Abstract
In patients with CKD, not only renal but also, nonrenal clearance of drugs is altered. Uremic toxins could modify the expression and/or activity of drug transporters in the liver. We tested whether the uremic toxin indoxyl sulfate (IS), an endogenous ligand of the transcription factor aryl hydrocarbon receptor, could change the expression of the following liver transporters involved in drug clearance: SLC10A1, SLC22A1, SLC22A7, SLC47A1, SLCO1B1, SLCO1B3, SLCO2B1, ABCB1, ABCB11, ABCC2, ABCC3, ABCC4, ABCC6, and ABCG2. We showed that IS increases the expression and activity of the efflux transporter P-glycoprotein (P-gp) encoded by ABCB1 in human hepatoma cells (HepG2) without modifying the expression of the other transporters. This effect depended on the aryl hydrocarbon receptor pathway. Presence of human albumin at physiologic concentration in the culture medium did not abolish the effect of IS. In two mouse models of CKD, the decline in renal function associated with the accumulation of IS in serum and the specific upregulation of Abcb1a in the liver. Additionally, among 109 heart or kidney transplant recipients with CKD, those with higher serum levels of IS needed higher doses of cyclosporin, a P-gp substrate, to obtain the cyclosporin target blood concentration. This need associated with serum levels of IS independent of renal function. These findings suggest that increased activity of P-gp could be responsible for increased hepatic cyclosporin clearance. Altogether, these results suggest that uremic toxins, such as IS, through effects on drug transporters, may modify the nonrenal clearance of drugs in patients with CKD.
Keywords: chronic kidney disease, ABC transporter, drug transporter, cyclosporine
Patients with CKD often require the prescription of numerous medications related to the disease and its complications and comorbidities.1,2 Changes in drug disposition make dose adjustments challenging in these patients, with frequently occurring drug-related adverse events.3,4
CKD not only alters the elimination of drugs excreted by the kidneys, but it also affects the metabolism of drugs subject to nonrenal clearance, which involves mainly the liver and intestines.5,6 Biotransformation and transport across cellular membranes are two essential cellular processes that control drug tissue distribution from the entry in the body to its elimination.7,8 How an uremic environment could modify these two processes is poorly understood5 due to the multiplicity and interplay of processes leading to the drug distribution and the broad substrate specificity of metabolic enzymes and transporters. In vitro studies using hepatocytes cultured with human uremic serum and animal models of CKD showed a reduced expression and direct inhibition of cytochrome P450 enzymes.6,9–11 A modification of expression and/or activity of some drug transporters, such as OATP1B1, OATP1B3, MRP4, BCRP, and P-glycoprotein (P-gp), is also observed in the context of uremia.6,12–15 These changes could be attributed to circulating molecules associated with CKD, such as uremic toxins.
Uremic solutes or toxins are compounds normally eliminated by the kidneys but accumulated in the blood and tissues of patients with CKD (http://www.uremic-toxins.org).16 They display a large variety in chemical structures and are classified into three groups according to their size and dialysis clearance: the small water-soluble compounds (molecular mass <500 D), the middle molecules (molecular mass >500 D), and the protein-bound compounds.16 The indolic uremic toxin indoxyl sulfate (IS), an end product of tryptophan metabolism by gut microbiota, is a protein-bound toxin poorly removed by dialysis.16 IS is a nephrovascular toxin, and its levels correlate with mortality in patients with CKD.17–19 It displays deleterious effects on endothelial and smooth vascular muscle cells that might participate in the adverse cardiovascular outcomes in patients with CKD.20 IS may also accelerate CKD progression; it induces fibrosis in rat kidneys via an increased expression of profibrotic and proinflammatory genes in tubular cells.21 The cellular receptor for IS is the ubiquitous transcription factor aryl hydrocarbon receptor (AhR), well known to mediate the toxicity of the widespread environmental pollutant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD).22–24 We have shown that the activation of AhR by IS in primary endothelial cells (human umbilical vein endothelial cells) leads to the increased expression of a part of the prototypic pattern of AhR-induced genes, such as those involved in detoxification processes.24
The aim of this study was to determine if IS could modify the expression of transporters known to be involved in the hepatic clearance of drugs and whether the AhR pathway is involved in this process. We first tested this hypothesis in vitro in the hepatocarcinoma cell line HepG2. We then investigated the effect of CKD on the hepatic expression of P-gp using two mouse models of CKD (adenine diet and remnant kidney models). Finally, we highlight the clinical relevance of our observations in a cohort of patients with CKD.
Results
IS Upregulated the Gene Expression of ABCB1 in HepG2 Cells
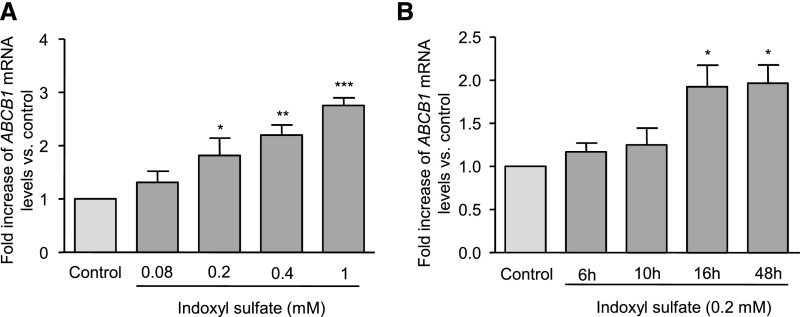
We tested whether IS altered the expression of genes encoding the drug liver transporters listed in Table 1.25–27 HepG2 cells were incubated for 16 hours with IS at the uremic concentration of 0.2 mM.28 Because IS is synthesized as a potassium salt, cells were also incubated with control medium containing KCl at 0.2 mM. IS only increased the expression of ABCB1, whereas no effect was observed on the other transporters screened (Table 1). ABCB1 gene encodes the transporter P-gp, which is also known as multidrug resistance gene 1. Dose-response experiments showed that IS increased the ABCB1 mRNA levels at 0.2, 0.4, and 1 mM by 81%, 120%, and 176%, respectively (Figure 1A). ABCB1 gene was overexpressed after 16 hours of incubation with IS and remained high until 48 hours (Figure 1B).
Table 1.
Expression of transporter genes after incubation of HepG2 cells with IS
| Gene (Transporter) | Drug Substrates25–27 | Fold Change of Expression after Incubation with IS |
|---|---|---|
| SLC10A1 (NTCP) | Atorvastatin, fluvastatin, pitavastatin, rosuvastatin | No change |
| SLC22A1 (OCT1) | Aciclovir, berberine, cimetidine, cis-diammine(pyridine) chloroplatin (ii), furamidine, ganciclovir, irinotecan, lamuvidine, metformin, oxaliplatin, palitacel, pentamidine, picoplatin, quinine, quinidine, zidovudine | No change |
| SLC22A7 (OAT2) | 5-Fluorouracil, allopurinol, bumetanide, cimetidine, erythromycin, methothrexate, paclitaxel, ranidine, salicylate, taxol, theophylline, tetracyclin, zidovudine | No change |
| SLC47A1 (MATE1) | Acyclovir, cephadrine, cephalexin, cimetidine, fexofenadine, gancyclovir, metformin, oxaliplatin, procainamide | No change |
| SLCO1B1 (OATP1B1) | Asunaprevir, bosentan, cerivastatin, danoprevir, enalapril, olmesartan, phalloidin, repaglinide, statins, temocaprilat, valsartan | No change |
| SLCO1B3 (OATP1B3) | Amanitin, bosentan, digoxin, docetaxel, enalapril, erythromycin, fexofenadine, fluvastatin, methotrexate, ouabain, paclitaxel, phaloidin, pitavastatin, rifampicin, rosuvastatin, statins, telmisartan, valsartan | No change |
| SLCO2B1 (OATP2B1) | Atorvastatin, benzylpenicillin, bosentan, ezetimibe-glucuronide, fexofenadine, fluvastatin, glibenclamide, montelukast, pitavastatin, pravastatin, rosuvastatin | No change |
| ABCB1 (MDR1; PGP) | Amiodarone, berberine, bisantrene, carbamazepine, celiprolol, chloroquine, colchicine, cyclosporin, daunorubicin, desipramine, digitoxin, digoxin, docetaxel, doxorubicin, erythromycin, etoposide, fexofenadine, grepafloxacin, imatinib, indinavir, ivermectin, levofloxacin, lidocaine, losartan, lovastatin, irinotecan, loperamide, methadone, methotrexate, mibefradil, mitoxantrone, morphine, nelfinavir, ortataxel, paclitaxel, ritonavir, saquinavir, seliciclib, sirolimus, sparfloxacin, sumatriptan, tacrolimus, talinolol, terfenadine, topotecan, vecuronium, vinblastine, vincristine | 2.5±0.2 |
| ABCB11 (BSEP) | 5-Fluorouracil, pravastatin, vinblastine | No change |
| ABCC2 (MRP2) | Cisplatin, doxorubicin, etoposide, glutathione and glucuronide conjugates, glucuronidated SN-38, methotrexate, mitoxantrone, olmesartan, valsartan, vinblastine | No change |
| ABCC3 (MRP3) | Acetaminophen glucuronide, etoposide, fexofenadine, methotrexate, teniposide, vincristine | No change |
| ABCC4 (MRP4) | Methotrexate | No change |
| ABCC6 (MRP6) | Etoposide | No change |
| ABCG2 (BCRP) | Abacavir, albendazole sulfoxide, anthracyclines, camptothecin, cerivastatin, cimetidine, ciprofloxacin, daunorubicin, dipyridamole, doxorubicin, edaravone, erlotinib, flavopiridol, gefitinib, glibenclamide, imatinib, irinotecan, lamivudine, methotrexate, mitoxantrone, nelfinavir, nitrofurantoin, norfloxacin, nucleoside analogs, ofloxacin, olmesartan, oxfendazole, pantoprazole, pitavastatin, prazosin, rosuvastatin, SN-38, statins, sulfasalazine, topotecan, zidovudine | No change |
Representative substrates for drug transporters in HepG2 cells and their expression evaluated by RT-PCR after exposure to IS (0.2 mM) for 16 hours. Analyses used a cutoff of twofold upregulated genes compared with control cells (KCl; 0.2 mM). Data are expressed as mean±SEM of four independent experiments. MDR1, multidrug resistance gene 1.
Figure 1.
IS increased the expression of ABCB1 in HepG2 cells. HepG2 cells were incubated with IS at (A) different concentrations and (B) different incubation times. mRNA levels of ABCB1 were quantified by quantitative comparative RT-PCR. Data are expressed as mean±SEM of (A) four and (B) six independent experiments. *P<0.05; **P<0.01; ***P<0.001.
IS Increased the Protein Expression and Activity of P-gp
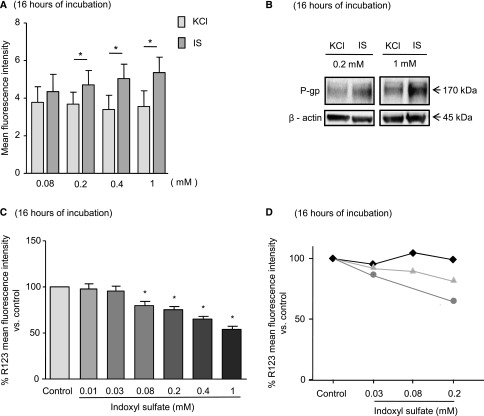
We tested whether IS increased the amount and activity of P-gp in HepG2 cells after 16 hours of incubation. Flow cytometry analysis revealed that IS at 0.2, 0.4, and 1 mM significantly increased the amounts of P-gp by 22%, 52%, and 51%, respectively (Figure 2A). By Western blot analysis, IS at 0.2 and 1 mM increased the amount of P-gp by 1.7- and 2.5-fold, respectively (Figure 2B). The efflux activity of P-gp was evaluated using its fluorescent substrate rhodamine 123 (R123). Flow cytometry analysis showed that IS at 0.08, 0.2, 0.4, and 1 mM decreased the intracellular concentrations of R123 by 13%, 19%, 34%, and 44%, respectively (Figure 2C), whereas at 0.03 and 0.01 mM, IS did not have an effect. The increase in P-gp activity was inhibited by P-gp inhibitors elacridar at 2 μM and verapamil at 50 μM (Supplemental Figure 1).
Figure 2.
IS increased the expression and activity of P-gp in HepG2 cells and the activity of P-gp in human primary hepatocytes. The expression of P-gp was evaluated in HepG2 by (A) flow cytometry and (B) Western blot. (C and D) The transporter efflux activity was evaluated by using its fluorescent substrate R123 (5 μM) in (C) HepG2 or (D) three samples of human primary hepatocytes. (A and C) Data are expressed as mean±SEM of six independent experiments. *P<0.05.
To confirm the effects observed on tumoral cell line, we used human primary hepatocytes from three donors to study the effect of IS on the R123 efflux. These primary cells are more sensitive to IS than HepG2, because mortality is high in the presence of IS at 1 mM. The responses of the donors displayed heterogeneity: one sample showed no response, the second showed a weak effect (18% of reduction at 0.2 mM IS), and the third showed a higher effect (36% of reduction with 0.2 mM IS) (Figure 2D).
Human Albumin Did Not Alter the Effects of IS during Short and Long Incubations
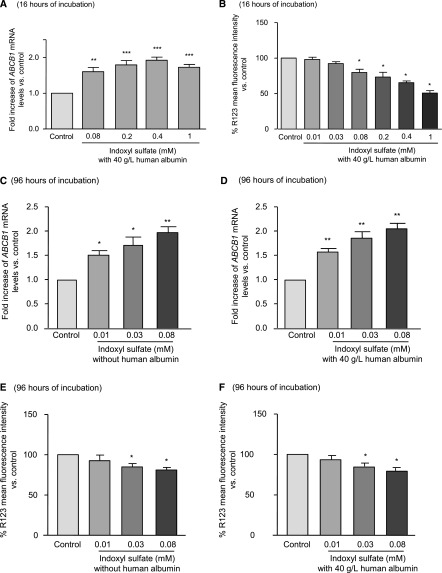
In blood, albumin binds to IS.29 Because culture medium contains only 2.5 g/L of albumin, we added human albumin at 40 g/L (Figure 3). Physiologic concentration of albumin did not affect the effects of IS on ABCB1 mRNA levels and P-gp activity after 16 hours of incubation (Figure 3, A and B).
Figure 3.
Human albumin did not modify the effects of IS during short or long incubations. Analysis of ABCB1 mRNA and P-gp activity in HepG2 cells incubated with IS or KCl (A and B) during 16 hours in the presence of human albumin or (C–F) for 96 hours with or without albumin. Results represent the mean±SEM of five independent experiments. *P<0.05; **P<0.01; ***P<0.001.
To mimic in vivo conditions (long-term exposure with lower concentrations of IS), HepG2 was incubated with lower concentrations of IS (0.08, 0.03, and 0.01 mM) for 96 hours instead of 16 hours with or without human albumin. IS at 0.03 and 0.08 mM increased both ABCB1 mRNA level (by 72% and 98%, respectively) and P-gp activity (intracellular concentration of R123 was decreased by 17% and 21%, respectively) (Figure 3, C and E). IS at 0.01 mM increased mRNA levels of P-gp but did not increase its activity. The same results were obtained in the presence of human albumin (Figure 3, D and F).
AhR Pathway Is Involved in the Induction of P-gp by IS
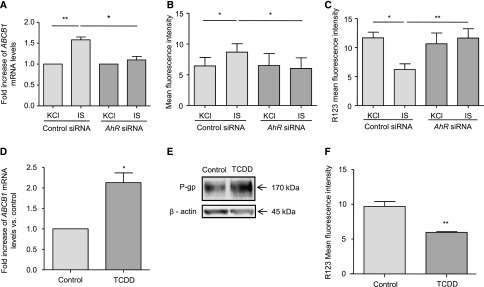
Because IS is an endogenous ligand of the transcription factor AhR, we studied the involvement of AhR pathway in the effect of IS on P-gp expression by using small interfering RNA (siRNA) targeted against AhR.24 AhR siRNA induced a significant decrease in AhR mRNA and protein levels (Supplemental Figure 2). The increased expression of P-gp induced by IS was completely abolished by AhR silencing at mRNA and protein levels, whereas scrambled control siRNA had no effect (Figure 4, A and B). Consequently, in cells treated with AhR siRNA, IS did not lead to an increased efflux activity of P-gp (Figure 4C). Furthermore, TCDD, the most well known agonist of AhR, increased gene expression of ABCB1, P-gp level (2.4-fold increase), and its efflux activity (Figures 4, D–F).
Figure 4.
Induction of P-gp expression and its activity by IS is dependent on the AhR pathway. (A–C) Analysis of ABCB1 mRNA, P-gp expression, and activity in HepG2 cells transfected with control siRNA or against AhR (AhR siRNA). (D–F) Analysis of ABCB1 mRNA, P-gp expression, and P-gp activity in HepG2 cells incubated with TCDD (30 nmol/L). Results represent the mean±SEM of (A) five and (B–D and F) four independent experiments. *P<0.05; **P<0.01.
P-gp Expression Is Increased in Two Mice Models of CKD
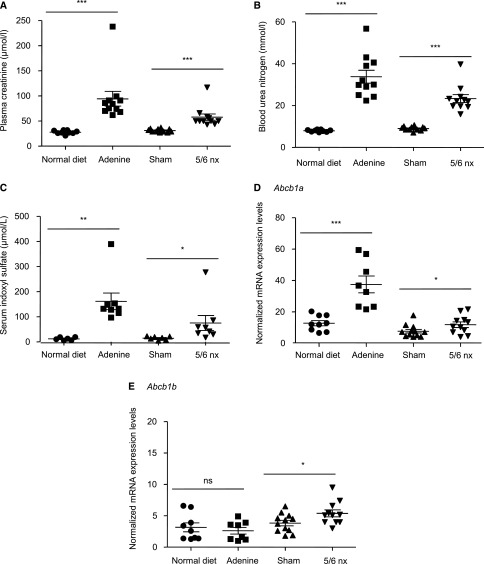
The adenine diet30 and the remnant kidney (5/6 nephrectomy)31 models of CKD were used to test whether the uremic environment could modify the liver expression of the ABCB1 mouse ortholog genes Abcb1a and Abcb1b. The two mice models of CKD showed significant increased plasma concentrations of creatinine, blood urea nitrogen, and IS (Figure 5, A–C), with a greater increase in the adenine diet model compared with the 5/6 nephrectomy model. In the adenine diet model, the kidney disease is induced by the deposition of adenine crystals in the kidneys, and we investigated whether liver could suffer from such damages. Histologic sections showed the presence of crystals in kidneys but not in livers (Supplemental Figure 3) as previously described.32 An increase in mRNA levels of Abcb1a was detected in the liver of mice with CKD compared with control mice, with an effect being more pronounced in adenine diet (Figure 5D). Abcb1b was only increased in 5/6-nephrectomized mice (Figure 5E).
Figure 5.
IS levels and abcb1 expression are increased in two mice models of CKD. Kidney function in mice was assessed using (A) plasma creatinine and (B) BUN, which indicates a reduction in GFR in adenine-treated mice and 5/6-nephrectomized (5/6 nx) mice. (C) Serum concentrations of IS in mice were increased in the two models of CKD. (D and E) mRNA expression levels of two genes, abcb1a and abcb1b, that encode to P-gp in mice. *P<0.05; **P<0.01; ***P<0.001.
Patients with Higher Levels of IS Needed to Take Higher Doses of Cyclosporin
We studied 109 patients with CKD (eGFR<60 ml/min per 1.73 m2; stages 3–5) under immunosuppressive treatment with cyclosporin after kidney or heart transplantation. Baseline characteristics of patients are listed in Table 2. Patients exhibited significantly increased levels of indolic uremic toxins (median [25th–75th percentile]; IS: 4.2 μM [0.06–11.7]; indole-3-acetic acid [IAA]: 1.9 μM [1.3–2.7]). Because of the large pharmacokinetic variability, treatment with cyclosporin needs obligatory therapeutic drug monitoring. To achieve the efficient blood target concentration, the correct dose of cyclosporin is then adjusted for each patient. Multivariate analysis of parameters that influence the dosage of cyclosporin showed that cyclosporin dosage requirement was significantly associated with levels of IS (P<0.05) (Table 3). We did not find a correlation between IAA levels and this ratio. Patients with higher levels of IS were observed to require higher doses of cyclosporin to reach the efficient blood target level needed to prevent transplant rejection.
Table 2.
Characteristics of heart and kidney transplant recipients with CKD
| Transplant Recipients Characteristics | Total, n=109 |
|---|---|
| Cyclosporin dosage, mg/d per 1 kg | 1.81 (1.51–2.13) |
| Sex ratio, women/men | 37/72 |
| Age, yr | 54.5 (47–62) |
| Body mass index, kg/m2 | 22.78 (20.5–25.9) |
| Cyclosporin blood concentration, mg/L | 87 (68.5–122.5) |
| Years after transplantation | 6 (4–8) |
| eGFR (CKD-EPI), ml/min per 1.73 m2 | 39.3 (32.2–50.1) |
| IS, μM | 4.2 (0.06–11.7) |
| IAA, μM | 1.9 (1.3–2.7) |
Results from recipient characteristics represent the medians and the interquartile ranges (25th–75th percentiles). CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Table 3.
Multiple linear regression modeling the relationship between cyclosporin dosage and IS levels
| Cyclosporin Dosage, mg/d per 1 kg | Coefficient | 95% CI | P Value |
|---|---|---|---|
| IS, μM | 0.01 | 0 to 0.03 | 0.03 |
| IAA, μM | −0.04 | 0.14 to 0.07 | 0.48 |
| Cyclosporin target blood concentration, mg/L | 0.010 | 0.006 to 0.015 | <0.001 |
| Recipient sex | 0.44 | 0.06 to 0.82 | 0.02 |
| Body mass index, kg/m2 | −0.09 | −0.14 to −0.05 | <0.001 |
| Age, yr | −0.01 | −0.03 to 0.01 | 0.18 |
| Time since transplant | 0 | −0.05 to 0.04 | 0.83 |
| eGFR (CKD-EPI), ml/min per 1.73 m2 | 0.01 | −0.01 to 0.02 | 0.33 |
R2 value of the model: 0.54. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Discussion
We showed that IS increases the expression and activity of P-gp in HepG2 cells in an AhR-dependent way. P-gp is an efflux pump exporting lipophilic substrates, including multiple drugs. It is expressed mainly in epithelia with a barrier function (brain, liver, intestine, and kidneys).33 It is involved in the decreased drug accumulation and development of resistance to various drugs. In mice models of CKD, the decline in renal function led to higher IS serum levels and an upregulation of liver Abcb1a expression. In patients with transplants and CKD treated by cyclosporin, which is a P-gp substrate, patients with higher serum levels of IS required higher doses of cyclosporin to achieve the serum target concentration.
A large gap exists between the observation of altered clearance of drugs in patients with CKD and the knowledge of mechanisms involved.5 We, therefore, tested the effect of IS on the expression of the main transporters known to be involved in drug clearance by the liver.6 We used the cell line HepG2 derived from a human hepatocellular carcinoma, because the expression of efflux transporters and cell polarity was maintained.34–36 These characteristics make HepG2 an adequate tool to study transporters expression.
We found that, except for P-gp, IS did not affect the mRNA levels of the other drug transporters analyzed. However, we could not exclude the possibility that IS can affect their activity in another way, such as by competitive inhibition. Indeed, IS and two other uremic toxins, kynurenic acid and IAA, have been shown to directly inhibit SCLO1B1- and SCLO1B3-mediated transport15 as well as MRP4 (ABCC4) and BCRP (ABCG2)–mediated efflux.12 Tsujimoto et al.37 showed that treatment of a hepatocellular carcinoma cell line (Hep3B) with uremic serum resulted in downregulation of the expression of OATP1B1 and OATP2B1 transporters. We were not able to confirm these results, but we evaluated here only the effect of IS. In addition, in CKD rats, MRP6 protein levels were reduced in the liver and kidney tissues, whereas its mRNA levels (ABCC6) were unchanged, pointing to post-translational mechanisms.38
To our knowledge, our study is the first to show that IS increases P-gp expression and its activity in HepG2 cells. We used a large range of IS concentrations (0.01–1 mM), because patients with CKD exhibit variable levels of IS (mean uremic concentration, 0.108 mM; highest value reported, 0.209 mM). Because in our cohort of patients with transplants, renal function is partly retained (39.3 ml/min per 1.73 m2), the mean concentration (0.010 mM) and the highest value of IS (0.126 mM) are lower. We observed an effect on ABCB1 mRNA level and P-gp for the highest concentrations of IS used (1, 0.4, and 0.2 mM). At 0.08 mM, a slight increase was observed, which was significant only for the activity of P-gp and was not significant for ABCB1 mRNA and P-gp levels. At 0.03 and 0.01 mM, IS did not affect P-gp. Thus, we ask if IS could act when used at these lower concentrations but after a longer time of incubation (96 hours instead of 16 hours) to mimic the in vivo conditions. After 96 hours of incubation at 0.08 and 0.03 mM, IS increased ABCB1 mRNA and P-gp activity. At 0.01 mM, no effect of IS was observed. The values of IS for which we observed an effect were in the range of those of our patients. Incubation for several days with concentrations found in patients certainly represents a more realistic situation than shorter incubations with supraphysiologic concentrations. Indeed, patients with CKD are continuously exposed systematically to uremic toxins, especially protein-bound compounds, which are badly removed by dialysis. In addition, we tested the effect of IS on three samples of human primary hepatocytes. The results obtained for the efflux of R123 display heterogeneity. Jigorel et al.39 found similar responses in terms of P-gp induction by TCDD, which extended from no response to threefold induction. Indeed, the high functional variability of these primary cells is well known and constitutes the obstacle for their use in routine.34 Despite this problem, we found that IS increases the efflux of R123 in human primary hepatocytes, because two of the three different samples tested have responded to it, suggesting that this effect of IS could take place in vivo.
This increase in P-gp expression induced by IS was AhR dependent, because AhR silencing abolished these effects. Indolic uremic toxins, such as IS, IAA, and indole-3-carbinol, are known to activate AhR.22,40,41 Schroeder et al.22 showed that IS activates AhR by direct binding. We have previously shown that IS and IAA increase cyclo-oxygenase 2 and tissue factor in an AhR-dependent way.24,42 P-gp is regulated by numerous transcription factors, among which are the xenosensor receptor pregnane X receptor, the constitutive androstane receptor, and the AhR.40,43 AhR is a transcription factor mainly located in the cytoplasm and associated with Hsp90, XAP2, and p23.44–46 Ligand binding to AhR leads to its activation and dissociation from these other partners. Then, AhR translocates to the nucleus, where in association with ARNT, it binds to specific DNA consensus sequences (the xenobiotic response element) in the promoters of target genes and induces their transcription.44,45 In addition to this genomic pathway, AhR has been shown to act as a signaling molecule without DNA binding.46,47 The involvement of AhR in the regulation of P-gp was not specific for IS, because TCDD has been shown to increase P-gp expression and activity in rat brain capillaries and reduce the accumulation of its substrates.48 We also showed that TCDD was able to increase the expression and activity of P-gp in HepG2. In human hepatocyte primary culture, the response to TCDD in terms of P-gp expression is highly variable, ranging from no modification to an increase.39 These results underline the complexity of P-gp regulation and could be explained by the facts that P-gp expression is under the control of multiple transcription factors and that each of them, like AhR, is involved in crosstalking with multiple signaling pathways.
We have shown that the effects of IS on P-gp expression are AhR dependent; however, the exact mechanism by which this is exerted is not elucidated. The increase in ABCB1 mRNA level was significantly detectable after 16 hours of incubation with IS, suggesting that direct binding of AhR to the promoter region of ABCB1 is not required. This is in accordance with the fact that xenobiotic response element was not found in its promoter region.49 Therefore, P-gp overexpression due to AhR activation must be related to the nongenomic pathway.
IS belongs to protein-bound uremic toxins. The degree of protein binding is high, because the free fraction represents 10% of total amount of IS.29 In vivo, albumin is the main binding protein for IS. The lack of albumin in culture medium can lead to an increase in the free fraction of IS. Thus, to approach the in vivo conditions of protein binding, we added to the culture medium: human albumin at 40 g/L. The presence of human albumin did not alter the effects of IS, suggesting that cellular uptake is not hampered by protein binding, which was recently shown for kidney proximal tubules50 as well, and that the overall amount of IS, the free and the bound states, is required to act on AhR and P-gp.
In rodents, P-gp exists as two isoforms displaying 90% of homologies that are encoded by two closely located genes: Abcb1a and Abcb1b.51 These isoforms are considered to fulfill the same function as the ABCB1 gene in humans.51 In the liver of two mice models of CKD, we found a significant increase in the mRNA expression of Abcb1a, whereas Abcb1b was only increased in 5/6-nephrectomized mice. The significant greater increase in mRNA level of Abcb1a in the adenine diet model could be related to the more advanced level of kidney disease in this model as suggested by the higher values of creatinine, urea, and consequently, IS. In addition, the fact that the Abcb1b mRNA level was only increased in nephrectomized mice suggested that metabolic modifications induced by adenine diet prevent this effect. Our results in CKD mice are in accordance with previous work showing an increase in P-gp expression and activity in the liver of 5/6-nephrectomized rats.52 Altogether, these results suggest that CKD is responsible for the increased expression of P-gp rather than the methods used to induce uremia (5/6 nephrectomy and adenine diet). In addition, because uremic sera from CKD rats52 and patients with CKD11 also led to an increase in P-gp activity in rat hepatocyte cell cultures, the authors suggested that seric factors may be involved. Because nothing was proven for other indole-derived compounds, we propose that uremic toxins, such as IS, could be some of the factors contributing to the upregulation of P-gp expression in CKD rodents.
In patients, we used an indirect method to analyze the activity of P-gp. The lipophilic nature of cyclosporin allows its entry into the cell by passive diffusion, but the exit of cyclosporin is dependent on the efflux transporter P-gp. Cyclosporin undergoes predominantly biliary clearance, with only 6% of renal excretion of unmodified cyclosporin.53 In this context, coupling of P-gp and cyclosporin is interesting to study in patients, because P-gp seems to be a determinant for cyclosporin bioavailability. In patients with transplants and CKD on cyclosporin treatment, we observed a correlation between the serum levels of IS and the need for higher dosage of the drug to achieve the target blood levels of cyclosporin. In other words, patients with higher levels of IS must take higher doses of cyclosporin to reach therapeutic levels to prevent transplant rejection. IS has been shown to slightly inhibit CYP3A4 at concentrations found in patients with CKD.54,55 However, this should lead to an increase in cyclosporin bioavailability, which was not observed. Together, these findings support our results and lead to the hypothesis that CKD conditions could cause an upregulation of the efflux transporter P-gp in liver. This upregulation could be responsible for increased liver clearance of cyclosporin and a decrease in its bioavailability. In accordance with our results, a recent meta-analysis revealed that one of the most widely described polymorphisms responsible for an increase in P-gp activity, C3435T, was associated with higher dose requirements in kidney transplant recipients.56,57 In addition, mice invalited for the Abcb1a gene, cannot eliminate cyclosporine and therefore display higher serum levels of this drug.58 The absence of correlation between IAA and dose of cyclosporin suggests that IAA cannot modify P-gp expression in an AhR-dependent way. This result seems, at first glance, unexpected, because these two toxins exhibit a similar structure and both are able to activate AhR; however, it can be explained in several ways. IAA has a carboxyl group attached to the indole ring, and IS has a sulfate group. This difference could lead to specificity in the pattern of gene expression between them other than the classic pattern of AhR activation. According to this, IAA and IS display different binding degrees for AhR22 that could be translated into differences in AhR activation and thus, differences in gene regulation. In addition, in clinical studies, where the levels of IS and IAA were measured simultaneously, common24,59 but also specific60–62 associations were found between these toxins and clinical parameters, arguing that not all indolic solutes act similarly. Finally, we could not exclude that IAA levels, which were lower than those of IS in our cohort, might have been too low to act in vivo on P-gp expression.
It is clearly established that CKD greatly influences the nonrenal clearance of many drugs, justifying dosing adjustment.4–6,11,63 Because of their importance in the overall elimination of drugs, transporters are decisive, even for drugs that are primarily metabolized by enzymes. However, drug distribution can also be affected by other factors, such as the drug’s physical and chemical characteristics, plasma protein binding, organelle sequestration, individual genetic conditions, and nutritional status. All of these factors may influence the pharmacokinetics of drugs, and they are exactly what makes it difficult to identify specific molecular mechanisms in patients.
In conclusion, we provide evidence that P-gp may be involved in an AhR-dependent detoxification process induced in response to the uremic toxin IS. It could lead to a possible subsequent reduction in the intracellular accumulation of P-gp substrates and changes in their elimination rates. We propose that the altered clearance of drugs associated with CKD could be due to the effects of some uremic toxins on transporter expression and activity. It is important to identify the transporters affected during CKD and the factors that can modulate their activities to improve the use of drugs in patients with CKD and reduce their secondary effects.
Concise Methods
Cell Culture and Treatments
HepG2 cells obtained from hepatocellular carcinoma (ATCC, Life Technologies, Saint Aubin, France) were grown in α-MEM (Life Technologies) containing 10% premium FBS (Life Technologies) and 400 mg/L of G418 (Geneticin; Life Technologies) under standard cell culture conditions (humidified atmosphere, 5% CO2, and 37°C). IS (Sigma-Aldrich, Saint Quentin Fallavier, France) was diluted from a stock solution prepared in water. The concentrations of IS were 0.01, 0.03, 0.08, 0.2, 0.4, and 1 mM, and they were in the range of those found in patients with CKD.28 Because IS is provided in a potassium salt form, we used KCl at equivalent concentrations as controls other than culture medium alone. We found no differences between cells treated with culture medium alone or KCl. For some experiments, 40 g/L of human albumin (VIABELEX; Laboratoire LFB Biomédicaments, Les Ulis, France) was added to the culture medium to mimic the in vivo conditions of human albumin blood concentration. For the incubation of 96 hours, the medium was changed three times. Three samples of human primary hepatocytes were purchased from Lonza (Levallois, France) and used according to the manufacturer’s instructions. Briefly, the cells were rapidly thawed and seeded at 106 cells per 1 ml for 2 days. The medium containing IS or KCl was then added for 16 hours. TCDD (Sigma-Aldrich), an AhR agonist, diluted in DMSO (Sigma-Aldrich) was used at 30 nM and compared with medium containing DMSO. Cells were treated during the indicated times.
Mice
C57BL/6J wild-type mice were purchased from The Jackson Laboratory and maintained as a breeding colony in the animal care facility at the Faculty of Medicine of Marseille. Male mice with CKD were used in this study. At 13 weeks of age, the mice were fed ad libitum with a 0.25% adenine-enriched diet (A04+0.25% adenine; SAFE, Augy, France) or a regular chow diet (A04 standard; SAFE) as controls. After adenine was administered for 2 weeks to induce uremia,30 it was withdrawn, and mice were fed with the regular chow diet for another week. By hematoxylin and eosin staining of kidney and liver sections (5 μm), we observed the presence of 2,8-dihydroxyadenine crystals in the kidney but did not in the liver of mice fed with adenine (Supplemental Figure 3) as previously described.32
As a second model of CKD, we performed 5/6 nephrectomy using a two-step procedure with minor modifications as previously described.31 Briefly, mice (9 weeks old) underwent 5/6 nephrectomy or sham surgery under general anesthesia (ketamine 125 mg/kg, xylazine 12.5 mg/kg, and atropine 0.25 mg/kg intraperitoneally). The left kidney was exposed through a flank incision, and we applied cortical electrocautery to the upper and lower poles (two thirds of the left kidney). One week later, we performed right total nephrectomy through a similar incision. Mice were euthanized at 6 weeks after induction of CKD. Sera were obtained from blood withdrawn via cardiac puncture during euthanasia. Creatinine and urea levels were measured with an Olympus AU400 auto analyzer at the Biochemistry Laboratory of the Centre de Recherche sur l’Inflammation (UMR 1149 INSERM, Université Paris Diderot, ERL CNRS 8252, Paris, France).
Total RNA Extraction from HepG2 Cells and Mouse Livers
Total RNA was extracted from HepG2 by RNeasy Mini Kit (Qiagen, Courtaboeuf, France). Mouse livers were lysed in TRIzol, and total RNA was purified by chloroform extraction and isopropanol precipitation. RNA concentration was determined using a NanoDrop Spectrophotometer (Thermoscientific, Wilmington, DE).
Comparative Quantification of mRNA Levels
Gene expression was analyzed by reverse transcription and comparative PCR. To analyze mRNA levels in HepG2 cells, primers were designed by the software primer3 (http://bioinfo.ut.ee/primer3-0.4.0/) to systematically encompass an intron to avoid amplification of contaminating genomic DNA. Reverse transcription using random primers and oligodT was performed on 500 ng of total RNA of each sample using the Takara PrimeScript RT Reagent Kit (Ozyme, Saint Quentin en Yvelines, France) followed by PCR on 12.5 ng of cDNA using the Takara SYBR qPCR Premix Ex Taq (Ozyme). PCR reactions were made with Mx3000P (Agilent, Massy, France) using comparative quantification of mRNA levels. All PCR reaction efficiencies were determined with Mx3000P software, and they were always superior to 90%. The results shown in this study were normalized with HPRT (housekeeping gene). The fusion curves were analyzed to assess the specificity of detected fluorescence. The sequences of the primers for HPRT1 and AhR are listed below:
HPRT (HPRT1; HGNC:5157)
HPRT-F 5′GGATTATACTGCCTGACCAAGGAAAGC3′
HPRT-R 5′GAGCTATTGTAATGACCAGTCAACAGG3′
AhR (AhR; HGNC:348)
AhR-F 5′TGTTGGACGTCAGCAAGTTC3′
AhR-R 5′TGGTGCCCAGAATAATGTGA3′.
The primers specific for transporters (SLC10A1, SLC22A1, SLC22A7, SLC47A1, SLCO1B1, SLCO1B3, SLCO2B1, ABCB1, ABCB11, ABCC2, ABCC3, ABCC4, ABCC6, and ABCG2) are listed in Supplemental Table 1.
To analyze mRNA levels in mouse livers, reverse transcription was performed from 500 ng of total RNA with the Takara PrimeScript RT Reagent Kit. Primers and MGB‐Taqman probes were purchased from Life Technologies (abcb1a: Mm00440761_m1; abcb1b: Mm00440736_m1; and the housekeeping gene Gusb: Mm01197698_m1). The PCR reaction mixture was prepared using the Brilliant II QPCR Master Mix (Agilent Technologies, Les Ulis, France). All PCR reactions were performed with the Mx3000P. The data were acquired and analyzed with the MxPro software (Agilent Technologies). Target gene expression was normalized on the basis of the Gusb content of each sample, and it was subsequently normalized to a basal mRNA level with the equation: N target =2ΔCt sample, where ΔCt is the Ct value of the target gene minus the Ct value of the Gusb gene. The results are reported as normalized mRNA levels (i.e., the N target value divided by the N target value of the smallest quantifiable amount of target gene mRNA [target gene Ct value =35]).
Uremic Toxins Measurement
IS was measured in human serum by HPLC using a reversed phase column, an ion-pairing mobile phase, and an isocratic flow as described.28 The sera of patients were collected, processed, and stored at −80°C until determination of uremic toxin concentrations.
R123 Accumulation Assay
Cells were cultured in the 24-well plates with a density of 15×104 per well for 24 hours. Then, cells were treated overnight with KCl or IS. Cells were washed once with HBSS; then, they were incubated for 5 minutes with HBSS containing or not containing 5 μM R123, a fluorescent substrate for P-gp.64 Cells were washed twice with HBSS in the presence or absence of the P-gp inhibitors verapamil and elacridar at 50 and 2 μM, respectively. Then, cells were subsequently incubated with HBSS with or without the P-gp inhibitors for 30 minutes. Cells were washed once with cold HBSS before the addition of 200 μl enzyme-free dissociation buffer at 37°C for about 2 minutes. The dissociated cell suspensions from each well were then placed in 15-ml tubes and subjected to centrifugation at 4°C for 3 minutes. The supernatant was discarded, and the cell pellet was reconstituted in ice cold HBSS-0.1% FBS for flow cytometric analysis immediately using a Gallios Flow Cytometer (Beckman-Coulter, Roissy, France).
Protein Extraction from HepG2
Cells were cultured in the T75 flasks with a density of 1×106 per flask for 24 hours. Then, cells were treated overnight with IS or TCDD and their controls KCl or DMSO, respectively. Cell culture flasks were placed in ice and washed once with cold PBS. Then, 500 μl cold RIPA buffer (50 mM Tris⋅HCl, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, and 1 mM EDTA, pH 7.4) containing protease inhibitors (Complete Mini; Roche Diagnostics France, Meylan, France) was added and kept at 4°C for 30 minutes. Cells were scraped, transferred to a microfuge tube, and maintained at constant agitation for 30 minutes at 4°C. Samples were sonicated for 1 minute and then kept on ice for another 1 minute between each pulse; this procedure was repeated three times. The homogenate was then centrifuged at 12,000×g for 15 minutes at 4°C, and the supernatant was collected. Protein concentrations were measured with the Bicinchoninic Acid Kit for Protein Determination (BCA1; Sigma-Aldrich).
Western Blot Analyses
Equal amounts of total protein (40 μg) from cell lysates were loaded on 4%–12% SDS-polyacrylamide electrophoretic gel and transferred onto a polyvinylidenedifluoride membrane. Nonspecific binding was blocked by immersing the membrane in PBS-5% BSA at room temperature for 1 hour. After saturation, the membrane was incubated overnight at 4°C with a primary antibody directed against P-gp (1:500 dilution; C219; Life Technologies) and actin (1:1000 dilution; D6A8; Cell Signaling, Yvelines, France) that was used as a loading control. After washing with PBST (PBS and 0.1% Tween-20) and PBS-5% BSA, the membrane was further incubated with the secondary peroxidase-conjugated antibody (Beckman-Coulter) at a 1:2000 dilution for 1 hour at room temperature. Membranes were washed with 5% BSA, and revelation was made by chemiluminescence (ECL Western blotting substrate; Pierce, Courtaboeuf, France); the gel image was captured using the Syngene GBox (Ozyme). Densitometry analyses of chemiluminescence staining were performed with the software geneSys (Ozyme). Results were expressed as a ratio between values obtained with IS or TCDD and the value obtained with control, and then, they were normalized with values obtained with actin.
Flow Cytometry Analyses of P-gp Expression
HepG2 were detached with a prewarmed cell dissociation buffer (Cell Dissociation Buffer, enzyme free, HBSS; Life Technologies) for 3 minutes at 37°C. Cells were washed with HBSS-20% FBS and saturated with HBSS-3% BSA-0.2% saponine for 15 minutes at 4°C. Cells were centrifuged and then incubated for 1 hour at 4°C with mAb against P-gp diluted at 20 μg/ml or irrelevant control mAb at 20 μg/ml in HBSS-1% BSA-0.2% saponine. After being washed three times with HBSS-1% BSA, cells were analyzed using a Gallios flow cytometer. Median fluorescence intensity on the whole cell population was determined by the Kaluza software (Beckman-Coulter) and expressed in arbitrary units. The median fluorescence intensity measured on cells labeled with the mAb against P-gp was corrected with isotype control value.
Gene Silencing
Downregulation of AhR mRNA levels was achieved by using a pool containing the three siRNAs directed against AhR (1200, 1999, and 1998; 40 pmol each; Applied Biosystems, Courtaboeuf, France) and the Cell Line Nucleofector Kit V (Lonza) according to the manufacturer’s instructions. Culture medium was changed 7 hours after the transfection. Because the decrease in AhR mRNA level was achieved 24 hours after transfection and remained stable until 96 hours, the effects of IS on AhR mRNA, protein, and activity were investigated 48 hours after transfection.
Patients
Patients were recruited in the Clinical Center of Investigation (APHM, Hôpitaux de Marseille, Marseille, France). We studied 109 patients with CKD (eGFR below 60 ml/min per 1.73 m2) and kidney or heart transplantation under immunosuppressive treatment with cyclosporin. Patients with kidney transplantation (n=62) are a subgroup of the FEG cohort, and patients with heart transplantation (n=47) are a subgroup of GRATEC cohort. The characteristics of patients are listed in Table 2.
Study Approval
All participants gave their written informed consent before any study procedure. The study was approved by the local ethics committees and conforms to the principles outlined in the Declaration of Helsinki. The animal experiments conform to Directive 2010/63/EU of the European Parliament and were approved by the local ethics committee (Comité d’Ethique en Expérimentation Animale de Marseille; C2EA-14).
Statistical Analyses
Data are expressed as mean±SEM or median and interquartile range (25th–75th percentiles) according to the normality of their distribution. For in vitro experiments, significant differences were determined using the Wilcoxon signed rank test or t test (GraphPad Software Inc., San Diego, CA). In mice experiments, significant differences were revealed by Mann–Whitney U test. Linear regression analysis was used to assess the relationship between serum cyclosporin dosage and levels of IS (Stata Software, Lakeway Drive, TX). A P value below 0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Karim Fallague and Christine Scagliarini for technical assistance.
This work was partially supported by a grant of The Coordination for the Improvement of Higher Education Personnel (CAPES Foundation), Ministry of Education of Brazil.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017030361/-/DCSupplemental.
References
- 1.Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R: Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol 4: 1089–1096, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manley HJ, Cannella CA: Nondialysis (home) medication utilization and cost in diabetic and nondiabetic hemodialysis patients. Nephrol News Issues 19: 27–38, 2005 [PubMed] [Google Scholar]
- 3.Mason NA: Polypharmacy and medication-related complications in the chronic kidney disease patient. Curr Opin Nephrol Hypertens 20: 492–497, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Verbeeck RK, Musuamba FT: Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur J Clin Pharmacol 65: 757–773, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Nolin TD: A synopsis of clinical pharmacokinetic alterations in advanced CKD. Semin Dial 28: 325–329, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeung CK, Shen DD, Thummel KE, Himmelfarb J: Effects of chronic kidney disease and uremia on hepatic drug metabolism and transport. Kidney Int 85: 522–528, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nigam SK: What do drug transporters really do? Nat Rev Drug Discov 14: 29–44, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyanagi T: Molecular mechanism of phase I and phase II drug-metabolizing enzymes: Implications for detoxification. Int Rev Cytol 260: 35–112, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Okabe H, Hashimoto Y, Inui KI: Pharmacokinetics and bioavailability of tacrolimus in rats with experimental renal dysfunction. J Pharm Pharmacol 52: 1467–1472, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Leblond FA, Petrucci M, Dubé P, Bernier G, Bonnardeaux A, Pichette V: Downregulation of intestinal cytochrome p450 in chronic renal failure. J Am Soc Nephrol 13: 1579–1585, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Nolin TD, Frye RF, Le P, Sadr H, Naud J, Leblond FA, Pichette V, Himmelfarb J: ESRD impairs nonrenal clearance of fexofenadine but not midazolam. J Am Soc Nephrol 20: 2269–2276, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutsaers HA, van den Heuvel LP, Ringens LH, Dankers AC, Russel FG, Wetzels JF, Hoenderop JG, Masereeuw R: Uremic toxins inhibit transport by breast cancer resistance protein and multidrug resistance protein 4 at clinically relevant concentrations. PLoS One 6: e18438, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mutsaers HA, Caetano-Pinto P, Seegers AE, Dankers AC, van den Broek PH, Wetzels JF, van den Brand JA, van den Heuvel LP, Hoenderop JG, Wilmer MJ, Masereeuw R: Proximal tubular efflux transporters involved in renal excretion of p-cresyl sulfate and p-cresyl glucuronide: Implications for chronic kidney disease pathophysiology. Toxicol In Vitro 29: 1868–1877, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Ryan JC, Dunn KW, Decker BS: Effects of chronic kidney disease on liver transport: Quantitative intravital microscopy of fluorescein transport in the rat liver. Am J Physiol Regul Integr Comp Physiol 307: R1488–R1492, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato T, Yamaguchi H, Kogawa T, Abe T, Mano N: Organic anion transporting polypeptides 1B1 and 1B3 play an important role in uremic toxin handling and drug-uremic toxin interactions in the liver. J Pharm Sci 17: 475–484, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jörres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W; European Uremic Toxin Work Group (EUTox) : Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int 63: 1934–1943, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Lin CJ, Wu V, Wu PC, Wu CJ: Meta-analysis of the associations of p-Cresyl Sulfate (PCS) and Indoxyl Sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS One 10: e0132589, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellis RJ, Small DM, Vesey DA, Johnson DW, Francis R, Vitetta L, Gobe GC, Morais C: Indoxyl sulphate and kidney disease: Causes, consequences and interventions. Nephrology (Carlton) 21: 170–177, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA; European Uremic Toxin Work Group (EUTox) : Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4: 1551–1558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sallée M, Dou L, Cerini C, Poitevin S, Brunet P, Burtey S: The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: A new concept to understand cardiovascular complications of chronic kidney disease. Toxins (Basel) 6: 934–949, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mutsaers HA, Stribos EG, Glorieux G, Vanholder R, Olinga P: Chronic kidney disease and fibrosis: The role of uremic retention solutes. Front Med (Lausanne) 2: 60, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroeder JC, Dinatale BC, Murray IA, Flaveny CA, Liu Q, Laurenzana EM, Lin JM, Strom SC, Omiecinski CJ, Amin S, Perdew GH: The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry 49: 393–400, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bock KW, Köhle C: The mammalian aryl hydrocarbon (Ah) receptor: From mediator of dioxin toxicity toward physiological functions in skin and liver. Biol Chem 390: 1225–1235, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Gondouin B, Cerini C, Dou L, Sallée M, Duval-Sabatier A, Pletinck A, Calaf R, Lacroix R, Jourde-Chiche N, Poitevin S, Arnaud L, Vanholder R, Brunet P, Dignat-George F, Burtey S: Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int 84: 733–744, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Russel FGM: Transporters: Importance in drug absorption, distribution, and removal. In: Enzyme- and Transporter-Based Drug-Drug Interactions: Progress and Future Challenges, 1st Ed., edited by Pang KS, Rodrigues AD, Peter RM, New York, Springer, 2010, pp 27–49 [Google Scholar]
- 26.Koepsell H: The SLC22 family with transporters of organic cations, anions and zwitterions. Mol Aspects Med 34: 413–435, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Liang Y, Li S, Chen L: The physiological role of drug transporters. Protein Cell 6: 334–350, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calaf R, Cerini C, Génovésio C, Verhaeghe P, Jourde-Chiche N, Bergé-Lefranc D, Gondouin B, Dou L, Morange S, Argilés A, Rathelot P, Dignat-George F, Brunet P, Charpiot P: Determination of uremic solutes in biological fluids of chronic kidney disease patients by HPLC assay. J Chromatogr B Analyt Technol Biomed Life Sci 879: 2281–2286, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Viaene L, Annaert P, de Loor H, Poesen R, Evenepoel P, Meijers B: Albumin is the main plasma binding protein for indoxyl sulfate and p-cresyl sulfate. Biopharm Drug Dispos 34: 165–175, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Schiavi SC, Tang W, Bracken C, O’Brien SP, Song W, Boulanger J, Ryan S, Phillips L, Liu S, Arbeeny C, Ledbetter S, Sabbagh Y: Npt2b deletion attenuates hyperphosphatemia associated with CKD. J Am Soc Nephrol 23: 1691–1700, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massy ZA, Ivanovski O, Nguyen-Khoa T, Angulo J, Szumilak D, Mothu N, Phan O, Daudon M, Lacour B, Drüeke TB, Muntzel MS: Uremia accelerates both atherosclerosis and arterial calcification in apolipoprotein E knockout mice. J Am Soc Nephrol 16: 109–116, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Feere DA, Velenosi TJ, Urquhart BL: Effect of erythropoietin on hepatic cytochrome P450 expression and function in an adenine-fed rat model of chronic kidney disease. Br J Pharmacol 172: 201–213, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM: Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 39: 361–398, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Donato MT, Lahoz A, Castell JV, Gómez-Lechón MJ: Cell lines: A tool for in vitro drug metabolism studies. Curr Drug Metab 9: 1–11, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Le Vee M, Jouan E, Noel G, Stieger B, Fardel O: Polarized location of SLC and ABC drug transporters in monolayer-cultured human hepatocytes. Toxicol In Vitro 29: 938–946, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Wojtal KA, de Vries E, Hoekstra D, van Ijzendoorn SC: Efficient trafficking of MDR1/P-glycoprotein to apical canalicular plasma membranes in HepG2 cells requires PKA-RIIalpha anchoring and glucosylceramide. Mol Biol Cell 17: 3638–3650, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsujimoto M, Hatozaki D, Shima D, Yokota H, Furukubo T, Izumi S, Yamakawa T, Minegaki T, Nishiguchi K: Influence of serum in hemodialysis patients on the expression of intestinal and hepatic transporters for the excretion of pravastatin. Ther Apher Dial 16: 580–587, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Lau WL, Liu S, Vaziri ND: Chronic kidney disease results in deficiency of ABCC6, the novel inhibitor of vascular calcification. Am J Nephrol 40: 51–55, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Jigorel E, Le Vee M, Boursier-Neyret C, Parmentier Y, Fardel O: Differential regulation of sinusoidal and canalicular hepatic drug transporter expression by xenobiotics activating drug-sensing receptors in primary human hepatocytes. Drug Metab Dispos 34: 1756–1763, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B: Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci 124: 1–22, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hankinson O: The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol 35: 307–340, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Dou L, Sallée M, Cerini C, Poitevin S, Gondouin B, Jourde-Chiche N, Fallague K, Brunet P, Calaf R, Dussol B, Mallet B, Dignat-George F, Burtey S: The cardiovascular effect of the uremic solute indole-3 acetic acid. J Am Soc Nephrol 26: 876–887, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller DS: Regulation of ABC transporters blood-brain barrier: The good, the bad, and the ugly. Adv Cancer Res 125: 43–70, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Denison MS, Nagy SR: Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol 43: 309–334, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Stockinger B, Hirota K, Duarte J, Veldhoen M: External influences on the immune system via activation of the aryl hydrocarbon receptor. Semin Immunol 23: 99–105, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Murray IA, Patterson AD, Perdew GH: Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat Rev Cancer 14: 801–814, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang G, Elferink CJ: A novel nonconsensus xenobiotic response element capable of mediating aryl hydrocarbon receptor-dependent gene expression. Mol Pharmacol 81: 338–347, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Hawkins BT, Miller DS: Activating PKC-β1 at the blood-brain barrier reverses induction of P-glycoprotein activity by dioxin and restores drug delivery to the CNS. J Cereb Blood Flow Metab 31: 1371–1375, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saeki M, Kurose K, Hasegawa R, Tohkin M: Functional analysis of genetic variations in the 5′-flanking region of the human MDR1 gene. Mol Genet Metab 102: 91–98, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Jansen J, Fedecostante M, Wilmer MJ, Peters JGP, Kreuser UM, van den Broek PH, Mensink RA, Boltje TJ, Stamatialis D, Wetzels JFM, van den Heuvel LP, Hoenderop JG, Masereeuw R: Bioengineered kidney tubules efficiently excrete uremic toxins. Sci Rep 6: 26715, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silva R, Vilas-Boas V, Carmo H, Dinis-Oliveira RJ, Carvalho F, de Lourdes Bastos M, Remião F: Modulation of P-glycoprotein efflux pump: Induction and activation as a therapeutic strategy. Pharmacol Ther 149: 1–123, 2015 [DOI] [PubMed] [Google Scholar]
- 52.Naud J, Michaud J, Leblond FA, Lefrancois S, Bonnardeaux A, Pichette V: Effects of chronic renal failure on liver drug transporters. Drug Metab Dispos 36: 124–128, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Burckart GJ, Starzl TE, Venkataramanan R, Hashim H, Wong L, Wang P, Makowka L, Zeevi A, Ptachcinski RJ, Knapp JE, Iwatsuki S, Esquivel C, Sanghvi A, Van Thiel DH: Excretion of cyclosporine and its metabolites in human bile. Transplant Proc 18[Suppl 5]: 46–49, 1986 [PMC free article] [PubMed] [Google Scholar]
- 54.Volpe DA, Tobin GA, Tavakkoli F, Dowling TC, Light PD, Parker RJ: Effect of uremic serum and uremic toxins on drug metabolism in human microsomes. Regul Toxicol Pharmacol 68: 297–303, 2014 [DOI] [PubMed] [Google Scholar]
- 55.Barnes KJ, Rowland A, Polasek TM, Miners JO: Inhibition of human drug-metabolising cytochrome P450 and UDP-glucuronosyltransferase enzyme activities in vitro by uremic toxins. Eur J Clin Pharmacol 70: 1097–1106, 2014 [DOI] [PubMed] [Google Scholar]
- 56.Lee J, Wang R, Yang Y, Lu X, Zhang X, Wang L, Lou Y: The effect of ABCB1 C3435T polymorphism on cyclosporine dose requirements in kidney transplant recipients: A meta-analysis. Basic Clin Pharmacol Toxicol 117: 117–125, 2015 [DOI] [PubMed] [Google Scholar]
- 57.Llaudó I, Colom H, Giménez-Bonafé P, Torras J, Caldés A, Sarrias M, Cruzado JM, Oppenheimer F, Sánchez-Plumed J, Gentil MÁ, Ekberg H, Grinyó JM, Lloberas N: Do drug transporter (ABCB1) SNPs and P-glycoprotein function influence cyclosporine and macrolides exposure in renal transplant patients? Results of the pharmacogenomic substudy within the symphony study. Transpl Int 26: 177–186, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P: Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest 96: 1698–1705, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gouroju S, Rao PVLNS, Bitla AR, Vinapamula KS, Manohar SM, Vishnubhotla S: Role of gut-derived uremic toxins on oxidative stress and inflammation in patients with chronic kidney disease. Indian J Nephrol 27: 359–364, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jourde-Chiche N, Dou L, Sabatier F, Calaf R, Cerini C, Robert S, Camoin-Jau L, Charpiot P, Argiles A, Dignat-George F, Brunet P: Levels of circulating endothelial progenitor cells are related to uremic toxins and vascular injury in hemodialysis patients. J Thromb Haemost 7: 1576–1584, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Suzuki Y, Itoh H, Fujioka T, Sato F, Kawasaki K, Sato Y, Sato Y, Ohno K, Mimata H, Kishino S: Association of plasma concentration of 4β-hydroxycholesterol with CYP3A5 polymorphism and plasma concentration of indoxyl sulfate in stable kidney transplant recipients. Drug Metab Dispos 42: 105–110, 2014 [DOI] [PubMed] [Google Scholar]
- 62.Karu N, McKercher C, Nichols DS, Davies N, Shellie RA, Hilder EF, Jose MD: Tryptophan metabolism, its relation to inflammation and stress markers and association with psychological and cognitive functioning: Tasmanian chronic kidney disease pilot study. BMC Nephrol 17: 171–184, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Momper JD, Venkataramanan R, Nolin TD: Nonrenal drug clearance in CKD: Searching for the path less traveled. Adv Chronic Kidney Dis 17: 384–391, 2010 [DOI] [PubMed] [Google Scholar]
- 64.Jouan E, Le Vée M, Mayati A, Denizot C, Parmentier Y, Fardel O: Evaluation of P-glycoprotein inhibitory potential using a rhodamine 123 accumulation assay. Pharmaceutics 8: pii:E12, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.