Abstract
The unique contributions of memory B cells and plasma cells in kidney diseases remain unclear. In this review, we evaluate the clinical experience with treatments directed at B cells, such as rituximab, and at plasma cells, such as proteasome inhibition, to shed light on the role of these two B lineage compartments in glomerular diseases. Specifically, analysis of these targeted interventions in diseases such as ANCA-associated vasculitis, SLE, and antibody-mediated transplant rejection permits insight into the pathogenetic effect of these cells. Notwithstanding the limitations of preclinical models and clinical studies (heterogeneous populations, among others), the data suggest that memory B and plasma cells represent two engines of autoimmunity, with variable involvement in these diseases. Whereas memory B cells and plasma cells appear to be key in ANCA-associated vasculitis and antibody-mediated transplant rejection, respectively, SLE seems likely to be driven by both autoimmune compartments. These conclusions have implications for the future development of targeted therapeutics in immune-mediated renal disease.
Keywords: ANCA, systemic lupus erythematosus, chronic allograft rejection, immunology
In health, memory B cells and plasma cells (PCs) comprise important but independently regulated compartments of our immunologic memory1 (Figure 1). Humoral immunity is key to the pathogenesis of many autoimmune renal diseases. Here B cells, when recognizing (auto)antigens and receiving appropriate T cell help, can differentiate into short-lived PCs, memory B or long-lived PCs also termed memory PCs2. However, their detailed contributions to different kidney diseases are not known.
Figure 1.
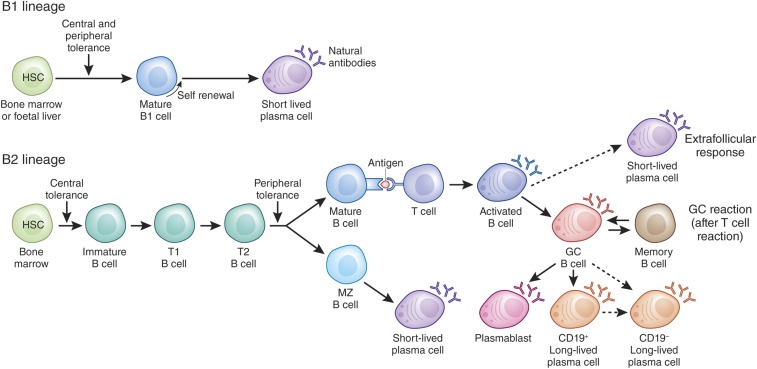
Distinct developmental and differentiation pathways of normal B cells. B1 and B2 B cell lineages appear to be independently regulated and undergo tightly controlled differentiation into certain memory B and PC subsets (Adapted from Dörner T et al.187
Long-lived PCs are considered ultimately differentiated, bone marrow (BM) resident cells secreting high-affinity antibodies that disseminate through the body, whereas memory B cells3 can rapidly proliferate in a clonally and antigen specific manner, and differentiate and recirculate upon antigen re-exposure. Overall, memory B cells and PCs form two immune defense lines that allow preservation of previous immune encounters by antibodies produced by long-lived PCs and a dynamic component to adapt humoral immunity by memory B cells. The two cellular subsets ensure stability and flexibility to maintain a sufficient B cell lineage defense. The extent to which these “two engines” of B cell lineage memory contribute to different renal autoimmune diseases is not fully understood, but is likely to differ between disorders.
In this context, clinical experiences with therapeutics selectively targeting CD20+ memory B cells but not CD20− PCs provided interesting lessons (Figure 2A), e.g., the response to rituximab in ANCA-associated vasculitis (AAV) and idiopathic membranous nephropathy (IMN). In contrast, SLE did not show similarly convincing responses to CD20 targeting. In chronic antibody-mediated rejection (ABMR), the addition of PC targeting agents (e.g., the proteasome inhibitor bortezomib) appears to be beneficial, with less evidence for rituximab.4 Currently available data from more selective immune targeting suggests that the pathogenic relevance of memory B cells and PCs may vary between autoimmune diseases (reviewed recently5), improving our understanding of individual diseases.
Figure 2.
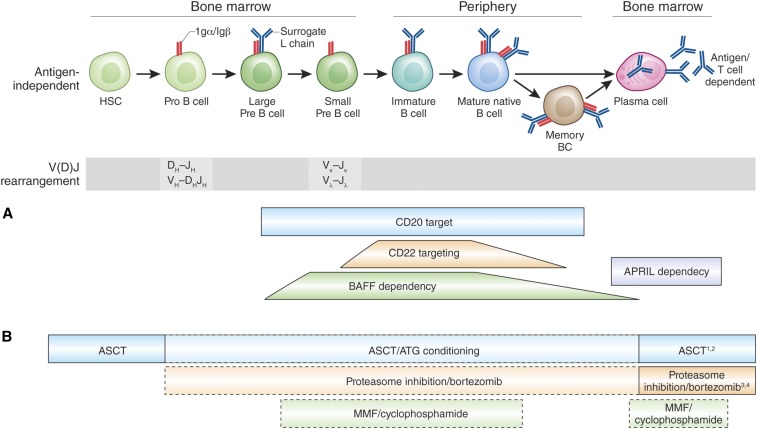
Interventions and their potential to target distinctly B lineage subsets and plasma cells. (A) Principles of direct (anti-CD20, anti-CD22) and indirect targeting of B cells and PCs (anti-BAFF or anti-APRIL strategies) have preferential effects on naïve versus memory B cells and PCs. (B) Principle of unspecific B cell and PC targeting, i.e., by proteasome inhibition or autologous stem cell transplantation (ASCT) with or without antithymocyte globulin (ATG) as well as mycophenolate mofetil (MMF) or cyclophosphamide. There appears to be a distinct susceptibility of memory B cell and PC dependent on the pharmacologic mechanisms.
Here we will take a reverse translational perspective to learn from the clinical use of B cell–directed therapies such as anti-CD20 or therapies that target the PC compartment in renal autoimmunity.
Induction of Memory B Cells and PCs
Distinct PC subsets can be induced via different pathways (Figure 1). First, B cells from the B1 cell lineage, which have been mainly studied in mice and lack a defined phenotype in humans, can form short-lived PCs, which produce polyreactive IgM for immediate defense. Second, B cells from the B2 cell lineage can form short-lived PCs in a T cell–independent manner (so-called marginal zone B2 cells), for example by stimulation with T cell-independent antigens such as pneumococcal capsular polysaccharides, and predominantly secrete low-affinity IgM antibodies.6,7 Moreover, B2 lineage cells are the main source of long-lived PCs and memory B cells upon activation by cognate T cells in germinal centers.8 The generation of long-lived PCs is the result of a two-step process. First, an extrafollicular response leads to the generation of short-lived activated B cells, of which some re-enter the B cell follicle and become plasmablasts in a T cell–dependent pathway. Subsequently, plasmablasts migrate through the blood stream and reside as long-lived PCs primarily in BM niches, possibly also in inflamed tissues.9,10 It is currently debated whether a small number of BM memory PCs can be induced independently of T cells.11,12 The germinal center response is a time-regulated developmental switch, first producing memory B cells and subsequently long-lived PCs13 with increasing affinity. It has long been suggested, but now experimentally proven, that memory B cells can also develop in a T cell–dependent, but germinal center–independent pathway.14 B cell lineage differentiation paths are summarized in Figure 1. The bulk of the data reported above has been obtained from preclinical models, although it remains to be delineated which differentiation pathway(s) and B cell lineages are involved in certain autoimmune conditions.
Memory B Cells
The definition of a memory B cell comprises an antigen-experienced, nonproliferating and, in the absence of antigen, persisting cell15 that responds more rapidly and efficiently when re-exposed to antigen. In contrast to sessile PCs, memory B cells recirculate and scan the body continuously. Their phenotype does not differ between certain lymphoid organs, and they do not depend on the presence of the spleen or tonsil.3
Most but not all memory B cells have undergone class-switch recombination, typically from IgM to IgG, and carry somatically hypermutated IgV gene rearrangements16,17 as one signature of previous T cell encounters. Loss of IgD expression (IgD−) identifies B cells that have undergone class-switch recombination. Switched memory B cells develop during T cell–dependent germinal center responses.13 In addition to Ig subclass B cell receptor (BCR) expression, other phenotypic B memory markers have been identified. Here, expression of CD27 serves as a universal marker for memory B cells in healthy donors.
The BCR of memory B cells shows a high affinity to the immunizing antigen compared with naïve B cells caused by using affinity-matured and somatically hypermutated BCR genes.18 Memory B cells that emerge from the T cell–dependent but germinal center–independent pathway do not show somatic hypermutations, and thus are not affinity matured.19 After a given immune response, memory B cells must repress their activation program and rest in a quiescent state for a long period to maintain their memory capacity.20 Upon antigen reactivation, memory B cells become activated and can differentiate either into PCs or further mature by re-entering the germinal center for additional affinity maturation.21 The involvement of memory B cells during resolution of an immune response and the mechanisms by which resident memory B cells are prevented from uncontrolled activation remain unclear for immunity as well as autoimmunity.
Notably, disturbances of circulating memory B cells in patients with chronic immune activation have been reported in chronic autoimmune conditions,22,23 such as SLE,22,24 rheumatoid arthritis (RA)25 or in HIV infection26 compared with healthy controls. In this context, enhanced CD27−IgD− B cells as well as CD27−IgD− coexpressing CD95+,27 CD21low28 or intracellular spleen tyrosine kinase Sykhigh29 were found. There is a possibility that these abnormalities result from an overly active immune system with enhanced B cell differentiation, but it is not clear if they result from incomplete germinal center responses, increased extrafollicular activity and/or enhanced T cell–independent B cell activation. Usually, these cells carry characteristics of B cell memory (e.g., mutated IgV genes), expressed costimulatory molecules, or lacked an active ABCB1 transporter as functional characteristic of memory B cells. It is currently not clear if these abnormalities of B cell memory are a source of autoimmunity or result of overly active immune activation.
The role of T cell–dependent memory B cell induction in SLE, AAV, and ABMR has been concluded from molecular data of Ig switched, hypermutated autoantibodies characteristic of these diseases,30–32 and histologic data of germinal center-like structures in affected tissues.33,34
PCs
PCs are responsible for maintaining serum antibody levels by continuously producing high-affinity antibodies and reside preferentially in the BM. Only a few plasmablasts that arise from the germinal center response migrate through the blood stream to become memory PCs.9 Crucial for the survival of a plasmablast to become an ultimately differentiated, long-lived PC is that they find appropriate soluble and insoluble (niche) survival conditions, whereas no postgerminal center selection processes of PCs are known. Long-lived PCs, also called memory PCs, find optimal survival conditions in the BM in healthy individuals at the end of a competitive journey starting as an early B lineage cell.
As direct PC precursors, plasmablasts express the C-X-C chemokine receptor type 4 (CXCR4)35 and downregulate CXCR5 and CXCR7 when they start to migrate toward the BM. The BM survival niches are composed of stromal cells expressing C-X-C motif chemokine ligand 12 (CXCL12) (ligand of CXCR4) and vascular cell adhesion protein 1 (VCAM1).36 Other cell types such as megakaryocytes, eosinophils, and basophils contribute to the production of survival factors such as a proliferation-inducing ligand (APRIL), B cell-activating factor (BAFF or BLyS), or IL-6, as well as adhesion molecules.37–39 The number of available niches in the BM or its capacity appears to be limited and serves as a regulatory factor for serum Ig levels. Recently, it has been debated if there is a physical or rather cell type–dependent functional BM niche.40
Besides the BM, other tissues can also offer survival niches in states of chronic inflammation. These niches disappear when the inflammation is resolved.41–43 Once it was thought that survival niches harbor almost exclusively highly-affinity matured PCs, but other studies revealed that immature PCs can access survival niches.44,45 Recent data suggest that there is heterogeneity of PCs in human BM. Here, a stable and highly differentiated CD19− PC fraction with a distinct phenotype (CD56+, HLA-DRnegative, CD44negative etc.) and a less mature CD19+ PC fraction exist in human BM, which suggests that further differentiation even within the BM PCs is a possibility.46 These human PC subsets in the BM have been confirmed subsequently.47,48 Because CD19− PCs usually enriched in human BM were also found in autoimmune tissues including kidney transplant rejection,46 it needs to be further delineated what factors are involved in their induction and/or maintenance in autoimmunity. Notably, a potential role of T cells within the BM has been suggested recently. Here, regulatory T cells (Treg) cells share a particular niche with PCs in CD11c+ BM cells and appear to regulate PC survival via CTLA-4.49 This suggests that T cells have distinct but largely unknown functions determining the lifestyle of PCs and mandates further studies.
Animal Models
Different animal models for SLE, the two AAV conditions (myeloperoxidase [MPO] or proteinase 3), and ABMR could provide evidence for the role of B lineage cells. Here analyses of T cells and relevant autoantibodies50–52 provide the basis that activation of B cells has taken place. However, the particular role of B cells independent of PC producing autoantibodies has only been clearly demonstrated in a lupus model.52,53 Tables 1 and 2 summarize key features, advantages, and disadvantages of the most instructive animal models in which B cell and PC contributions have been studied within AAV, lupus nephritis, and ABMR.
Table 1.
Indicators of the presumed pathogenic role of CD20+ B cells and CD20− PCs on the basis of available data of therapeutic responses to different B lineage cell directed agents
| Glomerular Disease | AAV | SLE | ABMR |
|---|---|---|---|
| Response to… | |||
| Anti-CD20 (rituximab) | Experience from RCTs leading to approval and several observational studies. | Experience from two RCTs and several observational studies. | Experience from one RCT and several cohort studies. |
| Effective in remission induction in and noninferior to cyclophosphamide.106,107 | RCTs failed to reach primary end points in renal140 and nonrenal SLE.188 | No difference in RCT of acute ABMR.189 | |
| Superior to azathioprine in maintenance of remission.108 | Meta-analysis of published data and EULAR and ERA-EDTA recommendations warrant the use of rituximab.142,144 | Partial success in retrospective cohort studies of acute ABMR.171 | |
| Meta-analysis of uncontrolled studies support data from RCTs. Rituximab might be superior in relapsing disease.190 | No effect of rituximab in chronic ABMR.171 | ||
| Proteasome inhibition (bortezomib) | Very limited experience. | Limited experience from small case series. | Limited experience from small cohort studies. |
| One patient with AAV refractory to rituximab successfully treated with bortezomib.119 | Report of five patients with lupus nephritis, three had complete remission after 6–12 mo.156 | Bortezomib is able to reduce DSA in most patients but graft survival remains very poor.181 | |
| Report of 12 patients with SLE, six with lupus nephritis. All patients responded to therapy.155 | |||
| Pathogenic relevance of … | |||
| (Memory) B cells | Regulatory B cells, but not memory B cells predict relapse after rituximab therapy.104,105 | Antibody-independent mechanisms e.g., autoantigen presentation, costimulation of T cells, production of regulatory cytokines.192 | Memory B cells can be reactivated in secondary transplantation upon antigen re-exposure.191 |
| Reconstitution of memory B cell subset can predict disease flares after rituximab therapy.136,137 | |||
| Long-lived PCs/autoantibodies | Materno-fetal transfer of anti-MPO antibodies led to vasculitis with pulmonary renal syndrome112 in neonates and can cause murine necrotizing crescentic GN.193 | Involved in the formation of immune complexes that lead to nephritis.192 | Responsible for the maintenance of serum DSA that cause transplant glomerulopathy.164 |
| Pathogenicity of anti-PR3 antibodies is not entirely proven.194 | |||
RCT, randomized, controlled trial; EULAR, European League Against Rheumatism; ERA-EDTA, European Renal Association/European Dialysis and Transplant Association.
Table 2.
Summary of the most relevant animal models of AAV, lupus nephritis, and ABMR with regard to B cell and PC function
| Animal Model | Kind of Intervention | Key Feature | Advantages and Disadvantages | Reference |
|---|---|---|---|---|
| AAV | ||||
| Mpo−/− mice/(Rag2−/−) mice | Transfer of anti-MPO antibodies or splenocytes, generated by MPO immunization of MPO deficient mice. | Induces necrotizing pauci-immune GN and, in some cases, pulmonary capillaritis. | Mainly B cell but not T cell–dependent, | Xiao et al.193 |
| Transfer of splenocytes: more severe disease with immune complex deposition in the glomeruli. | neutrophils are required. | |||
| Transfer of antibody alone: mild disease but pauci-immune. | Only mild disease without granuloma formation. | |||
| Disease induction strongly depends on genetic background.195 | ||||
| Depends on the passive transfer of antibodies. No cellular immunity. | ||||
| Wistar–Kyoto rats | Immunization of Wistar–Kyoto rats with human MPO and adjuvants. | Leads to crescentic nephritis and lung hemorrhage in all immunized rats. | Stable and severe disease induction. | Little et al.196 |
| Depends on the passive transfer of antibodies. | Chavelel et al.197 | |||
| No cellular immunity. | ||||
| Disease induction strongly depends on genetic background. | ||||
| Humanized NOD-SCID-IL-2Rγ−/− mice | Transfer of patients’ PR3-ANCA IgG into endotoxin-primed human-hematopoietic stem cell (HSC) mice, created using healthy donor (PR3) HSC. | Induction of hematuria, glomerular hypercellularity, and pulmonary capillaritis in a some of mice. | Very mild disease. | Little et al.198 |
| Technically challenging. | ||||
| Low level neutrophil reconstitution and absence of human T cells due to mixed chimerism. | ||||
| Lupus nephritis | ||||
| New Zealand Black (NZB)/New Zealand white (NZW) mice F1 intercross | Spontaneous polygenic. | Develop severe GN at 5–6 mo of age and kidney failure at 10–12 mo of age. Animals develop anti-nuclear antibodies without anti-Sm antibodies. | Difficult to use as a genetically modified model. Alleles have to be matched to NZB and NZW backgrounds. | Dixon et al.199 |
| Andrews et al.200 | ||||
| New Zealand mixes mice (NZM2328 and NZM2410) | Spontaneous polygenic. | Severe GN with an earlier onset compared with NZB/NZW F1. | No sex difference. | Rudofsky et al.201 |
| Morel202 | ||||
| MRL/MpJ-FASlpr (MRL/lpr) mice | MRL is a polygenetic mouse model. FASlpr mutation accelerates the time course of murine disease. | Production of class-switched autoantibodies (anti-DNA, Sm), end-organ disease including dermatitis, GN cardiovascular and lung disease. | Model meets all of the ACR criteria for SLE. | Dixon et al.199 |
| Mice that expressed a mutant transgene encoding surface Ig but are unable to secrete Ig developed nephritis in the absence of soluble autoantibodies. B cells with cognate BCR are essential for lupus nephritis via antigen-presentation to cognate T cells or other functions (cytokine, chemokine production, formation of germinal center). | Rapid and stable onset of disease. | Cohen et al.203 | ||
| Andrews et al.200 | ||||
| Herlands et al.204 | ||||
| Chan et al.53 | ||||
| ABMR | ||||
| Mouse heterotopic cardiac allografts | Repeated passive transfer of alloantibodies. | Animals develop chronic transplant arteriopathy. | Depends on the passive transfer of antibodies. | Uehara et al.205 |
| In B cell–deficient mice it was shown that chronic allograft vasculopathy was only observed in the presence of alloantibodies. | No cellular immunity. | |||
| No insights into the mechanism of antibody formation. | ||||
| Humanized CD52Tg (H-2K) mice | Transgenic mouse model in which CD52 is only expressed on T cells. | Animals show cardiac allograft vasculopathy, whereas animals without DSA show normal grafts.206 | Offers the possibility to study outcomes of animals with continuous de novo. | Kwun et al.206 |
| T cells are depleted by alemtuzumab (anti-CD52) and de novo allospecific B cells and DSA but not alloreactive T cells arise in some animals. |
Targeting Memory B Cells versus Targeting Memory PCs in Human Disease
Because expression of certain surface markers on memory B cells and PCs are distinct, selective targeting by using specific agents is a clinical possibility. Memory B cells show the phenotype of mature B cells expressing CD19, CD20, and CD22 besides the above discussed surface markers. PCs differ on the basis of their larger size and granularity, as well as loss of CD20 and CD22 expression, downregulation of CD19, and expression of CD38 and, to large extent, CD138.8 Memory B cells express three B cell cytokine receptors, BAFF receptor (BAFF-R), transmembrane activator and CAML interactor (TACI), and B cell maturation antigen (BCMA), whereas PCs have very diminished BAFF-R and TACI expression but receive survival signals through BCMA. There is differential signaling of BLyS/BAFF and APRIL through these receptors. We will briefly summarize certain agents that have an effect on PCs or (memory) B cells.
Conventional Immunosuppressants
Although a number of agents have an effect on B cell subsets, nonproliferating memory B cells have been shown to be more resistant to conventional immunosuppressants such as cyclophosphamide,54 as well as to mycophenolate mofetil55 (Figure 2B). Further, generation of plasmablasts and short-lived PCs are sufficiently inhibited by conventional immunosuppressants, and nondividing PC memory is not affected by therapies dependent on proliferation.56
Corticosteroids
Corticosteroids interrupt multiple steps of the immune response with genomic and nongenomic effects, but are mainly related to the inhibition of cytokine transcription via blocking transcription factors. This leads to the inhibition of certain ILs, thereby depleting T cells (IL-2) and eosinophils (IL-5), interrupting macrophage function (IL-1, TNF-α), and increasing neutrophil release from the BM and neutrophil migration to the sites of inflammation.57 Less is known about the detailed effect of corticosteroids on B cells. It has been suggested that B cells are not significantly inhibited by steroids and Ig levels are only slightly decreased.58 However, high-dose glucocorticoids affects PCs and has been widely used in myeloma, especially dexamethasone. Recent data from children with steroid-responsive nephrotic syndrome show that B cell numbers decline under corticosteroid therapy and that especially peripheral memory B cells further decline after corticosteroid cessations.59 In patients with autoimmune hemolytic anemia, splenic germinal center B cells and circulating plasmablasts were largely suppressed in patients under long-term corticosteroid therapy.60
Anti-CD20 Monoclonal Antibodies
Anti-CD20 monoclonal antibodies, such as the type 1 antibodies rituximab, ofatumumab, and ocrelizumab, largely deplete peripheral B cells including memory B cells from the peripheral blood but not always in the BM,61 lymph nodes,62 or synovium.63 CD20− pro-B cells and PCs are not targeted by these antibodies (Figure 2A), but they lead to impaired generation of plasmablasts and short-lived PCs. These monoclonals act by several mechanisms, including antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, and apoptosis induction.64 Novel type 2 monoclonal anti-CD20 antibodies, such as obinutuzumab (GA 101), are primarily inducing cell death with a more profound and lasting B cell depletion and are currently under investigation in autoimmune diseases.65
Targeting B Cell Survival Factors
There are several approaches indirectly targeting B cell and PC survival via cytokine targeting. One is blocking BAFF or the related cytokine APRIL (Figure 2B). These cytokines bind to three different receptors expressed on B cells: BAFF-R, TACI, and BCMA. BAFF-R exclusively binds BAFF,66 whereas both cytokines can bind to TACI and BCMA.67 APRIL, binding to BCMA is crucial for the survival of PCs,68 whereas memory B cells do not depend on these survival factors.69,70 Several biologic drugs targeting the BAFF/BLyS and APRIL axis have been studied over the last years, including belimumab, tabalumab, atacicept, and blisibimod. Belimumab binds to soluble BAFF and has been approved for the treatment of SLE.71 Tabalumab is a human mAb whereas blisibimod is a fusion polypeptide protein. Both block biologically active BAFF72–74 but development programs of tabalumab in SLE have been cancelled after poor efficacy results in clinical trials. Atacicept is a TACI− Ig fusion protein that blocks both BAFF and APRIL.75 So far, the effect of these compounds on PCs becomes evident by the substantial reduction of all Ig classes to different degrees, whereas the effect on memory B cells is largely confined to the initial increase in peripheral blood likely related to their mobilization.76,77
Proteasome Inhibitors
Proteasome inhibitors are prototypic for their effect on highly differentiated PCs with substantial endoplasmic reticulum stress. They selectively target the 26S proteasome,78,79 which is needed for the degradation of ubiquinated proteins.80 Their cytotoxic effect causes accumulation of misfolded proteins in the endoplasmic reticulum, leading to the activation of the terminal unfolded protein response causing apoptosis81 and prevention of the activation of NF-κ light chain enhancer of activated B cells (NF-κB), which is crucial for PC survival.82 Initial data were obtained for multiple myeloma but proteasome inhibitors are toxic to non-neoplastic cells as well.83 Further, cells with high protein production, such as PCs, are susceptible to proteasome inhibition.84 Bortezomib is the most widely used proteasome inhibitor approved for the treatment of mantle cell lymphoma and myeloma.85 The main limitation of bortezomib is severe neurotoxicity that occurs in up to 30% of all patients, resulting in therapy discontinuation,86 which is less with the new generation compounds carfilzomib87 and delanzomib.88
Anti-CD38
The monoclonal daratumumab targets CD38, which is highly expressed on the surface of short-lived PCs, long-lived PCs, and myeloma cells,89 but only expressed at low levels on lymphoid or myeloid cells and some other tissues.90 Daratumumab monotherapy was recently approved for the treatment of refractory multiple myeloma on the basis of two studies.91–93 Also, the addition of daratumumab to standard care has shown favorable results,94,95 with an overall acceptable safety profile.96 Nonetheless, a high number (71% of patients) of adverse reactions of cough, bronchospasm, and dyspnea were observed upon infusion of daratumumab.93 Patients with autoimmune diseases have not yet been treated with this antibody but it appears to lead to a profound depletion of plasma cells.
Targeting PC Homing and Survival
A number of recent efforts have been made to target certain factors involved in PC homing/migration or interfere with their survival in order to affect long-lived PCs otherwise refractory to treatment (reviewed recently2), including autologous stem cell transplantation.97 Clinical experiences are very limited. In contrast, the extrinsic and intrinsic characteristics of memory B cell maintenance are far less understood.
Clinical Experiences Suggest Distinct Role of Memory B and PCs in Different Glomerular Diseases
AAV
Small-vessel vasculitis, which is associated with autoantibodies against neutrophil cytoplasmic antigens, manifests as a necrotizing inflammation of vessels with little or no Ig and complement deposition in the vessel wall. This lack of Ig deposit distinguishes ANCA-associated GN (directed against MPO or proteinase 3) from immune complex-mediated GN, e.g., found in lupus nephritis.98 In human kidney specimens of patients with AAV, B cell clusters with IgD− B cells forming germinal center-like structures were found, whereas CD138-positive plasma was almost absent.99 T cells that are present in the inflamed kidney tissue mostly belong to a senescent memory T cell population, whereas the number of memory T cells in circulation is decreased, indicating their migration as effectors into the kidney.100,101
Disturbances of peripheral B cell subsets have been described in patients with AAV. Here memory B cells are reduced in patients with active disease,102 possibly by recruiting B cells to the sites of inflammation or their differentiation into PCs. During remission memory B cell levels are restored.102 An imbalance of regulatory B cells, a B cell fraction that is commonly defined by the production of IL 10, a cytokine able to suppress T cells, has been reported.103 These regulatory B cells are decreased in patients with AAV. Diminished regulatory B cells were found to predict relapse after rituximab therapy.103,104 The overall memory B cell population did not correlate with disease activity or the time to flare after B cell depletion,104 whereas CD38++ peripheral PC and B cell activation have been reported to correlate with active ANCA vasculitis.105
Anti-CD20 therapy with rituximab has been approved on the basis of clinical studies for remission induction,106,107 and is also a valuable maintenance therapy,108 especially in refractory disease or patients not responding to cyclophosphamide.109 Because the ANCA titers typically, but not always correlate with disease activity and are reliably reduced by rituximab to a greater extent than seen with cyclophosphamide, they appear to be the product of short-lived PCs,110 which provides the basis for the key role of autoreactive B cells in AAV. However, rituximab has also been effective in ANCA-negative patients with granulomatosis and polyangiitis,111 and the presence of B cells in close proximity to T cells and macrophages at sites of inflammation indicates alternative roles, such as antigen presentation, which support the disease process. The hypothesis of the pathogenicity of ANCA is also supported by a case report in which fetomaternal transfer of anti-MPO autoantibodies led to vasculitis with pulmonary renal syndrome112 in the offspring. Another case, however, did not develop GN or vasculitis upon transplacental transfer of MPO-ANCA.113 A more direct correlation of autoantibody titers and response to therapy is present in IMN, where a fall in phospholipase A2 receptor (PLA2R) antibody titers exactly predicts reductions in proteinuria and correlates with the extent of proteinuria.114
Circulating BAFF levels correlate with disease activity in AAV and therefore this cytokine has been suggested as potential treatment target.115–117 An ongoing trial is addressing the role of BAFF inhibition in patients with AAV: BREVAS is evaluating belimumab in combination with azathioprine as maintenance of remission after a standard induction regimen (Clinicaltrials.gov identifier NCT01663623). This approach follows the hypothesis to selectively block enhanced BAFF and reduce ANCA producing peripheral PCs and thereby affect AAV activity. A further study, COMBIVAS, is addressing the combination of BLyS/BAFF blockade and anti-CD20 on the basis of two hypotheses: that local BLyS/BAFF impairs B cell depletion at inflammatory sites and that the BLyS/BAFF surge seen after rituximab, due to the reduction of B cell BAFF-R clearance, promotes autoreactivity in the reconstituting B cell repertoire. BLyS/BAFF inhibition is also effective in IMN, where it significantly reduces anti-PLA2R antibodies titers within 28 weeks (Clinicaltrials.gov identifier NCT01610492).
There is little experience with agents targeting PCs in AAV. Bontscho et al.118 reported that bortezomib is able to inhibit anti-MPO–mediated necrotizing crescentic GN in mice by depleting MPO-specific PCs in the spleen and BM. A favorable effect of proteasome inhibition was also reported in one patient with AAV,119 but precludes any firm conclusion in the absence of larger experiences.
SLE
Lupus nephritis is the most common severe organ manifestation in SLE, seen in up to 50% of cases.120 Histologically, it can present with a spectrum of glomerular, vascular, and tubulointerstitial lesions, including germinal center-like structures in the interstitium. Cytokine-mediated activation of glomerular mesangial cells and in situ tubulointerstitial macrophages promotes the deposition of anti-dsDNA, anti-C1q, anti-nucleosome, and anti-glomerular antibodies recognizing glomerular structures—the formation of immune complexes with complement deposition initiating renal damage.121,122 Circulating neutrophil activation with neutrophil extracellular trap deposition in the glomeruli may also be an initiating event in renal inflammation. Anti-dsDNA antibodies represent a risk factor for lupus nephritis, with negative predictive value for renal outcome in some studies.123 In addition to the typical presence of autoantibodies, antibody-independent mechanisms contribute to lupus pathogenesis. Lupus nephritis biopsies contain interstitial B cell lymphocyte infiltrates that are organized along with T cells and dendritic cells,124–126 and undergo further differentiation as in germinal centers. Within these aggregates, T follicular helper cells are important for establishing germinal centers to promote activation and differentiation of B cells.126 T follicular helper cells are expanded in patients with SLE and the expression of programmed death 1 correlates with circulating plasmablasts and anti-dsDNA antibodies.127,128 The presence of these tertiary lymphoid structures is associated with the local formation of autoantibodies and tubular basement membrane immune complexes in more active disease.124
All these data show that the interaction of B and T cells is critical in lupus nephritis. In addition to the footprints of B cell abnormalities (autoantibodies and immune complexes), continuous activation of innate and the adaptive immunity is operative. Here, T cells provide help to B cells or are cytotoxic to glomeruli or tubular cells.129 A key characteristic of SLE T cells is their reduced IL-2 production, which inversely correlates with enhanced IL-17 regulated by the transcription factor CREM-α. Moreover, IL-2 as an essential growth and survival factor for Treg cells has been found to be functionally deficient in patients with SLE.130 A number of genetic risk alleles and epigenetic modifications altering the gene expression and the function of certain T cell subsets in SLE appear to be involved.131 These abnormal T cell responses can result in disturbances of B cells in SLE. Because SLE pathology apparently comprises pathogenic contributions of memory B cells and PCs dependent on T helper cells, abnormal T cell activation may represent a critical link. Proof for this idea is very small, but a recent phase 2 trial is using voclosporine in lupus nephritis.132 Alternatively, therapies with an effect on T and B cell interaction (Figure 3) are attractive. In addition, low-dose IL-2 has been studied in non-renal SLE133 and demonstrated some clinical efficacy, leading to an increase of Treg cells in vitro134 and in vivo.135
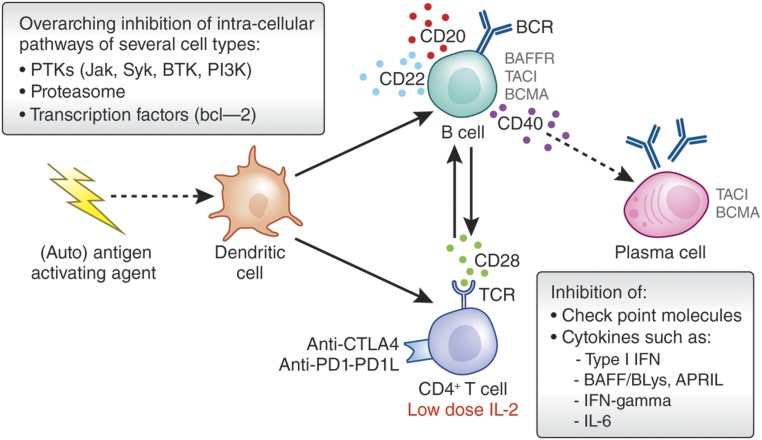
Figure 3.
Immune intervention at key nodes of immune interaction in SLE. Targeting key nodes of T cell and B cell interaction holds promise to improve abnormal T helper cell activity or enhanced regulatory T cell activity (i.e., using low-dose IL-2) that may also have the potential to normalize B lineage cell abnormalities.
The contribution of CD20+ and especially memory B cells became apparent by experience with B cell–depleting agents, such as rituximab. In several reports the reconstitution of the memory B cell subset after rituximab predicted recurrence of active disease.136,137 This phenomenon has not exclusively been observed in SLE but also in patients with rheumatoid arthritis25,138 and IMN, and suggests that memory B cells contribute to the pathogenesis of these diseases. A reduction in autoantibody titers, in contrast to AAV, did not always predict the effectiveness of rituximab therapy.139 Rituximab did not meet the primary efficacy end point in a lupus nephritis trial (LUNAR),140 partly due to aspects of the trial design. Clinical experiences141,142 in otherwise refractory patients support a clinical value that has led to recommendations as a second line agent by the American College of Rheumatology143 and European League Against Rheumatism-European Renal Association/European Dialysis and Transplant Association.144 Rituximab, in combination with mycophenolate mofetil, has been reported in newly diagnosed patients with lupus nephritis in a UK center,145 with a substantial rate of patients achieving complete remission without use of oral glucocorticoids. This study requires confirmation in treatment-naïve patients, whereas the currently available data suggest that anti-CD20 depletion alone may not be sufficient in patients with established disease. In this population, the additional role of PCs likely requires cotargeting.
Belimumab has been approved for general SLE on the basis of the successful phase 3 studies BLISS-52146 and BLISS-76.147 However, there are conflicting data about the interrelation of the cytokine levels of BAFF and APRIL with lupus activity.148 Notably, these studies found an association between response to belimumab and reductions in autoantibody titers, suggesting successful targeting of PCs. Although other compounds targeting BAFF, such as tabalumab149,150 or atacicept151 cotargeting APRIL, did not provide convincing clinical data, a more profound effect on PCs reflected by Ig reductions was noted. Selective inhibition of the PC-relevant cytokines without sufficient treatment of CD20+ memory B cells may hold more promise. In this context, a sequential approach of B cell depletion and BLyS/BAFF inhibition to influence PC activity is being evaluated in lupus nephritis, the Rituximab and Belimumab for Lupus Nephritis (CALIBRATE) study (clinicaltrials.gov identifier NCT02260934) and in extrarenal lupus, the BEAT-LUPUS study.152
Efforts to preferentially target the PC compartment have been undertaken. In a murine model of lupus-like nephritis, short- and long-lived PCs were depleted by bortezomib and lupus-like nephritis improved remarkably.83 These results were confirmed by Ichikawa et al.153 and Seavey et al.,154 using the less toxic proteasome inhibitors carfilzomib87 and delanzomib.88 Clinical experiences with proteasome inhibition in lupus nephritis rely only on bortezomib. Alexander et al.155 reported 12 patients with refractory SLE including six patients with nephritis. Musculoskeletal and mucocutaneous manifestations improved in all patients, and proteinuria significantly decreased in patients with nephritis. A recently published study reports a beneficial effect with a decrease in creatinine in three of five patients with lupus nephritis.156 A phase 2 study is currently investigating the safety and efficacy of bortezomib in patients with SLE (Clinicaltrials.gov identifier NCT02102594). A phase 1 study in lupus nephritis with the oral proteasome inhibitor ixazomib is underway (Clinicaltrials.gov identifier NCT02176486). Although proteasome inhibition is often considered a PC-targeted therapy, it has the advantage to cotarget PCs but also affect other immune cells.155 A potential difference of lupus nephritis versus AAV is that effective B lineage therapy requires cotargeting of memory B cells and PCs.
Kidney Transplantation
In the transplant setting, donor-specific antibodies (DSA) are likely the main biologic reason for allograft injury and loss.157 Their detection either pre- or post-transplant increases the risk of graft loss.158,159 DSA directly bind to the grafts and cause local inflammation and tissue damage through complement activation and human Fc gamma-mediated cytotoxicity, and also serve as opsonins that facilitate the activation of alloreactive T cells.160–162 These mechanisms lead to the development of transplant glomerulopathy, the histologic feature of chronic ABMR.163 Long-lived PCs are responsible for the maintenance of long-term circulating DSA, which are detectable in solid-organ transplant recipients and are associated with worse transplant outcomes.164 Quiescent memory B cells appear to be reactivated in cases of secondary transplantation in presensitized individuals. The best risk management to avoid transplant glomerulopathy comprises transplantation without preexisting DSA159 and avoidance of transplantation with HLA mismatches, especially as class 2 HLA mismatches are a strong predictive biomarker for DSA.165 The induction of DSA requires interaction between B cells and antigen-specific CD4 T helper cells. The presence of memory T cells before transplantation is associated with a poor transplant outcome.166,167 ABMR and the underlying importance of pathogenically relevant PCs producing DSA are a key target of immunotherapy to eradicate DSA. These strategies include plasmapheresis and immunoadsorption for the direct removal of DSA, intravenous Igs for immunomodulation,168 rituximab to deplete PC precursors, and targeting PCs by proteasome inhibition.169 Combined renal and allogeneic BM transplantation have also aimed to deplete PCs and induce tolerance without the need for antirejection therapy.170
B cell–directed therapy by rituximab seems to be partially effective in acute ABMR but ineffective in chronic ABMR.171 Jackson et al.172 provided interesting evidence about long-lived PCs in humans and the capacity of rituximab to interfere with relevant B cells as PC precursors when they compared DSA levels in patients who have been desensitized with or without rituximab. They found a greater reduction of DSA but a similar rate of DSA persistence, most likely explained by refractory long-lived PCs, whereas the difference in DSA titers can be ascribed to memory B cells undergoing differentiation into PCs.
Kidney transplantation permits comprehensive corresponding insights in tissue resident immunology. Just as in other glomerular diseases, tertiary lymphoid structures have been identified in kidney grafts.173 Within the tertiary lymphoid structures an increase of activated memory CD4+ T cells and a decrease in T regulatory subsets (IL-10–producing Tr1 cells and Foxp3-positive Tregs) has been observed.174 Also PC-rich infiltrates have been found in renal allografts and their occurrence was associated with an increased risk of allograft failure in the pediatric population.175 However, data on PC-rich infiltrates in the adult population are limited.176–178 To target the PC compartment, proteasome inhibition has been included in the treatment repertoire. In a model of chronic allograft nephropathy, bortezomib reduced antibody titers and depleted PCs in the BM.179 The reduction of PCs was also observed in BM aspirates of two patients treated with bortezomib for humoral rejection.180 Several small case series report the effect of bortezomib treatment on graft survival, kidney function, and DSA levels. Overall, patients responded together with a reduction in DSA levels and a stabilization of kidney function in refractory patients.181 The BORTEJECT (Clinicaltrials.gov identifier NCT01873157),182 the TRIBUTE study (Clinicaltrials.gov identifier NCT02201576), and the Effect of BM-MSCs on Chronic AMR after Kidney Transplantation study (Clinicaltrials.gov identifier NCT02563340), will evaluate the clinical value of bortezomib on chronic ABMR.
Notably, levels of BAFF were found to be associated with the occurrence of transplant glomerulopathy183 and enhanced ABMR,184 thus BAFF targeting appeared an attractive option. However, a study of tabalumab for desensitization did not lead to clinically significant reduction of DSA,185 which may be taken as another indicator that outstanding long-lived PCs are a key challenge in this clinical setting. Studies of belimumab for pretransplant desensitization and at the time of transplantation are ongoing (clinicaltrials.gov identifier NCT01025193). An innovative approach to reduce HLA antibodies in the setting of desensitization of highly sensitized patients is the use of the endopeptidase derived from Streptococcus pyogenes (IdeS), which cleaves IgG into F(ab′)2 and Fc fragments. IdeS leads to a complete IgG deficiency what makes it less attractive for long-term use in chronic ABMR.186 However, early clinical experiences with IdeS in renal transplants provide additional proof that targeting autoreactive PCs is key in targeting ABMR.
Concluding Remarks
A number of preclinical models have supported the role of B lineage cells in glomerulopathies, but do not reflect the complexity of human diseases. Interventions in human disease have inspired a revision of the role of B cells and long-lived (memory) PCs in these entities. Here, currently available data support a key role of B cells (likely memory and activated B cells) in AAV and IMN, and long-lived PCs as a pathogenic driver in chronic ABMR, whereas lupus nephritis appears to be under the influence of both B cells and PCs (Figure 4). As a consequence, the idea that distinct immunopathogenic contributions by memory B cells and PCs is also probably related to distinct T helper cell abnormalities requires consideration.
Figure 4.
Distinct role of B lineage and plasma cell functions in glomerular diseases. The sites of induction and residence, however, remain to be delineated.
Disclosures
None.
Acknowledgments
E.S. is supported by the Charité Junior Clinical Scientist Program (Charité-Universitätsmedizin Berlin and the Berlin Institute of Health). Related support has been provided by projects funded by the German Research Foundation (SFB650, 633, CRC “Immunobone,” TR130, and individual DFG projects Do491/7-4, 8-1,2, 10-1, all granted to T.D.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Ahuja A, Anderson SM, Khalil A, Shlomchik MJ: Maintenance of the plasma cell pool is independent of memory B cells. Proc Natl Acad Sci U S A 105: 4802–4807, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiepe F, Radbruch A: Plasma cells as an innovative target in autoimmune disease with renal manifestations. Nat Rev Nephrol 12: 232–240, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Giesecke C, Frölich D, Reiter K, Mei HE, Wirries I, Kuhly R, Killig M, Glatzer T, Stölzel K, Perka C, Lipsky PE, Dörner T: Tissue distribution and dependence of responsiveness of human antigen-specific memory B cells. J Immunol 192: 3091–3100, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Waiser J, Budde K, Schütz M, Liefeldt L, Rudolph B, Schönemann C, Neumayer H-H, Lachmann N: Comparison between bortezomib and rituximab in the treatment of antibody-mediated renal allograft rejection. Nephrol Dial Transplant 27: 1246–1251, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Dörner T, Lipsky PE: Beyond pan-B-cell-directed therapy - new avenues and insights into the pathogenesis of SLE. Nat Rev Rheumatol 12: 645–657, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Baumgarth N: Innate-like B cells and their rules of engagement. Adv Exp Med Biol 785: 57–66, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Defrance T, Taillardet M, Genestier L: T cell-independent B cell memory. Curr Opin Immunol 23: 330–336, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KGC, Dörner T, Hiepe F: Competence and competition: The challenge of becoming a long-lived plasma cell. Nat Rev Immunol 6: 741–750, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Tokoyoda K, Zehentmeier S, Chang H-D, Radbruch A: Organization and maintenance of immunological memory by stroma niches. Eur J Immunol 39: 2095–2099, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Tokoyoda K, Hauser AE, Nakayama T, Radbruch A: Organization of immunological memory by bone marrow stroma. Nat Rev Immunol 10: 193–200, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Bortnick A, Chernova I, Quinn WJ, Mugnier M, Cancro MP, Allman D: Long-lived bone marrow plasma cells are induced early in response to T cell-independent or T cell-dependent antigens. J Immunol 188: 5389–5396, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bortnick A, Allman D: What is and what should always have been: Long-lived plasma cells induced by T cell-independent antigens. J Immunol 190: 5913–5918, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weisel FJ, Zuccarino-Catania GV, Chikina M, Shlomchik MJ: A temporal switch in the germinal center determines differential output of memory B and plasma cells. Immunity 44: 116–130, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaji T, Ishige A, Hikida M, Taka J, Hijikata A, Kubo M, Nagashima T, Takahashi Y, Kurosaki T, Okada M, Ohara O, Rajewsky K, Takemori T: Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. J Exp Med 209: 2079–2097, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maruyama M, Lam KP, Rajewsky K: Memory B-cell persistence is independent of persisting immunizing antigen. Nature 407: 636–642, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Klein U, Küppers R, Rajewsky K: Evidence for a large compartment of IgM-expressing memory B cells in humans. Blood 89: 1288–1298, 1997 [PubMed] [Google Scholar]
- 17.Klein U, Rajewsky K, Küppers R: Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med 188: 1679–1689, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajewsky K: Clonal selection and learning in the antibody system. Nature 381: 751–758, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Takemori T, Kaji T, Takahashi Y, Shimoda M, Rajewsky K: Generation of memory B cells inside and outside germinal centers. Eur J Immunol 44: 1258–1264, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Good KL, Avery DT, Tangye SG: Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J Immunol 182: 890–901, 2009 [DOI] [PubMed] [Google Scholar]
- 21.McHeyzer-Williams LJ, Milpied PJ, Okitsu SL, McHeyzer-Williams MG: Class-switched memory B cells remodel BCRs within secondary germinal centers. Nat Immunol 16: 296–305, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J, Lee E-H, Milner ECB, Sanz I: A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol 178: 6624–6633, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Fecteau JF, Côté G, Néron S: A new memory CD27-IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. J Immunol 177: 3728–3736, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Anolik JH, Barnard J, Cappione A, Pugh-Bernard AE, Felgar RE, Looney RJ, Sanz I: Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum 50: 3580–3590, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Roll P, Dörner T, Tony H-P: Anti-CD20 therapy in patients with rheumatoid arthritis: Predictors of response and B cell subset regeneration after repeated treatment. Arthritis Rheum 58: 1566–1575, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Cagigi A, Du L, Dang LVP, Grutzmeier S, Atlas A, Chiodi F, Pan-Hammarström Q, Nilsson A: CD27(-) B-cells produce class switched and somatically hyper-mutated antibodies during chronic HIV-1 infection. PLoS One 4: e5427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobi AM, Reiter K, Mackay M, Aranow C, Hiepe F, Radbruch A, Hansen A, Burmester G-R, Diamond B, Lipsky PE, Dörner T: Activated memory B cell subsets correlate with disease activity in systemic lupus erythematosus: Delineation by expression of CD27, IgD, and CD95. Arthritis Rheum 58: 1762–1773, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Wehr C, Eibel H, Masilamani M, Illges H, Schlesier M, Peter H-H, Warnatz K: A new CD21low B cell population in the peripheral blood of patients with SLE. Clin Immunol 113: 161–171, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Fleischer SJ, Daridon C, Fleischer V, Lipsky PE, Dörner T: Enhanced tyrosine phosphatase activity underlies dysregulated B cell receptor signaling and promotes survival of human lupus B cells. Arthritis Rheumatol 68: 1210–1221, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Schroeder K, Herrmann M, Winkler TH: The role of somatic hypermutation in the generation of pathogenic antibodies in SLE. Autoimmunity 46: 121–127, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Jethwa HS, Clarke SH, Itoh-Lindstrom Y, Falk RJ, Jennette JC, Nachman PH: Restriction in V kappa gene use and antigen selection in anti-myeloperoxidase response in mice. J Immunol 165: 3890–3897, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Winkler TH, Fehr H, Kalden JR: Analysis of immunoglobulin variable region genes from human IgG anti-DNA hybridomas. Eur J Immunol 22: 1719–1728, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Ferdman J, Porcheray F, Gao B, Moore C, DeVito J, Dougherty S, Thomas MV, Farkash EA, Elias N, Kawai T, Malek SK, Tullius SG, Wong W, Zorn E: Expansion and somatic hypermutation of B-cell clones in rejected human kidney grafts. Transplantation 98: 766–772, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng J, Torkamani A, Grover RK, Jones TM, Ruiz DI, Schork NJ, Quigley MM, Hall FW, Salomon DR, Lerner RA: Ectopic B-cell clusters that infiltrate transplanted human kidneys are clonal. Proc Natl Acad Sci U S A 108: 5560–5565, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odendahl M, Mei H, Hoyer BF, Jacobi AM, Hansen A, Muehlinghaus G, Berek C, Hiepe F, Manz R, Radbruch A, Dörner T: Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood 105: 1614–1621, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, Zou YR, Littman DR, Cyster JG: A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med 194: 45–56, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu VT, Fröhlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, Lee JJ, Löhning M, Berek C: Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol 12: 151–159, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Gomez MR, Talke Y, Goebel N, Hermann F, Reich B, Mack M: Basophils support the survival of plasma cells in mice. J Immunol 185: 7180–7185, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Winter O, Moser K, Mohr E, Zotos D, Kaminski H, Szyska M, Roth K, Wong DM, Dame C, Tarlinton DM, Schulze H, MacLennan ICM, Manz RA: Megakaryocytes constitute a functional component of a plasma cell niche in the bone marrow. Blood 116: 1867–1875, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Wilmore JR, Allman D: Here, there, and anywhere? Arguments for and against the physical plasma cell survival niche. J Immunol 199: 839–845, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cassese G, Lindenau S, de Boer B, Arce S, Hauser A, Riemekasten G, Berek C, Hiepe F, Krenn V, Radbruch A, Manz RA: Inflamed kidneys of NZB / W mice are a major site for the homeostasis of plasma cells. Eur J Immunol 31: 2726–2732, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Hutloff A, Büchner K, Reiter K, Baelde HJ, Odendahl M, Jacobi A, Dörner T, Kroczek RA: Involvement of inducible costimulator in the exaggerated memory B cell and plasma cell generation in systemic lupus erythematosus. Arthritis Rheum 50: 3211–3220, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Schröder AE, Greiner A, Seyfert C, Berek C: Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci U S A 93: 221–225, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slocombe T, Brown S, Miles K, Gray M, Barr TA, Gray D: Plasma cell homeostasis: The effects of chronic antigen stimulation and inflammation. J Immunol 191: 3128–3138, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chernova I, Jones DD, Wilmore JR, Bortnick A, Yucel M, Hershberg U, Allman D: Lasting antibody responses are mediated by a combination of newly formed and established bone marrow plasma cells drawn from clonally distinct precursors. J Immunol 193: 4971–4979, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mei HE, Wirries I, Frölich D, Brisslert M, Giesecke C, Grün JR, Alexander T, Schmidt S, Luda K, Kühl AA, Engelmann R, Dürr M, Scheel T, Bokarewa M, Perka C, Radbruch A, Dörner T: A unique population of IgG-expressing plasma cells lacking CD19 is enriched in human bone marrow. Blood 125: 1739–1748, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV, Popova L, Kaminiski D, Fucile CF, Albizua I, Kyu S, Chiang K-Y, Bradley KT, Burack R, Slifka M, Hammarlund E, Wu H, Zhao L, Walsh EE, Falsey AR, Randall TD, Cheung WC, Sanz I, Lee FE-H: Long-lived plasma cells are contained within the CD19(-)CD38(hi)CD138(+) subset in human bone marrow. Immunity 43: 132–145, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhoj VG, Arhontoulis D, Wertheim G, Capobianchi J, Callahan CA, Ellebrecht CT, Obstfeld AE, Lacey SF, Melenhorst JJ, Nazimuddin F, Hwang W-T, Maude SL, Wasik MA, Bagg A, Schuster S, Feldman MD, Porter DL, Grupp SA, June CH, Milone MC: Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood 128: 360–370, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glatman Zaretsky A, Konradt C, Dépis F, Wing JB, Goenka R, Atria DG, Silver JS, Cho S, Wolf AI, Quinn WJ, Engiles JB, Brown DC, Beiting D, Erikson J, Allman D, Cancro MP, Sakaguchi S, Lu L-F, Benoist CO, Hunter CA: T regulatory cells support plasma cell populations in the bone marrow. Cell Reports 18: 1906–1916, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salama AD, Little MA: Animal models of antineutrophil cytoplasm antibody-associated vasculitis. Curr Opin Rheumatol 24: 1–7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kenney LL, Shultz LD, Greiner DL, Brehm MA: Humanized mouse models for transplant immunology. Am J Transplant 16: 389–397, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shlomchik MJ, Craft JE, Mamula MJ: From T to B and back again: Positive feedback in systemic autoimmune disease. Nat Rev Immunol 1: 147–153, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Chan OT, Madaio MP, Shlomchik MJ: B cells are required for lupus nephritis in the polygenic, Fas-intact MRL model of systemic autoimmunity. J Immunol 163: 3592–3596, 1999 [PubMed] [Google Scholar]
- 54.Dörner T, Jacobi AM, Lipsky PE: B cells in autoimmunity. Arthritis Res Ther 11: 247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fassbinder T, Saunders U, Mickholz E, Jung E, Becker H, Schlüter B, Jacobi AM: Differential effects of cyclophosphamide and mycophenolate mofetil on cellular and serological parameters in patients with systemic lupus erythematosus. Arthritis Res Ther 17: 92, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, Radbruch A, Hiepe F, Manz RA: Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med 199: 1577–1584, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fauci AS, Dale DC, Balow JE: Glucocorticosteroid therapy: Mechanisms of action and clinical considerations. Ann Intern Med 84: 304–315, 1976 [DOI] [PubMed] [Google Scholar]
- 58.Matas AJ: Minimization of steroids in kidney transplantation. Transpl Int 22: 38–48, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baris HE, Baris S, Karakoc-Aydiner E, Gokce I, Yildiz N, Cicekkoku D, Ogulur I, Ozen A, Alpay H, Barlan I: The effect of systemic corticosteroids on the innate and adaptive immune system in children with steroid responsive nephrotic syndrome. Eur J Pediatr 175: 685–693, 2016 [DOI] [PubMed] [Google Scholar]
- 60.Mahévas M, Michel M, Vingert B, Moroch J, Boutboul D, Audia S, Cagnard N, Ripa J, Menard C, Tarte K, Mégret J, Le Gallou S, Patin P, Thai L, Galicier L, Bonnotte B, Godeau B, Noizat-Pirenne F, Weill J-C, Reynaud C-A: Emergence of long-lived autoreactive plasma cells in the spleen of primary warm auto-immune hemolytic anemia patients treated with rituximab. J Autoimmun 62: 22–30, 2015 [DOI] [PubMed] [Google Scholar]
- 61.Leandro MJ, Cooper N, Cambridge G, Ehrenstein MR, Edwards JCW: Bone marrow B-lineage cells in patients with rheumatoid arthritis following rituximab therapy. Rheumatology (Oxford) 46: 29–36, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Kamburova EG, Koenen HJPM, Borgman KJE, ten Berge IJ, Joosten I, Hilbrands LB: A single dose of rituximab does not deplete B cells in secondary lymphoid organs but alters phenotype and function. Am J Transplant 13: 1503–1511, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Thurlings RM, Vos K, Wijbrandts CA, Zwinderman AH, Gerlag DM, Tak PP: Synovial tissue response to rituximab: Mechanism of action and identification of biomarkers of response. Ann Rheum Dis 67: 917–925, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pescovitz MD: Rituximab, an anti-cd20 monoclonal antibody: History and mechanism of action. Am J Transplant 6: 859–866, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Reddy V, Dahal LN, Cragg MS, Leandro M: Optimising B-cell depletion in autoimmune disease: Is obinutuzumab the answer? Drug Discov Today 21: 1330–1338, 2016 [DOI] [PubMed] [Google Scholar]
- 66.Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, Hession C, Schneider P, Sizing ID, Mullen C, Strauch K, Zafari M, Benjamin CD, Tschopp J, Browning JL, Ambrose C: BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science 293: 2108–2111, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Schneider P: The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol 17: 282–289, 2005 [DOI] [PubMed] [Google Scholar]
- 68.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin L-L, Mantchev GT, Bram RJ, Noelle RJ: BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med 199: 91–98, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benson MJ, Dillon SR, Castigli E, Geha RS, Xu S, Lam KP, Noelle RJ: Cutting edge: The dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol 180: 3655–3659, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Scholz JL, Crowley JE, Tomayko MM, Steinel N, O’Neill PJ, Quinn WJ 3rd, Goenka R, Miller JP, Cho YH, Long V, Ward C, Migone T-S, Shlomchik MJ, Cancro MP: BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc Natl Acad Sci U S A 105: 15517–15522, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stohl W: Therapeutic targeting of the BAFF/APRIL axis in systemic lupus erythematosus. Expert Opin Ther Targets 18: 473–489, 2014 [DOI] [PubMed] [Google Scholar]
- 72.Hsu H, Khare SD, Lee F, Miner K, Hu Y-L, Stolina M, Hawkins N, Chen Q, Ho S-YJ, Min H, Xiong F, Boone T, Zack DJ: A novel modality of BAFF-specific inhibitor AMG623 peptibody reduces B-cell number and improves outcomes in murine models of autoimmune disease. Clin Exp Rheumatol 30: 197–201, 2012 [PubMed] [Google Scholar]
- 73.Genovese MC, Fleischmann RM, Greenwald M, Satterwhite J, Veenhuizen M, Xie L, Berclaz P-Y, Myers S, Benichou O: Tabalumab, an anti-BAFF monoclonal antibody, in patients with active rheumatoid arthritis with an inadequate response to TNF inhibitors. Ann Rheum Dis 72: 1461–1468, 2013 [DOI] [PubMed] [Google Scholar]
- 74.Manetta J, Bina H, Ryan P, Fox N, Witcher DR, Kikly K: Generation and characterization of tabalumab, a human monoclonal antibody that neutralizes both soluble and membrane-bound B-cell activating factor. J Inflamm Res 7: 121–131, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gatto B: Atacicept, a homodimeric fusion protein for the potential treatment of diseases triggered by plasma cells. Curr Opin Investig Drugs 9: 1216–1227, 2008 [PubMed] [Google Scholar]
- 76.Nestorov I, Papasouliotis O, Pena Rossi C, Munafo A: Pharmacokinetics and immunoglobulin response of subcutaneous and intravenous atacicept in patients with systemic lupus erythematosus. J Pharm Sci 99: 524–538, 2010 [DOI] [PubMed] [Google Scholar]
- 77.Stohl W, Hiepe F, Latinis KM, Thomas M, Scheinberg MA, Clarke A, Aranow C, Wellborne FR, Abud-Mendoza C, Hough DR, Pineda L, Migone T-S, Zhong ZJ, Freimuth WW, Chatham WW; BLISS-52 Study Group; BLISS-76 Study Group : Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum 64: 2328–2337, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adams J, Behnke M, Chen S, Cruickshank AA, Dick LR, Grenier L, Klunder JM, Ma YT, Plamondon L, Stein RL: Potent and selective inhibitors of the proteasome: Dipeptidyl boronic acids. Bioorg Med Chem Lett 8: 333–338, 1998 [DOI] [PubMed] [Google Scholar]
- 79.Gardner RC, Assinder SJ, Christie G, Mason GG, Markwell R, Wadsworth H, McLaughlin M, King R, Chabot-Fletcher MC, Breton JJ, Allsop D, Rivett AJ: Characterization of peptidyl boronic acid inhibitors of mammalian 20 S and 26 S proteasomes and their inhibition of proteasomes in cultured cells. Biochem J 346: 447–454, 2000 [PMC free article] [PubMed] [Google Scholar]
- 80.Ciechanover A: The ubiquitin-proteasome proteolytic pathway. Cell 79: 13–21, 1994 [DOI] [PubMed] [Google Scholar]
- 81.Obeng EA, Carlson LM, Gutman DM, Harrington WJ Jr., Lee KP, Boise LH: Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 107: 4907–4916, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, Munshi N, Dang L, Castro A, Palombella V, Adams J, Anderson KC: NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem 277: 16639–16647, 2002 [DOI] [PubMed] [Google Scholar]
- 83.Neubert K, Meister S, Moser K, Weisel F, Maseda D, Amann K, Wiethe C, Winkler TH, Kalden JR, Manz RA, Voll RE: The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med 14: 748–755, 2008 [DOI] [PubMed] [Google Scholar]
- 84.Meister S, Schubert U, Neubert K, Herrmann K, Burger R, Gramatzki M, Hahn S, Schreiber S, Wilhelm S, Herrmann M, Jäck H-M, Voll RE: Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res 67: 1783–1792, 2007 [DOI] [PubMed] [Google Scholar]
- 85.Kane RC, Farrell AT, Sridhara R, Pazdur R: United States Food and Drug Administration approval summary: Bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res 12: 2955–2960, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Schiff D, Wen PY, van den Bent MJ: Neurological adverse effects caused by cytotoxic and targeted therapies. Nat Rev Clin Oncol 6: 596–603, 2009 [DOI] [PubMed] [Google Scholar]
- 87.O'Connor OA, Stewart AK, Vallone M, Molineaux CJ, Kunkel LA, Gerecitano JF, Orlowski RZ: A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin Cancer Res 15: 7085–7091, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gallerani E, Zucchetti M, Brunelli D, Marangon E, Noberasco C, Hess D, Delmonte A, Martinelli G, Böhm S, Driessen C, De Braud F, Marsoni S, Cereda R, Sala F, D'Incalci M, Sessa C: A first in human phase I study of the proteasome inhibitor CEP-18770 in patients with advanced solid tumours and multiple myeloma. Eur J Cancer 49: 290–296, 2013 [DOI] [PubMed] [Google Scholar]
- 89.Lin P, Owens R, Tricot G, Wilson CS: Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol 121: 482–488, 2004 [DOI] [PubMed] [Google Scholar]
- 90.Deaglio S, Vaisitti T, Billington R, Bergui L, Omede’ P, Genazzani AA, Malavasi F: CD38/CD19: A lipid raft-dependent signaling complex in human B cells. Blood 109: 5390–5398, 2007 [DOI] [PubMed] [Google Scholar]
- 91.McKeage K: Daratumumab: First global approval. Drugs 76: 275–281, 2016 [DOI] [PubMed] [Google Scholar]
- 92.Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, Belch A, Krishnan A, Vescio RA, Mateos MV, Mazumder A, Orlowski RZ, Sutherland HJ, Bladé J, Scott EC, Oriol A, Berdeja J, Gharibo M, Stevens DA, LeBlanc R, Sebag M, Callander N, Jakubowiak A, White D, de la Rubia J, Richardson PG, Lisby S, Feng H, Uhlar CM, Khan I, Ahmadi T, Voorhees PM: Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): An open-label, randomised, phase 2 trial. Lancet 387: 1551–1560, 2016 [DOI] [PubMed] [Google Scholar]
- 93.Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, Minnema MC, Lassen U, Krejcik J, Palumbo A, van de Donk NWCJ, Ahmadi T, Khan I, Uhlar CM, Wang J, Sasser AK, Losic N, Lisby S, Basse L, Brun N, Richardson PG: Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med 373: 1207–1219, 2015 [DOI] [PubMed] [Google Scholar]
- 94.Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, Spicka I, Hungria V, Munder M, Mateos MV, Mark TM, Qi M, Schecter J, Amin H, Qin X, Deraedt W, Ahmadi T, Spencer A, Sonneveld P; CASTOR Investigators : Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 375: 754–766, 2016 [DOI] [PubMed] [Google Scholar]
- 95.Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, Rabin N, Orlowski RZ, Komarnicki M, Suzuki K, Plesner T, Yoon S-S, Ben Yehuda D, Richardson PG, Goldschmidt H, Reece D, Lisby S, Khokhar NZ, O’Rourke L, Chiu C, Qin X, Guckert M, Ahmadi T, Moreau P; POLLUX Investigators : Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 375: 1319–1331, 2016 [DOI] [PubMed] [Google Scholar]
- 96.Oliva S, Palumbo A: Monoclonal antibodies for treating multiple myeloma - a new era, new safety considerations? Expert Opin Drug Saf 15: 1295–1300, 2016 [DOI] [PubMed] [Google Scholar]
- 97.Alexander T, Schneider S, Hoyer B, Cheng Q, Thiel A, Ziemer S, Burmester G-R, Arnold R, Radbruch A, Hiepe F: Development and resolution of secondary autoimmunity after autologous haematopoietic stem cell transplantation for systemic lupus erythematosus: Competition of plasma cells for survival niches? Ann Rheum Dis 72: 1102–1104, 2013 [DOI] [PubMed] [Google Scholar]
- 98.Jennette JC: Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol 17: 603–606, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Steinmetz OM, Velden J, Kneissler U, Marx M, Klein A, Helmchen U, Stahl RAK, Panzer U: Analysis and classification of B-cell infiltrates in lupus and ANCA-associated nephritis. Kidney Int 74: 448–457, 2008 [DOI] [PubMed] [Google Scholar]
- 100.Abdulahad WH, van der Geld YM, Stegeman CA, Kallenberg CGM: Persistent expansion of CD4+ effector memory T cells in Wegener’s granulomatosis. Kidney Int 70: 938–947, 2006 [DOI] [PubMed] [Google Scholar]
- 101.Voswinkel J, Müller A, Lamprecht P: Is PR3-ANCA formation initiated in Wegener’s granulomatosis lesions? Granulomas as potential lymphoid tissue maintaining autoantibody production. Ann N Y Acad Sci 1051: 12–19, 2005 [DOI] [PubMed] [Google Scholar]
- 102.Lepse N, Abdulahad WH, Rutgers A, Kallenberg CGM, Stegeman CA, Heeringa P: Altered B cell balance, but unaffected B cell capacity to limit monocyte activation in anti-neutrophil cytoplasmic antibody-associated vasculitis in remission. Rheumatology (Oxford) 53: 1683–1692, 2014 [DOI] [PubMed] [Google Scholar]
- 103.Todd SK, Pepper RJ, Draibe J, Tanna A, Pusey CD, Mauri C, Salama AD: Regulatory B cells are numerically but not functionally deficient in anti-neutrophil cytoplasm antibody-associated vasculitis. Rheumatology (Oxford) 53: 1693–1703, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bunch DO, McGregor JG, Khandoobhai NB, Aybar LT, Burkart ME, Hu Y, Hogan SL, Poulton CJ, Berg EA, Falk RJ, Nachman PH: Decreased CD5+ B cells in active ANCA vasculitis and relapse after rituximab. Clin J Am Soc Nephrol 8: 382–391, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Tervaert JW: Differential B- and T-cell activation in Wegener’s granulomatosis. J Allergy Clin Immunol 103: 885–894, 1999 [DOI] [PubMed] [Google Scholar]
- 106.Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, Kallenberg CGM, St Clair EW, Fessler BJ, Ding L, Viviano L, Tchao NK, Phippard DJ, Asare AL, Lim N, Ikle D, Jepson B, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh K, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Mueller M, Sejismundo LP, Mieras K, Stone JH; RAVE-ITN Research Group : Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med 369: 417–427, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CGM, St Clair EW, Turkiewicz A, Tchao NK, Webber L, Ding L, Sejismundo LP, Mieras K, Weitzenkamp D, Ikle D, Seyfert-Margolis V, Mueller M, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh KA, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Specks U; RAVE-ITN Research Group : Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363: 221–232, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaître O, Cohen P, Maurier F, Decaux O, Ninet J, Gobert P, Quémeneur T, Blanchard-Delaunay C, Godmer P, Puéchal X, Carron P-L, Hatron P-Y, Limal N, Hamidou M, Ducret M, Daugas E, Papo T, Bonnotte B, Mahr A, Ravaud P, Mouthon L; French Vasculitis Study Group : Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med 371: 1771–1780, 2014 [DOI] [PubMed] [Google Scholar]
- 109.Jones RB, Ferraro AJ, Chaudhry AN, Brogan P, Salama AD, Smith KGC, Savage COS, Jayne DRW: A multicenter survey of rituximab therapy for refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 60: 2156–2168, 2009 [DOI] [PubMed] [Google Scholar]
- 110.Fussner LA, Specks U: Can antineutrophil cytoplasmic antibody levels be used to inform treatment of pauci-immune vasculitis? Curr Opin Rheumatol 27: 231–240, 2015 [DOI] [PubMed] [Google Scholar]
- 111.Shah S, Hruskova Z, Segelmark M, Morgan MD, Hogan J, Lee SK, Dale J, Harper L, Tesar V, Jayne DRW, Geetha D: Treatment of severe renal disease in ANCA positive and negative small vessel vasculitis with rituximab. Am J Nephrol 41: 296–301, 2015 [DOI] [PubMed] [Google Scholar]
- 112.Bansal PJ, Tobin MC: Neonatal microscopic polyangiitis secondary to transfer of maternal myeloperoxidase-antineutrophil cytoplasmic antibody resulting in neonatal pulmonary hemorrhage and renal involvement. Ann Allergy Asthma Immunol 93: 398–401, 2004 [DOI] [PubMed] [Google Scholar]
- 113.Silva F, Specks U, Sethi S, Irazabal MV, Fervenza FC: Successful pregnancy and delivery of a healthy newborn despite transplacental transfer of antimyeloperoxidase antibodies from a mother with microscopic polyangiitis. Am J Kidney Dis 54: 542–545, 2009 [DOI] [PubMed] [Google Scholar]
- 114.Hofstra JM, Beck LH Jr., Beck DM, Wetzels JF, Salant DJ: Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 6: 1286–1291, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Holden NJ, Williams JM, Morgan MD, Challa A, Gordon J, Pepper RJ, Salama AD, Harper L, Savage COS: ANCA-stimulated neutrophils release BLyS and promote B cell survival: A clinically relevant cellular process. Ann Rheum Dis 70: 2229–2233, 2011 [DOI] [PubMed] [Google Scholar]
- 116.Nagai M, Hirayama K, Ebihara I, Shimohata H, Kobayashi M, Koyama A: Serum levels of BAFF and APRIL in myeloperoxidase anti-neutrophil cytoplasmic autoantibody-associated renal vasculitis: Association with disease activity. Nephron Clin Pract 118: c339–c345, 2011 [DOI] [PubMed] [Google Scholar]
- 117.Krumbholz M, Specks U, Wick M, Kalled SL, Jenne D, Meinl E: BAFF is elevated in serum of patients with Wegener’s granulomatosis. J Autoimmun 25: 298–302, 2005 [DOI] [PubMed] [Google Scholar]
- 118.Bontscho J, Schreiber A, Manz RA, Schneider W, Luft FC, Kettritz R: Myeloperoxidase-specific plasma cell depletion by bortezomib protects from anti-neutrophil cytoplasmic autoantibodies-induced glomerulonephritis. J Am Soc Nephrol 22: 336–348, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Novikov P, Moiseev S, Bulanov N, Shchegoleva E: Bortezomib in refractory ANCA-associated vasculitis: A new option? Ann Rheum Dis 75: e9, 2016 [DOI] [PubMed] [Google Scholar]
- 120.Almaani S, Meara A, Rovin BH: Update on lupus nephritis. Clin J Am Soc Nephrol 12: 825–835, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sterner RM, Hartono SP, Grande JP: The Pathogenesis of lupus nephritis. J Clin Cell Immunol 5: 1–7, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Davidson A: What is damaging the kidney in lupus nephritis? Nat Rev Rheumatol 12: 143–153, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de Leeuw K, Bungener L, Roozendaal C, Bootsma H, Stegeman CA: Auto-antibodies to double-stranded DNA as biomarker in systemic lupus erythematosus: Comparison of different assays during quiescent and active disease. Rheumatology (Oxford) 56: 698–703, 2017 [DOI] [PubMed] [Google Scholar]
- 124.Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, Kaverina N, Utset TO, Meehan SM, Quigg RJ, Meffre E, Clark MR: In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol 186: 1849–1860, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Espeli M, Bökers S, Giannico G, Dickinson HA, Bardsley V, Fogo AB, Smith KGC: Local renal autoantibody production in lupus nephritis. J Am Soc Nephrol 22: 296–305, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liarski VM, Kaverina N, Chang A, Brandt D, Yanez D, Talasnik L, Carlesso G, Herbst R, Utset TO, Labno C, Peng Y, Jiang Y, Giger ML, Clark MR: Cell distance mapping identifies functional T follicular helper cells in inflamed human renal tissue. Sci Transl Med 6: 230ra46, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Choi J-Y, Ho JH-E, Pasoto SG, Bunin V, Kim ST, Carrasco S, Borba EF, Gonçalves CR, Costa PR, Kallas EG, Bonfa E, Craft J: Circulating follicular helper-like T cells in systemic lupus erythematosus: Association with disease activity. Arthritis Rheumatol 67: 988–999, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Le Coz C, Joublin A, Pasquali J-L, Korganow A-S, Dumortier H, Monneaux F: Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PLoS One 8: e75319, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Suárez-Fueyo A, Bradley SJ, Klatzmann D, Tsokos GC: T cells and autoimmune kidney disease. Nat Rev Nephrol 13: 329–343, 2017 [DOI] [PubMed] [Google Scholar]
- 130.Lyssuk EY, Torgashina AV, Soloviev SK, Nassonov EL, Bykovskaia SN: Reduced number and function of CD4+CD25highFoxP3+ regulatory T cells in patients with systemic lupus erythematosus. Adv Exp Med Biol 601: 113–119, 2007 [PubMed] [Google Scholar]
- 131.Suárez-Fueyo A, Bradley SJ, Tsokos GC: T cells in systemic lupus erythematosus. Curr Opin Immunol 43: 32–38, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Parikh SV, Pendergraft WF, Tumlin JA, Saxena R, Solomons N, Huizinga RB: Treatment of active lupus nephritis with voclosporin: 48 week data from the Aura-LV study. Am J Kidney Dis 69: A2, 2017 [Google Scholar]
- 133.Humrich JY, von Spee-Mayer C, Siegert E, Alexander T, Hiepe F, Radbruch A, Burmester GR, Riemekasten G: Rapid induction of clinical remission by low-dose interleukin-2 in a patient with refractory SLE. Ann Rheum Dis 74: 791–792, 2015 [DOI] [PubMed] [Google Scholar]
- 134.von Spee-Mayer C, Siegert E, Abdirama D, Rose A, Klaus A, Alexander T, Enghard P, Sawitzki B, Hiepe F, Radbruch A, Burmester GR, Riemekasten G, Humrich JY: Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann Rheum Dis 75: 1407–1415, 2016 [DOI] [PubMed] [Google Scholar]
- 135.He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, Jin Y, Gan Y, Hu X, Jia R, Xu C, Hou Z, Leong YA, Zhu L, Feng J, An Y, Jia Y, Li C, Liu X, Ye H, Ren L, Li R, Yao H, Li Y, Chen S, Zhang X, Su Y, Guo J, Shen N, Morand EF, Yu D, Li Z: Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med 22: 991–993, 2016 [DOI] [PubMed] [Google Scholar]
- 136.Vital EM, Dass S, Buch MH, Henshaw K, Pease CT, Martin MF, Ponchel F, Rawstron AC, Emery P: B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum 63: 3038–3047, 2011 [DOI] [PubMed] [Google Scholar]
- 137.Iwata S, Tanaka Y: B-cell subsets, signaling and their roles in secretion of autoantibodies. Lupus 25: 850–856, 2016 [DOI] [PubMed] [Google Scholar]
- 138.Sellam J, Rouanet S, Hendel-Chavez H, Abbed K, Sibilia J, Tebib J, Le Loët X, Combe B, Dougados M, Mariette X, Taoufik Y: Blood memory B cells are disturbed and predict the response to rituximab in patients with rheumatoid arthritis. Arthritis Rheum 63: 3692–3701, 2011 [DOI] [PubMed] [Google Scholar]
- 139.Leandro MJ, Edwards JC, Cambridge G, Ehrenstein MR, Isenberg DA: An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum 46: 2673–2677, 2002 [DOI] [PubMed] [Google Scholar]
- 140.Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, Maciuca R, Zhang D, Garg JP, Brunetta P, Appel G; LUNAR Investigator Group : Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: The Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 64: 1215–1226, 2012 [DOI] [PubMed] [Google Scholar]
- 141.Tony H-P, Burmester G, Schulze-Koops H, Grunke M, Henes J, Kötter I, Haas J, Unger L, Lovric S, Haubitz M, Fischer-Betz R, Chehab G, Rubbert-Roth A, Specker C, Weinerth J, Holle J, Müller-Ladner U, König R, Fiehn C, Burgwinkel P, Budde K, Sörensen H, Meurer M, Aringer M, Kieseier B, Erfurt-Berge C, Sticherling M, Veelken R, Ziemann U, Strutz F, von Wussow P, Meier FM, Hunzelmann N, Schmidt E, Bergner R, Schwarting A, Eming R, Hertl M, Stadler R, Schwarz-Eywill M, Wassenberg S, Fleck M, Metzler C, Zettl U, Westphal J, Heitmann S, Herzog AL, Wiendl H, Jakob W, Schmidt E, Freivogel K, Dörner T; GRAID investigators : Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: Experience from a national registry (GRAID). Arthritis Res Ther 13: R75, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Duxbury B, Combescure C, Chizzolini C: Rituximab in systemic lupus erythematosus: An updated systematic review and meta-analysis. Lupus 22: 1489–1503, 2013 [DOI] [PubMed] [Google Scholar]
- 143.Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, Karpouzas GA, Merrill JT, Wallace DJ, Yazdany J, Ramsey-Goldman R, Singh K, Khalighi M, Choi S-I, Gogia M, Kafaja S, Kamgar M, Lau C, Martin WJ, Parikh S, Peng J, Rastogi A, Chen W, Grossman JM; American College of Rheumatology : American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 64: 797–808, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bertsias GK, Tektonidou M, Amoura Z, Aringer M, Bajema I, Berden JHM, Boletis J, Cervera R, Dörner T, Doria A, Ferrario F, Floege J, Houssiau FA, Ioannidis JPA, Isenberg DA, Kallenberg CGM, Lightstone L, Marks SD, Martini A, Moroni G, Neumann I, Praga M, Schneider M, Starra A, Tesar V, Vasconcelos C, van Vollenhoven RF, Zakharova H, Haubitz M, Gordon C, Jayne D, Boumpas DT: Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis 71: 1771–1782, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Condon MB, Ashby D, Pepper RJ, Cook HT, Levy JB, Griffith M, Cairns TD, Lightstone L: Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis 72: 1280–1286, 2013 [DOI] [PubMed] [Google Scholar]
- 146.Navarra SV, Guzmán RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK-M, Thomas M, Kim H-Y, León MG, Tanasescu C, Nasonov E, Lan J-L, Pineda L, Zhong ZJ, Freimuth W, Petri MA; BLISS-52 Study Group : Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: A randomised, placebo-controlled, phase 3 trial. Lancet 377: 721–731, 2011 [DOI] [PubMed] [Google Scholar]
- 147.Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, Sanchez-Guerrero J, Schwarting A, Merrill JT, Chatham WW, Stohl W, Ginzler EM, Hough DR, Zhong ZJ, Freimuth W, van Vollenhoven RF; BLISS-76 Study Group : A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 63: 3918–3930, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vincent FB, Morand EF, Mackay F: BAFF and innate immunity: New therapeutic targets for systemic lupus erythematosus. Immunol Cell Biol 90: 293–303, 2012 [DOI] [PubMed] [Google Scholar]
- 149.Merrill JT, van Vollenhoven RF, Buyon JP, Furie RA, Stohl W, Morgan-Cox M, Dickson C, Anderson PW, Lee C, Berclaz P-Y, Dörner T: Efficacy and safety of subcutaneous tabalumab, a monoclonal antibody to B-cell activating factor, in patients with systemic lupus erythematosus: Results from ILLUMINATE-2, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis 75: 332–340, 2016 [DOI] [PubMed] [Google Scholar]
- 150.Isenberg DA, Petri M, Kalunian K, Tanaka Y, Urowitz MB, Hoffman RW, Morgan-Cox M, Iikuni N, Silk M, Wallace DJ: Efficacy and safety of subcutaneous tabalumab in patients with systemic lupus erythematosus: Results from ILLUMINATE-1, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis 75: 323–331, 2016 [DOI] [PubMed] [Google Scholar]
- 151.Isenberg D, Gordon C, Licu D, Copt S, Rossi CP, Wofsy D: Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann Rheum Dis 74: 2006–2015, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ehrenstein MR, Wing C: The BAFFling effects of rituximab in lupus: Danger ahead? Nat Rev Rheumatol 12: 367–372, 2016 [DOI] [PubMed] [Google Scholar]
- 153.Ichikawa HT, Conley T, Muchamuel T, Jiang J, Lee S, Owen T, Barnard J, Nevarez S, Goldman BI, Kirk CJ, Looney RJ, Anolik JH: Beneficial effect of novel proteasome inhibitors in murine lupus via dual inhibition of type I interferon and autoantibody-secreting cells. Arthritis Rheum 64: 493–503, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Seavey MM, Lu LD, Stump KL, Wallace NH, Ruggeri BA: Novel, orally active, proteasome inhibitor, delanzomib (CEP-18770), ameliorates disease symptoms and glomerulonephritis in two preclinical mouse models of SLE. Int Immunopharmacol 12: 257–270, 2012 [DOI] [PubMed] [Google Scholar]
- 155.Alexander T, Sarfert R, Klotsche J, Kühl AA, Rubbert-Roth A, Lorenz H-M, Rech J, Hoyer BF, Cheng Q, Waka A, Taddeo A, Wiesener M, Schett G, Burmester G-R, Radbruch A, Hiepe F, Voll RE: The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann Rheum Dis 74: 1474–1478, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang H, Liu Z, Huang L, Hou J, Zhou M, Huang X, Hu W, Liu Z: The short-term efficacy of bortezomib combined with glucocorticoids for the treatment of refractory lupus nephritis. Lupus 26: 952–958, 2017 [DOI] [PubMed] [Google Scholar]
- 157.Loupy A, Jordan SC: Transplantation: Donor-specific HLA antibodies and renal allograft failure. Nat Rev Nephrol 9: 130–131, 2013 [DOI] [PubMed] [Google Scholar]
- 158.Mohan S, Palanisamy A, Tsapepas D, Tanriover B, Crew RJ, Dube G, Ratner LE, Cohen DJ, Radhakrishnan J: Donor-specific antibodies adversely affect kidney allograft outcomes. J Am Soc Nephrol 23: 2061–2071, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen J-P, Mooney N, Suberbielle C, Frémeaux-Bacchi V, Méjean A, Desgrandchamps F, Anglicheau D, Nochy D, Charron D, Empana J-P, Delahousse M, Legendre C, Glotz D, Hill GS, Zeevi A, Jouven X: Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369: 1215–1226, 2013 [DOI] [PubMed] [Google Scholar]
- 160.Carroll MC, Isenman DE: Regulation of humoral immunity by complement. Immunity 37: 199–207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Burns AM, Chong AS: Alloantibodies prevent the induction of transplantation tolerance by enhancing alloreactive T cell priming. J Immunol 186: 214–221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Burns AM, Ma L, Li Y, Yin D, Shen J, Xu J, Chong AS: Memory alloreactive B cells and alloantibodies prevent anti-CD154-mediated allograft acceptance. J Immunol 182: 1314–1324, 2009 [DOI] [PubMed] [Google Scholar]
- 163.Schinstock CA, Stegall M, Cosio F: New insights regarding chronic antibody-mediated rejection and its progression to transplant glomerulopathy. Curr Opin Nephrol Hypertens 23: 611–618, 2014 [DOI] [PubMed] [Google Scholar]
- 164.Perry DK, Pollinger HS, Burns JM, Rea D, Ramos E, Platt JL, Gloor JM, Stegall MD: Two novel assays of alloantibody-secreting cells demonstrating resistance to desensitization with IVIG and rATG. Am J Transplant 8: 133–143, 2008 [DOI] [PubMed] [Google Scholar]
- 165.Wiebe C, Pochinco D, Blydt-Hansen TD, Ho J, Birk PE, Karpinski M, Goldberg A, Storsley LJ, Gibson IW, Rush DN, Nickerson PW: Class II HLA epitope matching-A strategy to minimize de novo donor-specific antibody development and improve outcomes. Am J Transplant 13: 3114–3122, 2013 [DOI] [PubMed] [Google Scholar]
- 166.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, Tary-Lehmann M: Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol 163: 2267–2275, 1999 [PubMed] [Google Scholar]
- 167.Gorbacheva V, Fan R, Fairchild RL, Baldwin WM 3rd, Valujskikh A: Memory CD4 T cells induce antibody-mediated rejection of renal allografts. J Am Soc Nephrol 27: 3299–3307, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kazatchkine MD, Kaveri SV: Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med 345: 747–755, 2001 [DOI] [PubMed] [Google Scholar]
- 169.Abu Jawdeh BG, Cuffy MC, Alloway RR, Shields AR, Woodle ES: Desensitization in kidney transplantation: Review and future perspectives. Clin Transplant 28: 494–507, 2014 [DOI] [PubMed] [Google Scholar]
- 170.Chen Y-B, Kawai T, Spitzer TR: Combined bone marrow and kidney transplantation for the induction of specific tolerance. Adv Hematol 2016: 6471901–6471908, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Macklin PS, Morris PJ, Knight SR: A systematic review of the use of rituximab for the treatment of antibody-mediated renal transplant rejection. Transplant Rev (Orlando) 31: 87–95, 2017 [DOI] [PubMed] [Google Scholar]
- 172.Jackson AM, Kraus ES, Orandi BJ, Segev DL, Montgomery RA, Zachary AA: A closer look at rituximab induction on HLA antibody rebound following HLA-incompatible kidney transplantation. Kidney Int 87: 409–416, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Koenig A, Thaunat O: Lymphoid neogenesis and tertiary lymphoid organs in transplanted organs. Front Immunol 7: 646, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Thaunat O, Graff-Dubois S, Brouard S, Gautreau C, Varthaman A, Fabien N, Field A-C, Louedec L, Dai J, Joly E, Morelon E, Soulillou J-P, Michel J-B, Nicoletti A: Immune responses elicited in tertiary lymphoid tissues display distinctive features. PLoS One 5: e11398, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dufek S, Khalil A, Mamode N, Sebire NJ, Marks SD: Plasma-cell-rich infiltrates in paediatric renal transplant biopsies are associated with increased risk of renal allograft failure. Pediatr Nephrol 32: 679–684, 2017 [DOI] [PubMed] [Google Scholar]
- 176.Hasegawa J, Honda K, Wakai S, Shirakawa H, Omoto K, Okumi M, Ishida H, Tanabe K: Plasma cell-rich rejection after kidney transplantation and the role of donor-specific antibodies: A case report and review of the literature. Transplant Proc 47: 2533–2536, 2015 [DOI] [PubMed] [Google Scholar]
- 177.Katsuma A, Yamamoto I, Komatsuzaki Y, Niikura T, Kawabe M, Okabayashi Y, Yamakawa T, Katsumata H, Nakada Y, Kobayashi A, Tanno Y, Miki J, Yamada H, Ohkido I, Tsuboi N, Yamamoto H, Yokoo T: Subclinical antibody-mediated rejection due to anti-human-leukocyte-antigen-DR53 antibody accompanied by plasma cell-rich acute rejection in a patient with cadaveric kidney transplantation. Nephrology (Carlton) 21[Suppl 1]: 31–34, 2016 [DOI] [PubMed] [Google Scholar]
- 178.Uppin MS, Gudithi S, Taduri G, Prayaga AK, Raju SB: Expanding the antibody-mediated component of plasma cell-rich acute rejection: A case series. Indian J Nephrol 26: 176–181, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vogelbacher R, Meister S, Gückel E, Starke C, Wittmann S, Stief A, Voll R, Daniel C, Hugo C: Bortezomib and sirolimus inhibit the chronic active antibody-mediated rejection in experimental renal transplantation in the rat. Nephrol Dial Transplant 25: 3764–3773, 2010 [DOI] [PubMed] [Google Scholar]
- 180.Perry DK, Burns JM, Pollinger HS, Amiot BP, Gloor JM, Gores GJ, Stegall MD: Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant 9: 201–209, 2009 [DOI] [PubMed] [Google Scholar]
- 181.Ejaz NS, Alloway RR, Halleck F, Dürr M, Budde K, Woodle ES: Review of bortezomib treatment of antibody-mediated rejection in renal transplantation. Antioxid Redox Signal 21: 2401–2418, 2014 [DOI] [PubMed] [Google Scholar]
- 182.Eskandary F, Bond G, Schwaiger E, Kikic Z, Winzer C, Wahrmann M, Marinova L, Haslacher H, Regele H, Oberbauer R, Böhmig GA: Bortezomib in late antibody-mediated kidney transplant rejection (BORTEJECT Study): Study protocol for a randomized controlled trial. Trials 15: 107, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sango C, Merino D, San Segundo D, Rodrigo E, Lopez-Hoyos M, Benito A, Ángeles Ramos M, Gómez-Román J, Arias M: B-cell-activating factor levels are associated with antibody-mediated histological damage in kidney transplantation. Transplant Proc 48: 2910–2912, 2016 [DOI] [PubMed] [Google Scholar]
- 184.Pongpirul W, Chancharoenthana W, Pongpirul K, Leelahavanichakul A, Kittikowit W, Jutivorakool K, Nonthasoot B, Avihingsanon Y, Eiam-Ong S, Praditpornsilpa K, Townamchai N: B-cell activating factor (BAFF), a predictor of antibody mediated rejection in kidney transplantation recipients [published online ahead of print Nov 26, 2016]. Nephrology (Carlton) 10.1111/nep.12972 [DOI] [PubMed]
- 185.Mujtaba MA, Komocsar WJ, Nantz E, Samaniego MD, Henson SL, Hague JA, Lobashevsky AL, Higgins NG, Czader M, Book BK, Anderson MD, Pescovitz MD, Taber TE: Effect of treatment with tabalumab, a B cell-activating factor inhibitor, on highly sensitized patients with end-stage renal disease awaiting transplantation. Am J Transplant 16: 1266–1275, 2016 [DOI] [PubMed] [Google Scholar]
- 186.Jordan SC, Lorant T, Choi J, Kjellman C, Winstedt L, Bengtsson M, Zhang X, Eich T, Toyoda M, Eriksson B-M, Ge S, Peng A, Järnum S, Wood KJ, Lundgren T, Wennberg L, Bäckman L, Larsson E, Villicana R, Kahwaji J, Louie S, Kang A, Haas M, Nast C, Vo A, Tufveson G: IgG endopeptidase in highly sensitized patients undergoing transplantation. N Engl J Med 377: 442–453, 2017 [DOI] [PubMed] [Google Scholar]
- 187.Dörner T, Radbruch A, Burmester GR: B-cell-directed therapies for autoimmune disease. Nat Rev Rheumatol 5: 433–441, 2009 [DOI] [PubMed] [Google Scholar]
- 188.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, Utset TO, Gordon C, Isenberg DA, Hsieh H-J, Zhang D, Brunetta PG: Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: The randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 62: 222–233, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sautenet B, Blancho G, Büchler M, Morelon E, Toupance O, Barrou B, Ducloux D, Chatelet V, Moulin B, Freguin C, Hazzan M, Lang P, Legendre C, Merville P, Mourad G, Mousson C, Pouteil-Noble C, Purgus R, Rerolle J-P, Sayegh J, Westeel P-F, Zaoui P, Boivin H, Le Gouge A, Lebranchu Y: One-year results of the effects of rituximab on acute antibody-mediated rejection in renal transplantation: RITUX ERAH, a multicenter double-blind randomized placebo-controlled trial. Transplantation 100: 391–399, 2016 [DOI] [PubMed] [Google Scholar]
- 190.Silva-Fernández L, Loza E, Martínez-Taboada VM, Blanco R, Rúa-Figueroa I, Pego-Reigosa JM, Muñoz-Fernández S; Systemic Autoimmune Diseases Study Group of the Spanish Society for Rheumatology (EAS-SER) : Biological therapy for systemic vasculitis: A systematic review. Semin Arthritis Rheum 43: 542–557, 2014 [DOI] [PubMed] [Google Scholar]
- 191.Chong AS, Sciammas R: Memory B cells in transplantation. Transplantation 99: 21–28, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jacob N, Stohl W: Autoantibody-dependent and autoantibody-independent roles for B cells in systemic lupus erythematosus: Past, present, and future. Autoimmunity 43: 84–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Jennette JC: Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 110: 955–963, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dumoitier N, Terrier B, London J, Lofek S, Mouthon L: Implication of B lymphocytes in the pathogenesis of ANCA-associated vasculitides. Autoimmun Rev 14: 996–1004, 2015 [DOI] [PubMed] [Google Scholar]
- 195.Xiao H, Heeringa P, Liu Z, Huugen D, Hu P, Maeda N, Falk RJ, Jennette JC: The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol 167: 39–45, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Little MA, Smyth L, Salama AD, Mukherjee S, Smith J, Haskard D, Nourshargh S, Cook HT, Pusey CD: Experimental autoimmune vasculitis: An animal model of anti-neutrophil cytoplasmic autoantibody-associated systemic vasculitis. Am J Pathol 174: 1212–1220, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chavele K-M, Shukla D, Keteepe-Arachi T, Seidel JA, Fuchs D, Pusey CD, Salama AD: Regulation of myeloperoxidase-specific T cell responses during disease remission in antineutrophil cytoplasmic antibody-associated vasculitis: The role of Treg cells and tryptophan degradation. Arthritis Rheum 62: 1539–1548, 2010 [DOI] [PubMed] [Google Scholar]
- 198.Little MA, Al-Ani B, Ren S, Al-Nuaimi H, Leite M Jr., Alpers CE, Savage CO, Duffield JS: Anti-proteinase 3 anti-neutrophil cytoplasm autoantibodies recapitulate systemic vasculitis in mice with a humanized immune system. PLoS One 7: e28626, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dixon FJ, Andrews BS, Eisenberg RA, McConahey PJ, Theofilopoulos AN, Wilson CB: Etiology and pathogenesis of a spontaneous lupus-like syndrome in mice. Arthritis Rheum 21[5 Suppl]: S64–S67, 1978 [DOI] [PubMed] [Google Scholar]
- 200.Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Roths JB, Dixon FJ: Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med 148: 1198–1215, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rudofsky UH, Evans BD, Balaban SL, Mottironi VD, Gabrielsen AE: Differences in expression of lupus nephritis in New Zealand mixed H-2z homozygous inbred strains of mice derived from New Zealand black and New Zealand white mice. Origins and initial characterization. Lab Invest 68: 419–426, 1993 [PubMed] [Google Scholar]
- 202.Morel L: Mapping lupus susceptibility genes in the NZM2410 mouse model. Adv Immunol 115: 113–139, 2012 [DOI] [PubMed] [Google Scholar]
- 203.Cohen MG, Pollard KM, Schrieber L: Relationship of age and sex to autoantibody expression in MRL-+/+ and MRL-lpr/lpr mice: Demonstration of an association between the expression of antibodies to histones, denatured DNA and Sm in MRL-+/+ mice. Clin Exp Immunol 72: 50–54, 1988 [PMC free article] [PubMed] [Google Scholar]
- 204.Herlands RA, William J, Hershberg U, Shlomchik MJ: Anti-chromatin antibodies drive in vivo antigen-specific activation and somatic hypermutation of rheumatoid factor B cells at extrafollicular sites. Eur J Immunol 37: 3339–3351, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Uehara S, Chase CM, Cornell LD, Madsen JC, Russell PS, Colvin RB: Chronic cardiac transplant arteriopathy in mice: Relationship of alloantibody, C4d deposition and neointimal fibrosis. Am J Transplant 7: 57–65, 2007 [DOI] [PubMed] [Google Scholar]
- 206.Kwun J, Oh BC, Gibby AC, Ruhil R, Lu VT, Kim DW, Page EK, Bulut OP, Song MQ, Farris AB, Kirk AD, Knechtle SJ, Iwakoshi NN: Patterns of de novo allo B cells and antibody formation in chronic cardiac allograft rejection after alemtuzumab treatment. Am J Transplant 12: 2641–2651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]