Abstract
Atypical HUS (aHUS) is a disorder most commonly caused by inherited defects of the alternative pathway of complement, or the proteins that regulate this pathway, and life-threatening episodes of aHUS can be provoked by pregnancy. We retrospectively and prospectively investigated 27 maternal and fetal pregnancy outcomes in 14 women with aHUS from the Vienna Thrombotic Microangiopathy Cohort. Seven pregnancies (26%) were complicated by pregnancy-associated aHUS (p-aHUS), of which three appeared to be provoked by infection, bleeding, and curettage, and three individuals were considered to have preeclampsia/HELLP syndrome before the definitive diagnosis of p-aHUS was made. Mutations in genes that encode the complement alternative pathway proteins or the molecules that regulate this pathway were detected in 71% of the women, with no relationship to pregnancy outcome. Twenty-one pregnancies (78%) resulted in a live birth, two preterm infants were stillborn, and four pregnancies resulted in early spontaneous abortions. Although short-term renal outcome was good in most women, long-term renal outcome was poor; among the 14 women, four had CKD stage 1–4, five had received a renal allograft, and three were dialysis-dependent at study end. We prospectively followed nine pregnancies of four women and treated six of these pregnancies with prophylactic plasma infusions (one pregnancy resulted in p-aHUS, one intrauterine fetal death occurred, and seven pregancies were uneventful). Our study emphasizes the frequency of successful pregnancies in women with aHUS. Close monitoring of such pregnancies for episodes of thrombotic microangiopathy is essential but, the best strategy to prevent these episodes remains unclear.
Keywords: atypical hemolytic uremic syndrome, aHUS, pregnancy, TMA, complement-mediated
Pregnancy presents a challenge for the maternal immune system because of the need to protect the fetus from pathogens while preventing alloimmune injury by facilitating tolerance to paternal antigens. The challenge is focused on the feto-maternal interface in the placenta and requires a precise control of adaptive and innate immunity, including the complement system as exemplified by women with atypical hemolytic uremic syndrome (aHUS).
aHUS is a disorder most commonly caused by inherited defects of the alternative pathway of complement, or of the proteins that regulate it, and pregnancy can provoke life-threatening episodes.1,2 Pregnancy-related aHUS (p-aHUS) affects one in 25,000 pregnancies in the general population,3 which contrasts with an incidence of 20% in women with preexisting aHUS in whom it causes significant maternal mortality and morbidity.4 Classically, p-aHUS presents in the postpartum period and was believed to have little effect on fetal outcome. However, preeclampsia, eclampsia, and HELLP syndrome (hemolysis–elevated liver enzymes–low platelet) are all strongly linked to complement dysregulation and consequently to aHUS that has been complicated by worse fetal outcomes.5
Despite the risks, current consensus proposes that each pregnancy should be considered individually and carefully planned.4 However, the knowledge base is small, and it needs to be expanded before women with aHUS can be given robust advice about pregnancy.6 Here we present data on pregnancies from well characterized patients with aHUS, recruited into the Vienna Thrombotic Microangiopathy (TMA) cohort.
In total, we document 27 pregnancies in 14 women, including nine pregnancies in four women that were followed prospectively.
Results
Clinical Aspects of p-aHUS
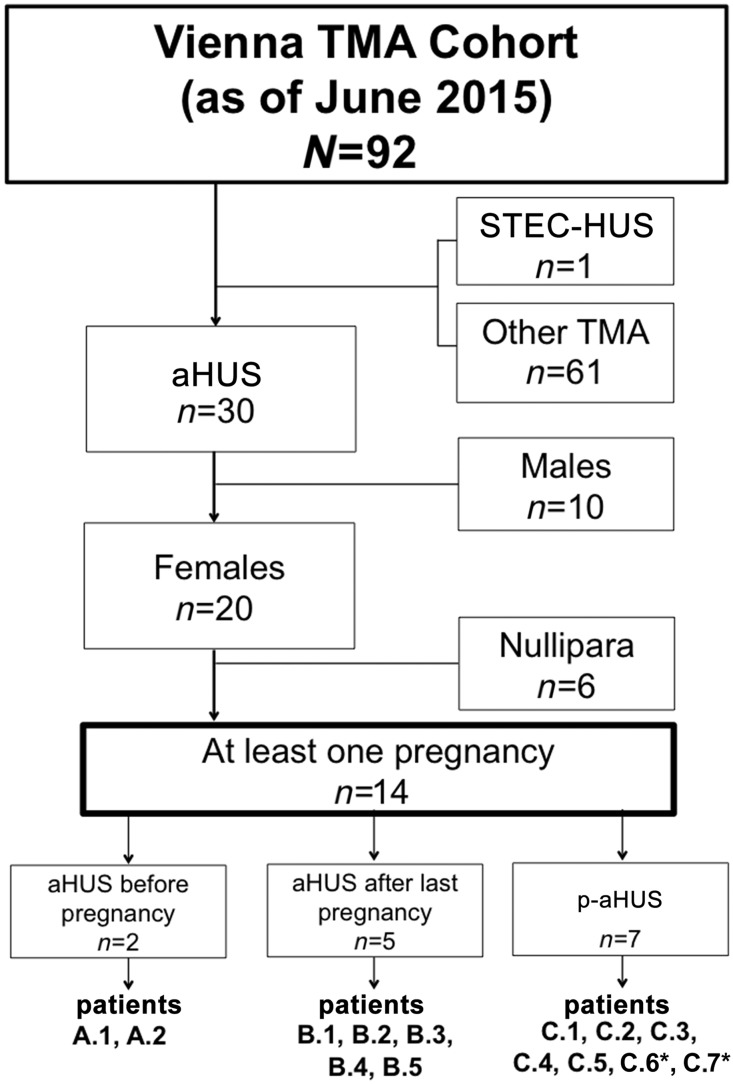
By the end of June of 2015, a total of 92 patients had been entered into the Vienna TMA Cohort. These included 30 (33%) with aHUS, of whom 20 were female; one with Shiga toxin–producing Escherichia coli (STEC)–HUS; and 61 with other causes of TMA (Figure 1). Fourteen of the 20 women with aHUS had been pregnant, and together they had had 27 pregnancies, of which nine (in four women) were followed prospectively and are described in detail in the Supplemental Data.
Figure 1.
Pregnancies among patients with aHUS. Flowchart of the Vienna TMA Cohort. *Developed p-aHUS in subsequent pregnancies.
The 14 women with aHUS who had pregnancies were aged 29±12 years (mean±SD) at diagnosis (Table 1). They segregated into three groups, depending on the timing of pregnancy in relation to diagnosis (Figure 1). Group A (14%) consisted of two individuals (A.1 and A.2) in whom aHUS was diagnosed before their first pregnancies, which occurred 6 and 14 years later. Group B (36%) contained five individuals (B.1 to B.6) who had had at least one uncomplicated pregnancy before their first episode of aHUS, that occurred a median of 6 years (range, 2–23 years) after their last pregnancy. Group C (50%) comprised seven women (C.1 to C.7) who presented with aHUS during or immediately after pregnancy. In five of these women, p-aHUS occurred during their first pregnancy, in the second trimester in two, the third trimester in two, and in the puerperium in one. Notably, patient C.6 initially presented with aHUS 1 year after the birth of her first baby and, after an uneventful second pregnancy, subsequently developed another episode during her third pregnancy. Patient C.7 presented with p-aHUS in the puerperium of her second pregnancy.
Table 1.
Manifestation of aHUS, treatment, and renal function during pregnancies in 14 women
| Patient | Onset of aHUS | Preg | Prior First Preg CKD Stage | Adverse Events | Maintenance Treatment | As of 2015 (End of Study) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Treatment | During Preg | After Preg | Prior First Preg | After Preg | Age | CKD Stage | Mainten. Treatment | |||
| A.1 | 3 | supportive, PEX | 2 | CKD-G1 | 1: none, with PI | 1: none | none | 1: none | 24 | CKD-G1 | none |
| 2: proteinuria, hypertension, with PI | 2: persistent proteinuria | 2: none | |||||||||
| A.2 | 24 | none | 3 | CKD-G1T | 1: none (KTX) | 1: none | none | 1: none | 33 | CKD-G2T | none |
| 2: none (KTX) | 2: none | 2: none | |||||||||
| 3: none (IUFD gw 31) | 3: none | 3: none | |||||||||
| B.1 | 28 | PEX, ecul | 1 | unknown | 1: none | 1: none | none | 1: none | 31 | CKD-G4 | none |
| B.2 | 39 | none | 2 | unknown | 1: none | 1: none | none | 1: none | 43 | CKD-G3bT | ecul |
| 2: hypertension | 2: none | ||||||||||
| B.3 | 37 | PEX | 2 | unknown | 1: none | 1: none | none | 1: none | 41 | normal | none |
| 2: none | 2: none | ||||||||||
| B.4 | 34 | PEX | 1 | unknown | 1: none | 1: none | none | 1: none | 42 | CKD-G4T | ecul |
| B.5 | 57 | none | 2 | unknown | 1: none | 1: none | none | 1: none | 60 | CKD-G2 | none |
| 2: none | 2: none | ||||||||||
| C.1 | 20 | PEX | 3 | normal | 1: none | 1: p-aHUS | none | 1: none | 23 | CKD-G1 | none |
| 2: none, with PI | 2: none | 2: none | |||||||||
| 3: none, with PI | 3: none | 3: none | |||||||||
| C.2 | 31 | none | 3 | CKD-G3b | 1: increasing creatinine, preeclampsia, fetal death | 1: p-aHUS | none | 1: none | 53 | CKD-G5D | none |
| 2: none, spont. abortion | 2: none | 2: none | |||||||||
| 3: none, spont. abortion | 3: none | 3: none | |||||||||
| C.3 | 28 | none | 1 | CKD-G3b/4T | 1: preeclampsia gw 26 | 1: p-aHUS | none | 1: none | 42 | CKD-G5D | none |
| C.4 | 24 | PEX | 1 | normal | 1: spont. abortion | 1: p-aHUS | none | 1: none | 27 | normal | none |
| C.5 | 33 | none | 1 | normal | 1: proteinuria, HELLP | 1: p-aHUS | none | 1: none | 42 | CKD-G5D | none |
| C.6 | 19 | PEX | 3 | normal | 1: none | 1: aHUS (+12 mo) | none | 1: PI | 32 | CKD-G4T | ecul |
| 2: none, with PI (KTX) | 2: none | 2: PI | |||||||||
| 3: none, with PI (KTX) | 3: p-aHUS (+1 d) | 3: ecul | |||||||||
| C.7 | 27 | PEX | 2 | unknown | 1: none | 1: none | none | 1: none | 33 | CKD-G1T | ecul |
| 2: none | 2: p-aHUS (+6 d) | 2: PEX | |||||||||
Preg, pregnancy; mainten., maintenance; PEX, plasma exchange; PI, plasma infusion; KTX, kidney transplant; IUFD, intrauterine fetal death; gw, gestational week; ecul, eculizumab; spont., spontaneous.
Overall, seven pregnancies (26%) were complicated by episodes of TMA (p-aHUS), whereas 20 (74%) were not. Accordingly, the incidence of p-aHUS was 6.94 episodes per 1000 weeks at risk (95% confidence interval [95% CI], 2.79 to 14.27). In three patients, episodes of TMA appeared to be provoked by recognized triggers: infection in patient C.6; bleeding in C.1; and curettage after a spontaneous abortion in patient C.4. In addition, three women (C.3, C.4, and C.5) were considered to have preeclampsia or HELLP syndrome before the definitive diagnosis of p-aHUS was made. One individual (C.7) had an induced preterm delivery, but we have no information about why this was considered necessary.
Complement Gene Mutations
Mutations in genes that encode complement alternative pathway proteins, or the molecules that regulate this pathway, were detected in 10 of 14 (71%) in our p-aHUS cohort, a frequency within the range of 50%–80% reported for aHUS more generally.7,8 Details of the mutations are shown in Table 2. On the basis of in silico analysis, functional studies, or previous association with aHUS, three of the patients (A.2, B.5, C.1) have mutations that are recognized as highly likely to be pathogenic and a further two (A.1, C.4) are recognized as probably pathogenic. The remaining five patients had rare variants that are also likely to be pathogenic.
Table 2.
Results of genetic investigations in 14 women with aHUS
| Patient | Gene | Alteration in Protein | Domain | In Silico Predictions on Possible Functional Effect | Minor Allele Frequencya | Functional Effect | Risk Haplotype | Copy Number of CFH, CFHR1,-R2, -R3, and -R5 |
|---|---|---|---|---|---|---|---|---|
| A.1 | CFI | p.G342E | serine protease | probably damaging,1 damaging,2 deleterious,3 disease causing4 | 0% | not characterized42 | het CFH H3 | no alteration |
| CD46 | p.D257Vfsa42 | SCR4 | disease causing4 | 0% | FS causing, damaging43 | |||
| A.2 | CFI | p.I416L | serine protease | benign,1 tolerated,2 neutral,3 disease causing4 | 0.01%–1.29% | quantitative Factor I Deficiency42,44–47 | none | het dele CFHR1,-R3 |
| B.1 | none | na | na | na | na | het CFH c.-331C>T | het dele CFHR1,-R3 | |
| B.2 | none | na | na | na | na | hom CFH H3 | no alteration | |
| B.3 | CD46 | p.E234K | SCR4 | benign,1 tolerated,2 neutral,3 poly4 | 0% | not characterized7 | het CFH H3 | no alteration |
| B.4 | C3 | p.D61N | MG1 | probably damaging,1 tolerated,2 neutral,3 poly4 | 0% | not characterizedb | het CFH H3 | no alteration |
| B.5 | CD46 | p.A353V | TM | benign,1 tolerated,2 neutral,3 poly4 | 0.29%–1.73% | deficient cell surface control of AP48–52 | none | no alteration |
| C.1 | C3 | p.K104E | MG1 | benign,1 tolerated,2 neutral,3 poly4 | 0% | not characterized | het CFH H3 | no alteration |
| C3 | p.D1457H | MG8 | probably damaging,1 damaging,2 deleterious,3 poly4 | 0.34% | not characterized | |||
| C.2 | THBD | p.E560Q | probably damaging,1 tolerated,2 neutral,3 disease causing4 | 0.01%–0.02% | not characterized | het CFH H3 | no alteration | |
| C.3 | none | na | na | na | na | het CFH H3/H8 | na | |
| C.4 | CFHR5 | p.E163Rfsa34 | SCR3 | disease causing4 | 0.09%–0.30% | FS causing, damaging53,54 | none | no alteration |
| C.5 | none | None | na | na | na | na | hom CFH H3 | no alteration |
| C.6 | CFH | p.N516K | SCR9 | probably damaging,1 tolerated,2 deleterious,3 poly4 | 0.02%–0.04% | not characterized42,44,48,55 | het CFH H3 hom p.E936D | no alteration |
| C.7 | CFH | p.D748Nfsa10 | SCR13 | disease causing4 | 0% | FS causing, damaging | het CFH H3 | |
| CD46 | p.A353V | TM | benign,1 tolerated,2 neutral,3 poly4 | 0.29%–1.73% | deficient cell surface control of AP48–52 | no alteration |
CFH, complement factor H; CFHR1–5, CFH related protein 1–5; CFI, complement factor I; het, heterogeneous; CD46, cluster of differentiation 46; SCR, short consensus repeat; FS, frameshift; dele, deletion; na, not applicable; hom, homogenous; poly, polymorphism; MG, macroglobulin; TM, transmembrane; AP, alternative pathway; THBD, thrombomodulin.
Minor allele frequency was included on the basis of the Exome Sequencing (http://evs.gs.washington.edu/EVS/) and 1000 Genomes Projects (reported by dbSNP https://www.ncbi.nlm.nih.gov/SNP/); for patient C.3 only CFH was analyzed and CFH c.-331C>T was not included (CFH-H3/H8); CFHR5 was sequenced only in patients C.4 and C.5.
p.K65Q at a near site affects the C3 binding to factor H and membrane cofactor protein.
The genes CFH, CFI, and CD46 are inherited together with other regulators of complement activation as part of a gene cluster on chromosome 1. Penetrance of pathogenic CFH, CFI, and CD46 mutations for aHUS is incomplete but is increased when they are inherited on a particular haplotype (CFH-H3).7 Ten of the 14 patients in the p-aHUS cohort inherited the CFH-H3 risk haplotype that included a complement mutation in seven individuals. Notably, the two patients without complement mutations were the only ones that were homozygous for CFH-H3 (Table 2).
Specific mutations had no discernable effect on the timing of p-aHUS in relation to pregnancy. Thus, recognized disease-causing mutations were present in both women who developed aHUS years before they first became pregnant (A.1 and A.2); in at least three of the five women who had uncomplicated pregnancies before developing clinically severe aHUS (group B); and in five of the seven women in group C who presented with p-aHUS.
Pregnancy in Women with aHUS
We analyzed 18 pregnancies retrospectively (patients B.1 to B.5 and C.1 to C.7), none of which were managed with prophylactic infusions of fresh frozen plasma (FFP) or other preventative therapy. Six of these pregnancies (33%) were complicated by p-aHUS (Table 1).
None of the eight pregnancies in individuals in group B were complicated by p-aHUS. However, patient B.2 developed hypertension without proteinuria (Table 1). During their first pregnancies, patients C.2 and C.3 (who had received a renal allograft) were both diagnosed clinically as having preeclampsia, but proved to have TMA on renal biopsy (p-aHUS), and subsequently progressed to ESRD. Patient C.4 developed p-aHUS with ARF triggered by curettage after spontaneous abortion. She was treated with eight therapeutic plasma exchanges, and her renal function recovered completely. Patient C.5 developed typical clinical signs of p-aHUS after a stillbirth and an initial diagnosis of HELLP syndrome; no specific treatment was given, and she developed progressive CKD. Finally, patient C.7 presented with p-aHUS after an uneventful second pregnancy and developed ESRD despite therapeutic plasma exchange.
Nine pregnancies were followed prospectively (A.1, A.2, C.1, and C.6), one of which led to p-aHUS (C.6). Patient A.1 was originally diagnosed as having STEC-HUS when she presented with TMA at the age of three. She subsequently had 22 further episodes before a definitive diagnosis of aHUS was made when she was shown to have inherited a CFI and CD46 mutation on the CFH-H3 susceptibility haplotype. She has had two pregnancies, both treated with prophylactic FFP (3 ml/kg twice monthly; Table 3). The first pregnancy was uncomplicated, but she developed hypertension and significant proteinuria during the second, although her serum creatinine concentration remained stable (Table 4). There were no signs of complement activation during either pregnancy.
Table 3.
Details about aHUS treatment of four women during pregnancies and postpartum period
| Patient | Preg | Year | Prospectively Followed | Maintenance Plasma Therapy before Pregnancy | Plasma Therapy during Pregnancy | Start (Gestational Week) | Stop (Postpartum Week) | TMA Episode |
|---|---|---|---|---|---|---|---|---|
| A.1 | 1 | 2009 | yes | none | 3 ml/kg twice monthly | 15 | continued | no |
| 2 | 2012 | yes | none | 3 ml/kg twice monthly | 13 | continued | no | |
| A.2 | 1 | 2010 | yes | none | 2.5 ml/kg twice monthly | 14 | continued | no |
| 2 | 2011 | yes | none | none | — | — | no | |
| 3 | 2014 | yes | none | none | — | — | no | |
| C.1 | 1 | 2011a | no | none | none | — | — | yes |
| 2 | 2014 | yes | none | 10 ml/kg twice monthly | 21 | 14 | no | |
| 3 | 2015 | yes | none | 10 ml/kg twice monthly | 15 | 24 | no | |
| C.6 | 1 | 2002a | no | none | none | — | — | nob |
| 2 | 2013 | yes | 25 ml/kg monthly | 25 ml/kg twice monthly | 14 | 19 | no | |
| 3 | 2015 | yes | 25 ml/kg monthly | 25 ml/kg monthly | — | 1c | yes |
Preg, pregnancy.
Pregnancy before follow-up at our department.
Presented with acute and chronic TMA in renal biopsy 12 mo after delivery.
Switch to eculizumab.
Table 4.
Renal function, BP, and signs of microangiopathic hemolytic anemia during 11 pregnancies
| Patient | Preg | Gestational/pp. weeka | Kidney Function | Signs of Microangiopathic Hemolytic Anemia | Hypertension | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A.1 | 1 | 13 | 28 | 36 | 3 | S-Crea | 0.67 | 0.69 | 0.69 | 0.93 | Hb | 11.1 | 10.4 | 10.1 | 11.7 | BP | 125/75 | 120/80 | 125/80 | 127/75 |
| UPE | 386 | 320 | 397 | 488 | PLC | 234 | 182 | 170 | 299 | Med | none | |||||||||
| LDH | 143 | 171 | 165 | 164 | ||||||||||||||||
| 2 | 14 | 28 | 36 | 3 | S-Crea | 0.71 | 0.66 | 0.71 | 0.85 | Hb | 11.8 | 11.5 | 10.6 | 11.8 | BP | 130/70 | 130/80 | 130/80 | 130/80 | |
| UPE | 776 | 727 | 2208 | 1178 | PLC | 237 | 254 | 225 | 317 | Med | none | N | N | N | ||||||
| LDH | 143 | 202 | 235 | 237 | ||||||||||||||||
| A.2 | 1 | 13 | 28 | 36 | 5 | S-Crea | 0.95 | 0.91 | 1.06 | 1.16 | Hb | 10.1 | 10.2 | 10.6 | 12.1 | BP | 125/88 | 125/79 | 147/105 | 120/80 |
| UPE | 56 | 112 | 155 | 91 | PLC | 263 | 172 | 137 | 182 | Med | M | M | M | none | ||||||
| LDH | 180 | 157 | 207 | 164 | ||||||||||||||||
| 2 | 14 | 27 | 39 | 2 | S-Crea | 0.86 | 0.85 | 0.85 | 1.15 | Hb | 10.5 | 10.6 | 11.2 | 10.9 | BP | 120/80 | 120/80 | 140/90 | 140/100 | |
| UPE | 47 | 164 | 280 | 65 | PLC | 308 | 254 | 194 | 332 | Med | N | N | N, M | N, M | ||||||
| LDH | 169 | 143 | 215 | 215 | ||||||||||||||||
| 3 | 12 | 28 | 33 | 1 | S-Crea | 0.71 | 0.8 | 0.78 | 0.92 | Hb | 10.4 | 10.7 | 11.9 | 9.3 | BP | 125/75 | 123/84 | 123/84 | 130/89 | |
| UPE | 104 | 127 | 145 | 102 | PLC | 349 | 233 | 258 | 304 | Med | N, M | N, M | N, M | N, M | ||||||
| LDH | 171 | 154 | 191 | 209 | ||||||||||||||||
| C.1 | 1 | 8 | 25 | 40 | 1 | S-Crea | na | 0.5 | 2.2 | Hb | 14.1 | 11.0 | 9.2 | 8.0 | BP | 115/75 | 110/70 | na | PRES | |
| UPE | neg | neg | na | 362 | PLC | na | na | 254 | 52 | Med | none | na | ||||||||
| LDH | na | 188 | 1964 | |||||||||||||||||
| 2 | 20 | 27 | 37 | 5 | S-Crea | 0.76 | 0.74 | 0.72 | 0.9 | Hb | 12.3 | 12.1 | 9.8 | 11.2 | BP | 110/90 | 125/85 | 125/78 | 125/78 | |
| UPE | 110 | 169 | 253 | 390 | PLC | 235 | 240 | 240 | 368 | Med | M | M | M | none | ||||||
| LDH | 184 | 164 | 268 | 194 | ||||||||||||||||
| 3 | 13 | 27 | 37 | 3 | S-Crea | 0.74 | 0.71 | 0.79 | 0.89 | Hb | 11.3 | 8.9 | 12.5 | 11.2 | BP | 138/102 | 123/65 | 126/81 | 121/71 | |
| UPE | 373 | 342 | 456 | 290 | PLC | 333 | 284 | 222 | 335 | Med | M | M | M | M | ||||||
| LDH | 185 | 205 | 191 | na | ||||||||||||||||
| C.6 | 1 | 8 | 23 | 36 | na | S-Crea | na | Hb | 14.1 | 12.3 | na | na | BP | 110/70 | 110/70 | 120/80 | na | |||
| UPE | neg | neg | neg | na | PLC | 272 | 243 | na | na | Med | none | |||||||||
| LDH | na | |||||||||||||||||||
| 2 | 14 | 28 | 38 | 3 | S-Crea | 1.46 | 1.71 | 2.01 | 1.96 | Hb | 10.0 | 8.4 | 8.9 | 9.3 | BP | 130/85 | 130/88 | 132/83 | 140/93 | |
| UPE | 101 | 85 | 202 | 366 | PLC | 279 | 236 | 191 | 398 | Med | U, H, N | U, L, N | U, L, N | U, Ca, A | ||||||
| LDH | 154 | na | 184 | 215 | ||||||||||||||||
| 3 | 13 | 29 | 37 | 1 | S-Crea | 1.66 | 2.06 | 2.42 | 7.53 | Hb | 9.9 | 8.5 | 9.6 | 6.8 | BP | 155/95 | 133/71 | 135/90 | 158/82 | |
| UPE | 212 | 244 | 2848 | 7653 | PLC | 263 | 177 | 178 | 108 | Med | U, L, N | U, L, N | U, L, N | U, Ca, A | ||||||
| LDH | 146 | 182 | 246 | 592 | ||||||||||||||||
Preg, pregnancy; pp., postpartum; S-Crea, serum creatinine (mg/dl); Hb, hemoglobin (mg/dl); BP, BP (mmHg); UPE, urinary protein excretion (mg/g creatinine); PLC, platelet count (G/L); Med, medication; LDH, lactate dehydrogenase (U/L); N, Nifedipin; M, Methyldopa; na, not available; PRES, posterior reversible encephalopathy syndrome; neg, urine dipstick negative; U, urapidil; H, hydrochlorothiazide; L, labetolol; Ca, carvedilol; A, amlodipine;
Columns 1–3 refer to gestational week; column 4 refers to postpartum week.
After repeated episodes of TMA according to renal biopsy, patient A.2, aged 24, presented with ESRD and later successfully received a renal allograft. After this she had three pregnancies all of which were followed prospectively. The first was managed with prophylactic FFP (2.5 ml/kg FFP twice monthly) and was uncomplicated. Prophylactic FFP was not given during either of the subsequent pregnancies: the second was completely uncomplicated, but the third resulted in a late intrauterine death, probably caused by in utero infection. There was no evidence of complement activation during any of the three pregnancies.
Patient C.1 has compound C3 mutations and has inherited the CFH-H3 susceptibility haplotype. Originally, she presented elsewhere with p-aHUS after her first pregnancy and was treated with 26 plasma exchanges over 41 weeks, but did not require maintenance therapy after she recovered. Her two subsequent pregnancies were managed by us with twice monthly prophylactic infusions of FFP (10 ml/kg), during the pregnancy and into the puerperium (Table 3). Neither pregnancy was complicated by p-aHUS, despite an infection with influenza H1N1—a known trigger for aHUS—during the third pregnancy (Table 4). However, she did have decreased serum C3c concentrations for at least 6 months postpartum, despite normal C3 concentrations and otherwise unremarkable laboratory values.
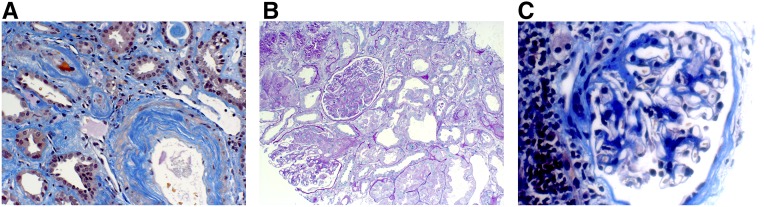
Patient C.6 has a CFH mutation on a CFH-H3 haplotype and developed aHUS complicated by renal failure 12 months after an apparently uncomplicated pregnancy. She received a renal allograft and was treated thereafter with monthly prophylactic infusions of FFP (25 ml/kg monthly). She had two further pregnancies. In the first, we increased the prophylactic FFP infusions from once to twice a month from the first trimester until the 19th postpartum week (Table 3), and supplemented this with 1600 ml FFP infusions on the day of her caesarian section and the day after (Table 4). She continued her maintenance prophylactic treatment with FFP during her third pregnancy and was again infused with 1600 ml FFP before and after her caesarean section. Despite this, she developed TMA within hours after surgery, possibly provoked by infection. The hemolysis and thrombocytopenia resolved rapidly with FFP infusions, but she developed ARF in the kidney transplant and a renal biopsy confirmed acute TMA (Figure 2). FFP alone has been reported to be ineffective in this situation,9–12 and so eculizumab was added, and this was followed by a steady improvement in serum creatinine. In retrospect, plasma haptoglobin had become undetectable around 4 weeks before the caesarian section, which might be suggestive of low levels of TMA despite the absence of symptoms or other evidence of microangiopathy (Table 4).
Figure 2.
Renal TMA in a patient with aHUS. Biopsy specimen of renal allograft of patient C.6 after third pregnancy showing (A) arteriolar TMA (acid fuchsin orange G stain, 200× magnification) and (B) glomerular TMA (Periodic Acid-Schiff stain, 200× magnification). In comparison, a renal biopsy after her second pregnancy showed (C) a histologically normal glomerulum with interstitial fibrosis (acid fuchsin orange G stain, 400× magnification).
Fetal Outcome of 27 Pregnancies in Women with aHUS
The fetal outcomes of all 27 pregnancies are documented in Table 5. The median gestational week at birth was 38 (interquartile range, 33–40 weeks). Nineteen pregnancies (70%) resulted in a term live birth and there were two preterm live births. In addition, two preterm infants were stillborn, and four pregnancies resulted in spontaneous abortions before the 21st gestational week (C.2, C.4).
Table 5.
Outcomes of 27 pregnancies in 14 women with aHUS
| Patient | Preg | Year | Outcome | Sex | Gestational Age at Birth, wk | Mode of delivery | Birth Weight, g | Birth Height, cm | Head Circumference at Birth, cm | Assessment |
|---|---|---|---|---|---|---|---|---|---|---|
| A.1 | 1 | 2009 | live birth | female | 40+0 | vaginal delivery | 3010 | 53 | 33 | AGA |
| 2 | 2012 | live birth | male | 38+2 | vaginal delivery | 3340 | 50 | 33 | AGA | |
| A.2 | 1 | 2010 | live birth | male | 37+3 | vaginal delivery | 3110 | 51 | 33 | AGA |
| 2 | 2011 | live birth | male | 40+3 | vaginal delivery | 3956 | 52 | 36 | AGA | |
| 3 | 2014 | stillbirth | male | 33+3 | vaginal delivery | 2230 | 46 | 35 | AGA | |
| B.1 | 1 | 2004 | live birth | female | 38+0 | C-section | 2900 | 40 | 33 | AGA |
| B.2 | 1 | 2003 | live birth | female | 41+3 | C-section | 4090 | 52 | 35 | AGA |
| 2 | 2005 | live birth | male | 40+2 | C-section | 3560 | 50 | 36 | AGA | |
| B.3 | 1 | 2001 | live birtha | unknown | unknown | C-section | unknown | unknown | unknown | unknown |
| 2 | 2006 | live birtha | unknown | unknown | C-section | unknown | unknown | unknown | unknown | |
| B.4 | 1 | 2005 | live birth | male | 38+3 | C-section | 3665 | 52 | 36.5 | AGA |
| B.5 | 1 | 1981 | live birth | male | 40+? | emergency C-section | 3270 | unknown | unknown | AGA |
| 2 | 1989 | live birth | female | 40+5 | vaginal delivery | 3500 | 50 | 34 | AGA | |
| C.1 | 1 | 2011 | live birth | female | 40+6 | emergency C-section | 4450 | 53 | 38 | LGA |
| 2 | 2014 | live birth | female | 37+3 | C-section | 3130 | 49 | 35 | AGAb | |
| 3 | 2015 | live birth | female | 38+0 | C-section | 3150 | 51 | 33.5 | AGA | |
| C.2 | 1 | 1993 | fetal death/abortion | na | 18 | vaginal delivery | na | na | na | na |
| 2 | 1999 | spontaneous abortion | na | 7 | vaginal delivery | na | na | na | na | |
| 3 | 2000 | spontaneous abortion | na | 9+6 | vaginal delivery | na | na | na | na | |
| C.3 | 1 | 2002 | live birth/premature | female | 26+? | emergency C-section | 888 | unknown | unknown | AGAc |
| C.4 | 1 | 2012 | spontaneous abortion | na | 18+3 | vaginal delivery | na | na | na | na |
| C.5 | 1 | 2006 | stillbirth | male | 31+1 | emergency C-section | 1210 | na | na | SGA |
| C.6 | 1 | 2002 | live birth | male | 39+0 | C-section | 3760 | 53 | 37 | AGA |
| 2 | 2013 | live birth | male | 38+3 | C-section | 3030 | 51 | 33 | AGA | |
| 3 | 2015 | live birth | female | 38+0 | C-section | 3240 | 49 | 34 | AGA | |
| C.7 | 1 | 2002 | live birth | male | 40+3 | vaginal delivery | unknown | unknown | unknown | unknown |
| 2 | 2009 | live birth | male | 35+? | vaginal delivery | unknown | unknown | unknown | unknown |
Preg, pregnancy; AGA, appropriate for gestational age; C-section, caesarian section; ?, unknown; LGA, large for gestational age; na, not applicable; SGA, small for gestational age.
Lost to follow-up.
Intermediate care unit stay.
Intensive care unit stay.
Seven pregnancies were complicated by p-aHUS: two were full term live births (C.1, C.6); three were preterm births, two live births (C.3, C.7) and one stillbirth (C.5); and there were two early spontaneous abortions due to fetal death (C.2, C.4).
In total there were 21 live infants: 16 with birth weights appropriate for gestational age, and one who was large for gestational age; the weights of the remaining four infants are unknown. The overall rate of adverse fetal outcomes in the aHUS cohort was 22 per 100 pregnancies (95% CI, 10 to 41) and was 70 per 100 for those whose pregnancies were complicated by p-aHUS (95% CI, 33 to 93).
Renal Outcome and Pregnancy in aHUS
At the end of the study in June of 2015, the renal status of the 14 women in our aHUS pregnancy cohort was as follows: six have native kidneys (normal kidney function, two; CKD-G1, two (one had had p-aHUS); and CKD-G2 and CKD-G4, one each), whereas the remaining eight progressed to ESRD (caused by p-aHUS in one). Seven of these received renal allografts, of which three have stable renal function with CKD-G1T to -G3T, two have CKD-G4T, and two developed ESRD (caused by p-aHUS in one) (Table 1). The rate of ESRD in all 14 women from first pregnancy until 2015 was 6.36 per 100 person-years (95% CI, 2.56 to 13.11; Table 1).
Discussion
Pregnancy is a critical condition for women with aHUS because of its well documented ability to trigger episodes of TMA with the attendant risk of irreversible renal failure. These risks led to many women being counseled not to have children and to a considerable number deciding to remain intentionally childless.13,14 Recently this approach has been criticized for being over-pessimistic,4 and this view is strongly re-enforced by our analysis of pregnancy in women with aHUS in the Vienna TMA cohort. Specifically, our results show that: (1) only a quarter of the 27 pregnancies studied were complicated by episodes of p-aHUS; (2) episodes of p-aHUS were three times more common in the retrospectively studied pregnancies (33%), which were managed like conventional low-risk pregnancies, compared with those who were studied prospectively (11%) in a specialized unit and in most cases treated with prophylactic FFP; and (3) fetal outcomes were excellent in those without antenatal episodes of p-aHUS. Our data show that the outcome of pregnancies in women with aHUS is much better than commonly appreciated.
The overall prevalence of p-aHUS was 26% in our cohort, with five out of seven presenting during the first pregnancy. Four were “classic” p-aHUS episodes presenting postpartum (three with an additional trigger) and three patients with an initial diagnosis of preeclampsia/HELLP. With an incidence of 3%–5%, preeclampsia is the most frequent complication after 20th gestational week,15 and its early clinical presentation can mimic p-aHUS, HELLP syndrome, thrombotic thrombocytopenic purpura, acute fatty liver of pregnancy, and SLE. Distinguishing between them can be challenging,4,16 but the most discriminatory laboratory tests have been summarized.17 Glomerular endotheliosis is the morphologic hallmark of preeclampsia,18 whereas thrombosis within vessels and glomeruli is the central feature in aHUS,19 and HELLP syndrome leads to acute tubular necrosis.20,21 The physiologic changes during pregnancy complicate interpretation of hematologic tests,22 proteinuria,23 and serum concentrations of complement factors, because the reference ranges established for nonpregnant subjects are inappropriate during pregnancy.24,25
In a large, multicenter study of women with p-aHUS, Bruel et al.26 reported that 58% of episodes occurred during first pregnancy, whereas in our study 71% of episodes of p-aHUS occurred in the first pregnancy. However, both studies document that a previously uncomplicated pregnancy does not reduce the probability of developing p-aHUS in subsequent pregnancies. Similarly, neither the presence nor the nature of complement gene mutations appeared to influence the risk of p-HUS in either cohort. Currently, there are no factors that can be used to predict the risk of developing p-aHUS in a given pregnancy, other than the presence of one of the disease triggers identified in nonpregnant patients with aHUS. Caesarian section was recently proposed as a particular risk factor for p-aHUS.12 Although the proportion of caesarian sections in our cohort was more than twice as high (65% for deliveries ≥21 weeks of gestation) compared with the general population,27 only three out of 15 (20%) caesarean sections were associated with postpartum p-aHUS, and all three had additional potential triggers (bleeding, infection, stillbirth).
The goal for the management of pregnancy in aHUS is to minimize the risk of an episode of TMA and mitigate the injury caused should one develop. Two approaches have been suggested: prophylactic infusions of FFP,6 perhaps coupled with plasma exchange as recommended for patients with thrombotic thrombocytopenic purpura28; or alternatively expectant management with immediate treatment with eculizumab should an episode of TMA occur. This is feasible because the safety of eculizumab in pregnancy has been established in patients with paroxysmal nocturnal hemoglobinuria,29,30 and in a small number of individuals with p-aHUS.31–38
In the absence of data from randomized, controlled trials, we have adopted a pragmatic approach and have increased the maintenance dose of prophylactic FFP for those already receiving it,39 and have introduced it for those who were not. Our standard regimen is to infuse FFP (10–20 ml/kg body wt every other week40), starting at 15th gestational week, and continue at the original dose for at least 12 weeks postpartum. Additionally, we have infused FFP on the day of delivery and again 1 day later. None of the six pregnancies in patients treated in this way were complicated by p-aHUS and none were accompanied by evidence of dysregulated complement activity. In addition, there were three pregnancies in which we did not use prophylactic FFP or increase maintenance dose; one of these was associated with p-aHUS and the two others were not. Accordingly, the prophylactic use of FFP during pregnancy in women with aHUS appears safe, but effectiveness to prevent p-aHUS needs yet to be determined.
Despite good outcome of pregnancies, the long-term renal prognosis was poor. However, it is impossible to know whether pregnancy influenced this prognosis or whether it simply reflects the natural history of aHUS more generally. The latter would be consistent with data from a recent international study in which women with complement gene mutations progressed more frequently to dialysis-dependent renal failure.26
In conclusion, our study emphasizes the frequency of successful pregnancies in women with aHUS. Close monitoring of such pregnancies for episodes of TMA is essential, but currently, the available data do not allow firm recommendations about the best strategy to prevent them.
Concise Methods
Patients
Patients presenting with laboratory signs of TMA or/and biopsy-proven TMA in renal tissue are eligible to be enrolled in the Vienna TMA cohort of the Division of Nephrology and Dialysis at the Medical University of Vienna. Our study includes all enrolled female patients with at least one reported pregnancy until 2015. All patients enrolled in the study gave written informed consent, investigations are in accordance with the Declaration of Helsinki, and the ethics committee of the Medical University of Vienna approved the study. Subjects were classified as aHUS in the case of: laboratory signs of TMA, sequence variations within complement regulatory protein genes, and absence of secondary forms of TMA.41 Disease definitions, classifications, and calculations are detailed in the Supplemental Material. Data collection was on the basis of chart review. With respect to pregnancies, data were obtained by personal interview, review of hospital charts, and the Austrian mother/child book.
Laboratory Methods
Laboratory work-up was carried out at the Department of Laboratory Medicine, Medical University of Vienna, and at the 3rd Department of Internal Medicine, Research Laboratory, Semmelweis University in Budapest. Genetic investigations were performed according to standard procedures and as detailed in the supplement (www.kimcl.at, http://semmelweis.hu/kutlab/en/introduction).
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to thank Prof. Andrew Rees and Prof. Lisa Schulkind for editing the manuscript.
This work was partly supported by the National Research Fund (Nemzeti Kutatási, Fejlesztési és Innovációs Hivatal) of Hungary, PD116119 (to D.C.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016090995/-/DCSupplemental.
References
- 1.Fakhouri F, Roumenina L, Provot F, Sallée M, Caillard S, Couzi L, Essig M, Ribes D, Dragon-Durey MA, Bridoux F, Rondeau E, Frémeaux-Bacchi V: Pregnancy-associated hemolytic uremic syndrome revisited in the era of complement gene mutations. J Am Soc Nephrol 21: 859–867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robson JS, Martin AM, Ruckley V, Macdonald MK: Irreversible post-partum renal failure. A new syndrome. Q J Med 37: 423–435, 1968 [PubMed] [Google Scholar]
- 3.Dashe JS, Ramin SM, Cunningham FG: The long-term consequences of thrombotic microangiopathy (thrombotic thrombocytopenic purpura and hemolytic uremic syndrome) in pregnancy. Obstet Gynecol 91: 662–668, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Fakhouri F, Vercel C, Frémeaux-Bacchi V: Obstetric nephrology: AKI and thrombotic microangiopathies in pregnancy. Clin J Am Soc Nephrol 7: 2100–2106, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Lynch AM, Salmon JE: Dysregulated complement activation as a common pathway of injury in preeclampsia and other pregnancy complications. Placenta 31: 561–567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodship TH, Cook HT, Fakhouri F, Fervenza FC, Frémeaux-Bacchi V, Kavanagh D, Nester CM, Noris M, Pickering MC, Rodríguez de Córdoba S, Roumenina LT, Sethi S, Smith RJ; Conference Participants : Atypical hemolytic uremic syndrome and C3 glomerulopathy: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) controversies conference. Kidney Int 91: 539–551, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez de Córdoba S, Hidalgo MS, Pinto S, Tortajada A: Genetics of atypical hemolytic uremic syndrome (aHUS). Semin Thromb Hemost 40: 422–430, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Nester CM, Thomas CP: Atypical hemolytic uremic syndrome: What is it, how is it diagnosed, and how is it treated? Hematology (Am Soc Hematol Educ Program) 2012: 617–625, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Loirat C, Garnier A, Sellier-Leclerc AL, Kwon T: Plasmatherapy in atypical hemolytic uremic syndrome. Semin Thromb Hemost 36: 673–681, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nürnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C: Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 368: 2169–2181, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Licht C, Greenbaum LA, Muus P, Babu S, Bedrosian CL, Cohen DJ, Delmas Y, Douglas K, Furman RR, Gaber OA, Goodship T, Herthelius M, Hourmant M, Legendre CM, Remuzzi G, Sheerin N, Trivelli A, Loirat C: Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int 87: 1061–1073, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huerta A, Arjona E, Portoles J, Lopez-Sanchez P, Rabasco C, Espinosa M, Cavero T, Blasco M, Cao M, Manrique J, Cabello-Chavez V, Suñer M, Heras M, Fulladosa X, Belmar L, Sempere A, Peralta C, Castillo L, Arnau A, Praga M, Rodriguez de Cordoba S: A retrospective study of pregnancy-associated atypical hemolytic uremic syndrome [published online ahead of print September 11, 2017]. Kidney Int doi:S0085-2538(17)30482-9 [DOI] [PubMed] [Google Scholar]
- 13.Tong A, Brown MA, Winkelmayer WC, Craig JC, Jesudason S: Perspectives on pregnancy in women with CKD: A semistructured interview study. Am J Kidney Dis 66: 951–961, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Tong A, Jesudason S, Craig JC, Winkelmayer WC: Perspectives on pregnancy in women with chronic kidney disease: Systematic review of qualitative studies. Nephrol Dial Transplant 30: 652–661, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Williams D, Davison J: Chronic kidney disease in pregnancy. BMJ 336: 211–215, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.August P: Obstetric nephrology: Pregnancy and the kidney--inextricably linked. Clin J Am Soc Nephrol 7: 2071–2072, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Sibai BM: Imitators of severe preeclampsia. Obstet Gynecol 109: 956–966, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Spargo BH, Lichtig C, Luger AM, Katz AI, Lindheimer MD: The renal lesion in preeclampsia. Perspect Nephrol Hypertens 5: 129–137, 1976 [PubMed] [Google Scholar]
- 19.Stillman IE, Karumanchi SA: The glomerular injury of preeclampsia. J Am Soc Nephrol 18: 2281–2284, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Abraham KA, Kennelly M, Dorman AM, Walshe JJ: Pathogenesis of acute renal failure associated with the HELLP syndrome: A case report and review of the literature. Eur J Obstet Gynecol Reprod Biol 108: 99–102, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Pourrat O, Touchard G, Robert R, Badia P, Bauwens M, Hauet T, Patte D: A kidney biopsy is clearly mandatory to confirm the indication of plasma exchanges in adult haemolytic uraemic syndrome. Ann Med Interne (Paris) 145: 369–372, 1994 [PubMed] [Google Scholar]
- 22.Townsley DM: Hematologic complications of pregnancy. Semin Hematol 50: 222–231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Airoldi J, Weinstein L: Clinical significance of proteinuria in pregnancy. Obstet Gynecol Surv 62: 117–124, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Baines MG, Millar KG, Mills P: Studies of complement levels in normal human pregnancy. Obstet Gynecol 43: 806–810, 1974 [PubMed] [Google Scholar]
- 25.Reggia R, Ziglioli T, Andreoli L, Bellisai F, Iuliano A, Gerosa M, Ramoni V, Tani C, Brucato A, Galeazzi M, Mosca M, Caporali R, Meroni PL, Tincani A: Primary anti-phospholipid syndrome: Any role for serum complement levels in predicting pregnancy complications? Rheumatology (Oxford) 51: 2186–2190, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Bruel A, Kavanagh D, Noris M, Delmas Y, Wong EKS, Bresin E, Provôt F, Brocklebank V, Mele C, Remuzzi G, Loirat C, Frémeaux-Bacchi V, Fakhouri F: Hemolytic uremic syndrome in pregnancy and postpartum. Clin J Am Soc Nephrol 12: 1237–1247, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heim K: Geburtenregister Österreich, Geburtsjahr 2012. Available at: http://www.iet.at/data.cfm?vpath=publikationen210/groe/groe-jahresbericht-2012&download=yes, 2014. Accessed August 1, 2017
- 28.Scully M, Thomas M, Underwood M, Watson H, Langley K, Camilleri RS, Clark A, Creagh D, Rayment R, Mcdonald V, Roy A, Evans G, McGuckin S, Ni Ainle F, Maclean R, Lester W, Nash M, Scott R, O Brien P; collaborators of the UK TTP Registry : Thrombotic thrombocytopenic purpura and pregnancy: Presentation, management, and subsequent pregnancy outcomes. Blood 124: 211–219, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Kelly RJ, Höchsmann B, Szer J, Kulasekararaj A, de Guibert S, Röth A, Weitz IC, Armstrong E, Risitano AM, Patriquin CJ, Terriou L, Muus P, Hill A, Turner MP, Schrezenmeier H, Peffault de Latour R: Eculizumab in pregnant patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med 373: 1032–1039, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Hallstensen RF, Bergseth G, Foss S, Jæger S, Gedde-Dahl T, Holt J, Christiansen D, Lau C, Brekke OL, Armstrong E, Stefanovic V, Andersen JT, Sandlie I, Mollnes TE: Eculizumab treatment during pregnancy does not affect the complement system activity of the newborn. Immunobiology 220: 452–459, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Servais A, Devillard N, Frémeaux-Bacchi V, Hummel A, Salomon L, Contin-Bordes C, Gomer H, Legendre C, Delmas Y: Atypical haemolytic uraemic syndrome and pregnancy: Outcome with ongoing eculizumab. Nephrol Dial Transplant 31: 2122–2130, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Ardissino G, Wally Ossola M, Baffero GM, Rigotti A, Cugno M: Eculizumab for atypical hemolytic uremic syndrome in pregnancy. Obstet Gynecol 122: 487–489, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Mussoni MP, Veneziano FA, Boetti L, Tassi C, Calisesi C, Nucci S, Rigotti A, Panzini I, Ardissino G: Innovative therapeutic approach: Sequential treatment with plasma exchange and eculizumab in a pregnant woman affected by atypical hemolytic-uremic syndrome. Transfus Apher Sci 51: 134–136, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Delmas Y, Bordes C, Loirat C, Frémeaux-Bacchi V, Combe C: Post-partum atypical haemolytic-uraemic syndrome treated with eculizumab: Terminal complement activity assessment in clinical practice. Clin Kidney J 6: 243–244, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zschiedrich S, Prager EP, Kuehn EW: Successful treatment of the postpartum atypical hemolytic uremic syndrome with eculizumab. Ann Intern Med 159: 76, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Cañigral C, Moscardó F, Castro C, Pajares A, Lancharro A, Solves P, de la Rubia J, Carpio N, Sanz MA: Eculizumab for the treatment of pregnancy-related atypical hemolytic uremic syndrome. Ann Hematol 93: 1421–1422, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Kourouklaris A, Ioannou K, Athanasiou I, Panagidou A, Demetriou K, Zavros M: Postpartum thrombotic microangiopathy revealed as atypical hemolytic uremic syndrome successfully treated with eculizumab: A case report. J Med Case Reports 8: 307, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Sousa Amorim E, Blasco M, Quintana L, Sole M, de Cordoba SR, Campistol JM: Eculizumab in pregnancy-associated atypical hemolytic uremic syndrome: Insights for optimizing management. J Nephrol 28: 641–645, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Thomas MR, Robinson S, Scully MA: How we manage thrombotic microangiopathies in pregnancy. Br J Haematol 173: 821–830, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Licht C, Weyersberg A, Heinen S, Stapenhorst L, Devenge J, Beck B, Waldherr R, Kirschfink M, Zipfel PF, Hoppe B: Successful plasma therapy for atypical hemolytic uremic syndrome caused by factor H deficiency owing to a novel mutation in the complement cofactor protein domain 15. Am J Kidney Dis 45: 415–421, 2005 [DOI] [PubMed] [Google Scholar]
- 41.George JN, Nester CM: Syndromes of thrombotic microangiopathy. N Engl J Med 371: 1847–1848, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Fremeaux-Bacchi V, Fakhouri F, Garnier A, Bienaimé F, Dragon-Durey MA, Ngo S, Moulin B, Servais A, Provot F, Rostaing L, Burtey S, Niaudet P, Deschênes G, Lebranchu Y, Zuber J, Loirat C: Genetics and outcome of atypical hemolytic uremic syndrome: A nationwide French series comparing children and adults. Clin J Am Soc Nephrol 8: 554–562, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullivan M, Rybicki LA, Winter A, Hoffmann MM, Reiermann S, Linke H, Arbeiter K, Patzer L, Budde K, Hoppe B, Zeier M, Lhotta K, Bock A, Wiech T, Gaspert A, Fehr T, Woznowski M, Berisha G, Malinoc A, Goek ON, Eng C, Neumann HP: Age-related penetrance of hereditary atypical hemolytic uremic syndrome. Ann Hum Genet 75: 639–647, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Le Quintrec M, Lionet A, Kamar N, Karras A, Barbier S, Buchler M, Fakhouri F, Provost F, Fridman WH, Thervet E, Legendre C, Zuber J, Frémeaux-Bacchi V: Complement mutation-associated de novo thrombotic microangiopathy following kidney transplantation. Am J Transplant 8: 1694–1701, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Maga TK, Nishimura CJ, Weaver AE, Frees KL, Smith RJ: Mutations in alternative pathway complement proteins in American patients with atypical hemolytic uremic syndrome. Hum Mutat 31: E1445–E1460, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Sellier-Leclerc AL, Fremeaux-Bacchi V, Dragon-Durey MA, Macher MA, Niaudet P, Guest G, Boudailliez B, Bouissou F, Deschenes G, Gie S, Tsimaratos M, Fischbach M, Morin D, Nivet H, Alberti C, Loirat C; French Society of Pediatric Nephrology : Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol 18: 2392–2400, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Bienaime F, Dragon-Durey MA, Regnier CH, Nilsson SC, Kwan WH, Blouin J, Jablonski M, Renault N, Rameix-Welti MA, Loirat C, Sautés-Fridman C, Villoutreix BO, Blom AM, Fremeaux-Bacchi V: Mutations in components of complement influence the outcome of Factor I-associated atypical hemolytic uremic syndrome. Kidney Int 77: 339–349, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Bresin E, Rurali E, Caprioli J, Sanchez-Corral P, Fremeaux-Bacchi V, Rodriguez de Cordoba S, Pinto S, Goodship TH, Alberti M, Ribes D, Valoti E, Remuzzi G, Noris M; European Working Party on Complement Genetics in Renal Diseases : Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype. J Am Soc Nephrol 24: 475–486, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caprioli J, Noris M, Brioschi S, Pianetti G, Castelletti F, Bettinaglio P, Mele C, Bresin E, Cassis L, Gamba S, Porrati F, Bucchioni S, Monteferrante G, Fang CJ, Liszewski MK, Kavanagh D, Atkinson JP, Remuzzi G; International Registry of Recurrent and Familial HUS/TTP : Genetics of HUS: The impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood 108: 1267–1279, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sartz L, Olin AI, Kristoffersson AC, Ståhl AL, Johansson ME, Westman K, Fremeaux-Bacchi V, Nilsson-Ekdahl K, Karpman D: A novel C3 mutation causing increased formation of the C3 convertase in familial atypical hemolytic uremic syndrome. J Immunol 188: 2030–2037, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Fang CJ, Fremeaux-Bacchi V, Liszewski MK, Pianetti G, Noris M, Goodship TH, Atkinson JP: Membrane cofactor protein mutations in atypical hemolytic uremic syndrome (aHUS), fatal Stx-HUS, C3 glomerulonephritis, and the HELLP syndrome. Blood 111: 624–632, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richards A, Kemp EJ, Liszewski MK, Goodship JA, Lampe AK, Decorte R, Müslümanoğlu MH, Kavukcu S, Filler G, Pirson Y, Wen LS, Atkinson JP, Goodship TH: Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc Natl Acad Sci U S A 100: 12966–12971, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vernon KA, Goicoechea de Jorge E, Hall AE, Fremeaux-Bacchi V, Aitman TJ, Cook HT, Hangartner R, Koziell A, Pickering MC: Acute presentation and persistent glomerulonephritis following streptococcal infection in a patient with heterozygous complement factor H-related protein 5 deficiency. Am J Kidney Dis 60: 121–125, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monteferrante G, Brioschi S, Caprioli J, Pianetti G, Bettinaglio P, Bresin E, Remuzzi G, Noris M: Genetic analysis of the complement factor H related 5 gene in haemolytic uraemic syndrome. Mol Immunol 44: 1704–1708, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Meyer NC, Wang K, Nishimura C, Frees K, Jones M, Katz LM, Sethi S, Smith RJ: Causes of alternative pathway dysregulation in dense deposit disease. Clin J Am Soc Nephrol 7: 265–274, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.