Abstract
This study sought to examine layer-specific longitudinal and circumferential systolic and diastolic strain, strain rate (SR) and diastolic time intervals in hypertensive patients with and without diastolic dysfunction. Fifty-eight treated hypertensive patients were assigned to normal diastolic function (NDF, N = 39) or mild diastolic dysfunction (DD, N = 19) group. Layer-specific systolic and diastolic longitudinal and circumferential strains and SR were assessed. Results showed no between-group difference in left ventricular mass index (DD: 92.1 ± 18.1 vs NDF: 88.4 ± 16.3; P = 0.44). Patients with DD had a proportional reduction in longitudinal strain across the myocardium (endocardial for DD −13 ± 4%; vs NDF −17 ± 3, P < 0.01; epicardial for DD −10 ± 3% vs NDF −13 ± 3%, P < 0.01; global for DD: −12 ± 3% vs NDF: −15 ± 3, P = 0.01), and longitudinal mechanical diastolic impairments as evidenced by reduced longitudinal strain rate of early diastole (DD 0.7 ± 0.2 L/s vs NDF 1.0 ± 0.3 L/s, P < 0.01) and absence of a transmural gradient in the duration of diastolic strain (DD endocardial: 547 ± 105 ms vs epicardial: 542 ± 113 ms, P = 0.24; NDF endocardial: 566 ± 86 ms vs epicardial: 553 ± 77 ms, P = 0.03). Patients with DD also demonstrate a longer duration of early circumferential diastolic strain (231 ± 71 ms vs 189 ± 58 ms, P = 0.02). In conclusion, hypertensive patients with mild DD demonstrate a proportional reduction in longitudinal strain across the myocardium, as well as longitudinal mechanical diastolic impairment, and prolonging duration of circumferential mechanical relaxation.
Keywords: diastolic dysfunction, hypertension, layer-specific strain, transmural gradient
Introduction
Chronic exposure to elevated afterload is associated with maladaptive left ventricular (LV) remodeling, which involves myocardial hypertrophy, collagen deposition and interstitial fibrosis, ultimately resulting in cardiac dysfunction (1, 2). Two-dimensional (2D) strain (ε) imaging can be employed to detect regional and global myocardial abnormalities not recognized by conventional echocardiography. Accordingly, impaired systolic and diastolic (ε) has been reported in patients with hypertension (3, 4). Nevertheless, global ε does not provide a comprehensive evaluation of LV mechanics, as it only measures global function and not myocardial layer-specific activity. The endocardium is the most susceptible layer to the early deleterious effects of hypertension, however, as the disease progresses, the pathology proliferates resulting in gradual deterioration of mid-myocardial and epicardial activity as well (5). Accordingly, different stages of hypertension may result in layer-specific dysfunction that cannot be detected from single-layer assessment.
Layer-specific strain is a new powerful tool that could circumvent such limitations (6) It provides a comprehensive examination of the three myocardial layers, and thus, is able to discern the source and progression of myocardial mechanical dysfunction. In this exploratory study, we sought to examine the effects of hypertension in patients with normal diastolic function (NDF) and mild DD on layer-specific circumferential and longitudinal systolic ε and strain rate (SR) as well as time intervals for layer-specific diastolic ε.
Methods
Patients
Fifty-eight patients (28 males, 30 females; age: 52 ± 8 years) with medicated essential hypertension were invited to take part at random from primary care practice. Patients had no evidence for cardiovascular disease, diabetes or secondary causes of hypertension that may affect cardiac function. Hypertension was defined as systolic blood pressure of >140 mmHg and diastolic blood pressure of >90 mmHg. All patients had a normal EF (>55%) and were divided into 2 groups: Group 1 (N = 39) had NDF while Group 2 (N = 19) had grade I (N = 16) and grade II DD (N = 3), based on joint criteria from the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) (7). The data were obtained from participants who were enrolled in a larger focused study comparing patients with hypertension and those with chronic kidney disease (8). The study was approved by the Black Country Research Ethics Committee (REC:09/H1202/113) and adhered to the Declaration of Helsinki. Informed consent was obtained from all individuals included in the study.
Echocardiography
All echocardiographic images were obtained using a commercially available ultrasound system (Vivid 7 or Vivid Q; GE Medical, Horten, Norway) with a 1.5–4 MHz phased array transducer. Images were acquired from participants in a left lateral decubitus position at the end respiration by a single experienced sonographer in accordance with ASE and EACVI guidelines (7, 9). Three consecutive cycles for each image were collected and stored for later offline analysis, these included parasternal long-axis, parasternal short-axis, (basal and papillary levels) and the apical 2-, 3- and 4-chamber orientations and were stored in a Raw Digital Imaging for Communications in Medicine (DICOM) format for offline analysis using a commercially available software (Echo-Pac version 7.1; GE Medical, Horten, Norway). Heart rate was determined from the electrocardiogram inherent to the ultrasound system.
Conventional M-mode, 2-dimensional, Doppler and tissue Doppler
LV dimensions were analyzed M-mode configuration, including septal thickness (IVSd, IVSs), posterior wall thickness (PWd, PWs) and LV internal dimension (LVIDd, LVIDs). LV and atrial volumes (LAV) were measured by Simpson’s biplane method as well as EF. LV mass was calculated using the ASE-corrected Deveraux formula (14), relative wall thickness (RWT) was calculated as (2*PWd)/LVIDd and LAV was indexed for body surface area.
Pulsed-wave and tissue Doppler were employed to assess diastolic function according to the recommendation of EACVI (12). Measures included early (E) and late (A) transmitral inflow velocities, their ratio (E/A), deceleration time (Dec T), isovolumic relaxation time (IVRT) and diastolic filling time (DFT). The duration of aortic valve closure (AVC) was measured from the pulsed-wave Doppler signal from the LV outflow tract while mitral valve opening (MVO) and closure (MVC) was taken from the trans-mitral pulsed-wave Doppler signal. Mitral annular velocities were obtained at the septum and lateral aspects for peak early (E′) and late (A′) myocardial diastolic velocities. In addition, E/E′ was averaged from the septal and lateral walls as a non-invasive index of LV filling pressures (10).
Myocardial speckle tracking
Images were acquired from the same orientations as for conventional imaging, but were optimized to a frame-rate within 40–90 frames per second. The focal zone was placed mid-LV cavity to reduce the impact of beam divergence while gain, dynamic range and reject were adjusted to maximize signal-to-noise ratio and further delineate the endocardial/epicardial borders.
Peak global transmural, endocardial and epicardial function
Peak (endocardial, mid-layer and epicardial) longitudinal ε and SR were calculated from the apical 4-chamber orientation as an average of the 6 regional segments (basal to apical). Circumferential ε was measured from the parasternal short-axis images at the basal and mid-levels and global peak ε and SR values were calculated as an average of all segments. During offline analysis, the endocardial border was manually traced, and the region of interest was adjusted to take the full thickness of the ventricle into account. The software automatically produced global ε and SR as well as separate ε curves (not SR) for the endocardial and epicardial layers. The raw data were exported to a spreadsheet (Microsoft Excel 2003) where all data points underwent cubic spline interpolation to provide 300 data points for both systole and diastole as previously described (11). Specific ε and SR were then calculated at 5% increments throughout the cardiac cycle allowing for a temporal assessment. Overall function was determined by the global peak ε from transmural, endocardial and epicardial layers as automatically determined by the EchoPac software (Version 13.1) for both longitudinal and circumferential planes. A peak strain endo-epi gradient was calculated as the difference between the two layers. Global peak systolic SR (SRS), peak early diastolic (SRE) and peak late diastolic SR (SRA) were obtained only from global longitudinal and circumferential planes.
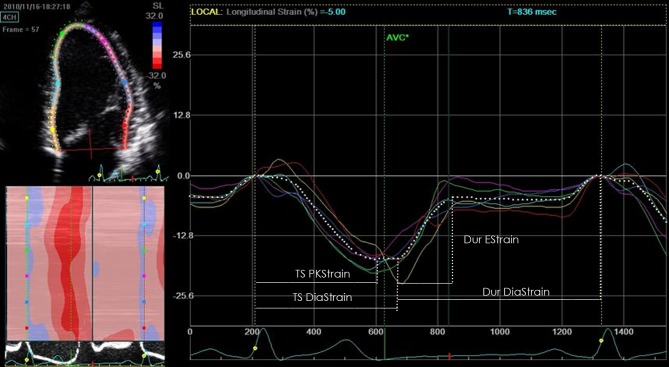
Time intervals for layer-specific diastolic ε data were calculated from longitudinal (4-chamber) and circumferential (basal and mid-levels) curves to allow global, endocardial and epicardial comparisons. Parameters included duration of early diastolic strain (Dur EStrain) measured from the onset of myocardial lengthening to the onset of diastasis and time of overall diastolic strain (Dur DiaStrain) defined as the time from the onset of myocardial lengthening to the point where the ε returns to baseline length (Fig. 1). Endo-epi gradients were calculated for all parameters as the difference between endocardial and epicardial timings. All time data were corrected for heartrate.
Figure 1.
Analysis of temporal systolic and diastolic strain.
Statistical analysis
A Student’s t test was used to determine between-group differences in demographics, conventional echocardiographic parameters, global ε measures and endo-epi strain gradient. A Kruskal–Wallis non-parametric test was used to determine differences in the amount of antihypertensive medications taken between groups. A two-way (group × layer) repeated measures analysis of variance (ANOVA) was performed to compare endocardial and epicardial ε parameters and timing parameters within and between groups. A Student’s t test post hoc was used if significant interactions or main effects were found. Data were reported as means ± s.d., and level of statistical significance was set at P ≤ 0.05. Statistical analyses were performed using Statistical Package for Social Sciences (SPSS 21.0) software.
Results
Participant demographics are presented in Table 1. Hypertensive patients with NDF were younger than patients with DD. There was no difference between the groups for weight, blood pressure or BMI. Both groups were taking the same amount of antihypertensive medications; however, patients with DD had significantly higher resting heart rates.
Table 1.
Participant characteristics.
| Normal diastolic function | Diastolic dysfunction | P-Value | |
|---|---|---|---|
| N | 39 | 19 | |
| Age (years) | 50 ± 7 | 57 ± 7 | <0.001 |
| Gender | 17 male, 22 female |
11 male, 8 female |
|
| Weight (kg) | 79 ± 14 | 78 ± 12 | 0.87 |
| Height (cm) | 168 ± 94 | 169 ± 11 | 0.75 |
| BMI (kg/m2) | 28 ± 4 | 28 ± 4 | 0.96 |
| Heart rate (bpm) | 64 ± 10 | 71 ± 11 | 0.01 |
| Blood pressure (mmHg) | |||
| Systolic | 141 ± 13 | 146 ± 12 | 0.19 |
| Diastolic | 85 ± 9 | 88 ± 9 | 0.32 |
| Antihypertensives (%) | |||
| ACE | 68 | 44 | 0.09 |
| Ca antagonists | 39 | 50 | 0.64 |
| Diuretics | 32 | 56 | 0.06 |
| B-blockers | 16 | 6 | 0.39 |
Conventional echocardiography
LV structural and functional data are presented in Table 2. Patients with DD had larger IVSd, LVPWd and RWT. Individuals with NDF had significantly larger LVIDd and LAVi. Patients with DD had lower E and higher A resulting in a lower E:A ratio. Dec T and IVRT were significantly longer in patients with DD; however, DFT was significantly longer in the NDF group. Patients with DD had significantly lower septal and lateral E′ velocities and higher E/E′. There were no between-group differences in AVC, MVC, MVO and EF.
Table 2.
Left ventricular structural and functional parameters.
| Normal diastolic function | Diastolic dysfunction | P-Value | |
|---|---|---|---|
| Structural parameters | |||
| IVSd (cm) | 1.1 ± 0.2 | 1.3 ± 0.2 | 0.001 |
| LVIDd (cm) | 4.7 ± 0.5 | 4.3 ± 0.5 | 0.01 |
| LVPWd (cm) | 0.9 ± 0.2 | 1.1 ± 0.1 | 0.03 |
| IVSs (cm) | 1.5 ± 0.2 | 1.7 ± 0.2 | 0.06 |
| LVIDs (cm) | 2.9 ± 0.4 | 2.8 ± 0.5 | 0.25 |
| LVPWs (cm) | 1.5 ± 0.2 | 1.6 ± 0.2 | 0.32 |
| LVMi (g/m2) | 88.4 ± 16.3 | 92.1 ± 18.1 | 0.44 |
| RWT | 0.41 ± 0.1 | 0.50 ± 0.1 | <0.001 |
| LVEDV (mL) | 89 ± 22 | 79 ± 24 | 0.38 |
| LAV (mL) | 52 ± 13 | 41 ± 18 | 0.01 |
| LAVi (mL/m2) | 27.8 ± 7.2 | 21.7 ± 8.9 | 0.007 |
| Functional parameters | |||
| E (m/s) | 0.76 ± 0.14 | 0.61 ± 0.13 | <0.001 |
| A (m/s) | 0.65 ± 0.15 | 0.74 ± 0.14 | 0.03 |
| E:A | 1.21 ± 0.27 | 0.82 ± 0.17 | <0.001 |
| E′ Sept (m/s) | 8 ± 1 | 5 ± 1 | <0.001 |
| E/E′ Sept | 9 ± 1 | 11 ± 2 | 0.003 |
| E′ Lat (m/s) | 12 ± 2 | 8 ± 1 | <0.001 |
| E/E′ Lat | 6 ± 1 | 7 ± 1 | 0.02 |
| E/E′ Ave | 7 ± 1 | 9 ± 1 | 0.002 |
| IVRT (ms) | 99 ± 21 | 111 ± 21 | 0.04 |
| Dec T (ms) | 184 ± 33 | 236 ± 56 | 0.01 |
| DFT (ms) | 555 ± 55 | 521 ± 51 | 0.02 |
| AVC (ms) | 355 ± 32 | 344 ± 31 | 0.21 |
| MVO (ms) | 438 ± 37 | 426 ± 54 | 0.33 |
| MVC (ms) | 24 ± 12 | 21 ± 12 | 0.42 |
| EF (%) | 66 ± 6 | 65 ± 6 | 0.32 |
A, late diastolic velocity; AVC, aortic valve closure; Dec T, deceleration time; DFT, diastolic filling time; E, early diastolic velocity; E′, mitral annular early diastolic velocity; EF, ejection fraction; IVRT, isovolumetric relaxation time; IVS, systolic interventricular septum; IVSD, diastolic interventricular septum; LAV volume, left atrial volume; LVEDV, left ventricular end diastolic volume; LVIDd, left ventricular diastolic internal dimension; LVIDs, left ventricular systolic internal dimension; LVMi, left ventricular mass index; LVPWd, left ventricular diastolic posterior wall thickness; LVPWs, left ventricular systolic posterior wall thickness; MVC, mitral valve opening; MVO, mitral valve closure.
Longitudinal strain
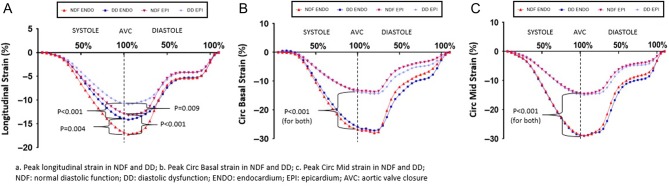
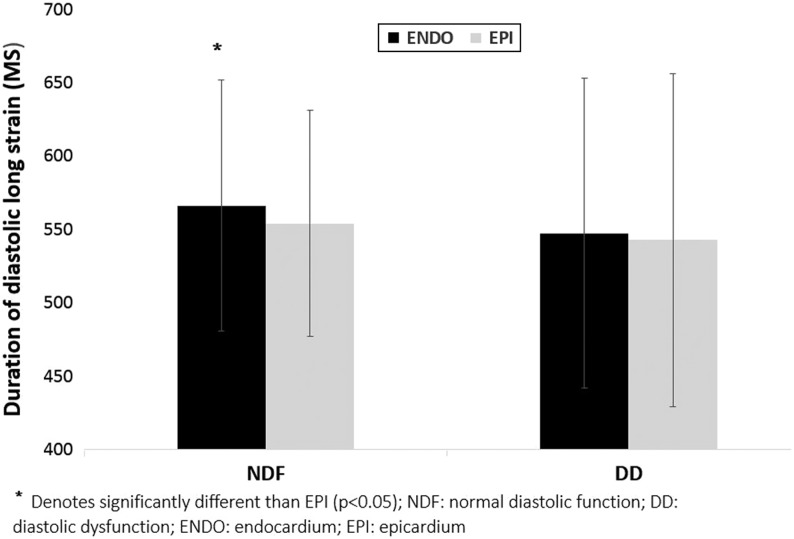
In both groups, endocardial ε was greater than epicardial ε resulting in a transmural gradient (Table 3). Absolute global, endocardial and epicardial ε were significantly lower in patients with DD, but there was no difference in the epi-endo gradient between groups (Fig. 2A and Table 3). The duration of overall diastolic ε was similar across the myocardium in patients with DD; however, in individuals with NDF, the duration of endocardial diastolic ε was longer than that of epicardial resulting in a transmural time gradient (Fig. 3). Patients with DD had lower SRE (Fig. 4). We performed an additional analysis controlling for the between-group differences in age, as age highly influences diastolic function, and found that the DD group still exhibited lower global strain compared to the NDF group (6.3 ± 1.0 vs 7.6 ± 1.5; P = 0.001).
Table 3.
Longitudinal and circumferential strain parameters across the myocardium.
| Normal diastolic function | Diastolic dysfunction | |||||||
|---|---|---|---|---|---|---|---|---|
| Global | Endocardial | Epicardial | Endo-Epi gradient | Global | Endocardial | Epicardial | Endo-Epi gradient | |
| Peak longitudinal strain (%) | −15 ± 3^ | −17 ± 3*,^ | −13 ± 3*,^ | 4 ± 2 | −12 ± 3^ | −13 ± 4*,^ | −10 ± 3*,^ | 3 ± 1 |
| Peak longitudinal SRS (L/s) | −0.80 ± 0.21 | − | − | − | −0.69 ± 0.15 | − | − | − |
| Peak longitudinal SRE (L/s) | 1.00 ± 0.32^ | − | − | − | 0.70 ± 0.23^ | − | − | − |
| Peak longitudinal SRA (L/s) | 0.70 ± 0.20 | − | − | − | 0.68 ± 0.25 | − | − | − |
| Peak Basal circumferential strain (%) | −19 ± 4 | −27 ± 5* | −13 ± 4* | 14 ± 5 | −19 ± 6 | −26 ± 7* | −14 ± 5* | 12 ± 3 |
| Peak basal circumferential SRS (L/s) | −0.95 ± 0.30 | − | − | − | −0.97 ± 0.34 | − | − | − |
| Peak basal circumferential SRE (L/s) | 1.43 ± 0.53 | − | − | − | 1.16 ± 0.48 | − | − | − |
| Peak basal circumferential SRA (L/s) | 0.66 ± 0.32 | − | − | − | 0.82 ± 0.47 | − | − | − |
| Peak mid-circumferential strain (%) | −20 ± 6 | −28 ± 7* | −14 ± 5* | 14 ± 4 | −21 ± 5 | −29 ± 6* | −14 ± 3* | 15 ± 4 |
| Peak mid-circumferential SRS (L/s) | −1.02 ± 0.45 | − | − | − | −0.95 ± 0.27 | − | ||
| Peak mid-circumferential SRE (L/s) | 1.35 ± 0.43 | − | − | − | 1.21 ± 0.36 | − | ||
| Peak mid-circumferential SRA (L/s) | 0.76 ± 0.36 | − | − | − | 0.89 ± 0.29 | − | − | − |
| Longitudinal dur EStrain (ms) | 247 ± 74 | 244 ± 68 | 244 ± 74 | − | 268 ± 63 | 265 ± 58 | 261 ± 64 | − |
| Longitudinal dur DiaStrain (ms) | 557 ± 76 | 566 ± 86* | 553 ± 77* | − | 547 ± 114 | 547 ± 105 | 542 ± 113 | − |
| Circumferential basal dur EStrain (ms) | 189 ± 58^ | 193 ± 60^ | 192 ± 56^ | − | 231 ± 78^ | 230 ± 71^ | 230 ± 74^ | − |
| Circumferential basal dur DiaStrain (ms) | 497 ± 98 | 501 ± 99 | 495 ± 100 | − | 504 ± 90 | 502 ± 88 | 502 ± 88 | − |
| Circumferential mid-dur EStrain (ms) | 228 ± 73 | 220 ± 73^ | 231 ± 73^ | − | 265 ± 70 | 259 ± 70^ | 274 ± 77^ | − |
| Circumferential mid-dur DiaStrain (ms) | 516 ± 118 | 514 ± 177 | 519 ± 110 | − | 515 ± 85 | 514 ± 89 | 513 ± 88 | − |
*Denotes statistically significant difference between endocardial and epicardial layers; ^denotes statistically significant difference between NDF and DF.
Dur DiaStrain, duration of overall diastolic strain; Dur EStrain, duration of early diastolic strain; SRA, late diastolic strain rate; SRE, early diastolic strain rate; SRS, systolic strain rate.
Figure 2.
(A, B and C) Peak longitudinal strain in NDF and DD (A), Peak Circ Basal strain in NDF and DD (B) and Peak Circ Mid strain in NDF and DD (C).
Figure 3.
Transmural gradient for duration of overall longitudinal diastolic strain.
Figure 4.
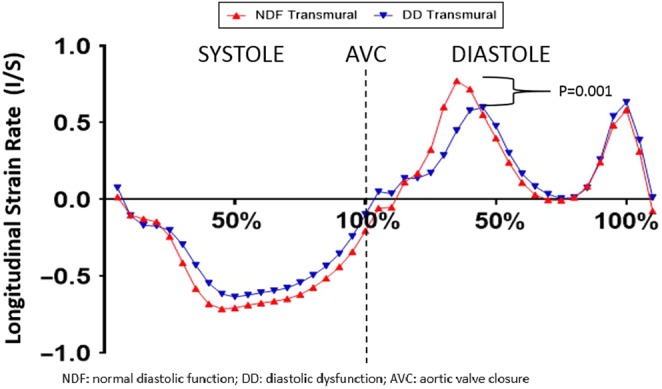
Longitudinal strain rate in patients with NDF and DD.
Circumferential strain (basal & mid-levels)
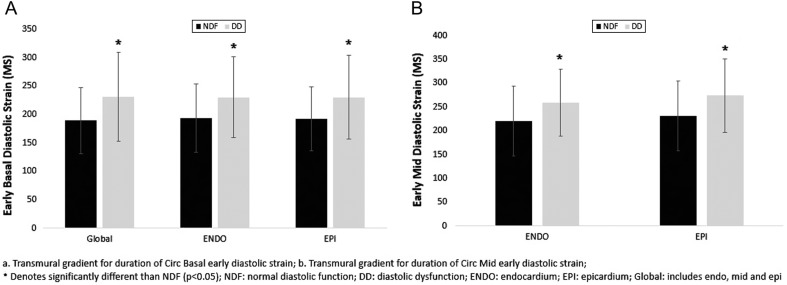
In both groups, endocardial ε was greater than epicardial ε at both basal and mid-levels, and there were no between-group differences in the endo-epi gradients (Fig. 2B and C and Table 3). There were no between-group differences in peak ε nor systolic SR at the basal and mid-layers. Patients with DD had prolonged duration of early diastolic ε across the myocardium at the basal level (Fig. 5A), whereas only endocardial and epicardial early diastolic ε were prolonged in the mid-level (Fig. 5B and Table 3). There was no between-group difference in the duration of overall diastolic ε.
Figure 5.
(A and B) Transmural gradient for duration of Circ Basal early diastolic strain (A) and Transmural gradient for duration of Circ Mid early diastolic strain (B).
Discussion
The key findings of this study are: (1) absolute peak longitudinal ε is proportionally reduced across the myocardium in patients with mild DD resulting in a maintained transmural gradient, (2) patients with mild DD exhibit longitudinal diastolic mechanical impairments, as evidenced by reduced longitudinal SRE and absence of longitudinal transmural gradient in the duration of overall diastolic ε and (3) duration of circumferential diastolic ε is prolonged in patients with DD as a potential compensatory mechanism for the longitudinal diastolic impairments.
Longitudinal transmural strain
Hypertensive patients with mild DD showed proportionally reduced peak longitudinal ε in all myocardial layers, resulting in a preserved transmural gradient. Although we did not have a normotensive control group for comparison, layer-specific longitudinal ε values from NDF and DD patients were markedly lower than previously reported values of healthy individuals (12). This suggests that in hypertension, longitudinal ε across the myocardium is deteriorated, while concomitant DD may further exacerbate such impairments. Global longitudinal ε is commonly thought of as an exclusive marker of endocardial deformation (3, 13). However, layer-specific ε analysis clearly demonstrates that all 3 myocardial layers contribute to longitudinal myocardial movement, and therefore, attributing translational planes to specific layers may be incorrect. In line with the current results, recent studies reported attenuated longitudinal ε across the myocardium in hypertensive patients, while global longitudinal ε failed to detect any impairments (14, 15). In contrast, attenuated longitudinal ε has been reported only in the endocardial (16) or endocardial and mid-myocardial layers of hypertensive patients (17). The discrepancy in the number of affected layers could potentially be explained by the duration of hypertension. Experimentally induced hypertension showed gradual spread of myocardial fibrosis and longitudinal impairment from the endocardium toward the epicardium, which took place over an 8-week period (5). The temporal progression of myocardial dysfunction is supported by human studies, where new or mildly hypertensive patients showed impaired strain only in the endocardium (15) or in the endocardium and mid-myocardium (16) while chronic patients had dysfunctional ε in all myocardial layers. It is important to note that in the aforementioned studies, patients did not have DD, and although both groups in the current study had chronic hypertension, the DD still experienced lower longitudinal strain compared to the NDF group. Suggesting that in addition to prolonged hypertension, LVDD could augment its detrimental effects on myocardial ε.
Circumferential transmural strain
Circumferential ε across the myocardium was similar between NDF and DD patients. Preserved global circumferential ε has been reported in hypertensive patients (3, 18), while more recently, layer-specific analysis revealed depressed endocardial and mid-myocardial circumferential ε only, with no alteration in epicardial function (16, 17). As mentioned, we did not include healthy controls in the study, however, compared to standard normative values (12), the current patients also showed lower endocardial circumfrential ε while epicardial activity was similar. Furthermore, it is implied that the main contributing factor to dysfunctional circumferential ε is an elevation in afterload, even if it is transient (17). This may explain the similar circumferential ε values between the DD and NDF groups, as both have similar average blood pressure. Moreover, similar to longitudinal ε, it is noteworthy to mention that circumferential ε often employed as an analog for mid-myocardial deformation (3, 13). However, this notion may not be correct, as layer-specific analysis shows that all 3 myocardial layers are involved in circumferential deformation.
Diastolic strain
Patients with DD showed impaired diastolic mechanical function, as evidenced by reduced longitudinal SRE, which is in agreement with previous studies (19). Furthermore, patients with NDF showed a transmural gradient in the duration of longitudinal diastolic ε, where the duration of endocardial diastolic ε was longer than epicardial. We must therefore consider this as normal physiology or at least a diastolic profile not affected by DD. In contrast, DD patients did not demonstrate such a transmural gradient in longitudinal diastolic ε, which may well reflect some degree of layer-specific diastolic impairment. In addition, the DD group showed prolonged circumferential early diastolic ε across the myocardium. We speculate that the prolonged circumferential diastolic ε may be a compensatory mechanism for the disordered longitudinal SRE. As longitudinal dysfunction results in impaired myocardial relaxation, there needs to be sustained relaxation elsewhere in order to maximize LV filling, and this may well occur by prolonging diastolic duration of the circumferential fibers. Compensatory increases in circumferential ε have been postulated as a means to preserve systolic function when longitudinal ε is compromised (13). However, to our knowledge, this is the first study to report compensatory diastolic circumferential ε in response to impaired compromised relaxation. As such, this observation requires further investigation.
Limitations
The primary limitation to this study is the small sample size of patients. However, calculations of effect size for transmural gradients were very small (data not shown), suggesting that the findings were of physiological basis and not limited by small power. Second, only 3 patients had advanced DD and the DD group showed smaller LAV compared to the NDF group; however, the majority of patients had mild DD and therefore, at this stage of the disease, LAV would not have increased, which is confirmed by normal E/E′ as well. Third, we did not include a normotensive group but the patients from the current study showed lower layer-specific ε compared to recently published normative values (12). This suggests that future studies with larger sample sizes, varying degrees of DD, as well as normal healthy participants are warranted in order to further elucidate the layer-specific pathology in hypertensive patients.
Conclusion
Hypertensive patients with mild DD demonstrate a proportional reduction in systolic longitudinal ε across the myocardium, thus maintaining the transmural gradient. Patients with mild DD also exhibit mechanical diastolic impairments, as evidenced by reduced longitudinal SRE and absence of a transmural gradient in overall diastolic ε, which may be compensated for by prolonging circumferential diastolic time.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This project was funded by The British Heart Foundation (BHF) Grant (PG/11/66/28982). The BHF did not have any role in study design; collection, analysis and interpretation of data; writing the report and the decision to submit the report for publication.
Acknowledgments
The authors would like to thank Adele Oxborough for providing technical support for data analysis.
References
- 1.Plaksej R, Kosmala W, Frantz S, Herrmann S, Niemann M, Stork S, Wachter R, Angermann CE, Ertl G, Bijnens B, et al Relation of circulating markers of fibrosis and progression of left and right ventricular dysfunction in hypertensive patients with heart failure. Journal of Hypertension 2009. 27 2483–2491. ( 10.1097/HJH.0b013e3283316c4d) [DOI] [PubMed] [Google Scholar]
- 2.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. Jounal of Clinical Investigation 2007. 117 568–575. ( 10.1172/JCI31044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szelényi Z, Fazakas Á, Szénási G, Tegze N, Fekete B, Molvarec A, Hadusfalvy-Sudár S, Jánosi O, Kiss M, Karádi I, et al The mechanism of reduced longitudinal left ventricular systolic function in hypertensive patients with normal ejection fraction. Journal of Hypertension 2015. 33 1962–1969. [DOI] [PubMed] [Google Scholar]
- 4.Saghir M, Areces M, Makan M. Strain rate imaging differentiates hypertensive cardiac hypertrophy from physiologic cardiac hypertrophy. Journal of the American Society of Echocardiography 2007. 20 151–157. ( 10.1016/j.echo.2006.08.006) [DOI] [PubMed] [Google Scholar]
- 5.Ishizu T, Seo Y, Kameda Y, Kawamura R, Kimura T, Shimojo N, Xu D, Murakoshi N, Aonuma K. Left ventricular strain and transmural distribution of structural remodeling in hypertensive heart disease. Hypertension 2014. 63 500–506. ( 10.1161/HYPERTENSIONAHA.113.02149) [DOI] [PubMed] [Google Scholar]
- 6.Tadic M, Cuspidi C. Multilayer strain: a powerful tool to be used with caution. Journal of Hypertension 2017. 35 198 ( 10.1097/HJH.0000000000001096) [DOI] [PubMed] [Google Scholar]
- 7.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Heart Journal – Cardiovascular Imaging 2016. 29 277–313. ( 10.1016/j.echo.2016.01.011) [DOI] [PubMed] [Google Scholar]
- 8.Ting SM, Hamborg T, McGregor G, Oxborough D, Lim K, Koganti S, Aldridge N, Imray C, Bland R, Fletcher S, et al Reduced cardiovascular reserve in chronic kidney failure: a matched cohort study. American Journal of Kidney Diseases 2015. 66 274–284. ( 10.1053/j.ajkd.2015.02.335) [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Bandano L, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Heart Journal – Cardiovascular Imaging 2015. 6 233–271. ( 10.1016/j.echo.2014.10.003) [DOI] [PubMed] [Google Scholar]
- 10.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and Doppler tissue imaging in the estimation of left ventricular filling pressures. Circulation 2000. 102 1788–1794. ( 10.1161/01.CIR.102.15.1788) [DOI] [PubMed] [Google Scholar]
- 11.Oxborough D, Shave R, Warburton D, Williams K, Oxborough A, Charlesworth S, Foulds H, Hoffman MD, Birch K, George K. Dilatation and dysfunction of the right ventricle immediately following ultra-endurance exercise: exploratory insights from conventional 2-dimensional and speckle tracking echocardiography. Circulation: Cardiovascular Imaging 2011. 4 256–263. ( 10.1161/CIRCINTERVENTIONS.110.959718) [DOI] [PubMed] [Google Scholar]
- 12.Shi J, Pan C, Kong D, Cheng L, Shu X. Left ventricular longitudinal and circumferential layer‐specific myocardial strains and their determinants in healthy subjects. Echocardiography 2015. 33 510–518. ( 10.1111/echo.13132) [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Khoury DS, Yue Y,, Torre-Amione G, Nagueh SF. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. European Heart Journal 2008. 29 1283–1290. ( 10.1093/eurheartj/ehn141) [DOI] [PubMed] [Google Scholar]
- 14.Lee WH, Liu YW, Yang LT, Tsai WC. Prognostic value of longitudinal strain of subepicardial myocardium in patients with hypertension. Journal of Hypertension 2016. 34 1195–1200. ( 10.1097/HJH.0000000000000903) [DOI] [PubMed] [Google Scholar]
- 15.Kim SA, Park SM, Kim MN, Shim WJ. Assessment of left ventricular function by layer-specific strain and its relationship to structure remodeling in patients with hypertension. Canadian Journal of Cardiology 2016. 32 211–216. ( 10.1016/j.cjca.2015.04.025) [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Yan ZN, Rui YF, Fan L, Shen D, Chen DL. Left ventricular systolic function changes in primary hypertension patients detected by the strain of different myocardium layers. Medicine 2016. 95 e2440 ( 10.1097/MD.0000000000002440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tadic M, Cuspidi C, Ivanovic B, Ilic I, Celic V, Kocijancic V. Influence of white-coat hypertension on left ventricular deformation 2-and 3-dimensional speckle tracking study: novelty and significance. Hypertension 2016. 67 592–596. ( 10.1161/HYPERTENSIONAHA.115.06822) [DOI] [PubMed] [Google Scholar]
- 18.Galderisi M, Lomoriello VS, Santoro A, Esposito R, Olibet M, Raia R, Di Minno MN, Guerra G, Mele D, Lombardi G. Differences of myocardial systolic deformation and correlates of diastolic function in competitive rowers and young hypertensives: a speckle-tracking echocardiography study. Journal of the American Society of Echocardiography 2010. 23 1190–1198. ( 10.1016/j.echo.2010.07.010) [DOI] [PubMed] [Google Scholar]
- 19.Kang SJ, Lim HS, Choi BJ, Hwang GS, Yoon MH, Tahk SJ, Shin JH. Longitudinal strain and torsion assessed by two-dimensional speckle tracking correlate with the serum level of tissue inhibitor of matrix metalloproteinase-1, a marker of myocardial fibrosis, in patients with hypertension. Journal of the American Society of Echocardiography 2008. 21 907–911. ( 10.1016/j.echo.2008.01.015) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a