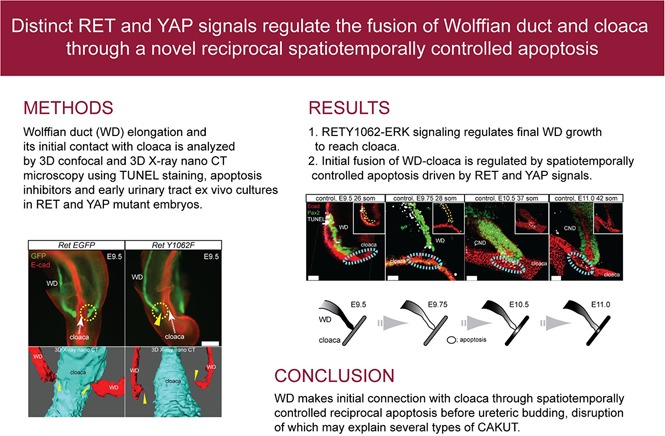
Abstract
The epithelial Wolffian duct (WD) inserts into the cloaca (primitive bladder) before metanephric kidney development, thereby establishing the initial plumbing for eventual joining of the ureters and bladder. Defects in this process cause common anomalies in the spectrum of congenital anomalies of the kidney and urinary tract (CAKUT). However, developmental, cellular, and molecular mechanisms of WD-cloaca fusion are poorly understood. Through systematic analysis of early WD tip development in mice, we discovered that a novel process of spatiotemporally regulated apoptosis in WD and cloaca was necessary for WD-cloaca fusion. Aberrant RET tyrosine kinase signaling through tyrosine (Y) 1062, to which PI3K- or ERK-activating proteins dock, or Y1015, to which PLCγ docks, has been shown to cause CAKUT-like defects. Cloacal apoptosis did not occur in RetY1062F mutants, in which WDs did not reach the cloaca, or in RetY1015F mutants, in which WD tips reached the cloaca but did not fuse. Moreover, inhibition of ERK or apoptosis prevented WD-cloaca fusion in cultures, and WD-specific genetic deletion of YAP attenuated cloacal apoptosis and WD-cloacal fusion in vivo. Thus, cloacal apoptosis requires direct contact and signals from the WD tip and is necessary for WD-cloacal fusion. These findings may explain the mechanisms of many CAKUT.
Keywords: apoptosis, kidney development, ureteric bud, CAKUT, nephric duct, Wolffian duct
The Wolffian duct (WD) is a critical structure for kidney formation and its connectivity with the cloaca (primitive bladder). Progenitors in the WD produce a ureteric bud (UB) from its distal end by embryonic day 10.5 in mice that gives rise to the entire collecting system of the kidney.1 These events are among the first steps necessary to form a contiguous connection between the upper and lower urinary tract. Developmental defects that disrupt UB or WD normal growth result in a variety of congenital anomalies of the kidney and urinary tract (CAKUT), including renal agenesis, hypoplasia, and ureter defects that can cause reflux or obstruction.2,3 Despite CAKUT being the largest cause of kidney failure in children, we have limited understanding of the specific signaling and developmental mechanisms that are fundamental to WD growth, its insertion into the cloaca, and UB induction.1
We and others have shown that GDNF, RET receptor tyrosine kinase mutations cause several types of CAKUT.4–6 RET activation results in the phosphorylation of key tyrosines, Y1015 and Y1062, which bind intracellular adaptors PLCγ and SHC, IGF, and FRS1 and 2ɑ, respectively. This results in activation of signaling cascades (PKC, MAPK, or PI3K) that regulate cellular processes such as proliferation, apoptosis, migration, and renewal.7,8 Mice with no or aberrant RET signaling, including through Y1062 or Y1015, cause a spectrum of distinct urinary tract defects reminiscent of CAKUT.8–13 Here we investigated specific RET-mediated pathways and cellular mechanisms that regulate distal WD elongation and its fusion with the cloaca.
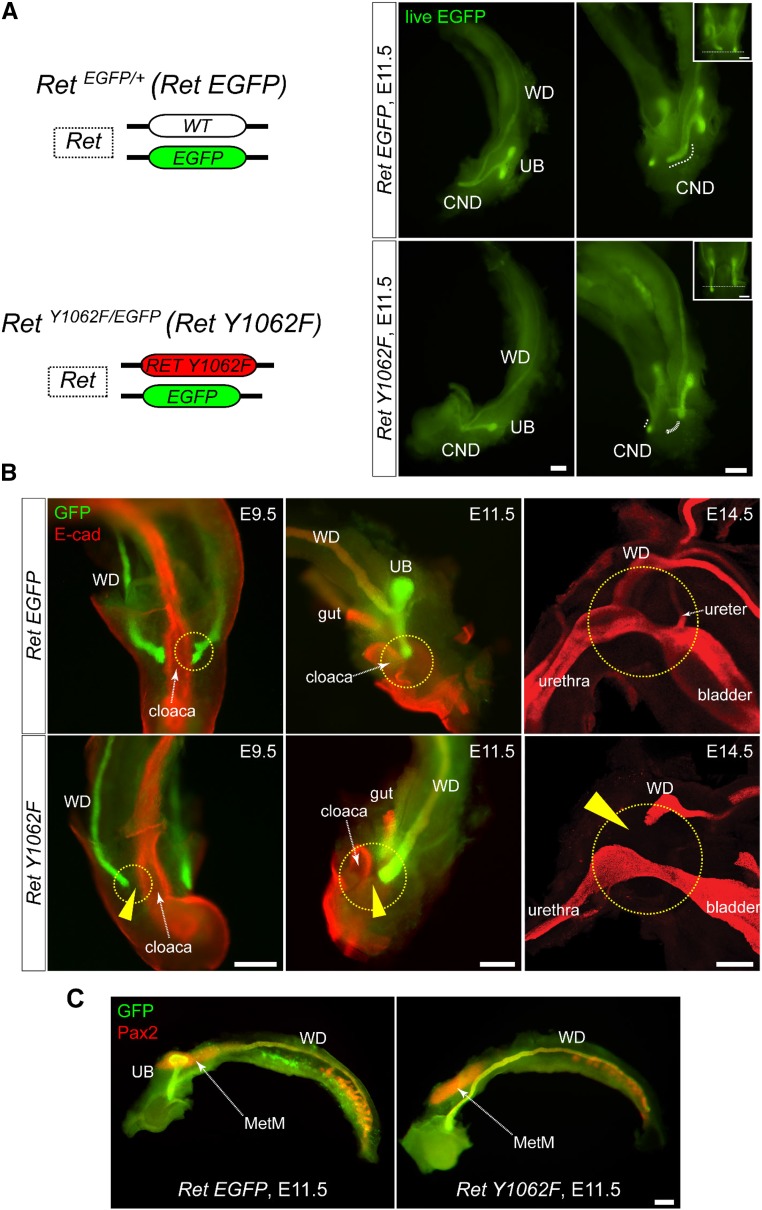
Previously we showed that RetY1015F mutant WDs were abnormally patterned, but surprisingly they were able to reach cloaca,14 unlike the Ret null mice15 (Supplemental Figure 1). Analysis of RetY1062F genitourinary tracts at different developmental time points beginning at embryonic day 9.5 showed failure of mutant WDs to reach cloaca, asymmetric WD growth, and UB induction defects at all ages examined, suggesting that RET-Y1062 signaling is important for WD to reach cloaca (Figure 1, A and B, Supplemental Movie 1). The metanephric mesenchyme and cloaca appeared normally developed in RetY1062F mice, indicating that the defects are WD autonomous and not because of the absence of these structures (Figure 1C).
Figure 1.
WDs of RetY1062F mutant mice fail to reach cloaca. (A) Left: Simplified illustration of modified Ret locus denoting RetEGFP reporter and RetY1062F mutant alleles. Right: Live EGFP (green) images of whole urinary tract of RetEGFP (RetEGFP/+) control and RetY1062F (RetY1062F/EGFP) embryos at embryonic day 11.5. Although the CND and T-shaped UB are normally developed in control (top), the UB is absent or rudimentary with no branching in RetY1062F mice (bottom, see also Supplemental Movie 1). WD length was asymmetric in 57% of the mutants (13 out of 23 embryos, inset) denoting defective elongation. (B) Whole-mount immunofluorescence images with GFP (green, WD) and E-cad (red, cloaca or bladder) antibodies reveal that the mutant WDs fail to reach cloaca or bladder during development. Images for embryonic days 9.5 and 11.5 are whole-mount images acquired by fluorescence stereomicroscope, and 14.5 images are three-dimensionally reconstructed confocal microscopy images. Arrowheads show the gap between the WD tip and cloaca or bladder. (C) Whole-mount immunofluorescence images with GFP (green, WD and UB) and Pax2 (red, WD, UB, and MetM) antibodies show normally developed MetM in RetY1062F mutant urinary tract, indicating that the WD defects seen in this mutant are autonomous. Scale bar, 200 μm. MetM; metanephric mesenchyme.
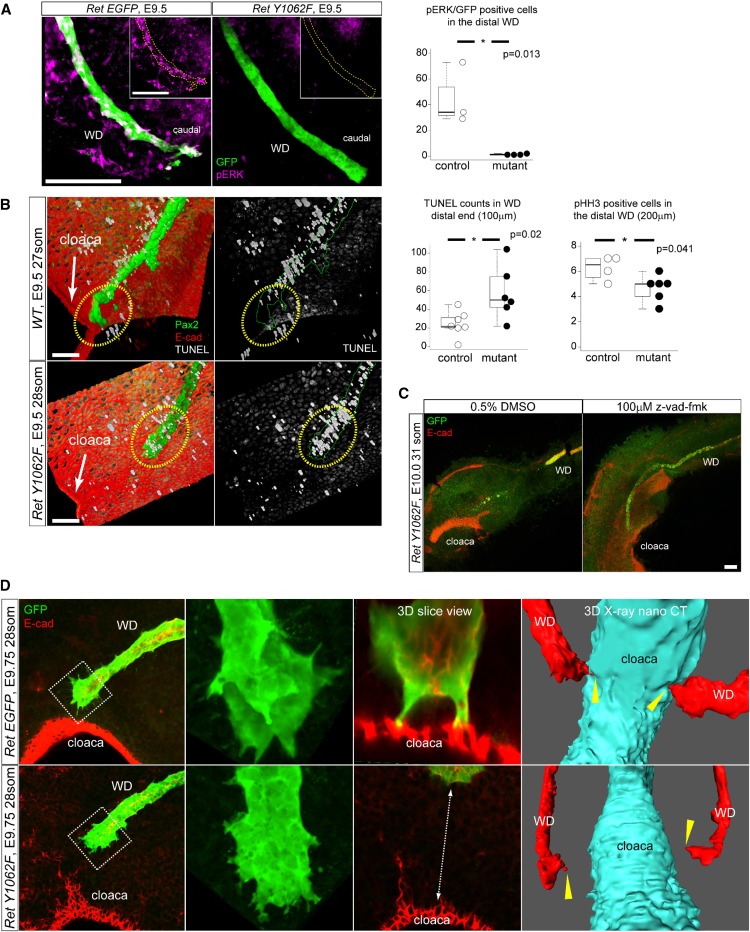
Because RET-Y1062 is a major docking site for activating MAPK and AKT signaling, we investigated which of these pathways is the mechanism of WD growth defects in RetY1062F mutant mice. The caudal RetY1062F WDs show markedly reduced ERK activity at embryonic day 9.5 compared with controls (Figure 2A), and rostral WDs (Supplemental Figure 2). We next examined how ERK activity affects WD growth to reach cloaca by analyzing proliferation and apoptosis. RetY1062F WDs exhibit markedly increased apoptosis in its tip and a modest reduction in proliferation compared with controls (Figure 2B, Supplemental Figure 2). The pAKT signal was sparse and not significantly different between the control and mutant WDs (Supplemental Figure 3). Inhibition of ERK activity in whole genitourinary cultures with ERK inhibitor U0126 prevented WD-cloaca fusion (Supplemental Figure 4). Inhibition of apoptosis with pan-caspase inhibitor z-vad-fmk in explant cultures of RetY1062F urinary tracts rescued WD elongation defects in these mutants (Figure 2C). In other systems, ERK activation by receptor tyrosine kinases regulates filopodia formation and cell migration to target sites.16 Control WDs exhibit filopodia-like cellular protrusions (CP) at their leading edge, with sharp spikes contacting cloaca, which were severely affected in RetY1062F WDs (Figure 2D, Supplemental Figure 5, Supplemental Movies 2 and 3). As an alternative method, we used whole-embryo three-dimensional x-ray nano-computed tomography imaging with submicron resolution to further confirm that RetY1062F mutant WDs have a defect in reaching cloaca and in CPs (Figure 2D, Supplemental Figure 5, Supplemental Movies 4 and 5).17 These results demonstrate that RET-Y1062-ERK signaling regulates terminal elongation of WD toward cloaca by controlling the survival of WD tip cells.
Figure 2.
Loss of ERK activity in RetY1062F WD tip causes increased apoptosis and migration defects to reach cloaca. (A) Whole-mount three-dimensional confocal immunofluorescence images with GFP (Ret-positive WD cells) and pERK antibodies reveal that the distal end of RetY1062F WDs shows lack of ERK activity at embryonic day 9.5. Yellow dotted lines outline the WD in the insets. Quantification of pERK/EGFP double-positive cells in the distal region (200 μm from the tip) of the WD shows significant reduction in RetY1062F mutant WD (control: 45.3±19.7, two litters, n=3; mutant: 1.3±0.4, three litters, n=4; P=0.01; mean±SD, t-test). (B) Three-dimensional reconstructed confocal z-stacked images of whole-mount immunofluorescence for TUNEL (white), Pax2 (green, WD), and E-cad (red, cloaca and body wall) in control (RetEGFP/+or WT) and RetY1062F (RetY1062F/EGFP) WDs. Note marked cell death in the distal end of WD in RetY1062F embryos at embryonic day 9.5. Control WD reaches cloaca and shows almost no TUNEL in the WD tip (top). Mutant WD tip shows extensive cell death preventing elongation and contact with cloaca (bottom). Yellow dashed circles represent WD tip region. Cell apoptosis measured by TUNEL staining in the distal region (100 μm from the tip) of WD is significantly increased in RetY1062F mutant (control: 24.6±12.3, two litters, n=6; mutant: 57.2±26.1, three litters, n=6; P=0.02; mean±SD, t-test). Cell proliferation measured by pHH3 immunofluorescence in the distal region (200 μm from the tip) of WD is significantly reduced in RetY1062F mice (control: 6.3±1.0, one litter, n=4; mutant: 4.7±1.0, one litter, n=6; P=0.04; mean±SD, t-test). (C) Inhibition of apoptosis in RetY1062F mutant WD at embryonic day 10.0 rescues defective elongation of WD toward cloaca. RetY1062F WDs treated with vehicle (DMSO) stop growing and fail to reach cloaca as observed in vivo (compare with Figure 1B). Growth defects of RetY1062F WD are rescued with z-vad-fmk treatment. Note that the 24-hour ex vivo culture is technically limited to assess if WD elongation is completely rescued, especially in WDs that are more severely affected. Images were acquired by confocal imaging with z-stacks of whole-mount immunofluorescence of cultured specimens with GFP (green, WD) and E-cad (red, WD and cloaca) antibodies. (D) RetEGFP WDs at embryonic day 9.5 show extensive sharp cellular protrusions in the advancing front as visualized by GFP (green, WD) and E-cad (red, cloaca) whole-mount three-dimensional confocal immunofluorescence microscopy maximal projection images. RetY1062F WDs lack terminal cellular protrusions and fail to reach cloacal epithelia (see also Supplemental Figure 5A, Supplemental Movies 2 and 3). Images on the right from embryonic day 9.75 whole three-dimensional x-ray nano-computed technology scanning further show WD-cloaca fusion and cellular protrusions defects in RetY1062F WDs (red, WD; cyan, cloaca). Yellow arrow heads represent the tip of the WD, which touched down on the cloaca in RetEGFP control embryo, whereas failed in RetY1062F mutant (see also Supplemental Figure 5B, Supplemental Movies 4 and 5). Scale bar, 100 μm. * denotes statistical significance.
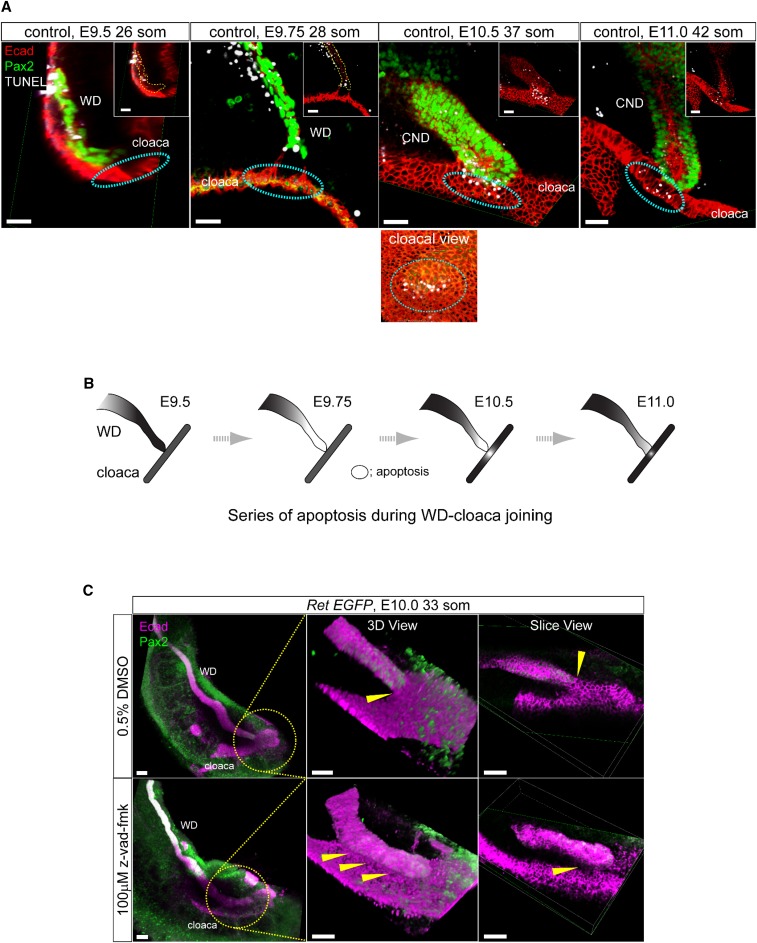
We next examined the role of apoptosis in WD-cloaca fusion. Systematic analysis of WD and cloaca between embryonic days 9.5 and 10.5 using whole-mount three-dimensional confocal microscopy with TUNEL staining revealed a highly ordered novel series of apoptosis events in both WD tip and cloaca (Figure 3A). At embryonic day 9.5 (26 somites) the WD tip harbors basal low-level apoptosis. At embryonic day 9.75 (28 somites), there is a notable increase in TUNEL-positive cells in the distal WD (reached cloaca) but not at the putative insertion site in cloaca. At embryonic days 10.0–10.5, TUNEL-positive cells appear in the cloaca at the point of contact with the WD tip. At embryonic day 11.0, apoptosis continues in cloaca and the distal WD (now called the common nephric duct [CND]); events beyond this time have been previously described in the process of ureter remodeling.18,19 To confirm that this spatiotemporal relay of apoptosis in WD and cloaca is critical for initial fusion, we performed whole urinary tract culture at embryonic day 10.0 with the pan-caspase inhibitor z-vad-fmk (Figure 3C). The WD tip fused to cloacal epithelia in vehicle-treated cultures, whereas WDs treated with z-vad-fmk failed to fuse with the cloaca. Taken together, these observations suggest that the initial fusion of WD-cloaca depends on highly precise interactions of apoptosis in these structures before ureteric budding.
Figure 3.
Novel interactive process of spatiotemporally restricted focal apoptosis is required to form the primary WD-cloaca contact before UB budding. (A) Images show time course analysis of WD-cloaca junction in control embryos using whole-mount immunofluorescence three-dimensional confocal microscopy with E-cad (red, cloaca and WD) and Pax2 (green, WD and MetM) antibodies combined with TUNEL staining (white). Cyan dashed circles represent the putative WD-cloaca fusion site; there is no apoptosis at this site in cloaca before its contact with WD (left). Note persistent focal apoptosis in cloaca at its site of contact with WD beginning at embryonic day 10.5 and until they fuse (middle and right). At embryonic day 11.5 onwards, CND begins to show further increase in apoptosis as previously reported to pave the way for later ureter insertion. Bottom image illustrates views from different angle depicting WD-cloaca contact and apoptosis. (B) The schematic illustrates the wave of interactive apoptosis between WD and cloaca during embryonic day 9.5 to 11.0 from the results shown in (A). (C) Inhibiting apoptosis prevents WD-cloacal fusion in whole urinary tract cultures. Whole-mount immunofluorescence analysis with E-cad (magenta, WD and cloacal epithelia) and Pax2 (green, WD) antibodies using three-dimensional reconstructed confocal imaging of whole urinary tract culture (starting at embryonic day 10.0) with pan-caspase inhibitor z-vad-fmk was performed. Vehicle treatment (0.5% DMSO) shows WD fusion with cloacal epithelia after 24 hours of culture (top, three-dimensional rendering and slice views, yellow arrowhead shows contact point). On the other hand, 100 μM z-vad-fmk treatment shows WD laying over cloacal epithelia but no clear fusion (bottom, yellow arrowheads point to no clear fusion between the WD tip and cloaca).
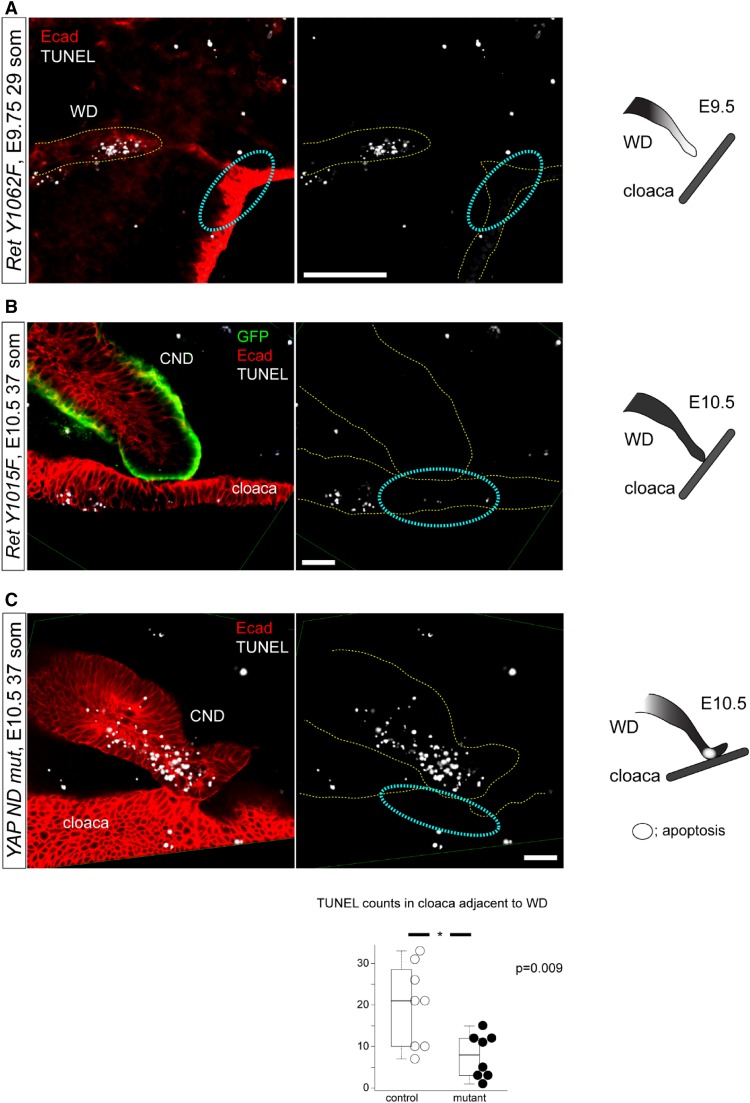
To determine how cloacal apoptosis is regulated, we examined if cloacal apoptosis is autonomous and does not depend on its contact with the WD. Analysis of RetY1062F mice in which WDs do not reach cloaca showed no cloacal apoptosis (Figure 4A), suggesting that WD tip contact is necessary for cloacal apoptosis. We then asked if WD tip apoptosis is required for cloacal apoptosis after it reached cloaca. To this end, we examined cloacal apoptosis in RetY1015F mutant WDs that fail to undergo CND apoptosis and are unable to fuse with the cloaca due to increased ERK activity.14 In RetY1015F mice, the WD tip reached the cloaca but neither cloacal nor WD tip apoptosis was observed suggesting that RET-Y1015–mediated signals from WD tip are necessary to induce cloacal apoptosis (Figure 4B). We previously showed that RET and YAP signaling pathways genetically interact in the WD tip and Yap-WD nulls initially contact cloaca but do not fuse properly with it.20 These mutant WDs exhibit a subset of abnormalities that are similar to the RetY1015F mutant WDs, including ectopic buds, abnormally located mesenchyme, increased proliferation, ERK activity, and elongated CNDs that suggest hyperactive RET signaling. Reducing Ret dosage in Yap-WD nulls corrects these defects. To determine if YAP signaling could be a mechanism of cloacal apoptosis from the WD tip, we examined cloacal apoptosis in Yap-WD nulls and found that cloacal apoptosis was markedly reduced (Figure 4C). These results demonstrate that differential ERK activity due to distinct RET docking tyrosine sites and related YAP signaling in the WD tip regulate final WD elongation and modulate cloacal apoptosis at their contact site to initiate WD tip-cloaca fusion.
Figure 4.
Ret docking tyrosines Y1062 and Y1015 signaling and YAP signaling in WD tip regulate induction of WD-cloacal apoptosis by distinct mechanism. (A) In RetY1062F embryos, WDs do not reach cloaca and cloacal apoptosis is not seen at the putative WD-cloaca fusion site (dashed cyan circles), indicating that cloacal cells at this site survive if WD does not reach it. (B) In RetY1015F embryos, WDs reach cloaca but both WD and cloaca fail to show apoptosis in the region of their contact, resulting in failure to form the initial WD-cloaca connection and proper ureter maturation. (C) In Yap-WD null mutant embryos, WD tip reaches cloaca but overshoots the potential fusion site with the cloaca. Apoptosis in the WD where it contacts cloaca (not the tip) is seen but extremely reduced in the contact site in cloaca; the graph shows quantification of cloacal apoptosis. This result suggests YAP signaling in the WD regulates cloacal apoptosis (control: 19.9±9.3, one litter, n=8; mutant: 7.8±5.0, one litter, n=8; P<0.01; mean±SD, t-test). All images were obtained by three-dimensional reconstructed z-stack images acquired by confocal microscopy using whole-mount immunofluorescence with E-cad (red, WD and cloaca) and GFP (green, WD) antibodies combined with TUNEL staining. Scale bar, 100 μm. * denotes statistical significance.
Our studies shed new light on current concepts in the pathogenesis of various forms of CAKUT, the mechanisms and fundamental knowledge of how infrastructure of the urinary system in mammals is established by delineating the mechanisms by which WD elongates and inserts into cloaca. The underlying pathogenetic mechanism of mutations in RET in CAKUT patients with agenesis, hypoplasia, and ureter defects may be because of aberrant ERK activity4–6 that affects elongation of the WD tip and its fusion with the cloaca through interdependent apoptosis. The studies in vitro provide supporting evidence for this model; however, one limitation is that they are not region selective and affect all structures. Although we have used RET signaling as a prototype to understand the mechanisms of WD development, RET is also a target of many upstream factors and regulates several molecules and downstream pathways that have roles in renal agenesis, reflux, or obstruction.21 Kidney formation or ureter connection defects with the bladder in other genetic mutants20,22 may be caused by some of the mechanisms delineated in abnormal WD growth, UB budding, and cloacal fusion in Ret individual signaling mutants.
Proper joining of epithelial tubes to their target structures is critical to the normal function of many organ systems. Different mechanisms are employed for joining of different epithelia. For example, cell invasion by the renal vesicle S-shaped bodies is used to fuse with the collecting duct,23–25 whereas spatiotemporally controlled apoptosis is required for the formation of anorectal opening.26 We delineated a novel focal cloacal apoptosis that depends on WD reaching the cloaca (via RET-Y1062) and the signals from the WD tip once a contact is made (via RET-Y1015 and YAP). The highly regulated apoptosis in both WD and cloaca is also necessary for subsequent CND degeneration and ureter remodeling between embryonic days 12.5 and 14.5,18,19 so the fusion between WD and cloaca continues to evolve to form a competent ureterovesical junction. In this regard, the mechanism of joining of these two different epithelia is apoptosis based end joining, and different from the connection between the distal tubule and the collecting ducts in the metanephros. Whether RET-mediated WD survival and elongation process are WD autonomous because of its interactions with other receptor tyrosine kinase pathways such as the FGF receptor, or dependent on factors that modulate RET activity in the urinary system such as GDNF or retinoic acid needs further studies.
The WD abnormalities in Yap-WD nulls and RetY105F mutants and previous observations that RET and YAP pathways interact support the view that normally YAP represses RET signaling, however, the underlying effector is unknown.20 The finding that loss of YAP in the WD blocks apoptosis in the cloaca is unexpected because YAP promotes proliferation and inhibits apoptosis in cell culture, and in cancer models. How might this unexpected observation be reconciled with existing data? Work with Drosophila has implicated a major role for the YAP homolog in cell competition, a process by which cells that are more fit promote the apoptosis of less fit cells.27 Cells with high levels of YAP or YAP homolog activity promote the apoptosis of cells with lower YAP activity. In Yap-WD nulls, the WD has no YAP and perhaps does not have competitive advantage over neighboring cells, and therefore cannot induce the death of adjacent cloacal cells. Thus, we hypothesize that during normal development, YAP activity is higher in the WD than in the cloaca. Further studies will help determine if cell competition underlies this RET-YAP dependent developmental apoptosis.
Our observations shed lights on the molecular and anatomic mechanisms of how WD makes initial connection with cloaca and understanding of pathogenesis of several types of CAKUT.
Concise Methods
Animal Models
All animal studies conducted were approved by the Animal Studies Committee at Washington University School of Medicine. We defined the day of the copulatory plug as embryonic day 0.5. The mice expressing EGFP reporter knocked-in the Ret locus were obtained as previously described (RetEGFP/+).14 Ret mutant mice were obtained by breeding parents harboring each mutant allele with Ret-EGFP reporter mice (RetY1062F, RetY1062F/EGFP; RetY1015F, RetY1015F/EGFP; Ret KO, RetEGFP/EGFP). For somite stage, we counted somites under a dissection microscope and confirmed with immunostaining. We used wild-type or Ret heterozygous mice as a control, unless indicated otherwise. Embryos of each genotype were chosen at random including sex for analysis as they became available from the breedings and littermate controls were used. RetY1062F and RetEGFP mice were maintained on C57BL/6 background and RETY1015F on C57B6–129SVJ background. Yap-WD nulls were generated by crossing Yapflox/flox mice with the Hoxb7:CreTg mouse line, and maintained on a mixed background.20 For quantitative analysis, the sample size was five or more from independent litters unless indicated otherwise (see legends of each figure). These numbers are adequate for morphologic analysis with the statistical tests used and logistically feasible for these early embryo stages and in compliance with humane use of animals. Prior power calculations were not done as variances within each group were not known in these early structures analyzed. Identical conditions were used to process control and mutant tissues.
Whole-Mount Immunofluorescence, Confocal Microscopy, and TUNEL Staining
Whole-mount immunofluorescence combined with TUNEL staining and confocal microscopy was performed as previously described.14 The antibodies used were GFP (GFP-1020, 1:200; Aves), E-cadherin (AF748, 1:50; R&D Systems), Pax2 (PRB-276P, 1:100; Covance), pERK1/2 (#4370, 1:50; Cell Signaling Technology), pAKT (#4060, 1:50; Cell Signaling Technology), and phospho-histone H3 (#9706, 1:100; Cell Signaling Technology). The visualization and analyses were done with a confocal microscopy system (Nikon C1, NIS elements BR and AR software).
Whole-Mount Urinary Tract Culture
Whole-mount urinary tract culture, including the culture using MEK inhibitor U0126 (Supplemental Figure 4), was performed as previously described.14 To confirm the role of apoptosis for initial WD-cloaca fusion, embryos at 33–37 somite stage (around embryonic day 10.5) were dissected and each sagittal half was cultured for 24 hours on an air-fluid interface on a transparent, 0.4 μm pore size, PET membrane (#353091; BD Biosciences) with either vehicle (DMSO, #154938; Sigma) or pan-caspase inhibitor–containing medium (100 μM z-vad-fmk, FMK001; R&D Systems). The specimens were fixed with 4% paraformaldehyde/PBS, pH 7.4, after the culture and subjected to whole-mount immunofluorescence and TUNEL staining.
Three-Dimensional X-Ray Nano-Computed Tomography Imaging
Embryos were stained with metal-enhanced DAB (#34065; Thermo Fisher Scientific) with an anti-RET antibody (#C31B4, 1:50; Cell Signaling Technology) and secondarily stained with osmium tetraoxide and uranyl acetate to provide intrinsic contrast and enhance the DAB stain. After staining, embryos were dehydrated and embedded in epoxy resin (Epon 812) for ultrarigid support necessary for long scan times. The embryos were then imaged in Zeiss Versa 520 x-ray microscope using 4× and 20× scintillator-coupled objectives to analyze tissues at 1.46 and 0.69 μm isometric voxels, respectively. Imaging was performed using 3201 projections for the 4× objective and 6001 projections for the 20× objective. Subsequent analysis and manual segmentation of the data were performed with Amira (Thermo Fisher Scientific).
Quantitative Analyses
For cell counts, all labeled cells with the respective antibodies were counted using three-dimensional reconstruction of the entire z-stack confocal images from whole-mount immunofluorescence experiments. We utilized NIS Elements BR software (Nikon) for quantifications. Statistical analysis results and data distribution are represented as whisker-boxplots (centered line is median) and dot plot by utilizing R software. The median values are indicated in each graph (first and third quartiles are represented by the lower and upper limits, the whiskers show the minimum and maximum values); statistical significance values (P<0.05 considered significant) and sample size are detailed in legends of each figure. Statistical significance was determined by two-tailed t-test.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Amanda Knoten for excellent technical assistance, especially with mouse breeding, care, and genotyping. M.H. and S.J. conceived the idea, designed the experiments, and wrote the manuscript. M.H. conducted the experiments. J.A.J.F. and M.S.J. designed and performed the three-dimensional x-ray nano-computed tomography imaging experiments. A.R. and H.M. provided the YAP-null WD embryos. All authors analyzed the data.
This work was supported by National Institutes of Health (NIH) grant R01DK082531 (S.J.). We thank the Kidney Translational Research Center (P30DK079333) and the Division of Nephrology for microscopy support, and the Murine Models Core (P30DK052574) of the Division of Gastroenterology, Digestive Diseases Research Cores Center for Ret mutant mice. We gratefully acknowledge support from Washington University School of Medicine, the Children’s Discovery Institute of Washington University, and St. Louis Children’s Hospital (CDI-CORE-2015-505) and the Foundation for Barnes-Jewish Hospital (3770). The Zeiss Xradia Versa 520 three-dimensional x-ray microscope was funded by the Office of Research Infrastructure Programs, a part of the NIH Office of the Director under grant 1S10OD021694-01.
The authors declare that all data supporting the findings of this study are available within the article and its Supplemental Material, or from the corresponding author on request.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017040380/-/DCSupplemental.
References
- 1.Costantini F, Kopan R: Patterning a complex organ: Branching morphogenesis and nephron segmentation in kidney development. Dev Cell 18: 698–712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pope JC 4th, Brock JW 3rd, Adams MC, Stephens FD, Ichikawa I: How they begin and how they end: Classic and new theories for the development and deterioration of congenital anomalies of the kidney and urinary tract, CAKUT. J Am Soc Nephrol 10: 2018–2028, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Woolf AS, Thiruchelvam N: Congenital obstructive uropathy: Its origin and contribution to end-stage renal disease in children. Adv Ren Replace Ther 8: 157–163, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Jeanpierre C, Macé G, Parisot M, Morinière V, Pawtowsky A, Benabou M, Martinovic J, Amiel J, Attié-Bitach T, Delezoide AL, Loget P, Blanchet P, Gaillard D, Gonzales M, Carpentier W, Nitschke P, Tores F, Heidet L, Antignac C, Salomon R; Société Française de Foetopathologie : RET and GDNF mutations are rare in fetuses with renal agenesis or other severe kidney development defects. J Med Genet 48: 497–504, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Skinner MA, Safford SD, Reeves JG, Jackson ME, Freemerman AJ: Renal aplasia in humans is associated with RET mutations. Am J Hum Genet 82: 344–351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee R, Ramos E, Hoffman M, VanWinkle J, Martin DR, Davis TK, Hoshi M, Hmiel SP, Beck A, Hruska K, Coplen D, Liapis H, Mitra R, Druley T, Austin P, Jain S: Traditional and targeted exome sequencing reveals common, rare and novel functional deleterious variants in RET-signaling complex in a cohort of living US patients with urinary tract malformations. Hum Genet 131: 1725–1738, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Airaksinen MS, Saarma M: The GDNF family: Signalling, biological functions and therapeutic value. Nat Rev Neurosci 3: 383–394, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Jain S: The many faces of RET dysfunction in kidney. Organogenesis 5: 177–190, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V: Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367: 380–383, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Jain S, Encinas M, Johnson EM Jr, Milbrandt J: Critical and distinct roles for key RET tyrosine docking sites in renal development. Genes Dev 20: 321–333, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain S, Naughton CK, Yang M, Strickland A, Vij K, Encinas M, Golden J, Gupta A, Heuckeroth R, Johnson EM Jr, Milbrandt J: Mice expressing a dominant-negative Ret mutation phenocopy human Hirschsprung disease and delineate a direct role of Ret in spermatogenesis. Development 131: 5503–5513, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Wong A, Bogni S, Kotka P, de Graaff E, D’Agati V, Costantini F, Pachnis V: Phosphotyrosine 1062 is critical for the in vivo activity of the Ret9 receptor tyrosine kinase isoform. Mol Cell Biol 25: 9661–9673, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jijiwa M, Fukuda T, Kawai K, Nakamura A, Kurokawa K, Murakumo Y, Ichihara M, Takahashi M: A targeting mutation of tyrosine 1062 in Ret causes a marked decrease of enteric neurons and renal hypoplasia. Mol Cell Biol 24: 8026–8036, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoshi M, Batourina E, Mendelsohn C, Jain S: Novel mechanisms of early upper and lower urinary tract patterning regulated by RetY1015 docking tyrosine in mice. Development 139: 2405–2415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chia I, Grote D, Marcotte M, Batourina E, Mendelsohn C, Bouchard M: Nephric duct insertion is a crucial step in urinary tract maturation that is regulated by a Gata3-Raldh2-Ret molecular network in mice. Development 138: 2089–2097, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendoza MC, Vilela M, Juarez JE, Blenis J, Danuser G: ERK reinforces actin polymerization to power persistent edge protrusion during motility. Sci Signal 8: ra47, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shearer T, Bradley RS, Hidalgo-Bastida LA, Sherratt MJ, Cartmell SH: Three-dimensional visualisation of soft biological structures by X-ray computed micro-tomography. J Cell Sci 129: 2483–2492, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Batourina E, Choi C, Paragas N, Bello N, Hensle T, Costantini FD, Schuchardt A, Bacallao RL, Mendelsohn CL: Distal ureter morphogenesis depends on epithelial cell remodeling mediated by vitamin A and Ret. Nat Genet 32: 109–115, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, Hensle T, Wang F, Niederreither K, McMahon AP, Carroll TJ, Mendelsohn CL: Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet 37: 1082–1089, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Reginensi A, Hoshi M, Boualia SK, Bouchard M, Jain S, McNeill H: Yap and Taz are required for Ret-dependent urinary tract morphogenesis. Development 142: 2696–2703, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis TK, Hoshi M, Jain S: To bud or not to bud: The RET perspective in CAKUT. Pediatr Nephrol 29: 597–608, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, Wilson PD, Costantini FD, Mason IJ, Licht JD: Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell 8: 229–239, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Airik R, Kispert A: Down the tube of obstructive nephropathies: The importance of tissue interactions during ureter development. Kidney Int 72: 1459–1467, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Kao RM, Vasilyev A, Miyawaki A, Drummond IA, McMahon AP: Invasion of distal nephron precursors associates with tubular interconnection during nephrogenesis. J Am Soc Nephrol 23: 1682–1690, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kao RM: The luminal connection: From animal development to lumopathies. Organogenesis 9: 111–117, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pyati UJ, Cooper MS, Davidson AJ, Nechiporuk A, Kimelman D: Sustained Bmp signaling is essential for cloaca development in zebrafish. Development 133: 2275–2284, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Menéndez J, Pérez-Garijo A, Calleja M, Morata G: A tumor-suppressing mechanism in Drosophila involving cell competition and the Hippo pathway. Proc Natl Acad Sci U S A 107: 14651–14656, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.