Close, 2007).
Various abiotic stresses induce overproduction of ROS, which are extremely reactive and toxic, causing damage to proteins, lipids, carbohydrates and DNA and ultimately resulting in oxidative stress (Zlatev and Lidon, 2012). Oxidative stress is commonly counteracted by two different processes, prevention or avoidance of ROS formation, including detoxifying/scavenging enzymes and several antioxidants to handle the toxic effect of ROS (Mittler, 2002; Gill and Tuteja, 2010). Concomitantly with ROS, plants also produce 2 to 6-fold higher methylglyoxal under abiotic stress (Yadav et al., 2005), which is a very reactive cytotoxic compound produced through different enzymatic and non-enzymatic reactions. Methylglyoxal damages cellular function and can even destroy DNA (Hasanuzzaman et al., 2017). Methylglyoxal is detoxified through the glyoxalase system composed of glyoxalase I and glyoxalase II, that catalyze methylglyoxal to D-lactate via reduced glutathione as a cofactor (Yadav et al., 2005).
Recently, new retrograde signals have been reported such as the metabolite 3′-phosphoadenosine 5′-phosphate that has been shown to accumulate during high light and drought, moving from chloroplast to nucleus to regulate ABA signaling and stomatal closure during the oxidative stress. This results in drought tolerance and the activation of the high light transcriptome (Pornsiriwong et al., 2017).
TFs play important roles in stress tolerance. Many abiotic stress-related genes and TFs have been isolated from different plant species and overexpressed in transgenic plants to improve stress tolerance (Table 1). The stress-inducible TFs include members of the DREB, ERF, WRKY, MYB, bHLH, bZIP, DOF, and NAC families (Shinozaki et al., 2003) (Figure 1). For example, the overexpression of Cycling Dof Factor 3 in Arabidopsis enhanced drought, cold and salt tolerance (Corrales et al., 2014, 2017), along with the up-regulation of a set of genes involved in cellular osmoprotection and ROS homeostasis (Corrales et al., 2017). In a recent review, Kulkarni et al. (2017) discussed the roles of other transcriptional regulators including ZFPs and EAR-motif containing repressors in abiotic stress regulation.
Table 1.
Summary of genes that were shown to have a role in heat and drought tolerance in wheat and maize.
| Gene/origin | Function/Mechanism | Type of promoter/Expression | Trait | Crop | Author |
|---|---|---|---|---|---|
| DREB1A/A. thaliana | TF | rd29A gene promoter/stress-inducible gene, upregulated | Improved drought salt and freezing tolerance | Wheat | Pellegrineschi et al., 2004 |
| Hsf6A (heat shock factor)/wheat | TF | Barley HVA1s promoter/Drought inducible, upregulated | Improved thermo tolerance | Wheat | Xue et al., 2014 |
| AtHDG11/A. thaliana | TF | Actin1 promoter/overexpression | Improved drought tolerance | Wheat | Li et al., 2016 |
| TOR/A. thaliana | Signaling Factor | CaMV35S promoter/overexpression | Improved drought tolerance | Wheat | Datla et al., 2014 (Patent application and unpublished results) |
| EF-Tu/maize | Elongation factor/chaperone like activity | Maize ubiquitin 1 promoter/overexpression | Improved thermos tolerance | Wheat | Fu et al., 2008 |
| Stress-responsive NAC gene/rice | TF | Maize ubiquitin 1 promoter/overexpression | Enhanced tolerance to drought and salt stresses | Wheat | Saad et al., 2013 |
| HVA1/barely | Group 3 LEA - HVA1 | Maize ubi1 promoter/overexpression | Improved drought tolerance and field evaluation for drought tolerance | Wheat | Sivamani et al., 2000; Bahieldin et al., 2005 |
| P5CS/Vigna aconitifolia | Proline Biosynthesis | Stress-induced promoter complex—AIPC/upregulation | Increased tolerance to water deficit | Wheat | Vendruscolo et al., 2007 |
| Phosphoenolpyruvate carboxylase (PEPC)/maize | C4, CAM and the citric acid cycles | Maize PEPC promoter/overexpression | Improved yield and drought tolerance | Wheat | Qin et al., 2016 |
| OsMYB55/rice | TF | Maize ubiquitin Ubi1 promoter/overexpression | Increased drought and heat stress tolerance | Maize | Casaretto et al., 2016 |
| ZmNF-YB2/maize | TF (Nuclear factor Y B subunit 2) | Rice actin 1 constitutive promoter/overexpression | Enhanced drought tolerance and photosynthetic capacity | Maize | Nelson et al., 2007 |
| ZmPIS gene/maize | Precursor of signal molecules | Maize ubiquitin promoter/overexpression | Enhanced drought tolerance | Maize | Liu et al., 2013 |
| NPK1/tobacco | Protein kinase | Constitutive 35 S promoter/overexpression | Enhanced drought tolerance | Maize | Shou et al., 2004 |
| CspA, CsB/bacteria | RNA chaperones, cold shock protein | Rice actin1 promoter/overexpression | Improved kernel yield under water limiting conditions in the field | Maize | Castiglioni et al., 2008 |
| 1-aminocyclopropane-1-carboxylic acid synthase 6/maize | Ethylene Biosynthesis | Maize ubiquitin promoter/downregulation | Improved grain yield under drought stress conditions in the field | Maize | Habben et al., 2014 |
| ZmARGOS1/maize | Down regulator of ethylene response and modulator of ethylene signal transduction | Maize ubiquitin promoter/overexpression | Drought tolerance enhancement in the field | Maize | Shi et al., 2015 |
| ZmARGOS1/maize | Down regulator of ethylene response and modulator of ethylene signal transduction | Genetic editing/downregulation | Drought tolerance enhancement in the field | Maize | Shi et al., 2017 |
| LOS5/A. thaliana | Cofactor sulfurase gene | “Super” promoter (manopine synthase)/overexpression | Enhanced drought tolerance | Maize | Lu et al., 2013 |
| betA gene/E. coli | Biosynthesis of glycine betaine | CaMV35S promoter/overexpression | Enhanced drought stress tolerance | Maize | Quan et al., 2004 |
| Trehalose-6-phosphate phosphatase (OsMADS6)/rice | Sucrose metabolism | OsMads6 promoter/overexpression | Enhance yield under well-watered and water stressed plants in the field | Maize | Nuccio et al., 2015 |
The production of a wide range of metabolites of low molecular mass can prevent the detrimental change in cellular components and restore homeostasis. These include soluble carbohydrates such as glucose and fructose, amino acids and a variety of sugars and sugar alcohols (Vinocur and Altman, 2005; Szabados and Savouré, 2010; Arbona et al., 2013; Morales et al., 2013). The accumulation of these compounds has been linked to abiotic stress tolerance and maintenance of cell turgor, protection of protein structures and stabilization of cellular membranes as cells dehydrate (Arbona et al., 2008, 2013; Zlatev and Lidon, 2012). Among amino acids, proline is the main effector contributing to osmotic adjustment in response to different abiotic stress conditions (Kaplan and Guy, 2004; De Campos et al., 2011; Morales et al., 2013; Sinay and Karuwal, 2014; Zandalinas et al., 2017). Therefore, the synthesis and accumulation of proline has been considered a tolerance trait for a significant amount of time (Janská et al., 2010).
Glycine betaine also plays an important role in abiotic stress tolerance in some plant species. Genes associated with glycine betaine synthesis in higher plants and microbes have been transferred into plants such as maize, which do not accumulate glycine betaine or enhance the level of synthesis upon stress (Quan et al., 2004). However, the mechanism of action of glycine betaine is unclear. Using a transgenic system, Su et al. (2006) observed that the apparent effects of glycine betaine biosynthesis on stress tolerance may be attributed to protective effects other than changes to the cellular osmotic balance.
Secondary metabolites are also essential compounds for plant acclimation and persistence under fluctuating environmental conditions and include: coumarins, lignin, anthocyanins, flavonoids and tannins (Fraser and Chapple, 2011). In citrus plants, increased levels of secondary metabolites such as phenylpropanoid, precursors of lignins, flavonols, and flavones were observed in response to drought and temperature stresses (Zandalinas et al., 2017).
Agronomic strategies for improving tolerance to drought and heat
Implementation of crop management practices can potentially alleviate the harmful effects of drought and heat stresses and include: soil management and culture practices, irrigation, crop residues and mulching, and selection of more appropriate crop varieties.
Under heat stress, the application of macronutrients such as K, Ca and micronutrients like B, Se, and Mn, which are known to modify stomatal function, can help activate the physiological and metabolic processes contributing to preserving high water potential in tissues thereby increasing heat stress tolerance (Waraich et al., 2012). The application of nutrients such as N, K, Ca, and Mg was also reported to reduce toxicity to ROS by increasing the concentration of antioxidant enzymes in plant cells (Waraich et al., 2012). On the other hand, several studies have shown that the application of fertilizer has no significant effect on drought stress and an optimal soil moisture content is required since water is critical for the mobility and metabolism of these nutrients (Lipiec et al., 2013).
The effects of drought have been alleviated through exogenous silicon (Si) application in wheat and rice (Gong et al., 2005; Gautam et al., 2016). Plants treated with Si displayed higher antioxidant activities (Gong et al., 2005; Ma et al., 2016), higher amounts of photosynthetic pigments (Gong et al., 2005), and expression changes of genes implicated in the ascorbate-reduced glutathione cycle, flavonoid biosynthesis and antioxidant response (Ma et al., 2016). Seed priming with Si was also efficient in the protection of maize plantlets from alkaline stress (Abdel Latef and Tran, 2016).
Attention has also been focused on the application of plant growth regulators known to be involved in the response to stress. Among the plant growth substances, salicylic acid, cytokinin and ABA have been reported to play a key role in drought tolerance. Under water stress conditions, plant growth regulator treatments can significantly increase the water potential and the chlorophyll content (Zhang et al., 2004). Exogenous application of ABA increased soybean yields under water deficit conditions (Zhang et al., 2004). New ABA formulations are currently available for commercial growers to delay drought-induced wilting symptoms and improve drought tolerance (Barrett and Campbell, 2006; Sharma et al., 2006; Blanchard et al., 2007; Huang et al., 2008; Waterland et al., 2010). Recent research has investigated the use of concentrated ABA or ABA analogs to maintain the marketability of horticulture crops by reducing drought stress symptoms (Monteiro et al., 2001; Sharma et al., 2006; Blanchard et al., 2007; Kim and van Iersel, 2008). ABA application during spring or summer also reduces transpiration in potted miniature rose (Rose hybrida L.) and results in better flower longevity (Monteiro et al., 2001). Perego et al. (2014) used another strategy to mitigate yield losses by bringing forward the date of sowing of maize in order to prevent heat stress during the flowering stage.
Genetics and genomics approaches
Conventional plant breeding has had limited success in mitigating the effects of abiotic stress on plant productivity. This may be due to the complexity associated with traits controlled by a number of genes present at multiple quantitative trait loci (QTL) (Parmar et al., 2017). However, there are cases of successes in conventional breeding for improved heat and drought tolerance traits. For example, Haley et al. (2007) developed a drought tolerant variety of wheat referred to as “Ripper.” This variety performed well with superior grain yields under non-irrigated conditions in Colorado. The variety also has superior milling and bread-baking quality. Badu-Apraku and Yallou (2009) developed maize varieties that had superior yield compared to the control varieties grown under drought conditions. These maize cultivars also performed well under biotic stress conditions. The use of quantitative traits locus markers in breeding programs with marker-assisted backcrossing and marker-assisted recurrent selection strategies have also had positive results (Mir et al., 2012). QTLs from wild emmer wheat were introgressed through marker-assisted selection, to improve drought resistance in elite durum (T. turgidum ssp. durum) and bread (T. aestivum) wheat cultivars (Merchuk-Ovnat et al., 2016). Three of the introgressed QTLs were confirmed, two in the durum wheat background and one in bread wheat. In most cases, the QTL x environment interaction was validated under drought with regard to grain yield and biomass improvement. Tahmasebi et al. (2016) used a recombinant inbred line population to map QTLs under well-irrigated, heat, drought, and a combination of drought and heat stress conditions for two years. They identified a QTL that explained up to 19.6% variation in grain yield in the drought, heat, and combined stress trials. The authors proposed that the marker could be used as a candidate for validation in other populations and identifying superior allelic variations in wheat cultivars to increase the efficiency of selection of high yielding lines adapted to heat and drought stress conditions.
Despite challenges due to genome size and polyploidy of crops such as wheat, significant progress has been made in genome sequencing, annotation and functional characterization of important genes (Uauy, 2017). Clavijo et al. (2017) have recently generated a new wheat whole-genome shotgun sequence assembly using a combination of optimized data types and an assembly algorithm intended to deal with huge and complex genomes. They identified 104,091 high-confidence protein-coding genes and 10,156 noncoding RNA genes. Recently, Luo et al. (2017) have sequenced the genome of the progenitor of the wheat D genome (Aegilops tauschii). The genome sequence data of both wheat and Aegilops should permit identification of structural variants, and assist with the annotation of gene models including those involved in complex traits such as heat and drought.
In addition to protein coding genes, some miRNAs are functionally preserved across plant species and have been shown to be regulated by drought stress. In drought-resistant wild emmer wheat, miR166 was shown to be down-regulated under drought stress (Kantar et al., 2011). Recently, Huang et al. (2017) have identified a long non-coding miRNA gene that controls β-diketone wax formation; these waxes play an important role in drought tolerance acting to minimize water loss. These observations propose that targeted miRNA-based genetic modifications have the potential to improve drought tolerance in cereal crops (Ferdous et al., 2015).
Genomic studies of species that are extremely drought tolerant such as desiccation tolerant (DT) or resurrection plants may serve as models for designing crops with enhanced drought tolerance (Giarola et al., 2017). Desiccation tolerance is common in seeds and other organisms, but only some angiosperm species possess vegetative desiccation tolerance that have evolved due to ecological constraints (Giarola et al., 2017). Xerophyta viscosa is a monocotyledonous plant species closely related to cereal crops. Therefore, it is an ideal model for understanding extreme dehydration in cereals. Reactivation of vegetative DT is based on the presence of genes associated with DT in reproductive structures, such as seeds, and hence the genomic information for DT was redirected toward vegetative tissues (Costa et al., 2017). Costa et al. showed that ABI5 may be a regulator of expression of the LEA4 family which may be an important factor in the longevity of X. viscosa in the dry state. These researchers also identified two structural orthologs of ABI3, a major regulator of seed maturation and DT along with the majority of the ABI3 regulated genes in leaves. The fact that LEA4 and ABI3 are involved in both drought tolerance and seed desiccation tolerance shows that these processes share similar mechanisms.
Epigenetics involves the study of gene expression changes caused by modifications of DNA structure without alterations to the nucleotide sequence. The modifications include changes in the methylation state of DNA, modification of histones and the expression of non-coding RNA. Epigenetics could be a potential new source for trait variation applicable for plant breeding (Mirouze and Paszkowski, 2011). Epigenetic control over plant response to stress is a complex phenomenon. Epigenetic modifications not only occur during plant exposure to stress, but can also establish epigenetic changes, resulting in modified gene expression, that can persist over several generations (Boyko and Kovalchuk, 2008). Zhu et al. (2008) showed that an imperfection in the deacetylase-like protein HOS15 produced an abnormal reaction to ABA and abiotic stress. Saez et al. (2008) identified SWI3B (a homolog of a chromatin remodeling complex) as one of the targets of an ABA-related PP2C, HAB1. These observations suggest that ABA and/or abiotic stress signaling modifies gene expression profiles and developmental programs through the modification of epigenetic status to cope with the stresses (Hirayama and Shinozaki, 2010).
Application of transgenic and genome editing tools and technologies
Understanding the mechanisms of abiotic stress damage is critical for the development of tolerant plant species and varieties. Application of transgenic-based approaches could help to introduce desirable abiotic stress tolerance traits into crop varieties. Toward this end, several studies have used genes and TFs associated with abiotic stress tolerance as target genes in the application of biotechnological strategies to develop drought tolerant plants.
Despite the benefits of commercial genetically engineered (GEn) plants (National Academies of Sciences, 2017) and the promising results shown with numerous GEn prototypes addressing abiotic stresses in crops, the broader application of this technology remains a major challenge because of the negative public perception regarding the intentional introduction of genes into plants, particularly in Europe. It is a complex issue and the lack of acceptance of GEns can be for different reasons. It is not the intent of this review to provide an in-depth discussion on this topic. However, given the significant potential of improving heat and drought through GEn, it is relevant to review some aspects of the applications of this potential technology.
Phytohormones are potential targets for genetic manipulation to obtain abiotic stress tolerant crops. Overexpression of ABA-pathway-related TFs, imparts an ABA-hypersensitive response and also improves the osmotic stress tolerance in transgenic plants (Abe et al., 2003; Gao et al., 2011). Similarly, transgenic plants overexpressing RD26, a stress-inducible NAC TF, have also shown high ABA-sensitivity and thus an up-regulation of ABA and stress-responsive genes (Fujita et al., 2004). Under moderate drought stress, during the flowering period, the yields of transgenic canola overexpressing a farnesyltransferase protein were significantly higher comparatively to the control (Wang et al., 2005). The overexpression of TFs that control root architecture induced drought tolerance in rice and transgenic Arabidopsis plants by promoting root growth and thus enhancing WUE (Redillas et al., 2012; He et al., 2016). Other TFs linked to WUE, such as those stimulating wax deposition in cuticle and suberin deposition (Legay et al., 2016), and other regulators able to modulate entire pathways, could be used for the same objective to activate stress-response genes and enhance tolerance (Casaretto et al., 2016; Landi et al., 2017).
Engineering of the glyoxalase pathway has been reported to enhance tolerance to abiotic stress in different plant species. Upregulation of both glyoxalase I and glyoxalase II and their overexpression in plant species revealed enhanced tolerance to various abiotic stresses including salinity, drought, metal toxicity, and extreme temperature (Singla-Pareek et al., 2003, 2008; Bhomkar et al., 2008; Lin et al., 2010; Tuomainen et al., 2011; Wu et al., 2013; Alvarez-Gerding et al., 2015; Hasanuzzaman et al., 2017).
Table 1 shows examples of GEn applications with drought and/or heat tolerance improvement in wheat and maize compared to non-transformed plants. These observations confirm that the genes introduced into plants have a role in stress tolerance. Generally, the transformed lines compared to the controls show less transpiration, higher metabolism of ROs, increased production of protective molecules such as proline, increased root mass and improved photosynthesis rate. These changes contribute to a higher yield of the transformed plants under abiotic stress compared to the control plants. It is worth noting that with the exception of one case (Habben et al., 2014), all the examples shown in Table 1 involve overexpression of the introduced gene. Over time, we notice that genes from the same species are used in transformation in combination with a different promoter (cisgenic) rather than using a gene from a different species (transgenic). This may be done so that there is a better public acceptance of GEn technology.
Demonstration of the beneficial effect of the genetic transformation under field condition compared to greenhouse conditions provides stronger evidence for the possible economic benefits of the GEn technology. Several studies are limited to greenhouse trials because of the regulatory requirement and additional cost for the confined trail with GEn plants. Nevertheless, evidence for improved abiotic stress tolerance has been shown under field conditions in several cases (Bahieldin et al., 2005; Nelson et al., 2007; Castiglioni et al., 2008; Nuccio et al., 2015).
Thus by applying GEn technologies, there is significant potential to improve drought and heat tolerance in important crops like wheat and corn. However, because of the stringent regulations for field trials of GEn plants it will be difficult to fully validate these observations toward improving crop productivity. Genome Editing (GEd) technologies may provide an acceptable alternative to address this issue. The advantage of GEd is that genetic modifications achieved using this technology produce minor genome changes that are equivalent to what is considered non-GEn, for example mutations generated through chemical and physical agents that are widely used in plant breeding. In GEd technologies, the targeted DNA sequence modifications are achieved by application of sequence-specific nucleases that create double-strand breaks in the target genomic loci selected for editing. The main methods used for gene editing in plants are Zinc Finger (ZF) nucleases, transcription activator–like effector nucleases (TALENs) and clustered, regularly interspaced, short palindromic repeats (CRISPR) (Voytas, 2013). Various strategies of GEd could be used to improve agronomic traits; for example, introduction of a premature stop codon to disable protein function or changes to a gene promoter motif to modulate gene expression. Wang et al. (2014) used TALEN and CRISPR-Cas9 technologies in hexaploid bread wheat to introduce targeted mutations in the three homoeoalleles that encode MILDEW-RESISTANCE LOCUS (MLO) proteins. Alternatively, to increase gene expression, Piatek et al. (2015) used a synthetic transcriptional repressor and activators as endogenous TFs to activate transcription of an endogenous genomic target. A better understanding of the physiological response to heat and drought stresses and connection with other biological processes will assist in the design of the most efficient means for GEd of plants toward improved stress resistance.
Future perspectives/conclusions
Plants have evolved sophisticated adaptive mechanisms to withstand diverse and complex abiotic stresses. With the advent of new technologies such as genomics and genetic transformation, significant progress has been made in understanding these complex traits in higher plants. However, the commercial application of positive research outputs requires further validation of products or prototypes in the field. There are further opportunities for research discoveries from new emerging frontiers such as epigenetics, GEd and plant interactions with the soil microbiome.
For example, the area of soil microbiome research offers opportunities for improving biotic and abiotic stress in crops. Plant mechanisms to escape from drought and/or heat stresses can be mediated by the microbes surrounding a plant, particularly the roots, and are associated with various plant developmental stages, physiological cascades, and biochemical and molecular reactions occurring at the cellular, tissue, or whole plant level. Advances made with the application of novel molecular and genomic tools and techniques have opened research avenues into plant microbiota and these encouraging developments are allowing the exploration of the biological functions of a wide diversity of microorganisms both inside and outside of plant host tissues.
Significant advancements in crop genome characterization and the optimization of genome editing technology in crops have and will continue to advance our understanding and capabilities toward development of stress tolerant crops. Ultimately, genome editing or transgenic approaches need to be combined with efforts using conventional and marker-assisted breeding activities to achieve the desired improved varieties. Further, it is important to take into account climate change models, which differ geographically, to guide breeding programs in target trait identification for selection and identification of new adapted germplasm (Harrison et al., 2014). These efforts will lead to tangible practical outcomes that may help mitigate the effects of climate change, especially with respect to drought and heat stresses, and will contribute to improved crop productivity and food security, particularly in areas such as Africa.
Author contributions
FB coordinated the review and drafted the abstract, the introduction and conclusions. ML contributed with the physiological and biochemical sections and editing the references. MJ contributed with the microorganisms sections. FB and RD contributed with the genetics and genomics components. FB, ML, MJ, and RD contributed to the revision of the whole manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Jitao Zou, Sateesh Kagale, Diana Bekkaoui, Abdallah Oukarroum, and Allan Feurtado for their valuable and critical suggestions to the manuscript.
Glossary
Abbreviations
- ABA
Abscisic acid
- DREB
dehydration-responsive element-binding proteins
- DT
Desiccation tolerance
- GEd
Genome Editing
- GEn
Genetic Engineering
- LEA protein
late embryogenesis abundant protein
- ROS
reactive oxygen species
- TF
Transcription Factor
- WUE
Water Use Efficiency.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2018.00026/full#supplementary-material
References
- Abdel Latef A. A., Tran L. S. (2016). Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front. Plant Sci. 7:243. 10.3389/fpls.2016.00243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H., Urao T., Ito T., Seki M., Shinozaki K., Yamaguchi-Shinozaki K. (2003). Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15, 63–78. 10.1105/tpc.006130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn Y. J., Zimmerman J. (2006). Introduction of the carrot HSP17. 7 into potato (Solanum tuberosum L.) enhances cellular membrane stability and tuberization in vitro. Plant Cell Environ. 29, 95–104. 10.1111/j.1365-3040.2005.01403.x [DOI] [PubMed] [Google Scholar]
- Alet A. I., Sánchez D. H., Cuevas J. C., Marina M., Carrasco P., Altabella T., et al. (2012). New insights into the role of spermine in Arabidopsis thaliana under long-term salt stress. Plant Sci. 182, 94–100. 10.1016/j.plantsci.2011.03.013 [DOI] [PubMed] [Google Scholar]
- Alleva K., Niemietz C. M., Maurel C., Parisi M., Tyerman S. D., Amodeo G. (2006). Plasma membrane of Beta vulgaris storage root shows high water channel activity regulated by cytoplasmic pH and a dual range of calcium concentrations. J. Exp. Bot. 57, 609–621. 10.1093/jxb/erj046 [DOI] [PubMed] [Google Scholar]
- Alvarez-Gerding X., Cortés-Bullemore R., Medina C., Romero-Romero J. L., Inostroza-Blancheteau C., Aquea F., et al. (2015). Improved salinity tolerance in carrizo citrange rootstock through overexpression of glyoxalase system genes. Biomed. Res. Int. 2015, 1–7. 10.1155/2015/827951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbona V., Flors V., Jacas J., García-Agustín P., Gómez-Cadenas A. (2003). Enzymatic and non-enzymatic antioxidant responses of Carrizo citrange, a salt-sensitive citrus rootstock, to different levels of salinity. Plant Cell Physiol. 44, 388–394. 10.1093/pcp/pcg059 [DOI] [PubMed] [Google Scholar]
- Arbona V., Hossain Z., López-Climent M. F., Pérez-Clemente R. M., Gómez-Cadenas A. (2008). Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol. Plant. 132, 452–466. 10.1111/j.1399-3054.2007.01029.x [DOI] [PubMed] [Google Scholar]
- Arbona V., Manzi M., Ollas Cd., Gómez-Cadenas A. (2013). Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 14, 4885–4811. 10.3390/ijms14034885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca R., Ferrante A., Vernieri P., Chrispeels M. J. (2006). Drought, abscisic acid and transpiration rate effects on the regulation of PIP aquaporin gene expression and abundance in Phaseolus vulgaris plants. Ann. Bot. 98, 1301–1310. 10.1093/aob/mcl219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M., Harris P. J. C. (2013). Photosynthesis under stressful environments: an overview. Photosynthetica 51, 163–190. 10.1007/s11099-013-0021-6 [DOI] [Google Scholar]
- Badu-Apraku B., Yallou C. G. (2009). Registration of-resistant and drought-tolerant tropical early maize populations TZE-W Pop DT STR C and TZE-Y Pop DT STR C. J. Plant Registr. 3, 86–90. 10.3198/jpr2008.06.0356crg [DOI] [Google Scholar]
- Bahieldin A., Mahfouz H. T., Eissa H. F., Saleh O. M., Ramadan A. M., Ahmed I. A., et al. (2005). Field evaluation of transgenic wheat plants stably expressing the HVA1 gene for drought tolerance. Physiol. Plant. 123, 421–427. 10.1111/j.1399-3054.2005.00470.x [DOI] [Google Scholar]
- Barrett J., Campbell C. (2006). S-ABA: Developing a new tool for the big grower. Big Grower 1, 26–29. [Google Scholar]
- Bauer H., Ache P., Lautner S., Fromm J., Hartung W., Al-Rasheid Khaled K. A., et al. (2013). The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr. Biol. 23, 53–57. 10.1016/j.cub.2012.11.022 [DOI] [PubMed] [Google Scholar]
- Bhomkar P., Upadhyay C. P., Saxena M., Muthusamy A., Prakash N. S., Pooggin M., et al. (2008). Salt stress alleviation in transgenic Vigna mungo L. Hepper (blackgram) by overexpression of the glyoxalase I gene using a novel Cestrum yellow leaf curling virus (CmYLCV) promoter. Mol. Breed. 22, 169–181. 10.1007/s11032-008-9164-8 [DOI] [Google Scholar]
- Bita C. E., Gerats T. (2013). Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 4:273. 10.3389/fpls.2013.00273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard M. G., Newton L. A., Runkle E. S., Woodlard D., Campbell C. A. (2007). Exogenous applications of abscisic acid improved the postharvest drought tolerance of several annual bedding plants. Acta Hort. 755, 127–132. 10.17660/ActaHortic.2007.755.16 [DOI] [Google Scholar]
- Blum A. (2017). Osmotic adjustment is a prime drought stress adaptive engine in support of plant production: osmotic adjustment and plant production. Plant Cell Environ. 40, 4–10. 10.1111/pce.12800 [DOI] [PubMed] [Google Scholar]
- Boursiac Y., Boudet J., Postaire O., Luu D. T., Tournaire-Roux C., Maurel C. (2008). Stimulus-induced downregulation of root water transport involves reactive oxygen species-activated cell signalling and plasma membrane intrinsic protein internalization. Plant J. 56, 207–218. 10.1111/j.1365-313X.2008.03594.x [DOI] [PubMed] [Google Scholar]
- Boursiac Y., Chen S., Luu D. T., Sorieul M., van den Dries N., Maurel C. (2005). Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 139, 790–805. 10.1104/pp.105.065029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko A., Kovalchuk I. (2008). Epigenetic control of plant stress response. Environ. Mol. Mutagen. 49, 61–72. 10.1002/em.20347 [DOI] [PubMed] [Google Scholar]
- Cairns J. E., Sonder K., Zaidi P. H., Verhulst N., Mahuku G., Babu R., et al. (2012). Maize production in a changing climate: impacts, adaptation, and mitigation strategies. Adv. Agron. 114, 1–65. 10.1016/B978-0-12-394275-3.00006-7 [DOI] [Google Scholar]
- Camejo D., Rodríguez P., Morales M. A., Dell'Amico J. M., Torrecillas A., Alarcón J. J. (2005). High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 162, 281–289. 10.1016/j.jplph.2004.07.014 [DOI] [PubMed] [Google Scholar]
- Casaretto J. A., El-kereamy A., Zeng B., Stiegelmeyer S. M., Chen X., Bi Y. M., et al. (2016). Expression of OsMYB55 in maize activates stress-responsive genes and enhances heat and drought tolerance. BMC Genomics 17:312. 10.1186/s12864-016-2659-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglioni P., Warner D., Bensen R. J., Anstrom D. C., Harrison J., Stoecker M., et al. (2008). Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in maize under water-limited conditions. Plant Physiol. 147, 446–455. 10.1104/pp.108.118828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervin C., Tira-Umphon A., Terrier N., Zouine M., Severac D., Roustan J. P. (2008). Stimulation of the grape berry expansion by ethylene and effects on related gene transcripts, over the ripening phase. Physiol. Plant. 134, 534–546. 10.1111/j.1399-3054.2008.01158.x [DOI] [PubMed] [Google Scholar]
- Clark S. E. (2001). Cell signaling at the shoot meristem. Nat. Rev. Mol. Cell Biol. 2, 276–284. 10.1038/35067079 [DOI] [PubMed] [Google Scholar]
- Clavijo B. J., Venturini L., Schudoma C., Accinelli G. G., Kaithakottil G., Wright J., et al. (2017). An improved assembly and annotation of the allohexaploid wheat genome identifies complete families of agronomic genes and provides genomic evidence for chromosomal translocations. Genome Res. 27, 885–896. 10.1101/gr.217117.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales A. R., Carrillo L., Lasierra P., Nebauer S. G., Dominguez-Figueroa J., Renau-Morata B., et al. (2017). Multifaceted role of cycling Dof Factor 3 (CDF3) in the regulation of flowering time and abiotic stress responses in Arabidopsis. Plant Cell Environ. 40, 748–764. 10.1111/pce.12894 [DOI] [PubMed] [Google Scholar]
- Corrales A. R., Nebauer S. G., Carrillo L., Fernández-Nohales P., Marqués J., Renau Morata B., et al. (2014). Characterization of tomato Cycling Dof Factors reveals conserved and new functions in the control of flowering time and abiotic stress responses. J. Exp. Bot. 65, 995–1012. 10.1093/jxb/ert451 [DOI] [PubMed] [Google Scholar]
- Costa M. D., Artur M. A., Maia J., Jonkheer E., Derks M. F., Nijveen H., et al. (2017). A footprint of desiccation tolerance in the genome of Xerophyta viscosa. Nat. Plants 3:17038. 10.1038/nplants.2017.38 [DOI] [PubMed] [Google Scholar]
- Daryanto S., Wang L., Jacinthe P. A. (2016). Global synthesis of drought effects on maize and wheat production. PLoS ONE 11:e0156362. 10.1371/journal.pone.0156362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datla R., Ren M., Qiu S., Selvaraj G. (2014). Tor-interacting Proteins (TIPs) and Genes Therefor. U.S. Patent No. 8,704,042. Washington, DC: U.S. Patent and Trademark Office. [Google Scholar]
- De Boeck H. J., Bassin S., Verlinden M., Zeiter M., Hiltbrunner E. (2015). Simulated heat waves affected alpine grassland only in combination with drought. New Phytol. 209, 531–541. 10.1111/nph.13601 [DOI] [PubMed] [Google Scholar]
- De Campos M. K. F., de Carvalho K., de Souza F. S., Marur C. J., Pereira L. F. P., Filho J. C. B., et al. (2011). Drought tolerance and antioxidant enzymatic activity in transgenic “Swingle” citrumelo plants over-accumulating proline. Environ. Exp. Bot. 72, 242–250. 10.1016/j.envexpbot.2011.03.009 [DOI] [Google Scholar]
- Devasirvatham V., Gaur P. M., Mallikarjuna N., Tokachichu R. N., Trethowan R. M., Tan D. K. Y. (2012). Effect of high temperature on the reproductive development of chickpea genotypes under controlled environments. Funct. Plant Biol. 39, 1009–1018. 10.1071/FP12033 [DOI] [PubMed] [Google Scholar]
- Djanaguiraman M., Prasad P. V. V., Murugan M., Perumal R., Reddy U. K. (2014). Physiological differences among sorghum (Sorghum bicolor L. Moench) genotypes under high temperature stress. Environ. Exp. Bot. 100, 43–54. 10.1016/j.envexpbot.2013.11.013 [DOI] [Google Scholar]
- Djoukeng J. D., Arbona V., Argamasilla R., Gomez-cadenas A. (2008). Flavonoid profiling in leaves of citrus genotypes under different environmental situations flavonoid profiling in leaves of Citrus genotypes. J. Agric. Food Chem. 56, 11087–11097. 10.1021/jf802382y [DOI] [PubMed] [Google Scholar]
- Dreesen F. E., De Boeck H. J., Janssens I. A., Nijs I. (2012). Summer heat and drought extremes trigger unexpected changes in productivity of a temperate annual/biannual plant community. Environ. Exp. Bot. 79, 21–30. 10.1016/j.envexpbot.2012.01.005 [DOI] [Google Scholar]
- Dwivedi R., Prasad S., Jaiswal B., Kumar A., Tiwari A., Patel S., et al. (2017). Evaluation of wheat genotypes (Triticum aestivum L.) at grain filling stage for heat tolerance. Int. J. Pure App. Biosci. 5, 971–975. 10.18782/2320-7051.2614 [DOI] [Google Scholar]
- Ellsworth P. Z., Cousins A. B. (2016). Carbon isotopes and water use efficiency in C4 plants. Curr. Opin. Plant Biol. 31, 155–161. 10.1016/j.pbi.2016.04.006 [DOI] [PubMed] [Google Scholar]
- Ende W. V., Peshev D. (2013). Sugars as antioxidants in plants, in Crop Improvement Under Adverse Condition, eds Tuteja N., Gill S. (New York, NY: Springer; ), 285–307. [Google Scholar]
- Epule E. T., Peng C., Lepage L., Chen Z. (2013). The causes, effects and challenges of Sahelian droughts: a critical review. Reg. Environ. Change 14, 145–156. 10.1007/s10113-013-0473-z [DOI] [Google Scholar]
- Farooq M., Bramley H., Palta J. A., Siddique K. H. M. (2011). Heat stress in wheat during reproductive and grain filling phases. Crit. Rev. Plant Sci. 30, 491–507. 10.1080/07352689.2011.615687 [DOI] [Google Scholar]
- Farooq M., Gogoi N., Barthakur S., Baroowa B., Bharadwaj N., Alghamdi S. S., et al. (2017). Drought stress in grain legumes during reproduction and grain filling. J. Agron. Crop Sci. 203, 81–102. 10.1111/jac.12169 [DOI] [Google Scholar]
- Fedoroff N. V. (2002). Cross-talk in abscisic acid signaling. Sci. STKE 140:re10 10.1126/stke.2002.140.re10 [DOI] [PubMed] [Google Scholar]
- Ferdous J., Hussain S. S., Shi B. J. (2015). Role of microRNAs in plant drought tolerance. Plant Biotechnol. J. 13, 293–305. 10.1111/pbi.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser C. M., Chapple C. (2011). The phenylpropanoid pathway in Arabidopsis. Arab. Book 9:e0152. 10.1199/tab.0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Momčilović I., Clemente T. E., Nersesian N., Trick H. N., Ristic Z. (2008). Heterologous expression of a plastid EF-Tu reduces protein thermal aggregation and enhances CO2 fixation in wheat (Triticum aestivum) following heat stress. Plant Mol. Biol. 68, 277–288. 10.1007/s11103-008-9369-6 [DOI] [PubMed] [Google Scholar]
- Fujii H., Verslues P. E., Zhu J. K. (2011). Arabidopsis decuple mutant reveals the importance of SnRK2 kinases in osmotic stress responses in vivo. Proc. Natl. Acad. Sci. U.S.A. 108, 1717–1722. 10.1073/pnas.1018367108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Fujita Y., Maruyama K., Seki M., Hiratsu K., Ohme-Takagi M., et al. (2004). A dehydration-induced NAC protein, RD26, is involved in a novel ABA dependent stress-signaling pathway. Plant J. 39, 863–876. 10.1111/j.1365-313X.2004.02171.x [DOI] [PubMed] [Google Scholar]
- Fujita M., Fujita Y., Noutoshi Y., Takahashi F., Narusaka Y., Yamaguchi-Shinozaki K., et al. (2006). Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 9, 436–442. 10.1016/j.pbi.2006.05.014 [DOI] [PubMed] [Google Scholar]
- Gao J. J., Zhang Z., Peng R. H., Xiong A. S., Xu J., Zhu B., et al. (2011). Forced expression of Mdmyb10, a myb transcription factor gene from apple, enhances tolerance to osmotic stress in transgenic Arabidopsis. Mol. Biol. Rep. 38, 205–211. 10.1007/s11033-010-0096-0 [DOI] [PubMed] [Google Scholar]
- Gautam P., Lal B., Tripathi R., Shahid M., Baig M. J., Raja R., et al. (2016). Role of silica and nitrogen interaction in submergence tolerance of rice. Environ. Exp. Bot. 125, 98–109. 10.1016/j.envexpbot.2016.02.008 [DOI] [Google Scholar]
- Gerbeau P., Amodeo G., Henzler T., Santoni V., Ripoche P., Maurel C. (2002). The water permeability of Arabidopsis plasma membrane is regulated by divalent cations and pH. Plant J. 30, 71–81. 10.1046/j.1365-313X.2002.01268.x [DOI] [PubMed] [Google Scholar]
- Giarola V., Hou Q., Bartels D. (2017). Angiosperm plant desiccation tolerance: hints from transcriptomics and genome sequencing. Trends Plant Sci. 22, 705–717. 10.1016/j.tplants.2017.05.007 [DOI] [PubMed] [Google Scholar]
- Gill S. S., Tuteja N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. 10.1016/j.plaphy.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Giorno F., Wolters-Arts M., Mariani C., Rieu I. (2013). Ensuring reproduction at high temperatures: the heat stress response during anther and pollen development. Plants 2, 489–506. 10.3390/plants2030489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H., Zhu X., Chen K., Wang S., Zhang C. (2005). Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 169, 313–321. 10.1016/j.plantsci.2005.02.023 [DOI] [Google Scholar]
- Goufo P., Moutinho-Pereira J. M., Jorge T. F., Correia C. M., Oliveira M. R., Rosa E. A. S., et al. (2017). Cowpea (Vigna unguiculata L. Walp.) metabolomics: osmoprotection as a physiological strategy for drought stress resistance and improved yield. Front. Plant Sci. 8:586. 10.3389/fpls.2017.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M. R., Jones J. D. G. (2009). Hormone (dis)harmony molds plant health and disease. Science 324, 750–752. 10.1126/science.1173771 [DOI] [PubMed] [Google Scholar]
- Gray S. B., Dermody O., Klein S. P., Locke A. M., McGrath J. M., Paul R. E., et al. (2016). Intensifying drought eliminates the expected benefits of elevated carbon dioxide for soybean. Nat. Plants 2:16132. 10.1038/nplants.2016.132 [DOI] [PubMed] [Google Scholar]
- Habben J. E., Bao X., Bate N. J., DeBruin J. L., Dolan D., Hasegawa D., et al. (2014). Transgenic alteration of ethylene biosynthesis increases grain yield in maize under field drought-stress conditions. Plant Biotechnol. J. 12, 685–693. 10.1111/pbi.12172 [DOI] [PubMed] [Google Scholar]
- Haley S. D., Johnson J. J., Peairs F. B., Quick J. S., Stromberger J. A., Clayshulte S. R., et al. (2007). Registration of “Ripper” wheat. J. Plant Reg. 1, 1–6. 10.3198/jpr2006.10.0689crc [DOI] [Google Scholar]
- Harper J. F., Breton G., Harmon A. (2004). Decoding Ca2+ signals through plant protein kinases. Annu. Rev. Plant Biol. 55, 263–288. 10.1146/annurev.arplant.55.031903.141627 [DOI] [PubMed] [Google Scholar]
- Harrison M. T., Tardieu F., Dong Z., Messina C. D., Hammer G. L. (2014). Characterizing drought stress and trait influence on maize yield under current and future conditions. Glob. Chang. Biol. 20, 867–878. 10.1111/gcb.12381 [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M., Nahar K., Hossain M. S., Mahmud J. A., Rahman A., Inafuku M., et al. (2017). Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int. J. Mol. Sci. 18:200. 10.3390/ijms18010200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G. H., Xu Y. J., Wang X. Y., Liu M. J., Li S. P., Chen M., et al. (2016). Drought-responsive WRKY transcription factor genes TaWRKY1 and TaWRKY33 from wheat confer drought and/or heat resistance in Arabidopsis. BMC Plant Biol. 16:116 10.1186/s12870-016-0806-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z. H., Fujiki M., Kohorn B. D. (1996). A cell wall-associated, receptor-like protein kinase. J. Biol. Chem. 271, 19789–19793. [DOI] [PubMed] [Google Scholar]
- Hedhly A. (2011). Sensitivity of flowering plant gametophytes to temperature fluctuations. Environ. Exp. Bot. 74, 9–16. 10.1016/j.envexpbot.2011.03.016 [DOI] [Google Scholar]
- Hirayama T., Shinozaki K. (2010). Research on plant abiotic stress responses in the post-genome era: past, present and future. Plant J. 61, 1041–1052. 10.1111/j.1365-313X.2010.04124.x [DOI] [PubMed] [Google Scholar]
- Hoque M. A., Uraji M., Banu M. N., Torii A., Mori I. C., Nakamura Y. (2012). Methylglyoxal inhibition of cytosolic ascorbate peroxidase from Nicotiana tabacum. J. Biochem. Mol. Toxicol. 26, 315–321. 10.1002/jbt.21423 [DOI] [PubMed] [Google Scholar]
- Howarth C. J. (2005). Genetic improvements of tolerance to high temperature, in Abiotic Stresses: Plant Resistance Through Breeding and Molecular Approaches, eds Ashraf M., Harris P. J. C. (New York, NY: Howarth Press Inc.), 277–300. [Google Scholar]
- Huang D., Feurtado J. A., Smith M. A., Flatman L. K., Koh C., Cutler A. J. (2017). Long noncoding miRNA gene represses wheat β-diketone waxes. Proc. Natl. Acad. Sci. U.S.A. 114, E3149–E3158. 10.1073/pnas.1617483114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D., Wu W., Abrams S. R., Cutler A. J. (2008). The relationship of drought-related gene expression in Arabidopsis thaliana to hormonal and environmental factors. J. Exp. Bot. 59, 2991–3007. 10.1093/jxb/ern155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A. E., Bauerle T. L. (2016). Long-distance plant signaling pathways in response multiple stressors: the gap in knowledge. J. Exp. Bot. 67, 2063–2079. 10.1093/jxb/erw099 [DOI] [PubMed] [Google Scholar]
- IPCC (2014). Summary for policymakers, in Climate Change 2014: impacts, adaptation, and vulnerability, in Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds Field C. B., Barros V. R., Dokken D. J., Mach K. J., Mastrandrea M. D., Bilir T. E., et al. (Cambridge; New York, NY: Cambridge University Press; ), 1–32. [Google Scholar]
- Janská A., Marsík P., Zelenková S., Ovesná J. (2010). Cold stress and acclimation - What is important for metabolic adjustment? Plant Biol. 12, 395–405. 10.1111/j.1438-8677.2009.00299.x [DOI] [PubMed] [Google Scholar]
- Johansson I., Karlsson M., Shukla V. K., Chrispeels M. J., Larsson C., Kjellbom P. (1998). Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell 10, 451–459. 10.2307/3870601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra N., Chakraborty D., Sharma A., Rai H. K., Jolly M., Chander S., et al. (2008). Effect of increasing temperature on yield of some winter crops in northwest India. Curr. Sci. 94, 82–88. [Google Scholar]
- Kantar M., Lucas S. J., Budak H. (2011). miRNA expression patterns of Triticum dicoccoides in response to shock drought stress. Planta 233, 471–484. 10.1007/s00425-010-1309-4 [DOI] [PubMed] [Google Scholar]
- Kaplan F., Guy C. L. (2004). β-Amylase induction and the protective role of maltose during temperature shock. Plant Physiol. 135, 1674–1684. 10.1104/pp.104.040808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N., Awasthi R., Gupta K., Gaur P., Siddique K. H., Nayyar H. (2013). Heat-stress-induced reproductive failures in chickpea (Cicer arietinum) are associated with impaired sucrose metabolism in leaves and anthers. Funct. Plant Biol. 40, 1334–1349. 10.1071/FP13082 [DOI] [PubMed] [Google Scholar]
- Keunen E., Peshev D., Vangronsveld J., Van Den Ende W., Cuypers A. (2013). Plant sugars are crucial players in the oxidative challenge during abiotic stress: extending the traditional concept. Plant Cell Environ. 36, 1242–1255. 10.1111/pce.12061 [DOI] [PubMed] [Google Scholar]
- Khan M. S., Kanwal B., Nazir S. (2015). Metabolic engineering of the chloroplast genome reveals that the yeast ArDH gene confers enhanced tolerance to salinity and drought in plants. Front. Plant Sci. 6:725. 10.3389/fpls.2015.00725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., van Iersel M. (2008). ABA drenches induce stomatal closure and prolong shelf life of Salvia splendens. SNA Res. Conf. 53, 107–111. [Google Scholar]
- Kirakosyan A., Kaufman P., Warber S., Zick S., Aaronson K., Bolling S., et al. (2004). Applied environmental stresses to enhance the levels of polyphenolics in leaves of hawthorn plants. Physiol. Plant. 121, 182–186. 10.1111/j.1399-3054.2004.00332.x [DOI] [PubMed] [Google Scholar]
- Kohler B., Blatt M. R. (2002). Protein phosphorylation activates the guard cell Ca2+ channel and is a prerequisite for gating by abscisic acid. Plant J. 32, 185–194. 10.1046/j.1365-313X.2002.01414.x [DOI] [PubMed] [Google Scholar]
- Kozłowska M., Rybus-Zajac M., Stachowiak J., Janowska B. (2007). Changes in carbohydrate contents of Zantedeschia leaves under gibberellin-stimulated flowering. Acta Physiol. Plant. 29, 27–32. 10.1007/s11738-006-0004-3 [DOI] [Google Scholar]
- Król A. (2013). The Growth and Water Uptake by Yellow Seed and Black Seed Rape Depending on the State of Soil Compaction. [dissertation]. Lublin: Bohdan Dobrzañski Institute of Agrophysics PAS. [Google Scholar]
- Kromdijk J., Głowacka K., Leonelli L., Gabilly S. T., Iwai M., Niyogi K. K., et al. (2016). Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354, 857–861. 10.1126/science.aai8878 [DOI] [PubMed] [Google Scholar]
- Kudla J., Batistic O., Hashimoto K. (2010). Calcium signals: the lead currency of plant information processing. Plant Cell 22, 541–563. 10.1105/tpc.109.072686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoyarova G., Veselova S., Hartung W., Farhutdinov R., Veselov D., Sharipova G. (2011). Involvement of root ABA and hydraulic conductivity in the control of water relations in wheat plants exposed to increased evaporative demand. Planta 233, 87–94. 10.1007/s00425-010-1286-7 [DOI] [PubMed] [Google Scholar]
- Kulkarni M., Soolanayakanahally R., Ogawa S., Uga Y., Selvaraj M. G., Kagale S. (2017). Drought response in wheat: key genes and regulatory mechanisms controlling root system architecture and transpiration efficiency. Front. Chem. 5:106. 10.3389/fchem.2017.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi S., Hausman J. F., Guerriero G., Esposito S. (2017). Poaceae vs. abiotic stress: focus on drought and salt stress, recent insights and perspectives. Front. Plant Sci. 8:1214. 10.3389/fpls.2017.01214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lata C., Prasad M. (2011). Role of DREBs in regulation of abiotic stress responses in plants. J. Exp. Bot. 62, 4731–4748. 10.1093/jxb/err210 [DOI] [PubMed] [Google Scholar]
- Lee S. B., Suh M. C. (2013). Recent advances in cuticular wax biosynthesis and its regulation in Arabidopsis. Mol. Plant. 6, 246–249. 10.1093/mp/sss159 [DOI] [PubMed] [Google Scholar]
- Legay S., Guerriero G., André C., Guignard C., Cocco E., Charton S., et al. (2016). MdMyb93 is a regulator of suberin deposition in russeted apple fruit skins. New Phytol. 212, 977–991. 10.1111/nph.14170 [DOI] [PubMed] [Google Scholar]
- Li L., Zheng M., Deng G., Liang J., Zhang H., Pan Z., et al. (2016). Overexpression of AtHDG11 enhanced drought tolerance in wheat (Triticum aestivum L.). Mol. Breed. 36:23 10.1007/s11032-016-0447-1 [DOI] [Google Scholar]
- Lin F., Xu J., Shi J., Li H., Li B. (2010). Molecular cloning and characterization of a novel glyoxalase I gene TaGly I in wheat (Triticum aestivum L.). Mol. Biol. Rep. 37, 729–735. 10.1007/s11033-009-9578-3 [DOI] [PubMed] [Google Scholar]
- Lipiec J., Doussan C., Nosalewicz A., Kondracka K. (2013). Effect of drought and heat stresses on plant growth and yield: a review. Int. Agrophys. 27, 463–477. 10.2478/intag-2013-0017 [DOI] [Google Scholar]
- Liu X., Zhai S., Zhao Y., Sun B., Liu C., Yang A., et al. (2013). Overexpression of the phosphatidylinositol synthase gene (ZmPIS) conferring drought stress tolerance by altering membrane lipid composition and increasing ABA synthesis in maize. Plant Cell Environ. 36, 1037–1055. 10.1111/pce.12040 [DOI] [PubMed] [Google Scholar]
- Loveys B. R., Scheurwater I., Pons T. L., Fitter A. H., Atkin O. K. (2002). Growth temperature influences the underlying components of relative growth rate: an investigation using inherently fast- and slow-growing plant species. Plant Cell Environ. 25, 975–987. 10.1046/j.1365-3040.2002.00879.x [DOI] [Google Scholar]
- Lu Y., Li Y., Zhang J., Xiao Y., Yue Y., Duan L., et al. (2013). Overexpression of Arabidopsis molybdenum cofactor sulfurase gene confers drought tolerance in maize (Zea mays L). PLoS ONE 8:e52126. 10.1371/journalpone0052126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A. A., Romeis T., Jones J. D. G. (2004). CDPK-mediated signaling pathways: specificity and cross-talk. J. Exp. Bot. 55, 181–188. 10.1093/jxb/erh008 [DOI] [PubMed] [Google Scholar]
- Luo M. C., Gu Y. Q., Puiu D., Wang H., Twardziok S. O., Deal K. R., et al. (2017). Genome sequence of the progenitor of the wheat D genome Aegilops tauschii. Nature 551, 498–502. 10.1038/nature24486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luomala E. M., Laitinen K., Sutinen S., Kellomaki S., Vapaavuori E. (2005). Stomatal density, anatomy and nutrient concentrations of Scots pine needles are affected by elevated CO2 and temperature. Plant Cell Environ. 28, 733–749. 10.1111/j.1365-3040.2005.01319.x [DOI] [Google Scholar]
- Ma D., Sun D., Wang C., Qin H., Ding H., Li Y., et al. (2016). Silicon application alleviates drought stress in wheat through transcriptional regulation of multiple antioxidant defense pathways. J. Plant Growth Regul. 35, 1–10. 10.1007/s00344-015-9500-2 [DOI] [Google Scholar]
- Maurel C., Verdoucq L., Luu D. T., Santoni V. (2008). Plant aquaporins: membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 59, 595–624. 10.1146/annurev.arplant.59.032607.092734 [DOI] [PubMed] [Google Scholar]
- McCubbin A. G., Kao T. (2000). Molecular recognition and response in pollen and pistil interactions. Annu. Rev. Cell Dev. Biol. 16, 333–364. 10.1146/annurev.cellbio.16.1.333 [DOI] [PubMed] [Google Scholar]
- Merchuk-Ovnat L., Barak V., Fahima T., Ordon F., Lidzbarsky G. A., Krugman T., et al. (2016). Ancestral QTL alleles from wild emmer wheat improve drought resistance and productivity in modern wheat cultivars. Front. Plant Sci. 7:452. 10.3389/fpls.2016.00452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir R. R., Zaman-Allah M., Sreenivasulu N., Trethowan R., Varshney R. K. (2012). Integrated genomics, physiology and breeding approaches for improving drought tolerance in crops. Theor. Appl. Genet. 125, 625–645. 10.1007/s00122-012-1904-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirouze M., Paszkowski J. (2011). Epigenetic contribution to stress adaptation in plants. Curr. Opin. Plant Biol. 14, 267–274. 10.1016/j.pbi.2011.03.004 [DOI] [PubMed] [Google Scholar]
- Mittler R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410. 10.1016/S1360-1385(02)02312-9 [DOI] [PubMed] [Google Scholar]
- Mittler R., Blumwald E. (2015). The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 27, 64–70. 10.1105/tpc.114.133090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro J. A., Nell T. A., Barrett J. E. (2001). Postproduction of potted miniature rose: flower respiration and single flower longevity. J. Amer. Soc. Hort. Sci. 126, 134–139. [Google Scholar]
- Morales C. G., Pino M. T., del Pozo A. (2013). Phenological and physiological responses to drought stress and subsequent rehydration cycles in two raspberry cultivars. Sci. Hortic. 162, 234–241. 10.1016/j.scienta.2013.07.025 [DOI] [Google Scholar]
- Nakashima K., Takasaki H., Mizoi J., Shinozaki K., Yamaguchi-Shinozaki K. (2012). NAC transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 1819, 97–103. 10.1016/j.bbagrm.2011.10.005 [DOI] [PubMed] [Google Scholar]
- Nakashima K., Yamaguchi-Shinozaki K. (2013). ABA signaling in stress-response and seed development. Plant Cell Rep. 32, 959–970. 10.1007/s00299-013-1418-1 [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. (2017). Genetically Engineered Crops: Experiences and Prospects. Washington, DC: National Academies Press; 10.17226/23395 [DOI] [PubMed] [Google Scholar]
- Nelson D. E., Repetti P. P., Adams T. R., Creelman R. A., Wu J., Warner D. C., et al. (2007). Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc. Natl. Acad. Sci. U.S.A. 104, 16450–16455. 10.1073/pnas.0707193104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü. (2015). Uncovering the hidden facets of drought stress: secondary metabolites make the difference. Tree Physiol. 36, 129–132. 10.1093/treephys/tpv128 [DOI] [PubMed] [Google Scholar]
- Nuccio M. L., Wu J., Mowers R., Zhou H. P., Meghji M., Primavesi L. F., et al. (2015). Expression of trehalose-6-phosphate phosphatase in maize ears improves yield in well-watered and drought conditions. Nat. Biotechnol. 33, 862–869. 10.1038/nbt.3277 [DOI] [PubMed] [Google Scholar]
- Oceanic Atmospheric Administration website (2015). Available online at: https://www.ncdc.noaa.gov/billions/events/US/1980-2017
- Ort D. R., Merchant S. S., Alric J., Barkan A., Blankenship R. E., Bock R., et al. (2015). Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. U.S.A. 112, 8529–8536. 10.1073/pnas.1424031112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek T., Zazímalová E., Ruthardt N., Petrásek J. (2005). Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435, 1251–1256. 10.1038/nature03633 [DOI] [PubMed] [Google Scholar]
- Parent B., Turc O., Gibon Y., Stitt M., Tardieu F. (2010). Modelling temperature-compensated physiological rates, based on the co-ordination of responses to temperature of developmental processes. J. Exp. Bot. 61, 2057–2069. 10.1093/jxb/erq003 [DOI] [PubMed] [Google Scholar]
- Parmar N., Singh K. H., Sharma D., Singh L., Kumar P., Nanjundan J., et al. (2017). Genetic engineering strategies for biotic and abiotic stress tolerance and quality enhancement in horticultural crops: a comprehensive review. 3 Biotech. 7:239. 10.1007/s13205-017-0870-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrineschi A., Reynolds M., Pacheco M., Brito R. M., Almeraya R., Yamaguchi-Shinozaki K., et al. (2004). Stress-induced expression in wheat of the Arabidopsis thaliana DREB1A gene delays water stress symptoms under greenhouse conditions. Genome 47, 493–500. 10.1139/g03-140 [DOI] [PubMed] [Google Scholar]
- Perego A., Sanna M., Giussani A., Chiodini M. E., Fumagalli M., Pilu S. R., et al. (2014). Designing a high-yielding maize ideotype for a changing climate in Lombardy plain (northern Italy). Sci. Total Environ. 499, 497–509. 10.1016/j.scitotenv.2014.05.092 [DOI] [PubMed] [Google Scholar]
- Péret B., Li G., Zhao J., Band L. R., Voß U., Postaire O., et al. (2012). Auxin regulates aquaporin function to facilitate lateral root emergence. Nat. Cell Biol. 14, 991–998. 10.1038/ncb2573 [DOI] [PubMed] [Google Scholar]
- Piatek A., Ali Z., Baazim H., Li L., Abulfaraj A., Al-Shareef S., et al. (2015). RNA-guided transcriptional regulation in planta via synthetic dCas9-based transcription factors. Plant Biotechnol. J. 13, 578–589. 10.1111/pbi.12284 [DOI] [PubMed] [Google Scholar]
- Pieterse C. M., Leon-Reyes A., Van der Ent S., Van Wees S. C. (2009). Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 5, 308–316. 10.1038/nchembio.164 [DOI] [PubMed] [Google Scholar]
- Poorter H., Niinemets U., Poorter L., Wright I. J., Villar R. (2009). Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol. 182, 565–588. 10.1111/j.1469-8137.2009.02830.x [DOI] [PubMed] [Google Scholar]
- Pornsiriwong W., Estavillo G. M., Chan K. X., Tee E. E., Ganguly D., Crisp P. A., et al. (2017). A chloroplast retrograde signal, 3”-phosphoadenosine 5”-phosphate, acts as a secondary messenger in abscisic acid signaling in stomatal closure and germination. eLife 6:e23361 10.7554/eLife.23361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N., Xu W., Hu L., Li Y., Wang H., Qi X., et al. (2016). Drought tolerance and proteomics studies of transgenic wheat containing the maize C4 phosphoenolpyruvate carboxylase (PEPC) gene. Protoplasma 253, 1503–1512. 10.1007/s00709-015-0906-2 [DOI] [PubMed] [Google Scholar]
- Quan R., Shang M., Zhang H., Zhao Y., Zhang J. (2004). Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant Biotechnol. J. 2, 477–486. 10.1111/j.1467-7652.2004.00093.x [DOI] [PubMed] [Google Scholar]
- Rahnama A., Poustini K., Tavakkol-Afshari R., Tavakoli A. (2010). Growth and stomatal responses of bread wheat genotypes in tolerance to salt stress. Int. J. Biol. Life Sci. 6, 216–221. [Google Scholar]
- Reddy A. S., Ali G. S., Celesnik H., Day I. S. (2011). Coping with stresses: roles of calciumand calcium/calmodulin-regulated gene expression. Plant Cell 23, 2010–2032. 10.1105/tpc.111.084988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redillas M. C., Jeong J. S., Kim Y. S., Jung H., Bang S. W., Choi Y. D., et al. (2012). The overexpression of OsNAC9 alters the root architecture of rice plants enhancing drought resistance and grain yield under field conditions. Plant Biotechnol. J. 10, 792–805. 10.1111/j.1467-7652.2012.00697.x [DOI] [PubMed] [Google Scholar]
- Rexroth S., Mullineaux C. W., Ellinger D., Sendtko E., Rögner M., Koenig F. (2011). The plasma membrane of the cyanobacterium Gloeobacter violaceus contains segregated bioenergetic domains. Plant Cell 23, 2379–2390. 10.1105/tpc.111.085779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds M., Tuberosa R. (2008). Translational research impacting on crop productivity in drought-prone environments. Curr. Opin. Plant Biol. 11, 171–179. 10.1016/j.pbi.2008.02.005 [DOI] [PubMed] [Google Scholar]
- Rodrigues O., Reshetnyak G., Grondin A., Saijo Y., Leonhardt N., Maurel C., et al. (2017). Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA-and pathogen-triggered stomatal closure. Proc. Natl. Acad. Sci. U.S.A. 114, 9200–9205. 10.1073/pnas.1704754114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald P. C. (1998). Resistance gene evolution. Curr. Opin. Plant Biol. 1, 294–298. 10.1016/1369-5266(88)80049-9 [DOI] [PubMed] [Google Scholar]
- Ruggiero A., Punzo P., Landi S., Costa A., Van Ooosten M., Grillo S. (2017). Improving plant water use efficiency through molecular genetics. Horticulturae 3:31 10.3390/horticulturae3020031 [DOI] [Google Scholar]
- Saad A. S., Li X., Li H. P., Huang T., Gao C. S., Guo M. W., et al. (2013). A rice stress-responsive NAC gene enhances tolerance of transgenic wheat to drought and salt stresses. Plant Sci. 203, 33–40. 10.1016/j.plantsci.2012.12.016 [DOI] [PubMed] [Google Scholar]
- Saez A., Rodrigues A., Santiago J., Rubio S., Rodriguez P. L. (2008). HAB1–SWI3B interaction reveals a link between abscisic acid signaling and putative SWI/SNF chromatin-remodeling complexes in Arabidopsis. Plant Cell 20, 2972–2988. 10.1105/tpc.107.056705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah S. K., Reddy K. R., Li J. (2016). Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 7:571. 10.3389/fpls.2016.00571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saibo N. J., Lourenço T., Oliveira M. M. (2009). Transcription factors and regulation of photosynthetic and related metabolism under environmental stresses. Ann. Bot. 103, 609–623. 10.1093/aob/mcn227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto A., Murata N. (2002). The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ. 25, 163–171. 10.1046/j.0016-8025.2001.00790.x [DOI] [PubMed] [Google Scholar]
- Schachtman D. P., Goodger J. Q. (2008). Chemical root to shoot signaling under drought. Trends Plant Sci. 13, 281–287. 10.1016/j.tplants.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Schieber M., Chandel N. S. (2014). ROS Function in redox signaling and oxidative stress. Curr. Biol. 24, 453–462. 10.1016/j.cub.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher K., Chory J. (2000). Brassinosteroid signal transduction: still casting the actors. Curr. Opin. Plant Biol. 3, 79–84. 10.1016/S1369-5266(99)00038-2 [DOI] [PubMed] [Google Scholar]
- Sessa G., Martin G. B. (2000). Signal recognition and transduction mediated by the tomato Pto kinase: a paradigm of innate immunity in plants. Microbes Infect. 2, 1591–1597. 10.1016/S1286-4579(00)01315-0 [DOI] [PubMed] [Google Scholar]
- Shahinnia F., Le Roy J., Laborde B., Sznajder B., Kalambettu P., Mahjourimajd S., et al. (2016). Genetic association of stomatal traits and yield in wheat grown in low rainfall environments. BMC Plant Biol. 16:150. 10.1186/s12870-016-0838-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam S., Kjaer K. H., Ottosen C. O., Rosenqvist E., Sharma D. K., Wollenweber B. (2013). The alleviating effect of elevated CO2 on heat stress susceptibility of two wheat (Triticum aestivum L.) cultivars. J. Agron. Crop Sci. 199, 340–350. 10.1111/jac.12023 [DOI] [Google Scholar]
- Sharma N., Waterer D. R., Abrams S. R. (2006). Evaluation of abscisic acid analogs as holding agents for bedding plant seedlings. Hort. Technol. 16, 71–77. [Google Scholar]
- Shi J., Gao H., Wang H., Lafitte H. R., Archibald R. L., Yang M., et al. (2017). ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol. J. 15, 207–216. 10.1111/pbi.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Habben J. E., Archibald R. L., Drummond B. J., Chamberlin M. A., Williams R. W., et al. (2015). Overexpression of ARGOS genes modifies plant sensitivity to ethylene, leading to improved drought tolerance in both Arabidopsis and maize. Plant Physiol. 169, 266–282. 10.1104/pp.15.00780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Yamaguchi-Shinozaki K., Seki M. (2003). Regulatory network of gene expression in the drought and cold stress responses. Curr. Opin. Plant Biol. 6, 410–417. 10.1016/S1369-5266(03)00092-X [DOI] [PubMed] [Google Scholar]
- Shou H., Bordallo P., Wang K. (2004). Expression of the Nicotiana protein kinase (NPK1) enhanced drought tolerance in transgenic maize. J. Exp. Bot. 55, 1013–1019. 10.1093/jxb/erh129 [DOI] [PubMed] [Google Scholar]
- Shulaev V., Cortes D., Miller G., Mittler R. (2008). Metabolomics for plant stress response. Physiol. Plant. 132, 199–208. 10.1111/j.1399-3054.2007.01025.x [DOI] [PubMed] [Google Scholar]
- Sicher R. C., Timlin D., Bailey B. (2012). Responses of growth and primary metabolism of water-stressed barley roots to rehydration. J. Plant Physiol. 169, 686–695. 10.1016/j.jplph.2012.01.002 [DOI] [PubMed] [Google Scholar]
- Sikuku P. A., Netondo G. W., Onyango J. C., Musyimi D. M. (2010). Chlorophyll fluorescence, protein and chlorophyll content of three NERICA rainfed rice varieties under varying irrigation regimes. ARPN J. Agr. Biol. Sci. 5, 19–25. [Google Scholar]
- Sinay H., Karuwal R. S. (2014). Proline and total soluble sugar content at the vegetative phase of six corn cultivars from Kisar island Maluku, grown under drought stress conditions. Int. J. Adv. Agri. Res. 2, 77–82. [Google Scholar]
- Singh V., Nguyen C. T., Yang Z., Chapman C., van Oosterom E. J., Hammer G. L. (2016). Genotypic differences in effects of short episodes of high-temperature stress during reproductive development in sorghum. Crop Sci. 56, 1561–1572. 10.2135/cropsci2015.09.0545 [DOI] [Google Scholar]
- Singla-Pareek S. L., Reddy M. K., Sopory S. K. (2003). Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance. Proc. Natl. Acad. Sci. U.S.A. 100, 14672–14677. 10.1073/pnas.2034667100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla-Pareek S. L., Yadav S. K., Pareek A., Reddy M. K., Sopory S. K. (2008). Enhancing salt tolerance in a crop plant by overexpression of glyoxalase II. Transgenic Res. 17, 171–180. 10.1007/s11248-007-9082-2 [DOI] [PubMed] [Google Scholar]
- Sivamani E., Bahieldin A., Wraith J. M., Al-Niemi T., Dyer W. E., Ho T. D., et al. (2000). Improved biomass productivity and water use efficiency under water deficit conditions in transgenic wheat constitutively expressing the barley HVA1 gene. Plant Sci. 155, 1–9. 10.1016/S0168-9452(99)00247-2 [DOI] [PubMed] [Google Scholar]
- Stocker T. F., Qin D., Plattner G. K., Alexander L. V., Allen S. K., Bindoff N. L., et al. (2013). Technical summary, in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge: University Press; ), 33–115. [Google Scholar]
- Su J., Hirji R., Zhang L., He C., Selvaraj G., Wu R. (2006). Evaluation of the stress-inducible production of choline oxidase in transgenic rice as a strategy for producing the stress-protectant glycine betaine. J. Exp. Bot. 57, 1129–1135. 10.1093/jxb/erj133 [DOI] [PubMed] [Google Scholar]
- Suzuki N., Miller G., Salazar C., Mondal H. A., Shulaev E., Cortes D. F., et al. (2013). Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25, 3553–3569. 10.1105/tpc.113.114595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabados L., Savouré A. (2010). Proline: a multifunctional amino acid. Trends Plant Sci. 15, 89–97. 10.1016/j.tplants.2009.11.009 [DOI] [PubMed] [Google Scholar]
- Tahmasebi S., Heidari B., Pakniyat H., McIntyre C. L. (2016). Mapping QTLs associated with agronomic and physiological traits under terminal drought and heat stress conditions in wheat (Triticum aestivum L.). Genome 60, 26–45. 10.1139/gen-2016-0017 [DOI] [PubMed] [Google Scholar]
- Tisné S., Schmalenbach I., Reymond M., Dauzat M., Pervent M., Vile D., et al. (2010). Keep on growing under drought: genetic and developmental bases of the response of rosette area using a recombinant inbred line population. Plant Cell Environ. 33, 1875–1887. 10.1111/j.1365-3040.2010.02191.x [DOI] [PubMed] [Google Scholar]
- Tungngoen K., Kongsawadworakul P., Viboonjun U., Katsuhara M., Brunel N., Sakr S., et al. (2009). Involvement of HbPIP2;1 and HbTIP1;1 aquaporins in ethylene stimulation of latex yield through regulation of water exchanges between inner liber and latex cells in Hevea brasiliensis. Plant Physiol. 151, 843–856. 10.1104/pp.109.140228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomainen M., Ahonen V., Kärenlampi S. O., Schat H., Paasela T., Svanys A., et al. (2011). Characterization of the glyoxalase 1 gene TcGLX1 in the metal hyperaccumulator plant Thlaspi caerulescens. Planta 233, 1173–1184. 10.1007/s00425-011-1370-7 [DOI] [PubMed] [Google Scholar]
- Uauy C. (2017). Wheat genomics comes of age. Curr. Opin. Plant Biol. 36, 142–148. 10.1016/j.pbi.2017.01.007 [DOI] [PubMed] [Google Scholar]
- Vandeleur R. K., Sullivan W., Athman A., Jordans C., Gilliham M., Kaiser B. N., et al. (2014). Rapid shoot-to-root signalling regulates root hydraulic conductance via aquaporins. Plant Cell Environ. 37, 520–538. 10.1111/pce.12175 [DOI] [PubMed] [Google Scholar]
- Vendruscolo E. C., Schuster I., Pileggi M., Scapim C. A., Molinari H. B., Marur C. J., et al. (2007). Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J. Plant Physiol. 164, 1367–1376. 10.1016/j.jplph.2007.05.001 [DOI] [PubMed] [Google Scholar]
- Verma V., Ravindran P., Kumar P. P. (2016). Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 16:86. 10.1186/s12870-016-0771-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinocur B., Altman A. (2005). Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr. Opin. Biotechnol. 16, 123–132. 10.1016/j.copbio.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Voytas D. F. (2013). Plant genome engineering with sequence-specific nucleases. Annu. Rev. Plant Biol. 64, 327–350. 10.1146/annurev-arplant-042811-105552 [DOI] [PubMed] [Google Scholar]
- Wahid A., Close T. J. (2007). Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol. Plantarum. 51, 104–109. 10.1007/s10535-007-0021-0 [DOI] [Google Scholar]
- Wahid A., Gelani S., Ashraf M., Foolad M. R. (2007). Heat tolerance in plants: an overview. Environ. Exp. Bot. 61, 199–223. 10.1016/j.envexpbot.2007.05.011 [DOI] [Google Scholar]
- Walker J. C., Zhang R. (1990). Relationship of a putative receptor protein kinase from maize to the S-locus glycoproteins of Brassica. Nature 345, 743–746. 10.1038/345743a0 [DOI] [PubMed] [Google Scholar]
- Wang Y., Cheng X., Shan Q., Zhang Y., Liu J., Gao C., et al. (2014). Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 32, 947–951. 10.1038/nbt.2969 [DOI] [PubMed] [Google Scholar]
- Wang Y., Ying J., Kuzma M., Chalifoux M., Sample A., McArthur C., et al. (2005). Molecular tailoring of farnesylation for plant drought tolerance and yield protection. Plant J. 43, 413–424. 10.1111/j.1365-313X.2005.02463.x [DOI] [PubMed] [Google Scholar]
- Waraich E. A., Ahmad R., Halim A., Aziz T. (2012). Alleviation of temperature stress by nutrient management in crop plants: a review. J. Soil Sci. Plant Nut. 12, 221–244. 10.4067/S0718-95162012000200003 [DOI] [Google Scholar]
- Waterland N. L., Campbell C. A., Finer J. J., Jones M. L. (2010). Abscisic acid application enhances drought stress tolerance in bedding plants. Hort. Sci. 45, 409–413. [Google Scholar]
- Wheaton E., Kulshreshtha S., Wittrock V., Koshida G. (2008). Dry times: hard lessons from the Canadian drought of 2001 and 2002. Can. Geograph. 52, 241–262. 10.1111/j.1541-0064.2008.00211.x [DOI] [Google Scholar]
- Wu C., Ma C., Pan Y., Gong S., Zhao C., Chen S., et al. (2013). Sugar beet M14 glyoxalase I gene can enhance plant tolerance to abiotic stresses. J. Plant Res. 126, 415–425. 10.1007/s10265-012-0532-4 [DOI] [PubMed] [Google Scholar]
- Xue G. P., Drenth J., McIntyre C. L. (2014). TaHsfA6f is a transcriptional activator that regulates a suite of heat stress protection genes in wheat (Triticum aestivum L.) including previously unknown Hsf targets. J. Exp. Bot. 66, 1025–1039. 10.1093/jxb/eru462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S. K., Singla-Pareek S. L., Ray M., Reddy M. K., Sopory S. K. (2005). Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem. Biophys. Res. Commun. 337, 61–67. 10.1016/j.bbrc.2005.08.263 [DOI] [PubMed] [Google Scholar]
- Zandalinas S. I., Rivero R. M., Martínez V., Gómez-Cadenas A., Arbona V. (2016). Tolerance of citrus plants to the combination of high temperatures and drought is associated to the increase in transpiration modulated by a reduction in abscisic acid levels. BMC Plant Biol. 16:105. 10.1186/s12870-016-0791-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas S. I., Sales C., Beltrán J., Gómez-Cadenas A., Arbona V. (2017). Activation of secondary metabolism in citrus plants is associated to sensitivity to combined drought and high temperatures. Front. Plant Sci. 7:1954. 10.3389/fpls.2016.01954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Jia W., Yang J., Ismail A. M. (2006). Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res. 97, 111–119. 10.3390/ijms13033189 [DOI] [Google Scholar]
- Zhang M., Duan L., Zhai Z., Li J., Tian X., Wang B., et al. (2004). Effects of plant growth regulators on water deficit-induced yield loss in soybean, in Proceedings of the 4th International Crop Science Congress (Brisbane, QLD: ). [Google Scholar]
- Zhao C., Liu B., Piao S., Wang X., Lobell D. B., Huang Y., et al. (2017). Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. U.S.A. 114, 9326–9331. 10.1073/pnas.1701762114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Jeong J. C., Zhu Y., Sokolchik I., Miyazaki S., Zhu J. K., et al. (2008). Involvement of Arabidopsis HOS15 in histone deacetylation and cold tolerance. Proc. Natl. Acad. Sci. U.S.A. 105, 4945–4950. 10.1073/pnas.0801029105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatev Z., Lidon F. C. (2012). An overview on drought induced changes in plant growth, water relations and photosynthesis. Emir. J. Food Agric. 24, 57–72. 10.9755/ejfa.v24i1.10599 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.