Abstract
Oncolytic viruses (OVs) are an emerging cancer therapeutic, with a near complete absence of serious adverse effects. However, clinical efficacy is relatively modest, related to poor tumor penetration, failure to lyse cancer stem cells (CSCs) and blockade of immunogenic cell death by the immunosuppressive tumor microenvironment. To overcome such limitations, we developed an OV (known as ΔPK) with multimodal anti-tumor activity. ΔPK has potent anti-tumor activity both in melanoma cell lines and xenograft animal models, associated with virus replication and the induction of multiple independent programmed cell death pathways. It lyses CSCs through autophagy modulation and it reverses the immunosuppressive tumor microenvironment by altering the balance of cytokines secreted by the tumor cells. This includes decreased tumor cell secretion of the immunosuppressive and procancerous cytokines IL-10 and IL-18 and concomitant increased secretion of the proinflammatory cytokines TNF-α, GM-CSF, IL-6 and IL-1β. ΔPK also upregulates the NKG2D ligand, MICA expressed by cytotoxic NK and T cells, and downregulates the negative immune checkpoint regulator cytotoxic T-lymphocyte antigen-4 (CTLA-4). ΔPK is well tolerated in human patients in whom it also alters the Th1/Th2 balance. Further studies are designed to elucidate the role of these contributions in different tumor types.
Keywords: HSV, oncolytic virotherapy, melanoma, immunogenic cell death, inflammatory cytokines, MICA
A review of current data on the multimodal anti-tumoral activity of the promising oncolytic virus ΔPK including inhibition of the immunosuppressive tumor microenvironment and activation of multiple programmed cell death pathways.
INTRODUCTION
The last two decades have witnessed increased interest in oncolytic viruses (OVs) as cancer therapeutics. To date, 20 virus platforms have been developed, and new candidates continue to emerge. On 27 October 2015, the US Food and Drug Administration (FDA) approved an OV derived from Herpes simplex virus type 1 (oHSV) known as T-Vec for the treatment of advanced metastatic melanoma under the name of IMLYGIC. Other OVs have also entered/completed phase I/II clinical trials for glioblastoma multiforme (GBM) (Wollmann, Ozduman and van den Pol 2012) and other solid tumors including bladder, liver, colorectal, head and neck, prostate, ovarian and lung (Turnbull et al. 2015). Importantly, the OVs have a near complete absence of serious adverse events. Unfortunately, however, efficacy was limited and often did not exceed that seen for gold standard chemotherapy (Weller et al. 2013), as also recognized in the recent FDA approval. An important aspect that is often ignored in the effort to overcome this limitation is the recognition that clinical efficacy is a delicate balance of forces, between (i) effective OV replication and virus clearance by the induced antiviral immunity, (ii) antitumor immunity and factors promoting tumor growth, and (iii) immune stimulation and the immunosuppressive characteristics of the tumor microenvironment. As altering any one of these parameters may counteract the positive effect of the other parameters, the development of OVs with multimodal death-inducing activity has become particularly desirable.
Our studies were designed to address this question. They focus on an OV, known as ΔPK, the development of which follows on the finding that the large subunit of the Herpes simplex virus type 2 (HSV-2) ribonucleotide reductase (R1) has an independent protein kinase activity (ICP10PK), which is not conserved in HSV-1. ICP10PK stimulates cell proliferation through the activation of Ras signaling pathways and is required for virus growth in slowly replicating normal cells (Smith et al. 2000). ICP10PK overrides multiple programmed cell death (PCD) pathways, which are likely to be activated upon infection with an ICP10PK-deleted mutant (Perkins, Pereira and Aurelian 2003; Golembewski et al. 2007; Wales et al. 2007, 2008). Moreover, ΔPK induces CD4+ T helper type 1 (Th1) cells that override the Th2 type response characteristic of melanoma (Wachsman et al. 2001; Gyotoku, Ono and Aurelian 2002) and it has the distinct advantage that it was well tolerated in human patients in whom it also altered the Th1/Th2 balance (Casanova et al. 2002; Aurelian 2004). Here, we briefly review current and novel findings about the oncolytic activity of ΔPK, keeping in mind the question of OV multimodal anti-tumor activity.
ΔPK-induced melanoma cell death involves activation of multiple PCD pathways
Melanoma is a highly aggressive and drug-resistant cancer that accounts for approximately 75% of cancer skin deaths (Jemal et al. 2007). Resistance was also reported for canonical OVs that lyse tumor cells through virus replication (Vaha-Koskela et al. 2006) and it evades immune recognition through its highly immunosuppressive microenvironment (Polak et al. 2007). We found that in addition to its replication-related tumor cell lysis, ΔPK activates multiple PCD pathways in various genetically distinct melanoma cultures. These include caspases-3 and 7, which occupy non-redundant roles within the cell death machinery (Walsh et al. 2008) and calpains, which act through different and often independent PCD pathways (Chu et al. 2005; Wales et al. 2008). Calpain activation was first seen at 1 h post infection (pi) and it was followed by the activation of caspase-7, first seen at 4 h pi. Both were still activated at 24 h pi, when activated caspase-3 was also observed. Using specific inhibitors, we confirmed that these death pathways function independently and contribute to ΔPK-induced melanoma oncolysis in an additive fashion (Colunga, Laing and Aurelian 2010). While likely contributing to the ability of ΔPK to override genetic differences in the target tumors, this wide PCD function also increases its ability to penetrate the tumor, despite its relatively low virus titers, which are characteristic of all OVs.
ΔPK lyses cancer stem cells
One interpretation of the poor clinical efficacy of OV therapy is its failure to effectively eradicate cancer stem cells (CSCs), which are believed to initiate tumor formation (Schatton and Frank 2008). CSCs are a small subset of cancer-initiating cells that are identified by an assortment of phenotypic and molecular markers and growth in spheroid culture. They are also involved in relapse, drug resistance and the modulation of the immune system. The upregulation of ATP-binding cassette drug exporting machinery by CSCs and their relative quiescence both contribute to drug resistance. Molecular data also indicate a multilayered relationship between CSCs and the immunosuppressive tumor microenvironment (Raggi et al. 2016). While OVs developed from various virus platforms were shown to lyse cells with CSC properties (Marcato et al. 2009; Smith et al. 2014), the relationship between CSC lysis and other death-associated mechanisms is still poorly understood.
We studied melanoma (A2058 and A375) and breast cancer (HS578T) CSC, defined physically by the expression of specific markers, namely CD44+CD24−/low (breast cancer) and CD271 (melanoma) (Al-Hajj et al. 2003; Pece et al. 2010; Perego et al. 2010; Quintana et al. 2010), and functionally by their ability to grow in 3D multicellular tumor spheroids and form colonies in agar. The 3D cultures include a gradient of proliferating cells similar to those found in tumor avascular microregions and reflect the tumor microenvironment (Grimshaw et al. 2008; Quintana et al. 2010; Dufau et al. 2012; Loessner et al. 2013). When infected with ΔPK, even at low virus titers [multiplicity of infection (moi) = 0.1)], the 3D cultures were largely reduced to debris and most of the cells (95%) stained with propidium iodide, confirming cell death. The cells that survived infection retained ΔPK susceptibility (no resistance). Moreover, ΔPK-infected 2D cultures lost their ability to grow into spheroids or clone in agar, further confirming CSC loss (Colunga et al. 2014). Evolving complexities, however, include the recent recognition that tumors can harbor multiple phenotypically and/or genetically distinct CSCs and that the CSC phenotype can vary substantially between patients (Aghi and Martuza 2005; Ribacka, Pesonen and Hemminki 2008), suggesting that oncolytic efficacy could differ for distinct tumor cell types and/or OVs. Additional studies are needed in order to address this question.
CSC lysis is through calpain-dependent clearance of the LC3-interacting protein p62/SQSTM1
CSC lysis is through ΔPK-induced calpain activation, as evidenced by the finding that 3D growth was restored by treatment with the calpain inhibitor PD150606. Significantly, 3D growth was not restored by treatment with the pancaspase inhibitor z-VAD-fmk, underscoring the therapeutic advantage of an OV that can activate distinct PCD pathways (Colunga et al. 2014). In addition, we found that chloroquine, an established inhibitor of autophagy (Egger et al. 2013) that does not affect virus replication, rescued the 3D growth potential of the ΔPK-infected melanoma cells, indicating that autophagy contributes to the CSC-lytic activity of ΔPK. Consistent with this interpretation, ΔPK caused a significant increase in the levels of the membrane-bound phosphatidyl–ethanolamine-conjugated form of microtubule-associated protein 1 light chain 3 (LC3II) (34-fold higher LC3II/LC3-I ratio) which becomes imbedded in autophagosome membranes and is an established autophagy marker (Mehrpour et al. 2010; Mizushima, Yoshimori and Levine 2010). However, the mechanism responsible for the effect of ΔPK on autophagy did not involve modulation of other autophagy proteins (namely Atg5). Rather, ΔPK caused the clearance of p62/SQSTM1, a stress-inducible protein that interacts with LC3, functions as a signaling hub in life and death pathways and is implicated in tumorigenesis (Parkhitko et al. 2011; Inoue et al. 2012; Moscat and Diaz-Meco 2012). Interestingly, p62/SQSTM1 expression was restored by treatment with the calpain inhibitor PD150606, indicating that its clearance is calpain-dependent (Colunga et al. 2014).
Challenges of OVs and immunotherapy
Recent efforts have focused on the ability of OVs to stimulate anti-tumor immunity, a new and potentially promising aspect of their therapeutic activity. Specifically, OV-lysed tumor cells were shown to release damage-associated molecular patterns (DAMPs) and tumor-associated antigens (TAAs), which are cross-presented to the immune system, leading to the activation of antitumor immunity (Aymeric et al. 2010; Krysko et al. 2012; Kroemer et al. 2013). Major DAMPs include high mobility group box protein-1 (HMGB1), which has proinflammatory properties, heat shock proteins (namely Hsp70) and uric acid that is also increased by stress-induced nucleic acid degradation. This property, known as immunogenic cell death (ICD) was reported for various OVs developed from distinct virus platforms (Bartlett et al. 2013; Chiocca and Rabkin 2014; Koks et al. 2015), but the extent of its contribution to the OV clinical efficacy is still unclear. Because autophagy improves TAAs cross-presentation (Meng et al. 2013), likely contributing to OV therapy (Uhl et al. 2009; Duffy et al. 2015), and ΔPK modulates autophagy, we wanted to know whether ΔPK has immunomodulatory activity. This is particularly important, because DAMPs and TAAs are not always immunostimulatory. Indeed, the major DAMP, HMGB1, was recently shown to promote the development of myeloid suppressor cells (MDSC) and contribute to their ability to suppress T-cell activation (Parker et al. 2014). Also, inflammatory cytokines, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) that are often used to enhance the OV therapeutic activity, can also have tolerogenic activity depending on dose and the presence of other cytokines (Bhattacharya et al. 2015).
ΔPK alters the balance of melanoma-secreted cytokines from immunosuppressive to inflammatory
The immunosuppressive tumor microenvironment contributes to the relatively poor clinical efficacy of OV therapy (de Aquino et al. 2015). The microenvironment contains infiltrating Tregs and MDSC, which maintain an immunosuppressive environment and promote tumor growth, but the contribution of direct melanoma cell secretion of immunosuppressive cytokines is still poorly understood. Targeting only one of the immunosuppressive mechanisms that define the tumor microenvironment is unlikely to be therapeutically effective, unless it can be identified as dominant in the specific cancer being treated (Marvel and Gabrilovich 2015). Indeed, it is becoming increasingly evident that OVs, which can naturally modulate the tumor microenvironment, are likely to be most effective.
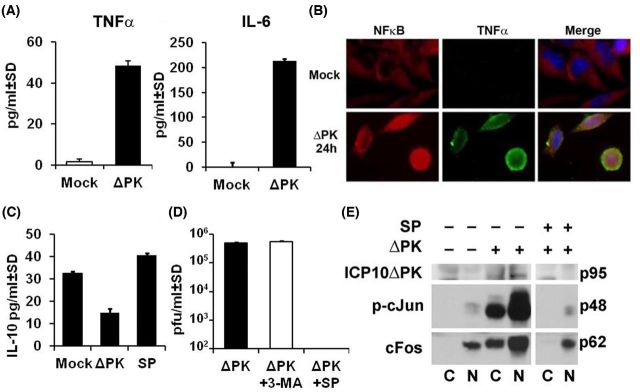
Our studies focused on the immunosuppressive cytokine IL-10 (Chen et al. 2014), and multiple inflammatory cytokines (TNF-α, IL-1β, IL-6, GM-CSF). Specifically, melanoma cells were mock infected with PBS or infected with ΔPK (moi = 1) and the conditioned media (24 h p.i.) were assayed for cytokine secretion by ELISA. The mock-infected cultures secreted IL-10 (32.5 ± 0.84 and 25.9 ± 0.62 ng/ml for A375 and A2058 cells, respectively), but not TNF-α, IL-1β, IL-6 or GM-CSF. The balance of secreted cytokines was reversed upon ΔPK infection, with a simultaneous decrease in the levels of IL-10 and a significant increase in the secretion of all the inflammatory cytokines, as shown in Fig. 1A for TNF-α and IL-6.
Figure 1.
ΔPK inhibits the immunosuppressive melanoma microenvironment through altered cytokine secretion. (A) Conditioned media from melanoma A2058 cells mock or ΔPK infected (moi = 1; 24 h) were assayed for TNF-α and IL-6 by ELISA. ΔPK increases the secretion of both cytokines. (B) A2058 cells were mock or ΔPK infected (moi = 1; 24 h) and stained in double immunofluorescence with Alexafluor 594-labeled NFκB p50 (red) and Alexafluor 488-labeled TNF-α (green) antibodies and counterstained with DAPI to visualize nuclei. ΔPK activates NFκB (intranuclear) and it colocalizes with TNF-α. (C) Conditioned media from A2058 melanoma cells mock or ΔPK infected (moi = 1; 24 h) in the presence or absence of the JNK-specific inhibitor SP600125 (100 μM) were assayed for IL-10 by ELISA. IL-10 secretion is inhibited by ΔPK and restored by JNK inhibition. (D) ΔPK replication, determined by plaque assay [(plaque forming units (pfu)/ml)] is inhibited by SP600125 (100 μM), but not 3-MA (5 mM). (E) ΔPK activates AP-1 signaling as measured by immunoblotting of cytoplasmic and nuclear fractions from infected melanoma cells with antibodies to p-c-Jun and c-Fos. SP600125 (100 μM) inhibits AP-1 signaling activation.
ΔPK upregulates/modulates the tumor microenvironment through autophagy-dependent pathways and virus replication
Consistent with previous findings that link autophagy to cytokine secretion (Crisan et al. 2011; Harris 2011), the levels of secreted proinflammatory cytokines were significantly reduced in cells infected with ΔPK in the presence of the autophagy inhibitor 3-MA (Bollino et al. 2016). Upregulation was through stimulation of Toll-like receptor factors (TLRs), which are pattern recognition receptors that promote T-cell-mediated adaptive immunity (Akira, Takeda and Kaisho 2001; Bergsbaken, Fink and Cookson 2009; Inoue and Tani 2014). One of these, TLR2, had been previously shown to induce inflammatory cytokine production by microglia, astrocytes, neutrophils and monocytes following HSV-1 intracranial infection (Aravalli et al. 2005; Villalba et al. 2012; Wang et al. 2012), but the role of TLRs in tumor cell secretion of inflammatory cytokines is unknown. We found that ΔPK stimulates TLR2 pathways in melanoma cells, which are also autophagy dependent (inhibited by 3-MA) (Bollino et al. 2016). Moreover, double immunofluorescent staining with antibodies to TNF-α and NFκB p50 indicates that TNF-α colocalizes with intranuclear (activated) NFκB in ΔPK- but not mock-infected cells (Fig. 1B).
By contrast, inhibition of the immunosuppressive cytokine IL-10 is due to virus replication-associated activation of JNK pathways (Bollino et al. 2016) and AP-1 transcriptional activation. Indeed, IL-10 expression was restored by treatment with the JNK-specific inhibitor SP600125 (100 μM) (Fig. 1C) that also inhibited virus replication as determined by plaque assay (Fig. 1D). We conclude that signaling via activator protein-1 (AP-1) transcription factors is involved in this process, because the levels of phosphorylated c-Jun (p-c-Jun) and c-Fos were significantly increased in ΔPK- as compared to mock-infected cultures, primarily in the nuclear fraction (Fig. 1E). Indeed, AP-1 transcription factors control a wide range of cellular processes and it is well established that c-Jun and c-Fos have strong transactivation ability. Collectively, the data indicate that autophagy-dependent TLR2/NFκB pathways and virus replication-associated JNK and AP-1 signaling pathways jointly contribute to the ability of ΔPK to alter the tumor microenvironment.
ΔPK upregulates the inflammatory cytokine IL-1β through pyroptosis stimulation
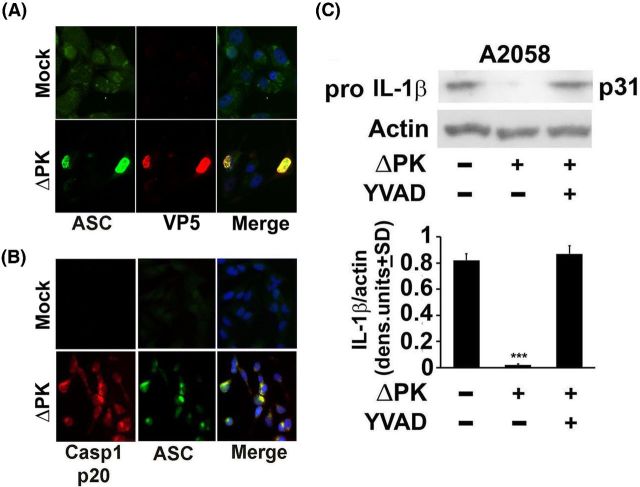
Pyroptosis is a caspase 1-dependent form of inflammatory cell death, which is activated after an initial NFκB-dependent priming step (Fernandes-Alnemri et al. 2007; Sutterwala, Haasken and Cassel 2014). It is characterized by the oligomerization of apoptosis-associated speck-like protein containing a CARD (ASC) and procaspase 1 (pyroptosome formation) leading to the cleavage (activation) of caspase 1 and the resulting activation of proinflammatory cytokines, such as IL-1β (Fernandes-Alnemri et al. 2007). Having seen that the levels of IL-1β are increased in ΔPK-infected cells, we wanted to know whether this involves pyroptosis-related caspase-1 activation. In a first series of experiments, A375 and A2058 melanoma cells were mock or ΔPK infected (moi = 1; 24 h) and stained in double immunofluorescence with antibodies to ASC and the major virus capsid protein VP5. The data summarized in Fig. 2A indicate that ASC is upregulated in the ΔPK as compared to mock-infected cells and it colocalizes with VP5, confirming that it is associated with virus replication. In a second series of experiments, the mock- and ΔPK infected cultures were stained in double immunofluorescence with antibodies to ASC and activated caspase 1 (p20). Staining was virtually absent from the mock-infected cells, whereas ASC and activated caspase 1 colocalized in the ΔPK-infected cells (Fig. 2B). Caspase-1 activation was accompanied by IL-1β production as evidenced by the loss of pro-IL-1β, and its restored expression in cells infected with ΔPK in the presence of the caspase 1-specific inhibitor z-YVAD-fmk (YVAD) (Fig. 2C). Collectively, the data indicate that ΔPK-induced IL-1β expression involves pyroptosome-dependent caspase-1 activation and pro-IL-1β cleavage.
Figure 2.
ΔPK-induced pyroptosis activates caspase-1 resulting in IL-β production. (A) Mock and ΔPK-infected A2058 cells were stained (moi = 1; 24 h pi) in double immunofluorescence with Alexafluor 488-labeled ASC antibody (green) and Alexafluor 594 labeled antibody to the major virus capsid protein VP5 (red). Coverslips were mounted in Vectashield with DAPI to visualize nuclei. (B) Parallel mock or ΔPK-infected A2058 cells grown on coverslips were fixed 24 h pi and stained in double immunofluorescence with Alexafluor 488-labeled ASC (green) and Alexafluor 594 labeled antibody to activated caspase-1 (p20)(red), as in (A). Coverslips were mounted in Vectashield with DAPI to visualize nuclei. (C) Extracts from A2058 cultures mock or ΔPK infected (moi = 1; 24 h) in the absence or presence of the caspase-1-specific inhibitor z-YVAD-fmk (YVAD) (100 μM) were immunoblotted with antibody to pro-IL-β. Blots were stripped and reprobed with actin as a loading control. Data were quantified by densitometric scanning and are expressed as densitometric units ± SD. (***P < 0.001 vs mock, by one-way ANOVA).
ΔPK inhibits expression of the negative immune checkpoint regulator CTLA-4
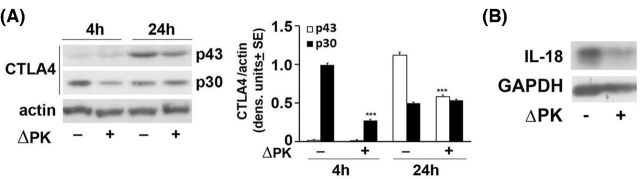
CTLA-4 is a glycoprotein of the immunoglobulin superfamily that functions as an inhibitory receptor of T-cell activation and effector functions and is implicated in the maintenance of immune tolerance (immune checkpoint) (Perez et al. 1997). Recent studies have shown that CTLA-4 is constitutively expressed in several solid tumors, including melanoma (Contardi et al. 2005), and its blockade results in impressive tumor regression (Leach, Krummel and Allison 1996). We have recently shown that ΔPK inhibits the expression of CTLA-4 (as represented by the p30 cytosolic protein) in distinct melanoma cultures (Bollino et al. 2016). Importantly, this results in a significant reduction in the levels of the glycosylated membrane-associated cell surface p43 form (Fig. 3A). Collectively, the data indicate that ΔPK inhibits CTLA-4 expression and its cell surface localization, potentially contributing to increased susceptibility to immune destruction. However, the mechanism of ΔPK-mediated inhibition is still unclear.
Figure 3.
ΔPK inhibits expression of CTLA-4 and IL-18. (A) Cell extracts from mock and ΔPK-infected A2058 cells (moi = 1) were collected at 4 and 24 h pi and immunoblotted with antibody to CTLA-4 [recognizes the glycosylated (p43) and cytosolic (p30) protein forms]. Blots were stripped and reprobed with antibody to β-actin used as gel loading control. Data were quantified by densitometric scanning and the results are expressed as densitometric units ± SE. (B) Cell extracts from mock and ΔPK-infected breast cancer cells MDA-MB-231 were collected at 24 h pi and immunoblotted with antibody to IL-18.
ΔPK inhibits IL-18 expression
Interleukin-18 (IL-18) is a proinflammatory cytokine produced by a variety of cell types and tissues. However, recent studies have shown that it also has a procancerous effect, including stimulation of tumor cell migration (Yang et al. 2015) and escape from immune surveillance (Kang et al. 2009). Its expression and secretion are elevated in patients with breast and gastric cancer with rapid tumor progression, metastasis and unfavorable outcomes (Gunel et al. 2002). To examine whether ΔPK can also function at the level of tumor cell migration and metastasis, we asked whether it can also inhibit the expression of IL-18. The data summarized in Fig. 3B indicate that IL-18 is expressed in mock-infected breast cancer cells (MDA-MB-231), but its expression is inhibited by infection with ΔPK (moi = 1; 24 h), as determined by immunoblotting. The data confirm previous findings associating tumor migration with IL-18 expression in breast cancer (Yang et al. 2015) and indicate that its expression is inhibited by ΔPK, further supporting its strong therapeutic potential.
ΔPK inhibits tumor growth involving multiple PCD pathways
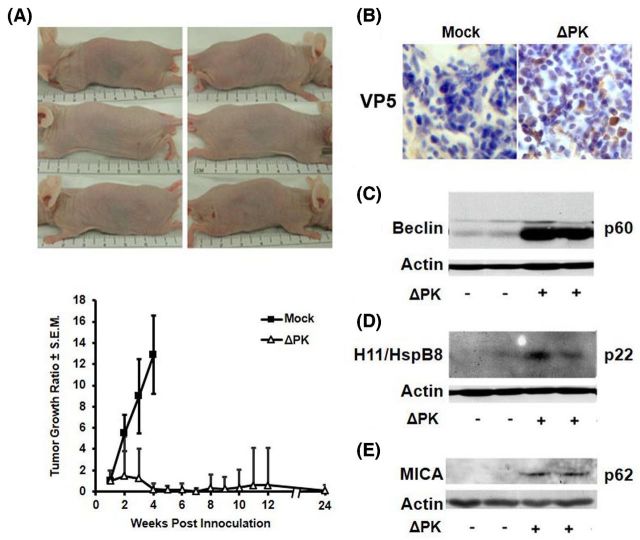
Significantly, ΔPK inhibits melanoma tumor growth in xenograft models (Colunga, Laing and Aurelian 2010; Bollino et al. 2016). In a xenograft established from a primary melanoma that was passed in culture for only six to eight times prior to study (LM), ΔPK intratumoral injection (106 pfu/injection) caused a significant inhibition of tumor growth. Injections were given at weekly intervals beginning when the tumors became palpable (approximately 200 mm3) for a total of four injections. Minimum and maximum perpendicular tumor axes were measured every other day and tumor volume was calculated using the formula: volume = [(length × width2)/2]. Data are expressed as tumor growth ratio, calculated by dividing each tumor volume measured over time by the initial tumor volume on day 7. The mock-treated xenografts evidenced time-dependent growth, but ΔPK caused a significant (p < 0.001) decrease in tumor growth with complete remission seen for 7/8 tumors (87.5%) followed for 5 months after the last ΔPK injection (Fig. 4A). The lone recurrent tumor (seen in one animal) did not reach endpoint criteria (1.5 cm in diameter) by this time and survival was 100%.
Figure 4.
ΔPK inhibits the growth of LM melanoma xenografts. (A) LM melanoma cells (107) were implanted subcutaneously into both flanks of Balb/c nude mice and given four intratumoral injections of ΔPK (n = 4; 106 pfu) or growth medium (n = 4; mock) at weekly intervals beginning on day 7, when the tumors were palpable. Tumor volume was monitored for 5 months after the last ΔPK injection. The difference between mock and ΔPK treatment became statistically significant on day 14 (p < 0.001 by two-way ANOVA) and remained significant to the end of the study. Three ΔPK-treated mice showing complete tumor eradication were photographed at day 35. Data are expressed as tumor growth ratio, calculated by dividing each tumor volume measured over time by the initial tumor volume on day 7. (B) A2058 xenografts mock or ΔPK infected were collected at 7 days after the last ΔPK injection and stained with antibody to the major virus capsid protein VP5 by immunohistochemistry and counterstained with Mayer's Hematoxylin. Duplicates of the A2058 xenografts were immunoblotted with antibodies to Beclin-1 (C), H11/HspB8 (D) or MICA (E), and then stripped and re-probed with antibody to actin. Each lane represents a different tumor. Representatives are shown for each antibody. IACUC approved protocol #0513003.
Virus titers in the ΔPK-treated tissues ranged between 2×102 and 1.5×105 pfu/ml and serial sections encompassing the entire tumor mass stained with antibody to the major virus capsid protein VP5, with approximately 18%–25% VP5+ cells/section (Fig. 4B). As previously described (Colunga, Laing and Aurelian 2010), calpain and caspases-7 and -3 were also activated in the ΔPK- but not mock-treated LM tissues, and the critical autophagy protein Beclin-1 was upregulated (Fig. 4C). The ΔPK-treated tissues also evidenced increased expression of the small heat shock protein H11/HspB8 that has tumor suppressor activity and is silenced in most melanomas, triggering apoptosis upon forced expression (Li et al. 2007) (Fig. 4D). Finally, the ΔPK-infected xenografts evidenced expression of MICA (Fig. 4E), a ligand for the activating receptor NKG2D expressed on NK, γδ T, cytotoxic αβ CD8+ T and NKT cells, the expression of which on the tumor cells engages NKG2D, resulting in their cytotoxic killing (Bauer et al. 1999). In this context, it is important to point out that ΔPK-mediated MICA upregulation is through IL-10 inhibition (Bollino et al. 2016), further confirming the importance of the appropriate tumor microenvironment regulation in the OV clinical efficacy.
CONCLUDING REMARKS
OVs are engineered and selected to exploit genetic defects in tumor cells that enable selective virus replication. They are designed to reduce the tumor burden by cell lysis resulting from virus replication and the generation of infectious virus progeny that spreads throughout the tumor mass. HSV is a particularly promising OV platform because it has a broad host spectrum, is cytolytic, its genome does not integrate into the cellular genome precluding insertional mutagenesis and antiviral drugs are available to safeguard against unfavorable virus replication. Indeed, an HSV-1-based OV, known as T-VEC, was recently FDA approved for the treatment of advanced metastatic melanoma. However, while they are well tolerated, OVs have limited clinical efficacy, as also recognized by the FDA in this approval.
OVs are expected to spread through the tumor mass lysing the cells through productive replication. Accordingly, their limited efficacy was attributed to inhibition of replication by antiviral immunity, incomplete dissemination in the tumor mass due to the failure to lyse CSC, and the failure to appropriately modulate antitumor immunity. Ongoing efforts are focused on improving each one of the potential mechanisms responsible for OV anti-tumor activity. However, it is becoming increasingly evident that OVs with multimodal mechanisms of action are likely to have better clinical efficacy, because they avoid limitations independently ascribed to each specific mechanism.
ΔPK is deleted in the R1 (ICP10) kinase catalytic domain but it retains its transmembrane domain, which is required for membrane localization, protein function and virion stability. The kinase deleted ICP10 protein (also known as p95) is present in the virion tegument preserving the structural integrity required for optimal virus uptake and thereby, tumor penetration (Smith 2005). p95 expression is directed by the authentic ICP10 promoter that has IE kinetics and responds to AP-1 transcription factors (Gober et al. 2005) that regulate genes involved in tumor cell apoptosis (Royuela et al. 2008). ΔPK does not have any genetic defect other than this deletion (Smith et al. 2000). It has the distinct advantages of (i) inducing a Th1 response (Wachsman et al. 2001; Gyotoku, Ono and Aurelian 2002) that can override the melanoma Th2-based immunosuppressive microenvironment (Polak et al. 2007) and (ii) being tolerated well in humans (Casanova et al. 2002; Aurelian 2004). Importantly, although the mutational landscape determines therapeutic sensitivity (Rizvi et al. 2015), ΔPK had robust oncolytic activity in heterogeneous melanoma cultures that are molecularly distinct. In addition to virus replication, oncolytic activity includes the independent induction of calpain, caspase-3 and caspase-7-related PCD pathways, autophagy modulation, restored expression of tumor suppressor functions and lysis of melanoma and breast cancer CSC through calpain-dependent autophagy modulation (Colunga, Laing and Aurelian 2010; Colunga et al. 2014).
Our more recent studies focused on the ability of ΔPK to modulate the immunosuppressive tumor microenvironment that is responsible, at least in part, for the limited OV clinical efficacy (Devaud et al. 2013). Current efforts had focused on arming the OVs with inflammatory cytokines, such as GM-CSF or cotreatment with various immunomodulatory factors (namely TLR ligands) but the delivered immunostimulatory signals were relatively non-specific and they often increased toxicity and even contributed to the establishment of immune tolerance (Rommelfanger et al. 2013; Kaufman et al. 2014). As schematically represented in Fig. 5, ΔPK has multimodal anti-tumor activity. Specifically, in addition to specific tumor cell lysis through virus replication and the induction of multiple independent PCD pathways, ΔPK alters the immunosuppressive tumor microenvironment. It inhibits the ability of the tumor cells to secrete immunosuppressive and tumor promoting cytokines (namely IL-10 and IL-18, respectively) while simultaneously increasing the secretion of multiple inflammatory cytokines (namely TNF-α, GM-CSF, IL-6 and IL-1β). IL-10 inhibition is particularly relevant, because IL-10 inhibits antigen presentation (Buelens et al. 1997) and expression of MHC class II and costimulatory molecules (de Waal Malefyt et al. 1991), and it promotes MDSC and Tregs recruitment to the tumor microenvironment (Marvel and Gabrilovich 2015). ΔPK-mediated IL-10 inhibition also resulted in the upregulation of MICA, the ligand for the activating receptor NKG2D expressed on cytotoxic NK and T cells, thereby enhancing cytotoxic tumor cell killing (Bauer et al. 1999). Inhibition of IL-18 is also particularly important, because IL-18 is a potent stimulator of tumor cell migration and metastasis, and it allows escape from immune surveillance (Cho et al. 2000; Yang et al. 2015). Significantly, the ΔPK-upregulated inflammatory cytokines TNF-α and GM-CSF were individually associated with improved efficacy of virotherapy (Hersey and Gallagher 2014; Hirvinen et al. 2015). Notably, however, ΔPK also upregulated IL-1β, which induces robust and durable primary and secondary CD4+ T-cell responses (Ben-Sasson et al. 2009) and IL-6, which was shown to enhance the ability of dendritic cells to stimulate cytotoxic T-cell responses and therapeutic immunity in melanoma by counteracting Treg-mediated immunosuppression (Kalyanasundaram Bhanumathy et al. 2015). In addition, ΔPK also inhibits the expression of CTLA-4 that is constitutively expressed by melanoma cells (Contardi et al. 2005; Shah et al. 2008) and behaves as a negative regulator of T-cell function (Teft, Kirchhof and Madrenas 2006). All these activities were also seen in melanoma xenograft models and were associated with long-term survival, further documenting the multimodal anti-tumor activity of ΔPK. However, it must be remembered that the findings were primarily in melanoma. The anti-tumor efficacy of ΔPK in other tumor cell types and in immunocompetent animals, the mechanisms that contribute to tumor cell death and the role of the site and extent of disease in the therapeutic efficacy of ΔPK remain to be better elucidated.
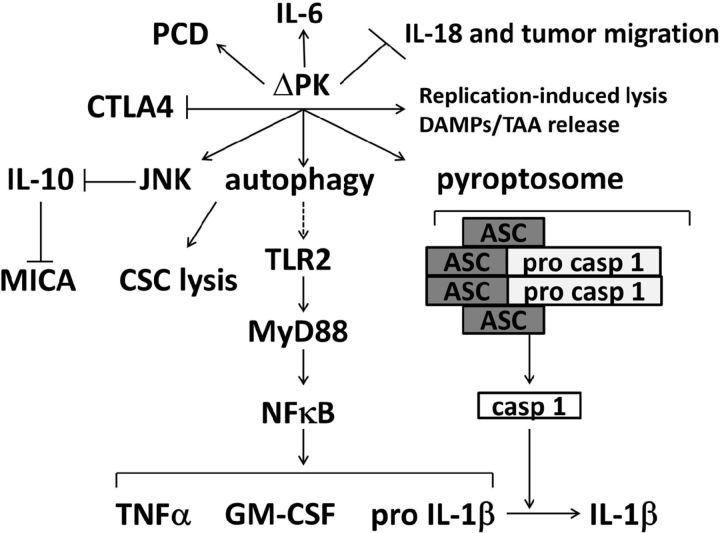
Figure 5.
Schematic representation of the ΔPK multimodal activity in melanoma. ΔPK lyses the tumor cells through virus replication, and this results in the release of DAMPs and TAAs that trigger ICD. It also induces multiple PCD pathways and autophagy-related CSC lysis. In addition, ΔPK alters the tumor microenvironment by inhibiting IL-10 through JNK/c-Jun activation while stimulating the expression of inflammatory cytokines through TLR2 activation and pyroptosis. This is shown for TNF-α, GM-CSF and IL-1β, but ΔPK also stimulates expression/secretion of the proinflammatory cytokine IL-6, through a still unresolved mechanism. IL-10 inhibition upregulates the expression of MICA and thereby presumably stimulates NK and T-cell-mediated cytotoxicity. Simultaneously, ΔPK inhibits the expression of IL-18 and thereby presumably interferes with tumor cell migration. It also inhibits the negative immune checkpoint regulator CTLA-4, further favoring appropriate immunoregulatory anti-tumor activity.
Conflict of interest. None declared.
Acknowledgments
The studies were supported by Grant AR053512 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and by Grant AA021261 from the National Institute on Alcohol Abuse and Alcoholism. AC was supported by grant ES07263 from National Institute of Environmental Health Sciences, NIH.
REFERENCES
- Aghi M, Martuza RL. Oncolytic viral therapies - the clinical experience. Oncogene. 2005;24:7802–16. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, et al. Prospective identification of tumorigenic breast cancer cells. P Natl Acad Sci USA. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravalli RN, et al. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J Immunol. 2005;175:4189–93. doi: 10.4049/jimmunol.175.7.4189. [DOI] [PubMed] [Google Scholar]
- Aurelian L. Herpes simplex virus type 2 vaccines: new ground for optimism? Clin Diagn Lab Immunol. 2004;11:437–45. doi: 10.1128/CDLI.11.3.437-445.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymeric L, et al. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 2010;70:855–8. doi: 10.1158/0008-5472.CAN-09-3566. [DOI] [PubMed] [Google Scholar]
- Bartlett DL, et al. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer. 2013;12:103. doi: 10.1186/1476-4598-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson SZ, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. P Natl Acad Sci USA. 2009;106:7119–24. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P, et al. Dual role of GM-CSF as a pro-inflammatory and a regulatory cytokine: implications for immune therapy. J Interf Cytok Res. 2015;35:585–99. doi: 10.1089/jir.2014.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollino D, et al. PK oncolytic activity includes modulation of tumor cell milieu. J Gen Virol. 2016;97:496–508. doi: 10.1099/jgv.0.000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buelens C, et al. Interleukin-10 prevents the generation of dendritic cells from human peripheral blood mononuclear cells cultured with interleukin-4 and granulocyte/macrophage-colony-stimulating factor. Eur J Immunol. 1997;27:756–62. doi: 10.1002/eji.1830270326. [DOI] [PubMed] [Google Scholar]
- Casanova G, et al. A double-blind study of the efficacy and safety of the ICP10deltaPK vaccine against recurrent genital HSV-2 infections. Cutis. 2002;70:235–9. [PubMed] [Google Scholar]
- Chen L, et al. Cotransfection with IL-10 and TGF-beta1 into immature dendritic cells enhances immune tolerance in a rat liver transplantation model. Am J Physiol-Gastr L. 2014;306:G575–81. doi: 10.1152/ajpgi.00283.2013. [DOI] [PubMed] [Google Scholar]
- Chiocca EA, Rabkin SD. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res. 2014;2:295–300. doi: 10.1158/2326-6066.CIR-14-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D, et al. Endogenous interleukin-18 modulates immune escape of murine melanoma cells by regulating the expression of Fas ligand and reactive oxygen intermediates. Cancer Res. 2000;60:2703–9. [PubMed] [Google Scholar]
- Chu CT, et al. Apoptosis inducing factor mediates caspase-independent 1-methyl-4-phenylpyridinium toxicity in dopaminergic cells. J Neurochem. 2005;94:1685–95. doi: 10.1111/j.1471-4159.2005.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colunga A, et al. Calpain-dependent clearance of the autophagy protein p62/SQSTM1 is a contributor to DeltaPK oncolytic activity in melanoma. Gene Ther. 2014;21:371–8. doi: 10.1038/gt.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colunga AG, Laing JM, Aurelian L. The HSV-2 mutant DeltaPK induces melanoma oncolysis through nonredundant death programs and associated with autophagy and pyroptosis proteins. Gene Ther. 2010;17:315–27. doi: 10.1038/gt.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contardi E, et al. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int J Cancer. 2005;117:538–50. doi: 10.1002/ijc.21155. [DOI] [PubMed] [Google Scholar]
- Crisan TO, et al. Inflammasome-independent modulation of cytokine response by autophagy in human cells. PLoS One. 2011;6:e18666. doi: 10.1371/journal.pone.0018666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Aquino MT, et al. Challenges and future perspectives of T cell immunotherapy in cancer. Immunol Lett. 2015;166:117–33. doi: 10.1016/j.imlet.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud C, et al. Immune modulation of the tumor microenvironment for enhancing cancer immunotherapy. Oncoimmunology. 2013;2:e25961. doi: 10.4161/onci.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufau I, et al. Multicellular tumor spheroid model to evaluate spatio-temporal dynamics effect of chemotherapeutics: application to the gemcitabine/CHK1 inhibitor combination in pancreatic cancer. BMC Cancer. 2012;12:15. doi: 10.1186/1471-2407-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy A, et al. Autophagy modulation: a target for cancer treatment development. Cancer Chemoth Pharm. 2015;75:439–47. doi: 10.1007/s00280-014-2637-z. [DOI] [PubMed] [Google Scholar]
- Egger ME, et al. Inhibition of autophagy with chloroquine is effective in melanoma. J Surg Res. 2013;184:274–81. doi: 10.1016/j.jss.2013.04.055. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gober MD, et al. Stress up-regulates neuronal expression of the herpes simplex virus type 2 large subunit of ribonucleotide reductase (R1; ICP10) by activating activator protein 1. J Neurovirol. 2005;11:329–36. doi: 10.1080/13550280591002423. [DOI] [PubMed] [Google Scholar]
- Golembewski EK, et al. The HSV-2 protein ICP10PK prevents neuronal apoptosis and loss of function in an in vivo model of neurodegeneration associated with glutamate excitotoxicity. Exp Neurol. 2007;203:381–93. doi: 10.1016/j.expneurol.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimshaw MJ, et al. Mammosphere culture of metastatic breast cancer cells enriches for tumorigenic breast cancer cells. Breast Cancer Res. 2008;10:R52. doi: 10.1186/bcr2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunel N, et al. Clinical importance of serum interleukin-18 and nitric oxide activities in breast carcinoma patients. Cancer. 2002;95:663–7. doi: 10.1002/cncr.10705. [DOI] [PubMed] [Google Scholar]
- Gyotoku T, Ono F, Aurelian L. Development of HSV-specific CD4+ Th1 responses and CD8+ cytotoxic T lymphocytes with antiviral activity by vaccination with the HSV-2 mutant ICP10DeltaPK. Vaccine. 2002;20:2796–807. doi: 10.1016/s0264-410x(02)00199-8. [DOI] [PubMed] [Google Scholar]
- Harris J. Autophagy and cytokines. Cytokine. 2011;56:140–4. doi: 10.1016/j.cyto.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Hersey P, Gallagher S. Intralesional immunotherapy for melanoma. J Surg Oncol. 2014;109:320–6. doi: 10.1002/jso.23494. [DOI] [PubMed] [Google Scholar]
- Hirvinen M, et al. Immunological effects of a TNF-alpha armed oncolytic adenovirus. Hum Gene Ther. 2015;26:134–44. doi: 10.1089/hum.2014.069. [DOI] [PubMed] [Google Scholar]
- Inoue D, et al. Accumulation of p62/SQSTM1 is associated with poor prognosis in patients with lung adenocarcinoma. Cancer Sci. 2012;103:760–6. doi: 10.1111/j.1349-7006.2012.02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Tani K. Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ. 2014;21:39–49. doi: 10.1038/cdd.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Kalyanasundaram Bhanumathy K, et al. Potent immunotherapy against well-established thymoma using adoptively transferred transgene IL-6-engineered dendritic cell-stimulated CD8+ T-cells with prolonged survival and enhanced cytotoxicity. J Gene Med. 2015;17:153–60. doi: 10.1002/jgm.2836. [DOI] [PubMed] [Google Scholar]
- Kang JS, et al. Interleukin-18 increases metastasis and immune escape of stomach cancer via the downregulation of CD70 and maintenance of CD44. Carcinogenesis. 2009;30:1987–96. doi: 10.1093/carcin/bgp158. [DOI] [PubMed] [Google Scholar]
- Kaufman HL, et al. Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. J Immunother Cancer. 2014;2:11. doi: 10.1186/2051-1426-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koks CA, et al. Immune suppression during oncolytic virotherapy for high-grade glioma; yes or no? J Cancer. 2015;6:203–17. doi: 10.7150/jca.10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, et al. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- Krysko DV, et al. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–75. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- Li B, et al. Overload of the heat-shock protein H11/HspB8 triggers melanoma cell apoptosis through activation of transforming growth factor-beta-activated kinase 1. Oncogene. 2007;26:3521–31. doi: 10.1038/sj.onc.1210145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loessner D, et al. Growth of confined cancer spheroids: a combined experimental and mathematical modelling approach. Integr Biol. 2013;5:597–605. doi: 10.1039/c3ib20252f. [DOI] [PubMed] [Google Scholar]
- Marcato P, et al. Oncolytic reovirus effectively targets breast cancer stem cells. Mol Ther. 2009;17:972–9. doi: 10.1038/mt.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356–64. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrpour M, et al. Overview of macroautophagy regulation in mammalian cells. Cell Res. 2010;20:748–62. doi: 10.1038/cr.2010.82. [DOI] [PubMed] [Google Scholar]
- Meng S, et al. Targeting autophagy to enhance oncolytic virus-based cancer therapy. Expert Opin Biol Ther. 2013;13:863–73. doi: 10.1517/14712598.2013.774365. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–26. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT. p62: a versatile multitasker takes on cancer. Trends Biochem Sci. 2012;37:230–6. doi: 10.1016/j.tibs.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KH, et al. HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells. Cancer Res. 2014;74:5723–33. doi: 10.1158/0008-5472.CAN-13-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhitko A, et al. Tumorigenesis in tuberous sclerosis complex is autophagy and p62/sequestosome 1 (SQSTM1)-dependent. P Natl Acad Sci USA. 2011;108:12455–60. doi: 10.1073/pnas.1104361108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pece S, et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140:62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Perego M, et al. Heterogeneous phenotype of human melanoma cells with in vitro and in vivo features of tumor-initiating cells. J Invest Dermatol. 2010;130:1877–86. doi: 10.1038/jid.2010.69. [DOI] [PubMed] [Google Scholar]
- Perez VL, et al. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–7. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- Perkins D, Pereira EF, Aurelian L. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) functions as a dominant regulator of apoptosis in hippocampal neurons involving activation of the ERK survival pathway and upregulation of the antiapoptotic protein Bag-1. J Virol. 2003;77:1292–305. doi: 10.1128/JVI.77.2.1292-1305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak ME, et al. Mechanisms of local immunosuppression in cutaneous melanoma. Brit J Cancer. 2007;96:1879–87. doi: 10.1038/sj.bjc.6603763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana E, et al. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell. 2010;18:510–23. doi: 10.1016/j.ccr.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggi C, et al. Cancer stem cells and tumor-associated macrophages: a roadmap for multitargeting strategies. Oncogene. 2016;35:671–82. doi: 10.1038/onc.2015.132. [DOI] [PubMed] [Google Scholar]
- Ribacka C, Pesonen S, Hemminki A. Cancer, stem cells, and oncolytic viruses. Ann Med. 2008;40:496–505. doi: 10.1080/07853890802021342. [DOI] [PubMed] [Google Scholar]
- Rizvi NA, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelfanger DM, et al. The efficacy versus toxicity profile of combination virotherapy and TLR immunotherapy highlights the danger of administering TLR agonists to oncolytic virus-treated mice. Mol Ther. 2013;21:348–57. doi: 10.1038/mt.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royuela M, et al. TNF-alpha/IL-1/NF-kappaB transduction pathway in human cancer prostate. Histol Histopathol. 2008;23:1279–90. doi: 10.14670/HH-23.1279. [DOI] [PubMed] [Google Scholar]
- Schatton T, Frank MH. Cancer stem cells and human malignant melanoma. Pigment Cell Melanoma R. 2008;21:39–55. doi: 10.1111/j.1755-148X.2007.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah KV, et al. CTLA-4 is a direct target of Wnt/beta-catenin signaling and is expressed in human melanoma tumors. J Invest Dermatol. 2008;128:2870–9. doi: 10.1038/jid.2008.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC. The herpes simplex virus type 2 protein ICP10PK: a master of versatility. Front Biosci. 2005;10:2820–31. doi: 10.2741/1738. [DOI] [PubMed] [Google Scholar]
- Smith CC, et al. Ras-GAP binding and phosphorylation by herpes simplex virus type 2 RR1 PK (ICP10) and activation of the Ras/MEK/MAPK mitogenic pathway are required for timely onset of virus growth. J Virol. 2000;74:10417–29. doi: 10.1128/jvi.74.22.10417-10429.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TT, et al. Oncolytic viral therapy: targeting cancer stem cells. Oncolytic Virother. 2014;2014:21–33. doi: 10.2147/OV.S52749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319:82–95. doi: 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65–97. doi: 10.1146/annurev.immunol.24.021605.090535. [DOI] [PubMed] [Google Scholar]
- Turnbull S, et al. Evidence for oncolytic virotherapy: Where have we got to and where are we going? Viruses. 2015;7:6291–312. doi: 10.3390/v7122938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl M, et al. Autophagy within the antigen donor cell facilitates efficient antigen cross-priming of virus-specific CD8+ T cells. Cell Death Differ. 2009;16:991–1005. doi: 10.1038/cdd.2009.8. [DOI] [PubMed] [Google Scholar]
- Vaha-Koskela MJ, et al. Oncolytic capacity of attenuated replicative semliki forest virus in human melanoma xenografts in severe combined immunodeficient mice. Cancer Res. 2006;66:7185–94. doi: 10.1158/0008-5472.CAN-05-2214. [DOI] [PubMed] [Google Scholar]
- Villalba M, et al. Herpes simplex virus type 1 induces simultaneous activation of Toll-like receptors 2 and 4 and expression of the endogenous ligand serum amyloid A in astrocytes. Med Microbiol Immun. 2012;201:371–9. doi: 10.1007/s00430-012-0247-0. [DOI] [PubMed] [Google Scholar]
- Wachsman M, et al. A growth and latency compromised herpes simplex virus type 2 mutant (ICP10DeltaPK) has prophylactic and therapeutic protective activity in guinea pigs. Vaccine. 2001;19:1879–90. doi: 10.1016/s0264-410x(00)00446-1. [DOI] [PubMed] [Google Scholar]
- Wales SQ, et al. The herpes simplex virus type 2 gene ICP10PK protects from apoptosis caused by nerve growth factor deprivation through inhibition of caspase-3 activation and XIAP up-regulation. J Neurochem. 2007;103:365–79. doi: 10.1111/j.1471-4159.2007.04745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wales SQ, et al. ICP10PK inhibits calpain-dependent release of apoptosis-inducing factor and programmed cell death in response to the toxin MPP+ Gene Ther. 2008;15:1397–409. doi: 10.1038/gt.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JG, et al. Executioner caspase-3 and caspase-7 are functionally distinct proteases. P Natl Acad Sci USA. 2008;105:12815–9. doi: 10.1073/pnas.0707715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JP, et al. Role of specific innate immune responses in herpes simplex virus infection of the central nervous system. J Virol. 2012;86:2273–81. doi: 10.1128/JVI.06010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller M, et al. Standards of care for treatment of recurrent glioblastoma—are we there yet? Neuro Oncol. 2013;15:4–27. doi: 10.1093/neuonc/nos273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann G, Ozduman K, van den Pol AN. Oncolytic virus therapy for glioblastoma multiforme: concepts and candidates. Cancer J. 2012;18:69–81. doi: 10.1097/PPO.0b013e31824671c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, et al. Interleukin-18 enhances breast cancer cell migration via down-regulation of claudin-12 and induction of the p38 MAPK pathway. Biochem Bioph Res Co. 2015;459:379–86. doi: 10.1016/j.bbrc.2015.02.108. [DOI] [PubMed] [Google Scholar]