Abstract
Albuminuria is a key instigator of tubulointerstitial inflammation associated with CKD, but the mechanism through which filtered albumin propagates renal injury remains unclear. In this study, we explored the role in this process of exosome mRNA released from tubular epithelial cells (TECs). Compared with control mice, acute and chronic kidney injury models had more exosomes containing inflammatory cytokine mRNA, particularly the chemokine CCL2, in kidneys and urine. In vitro stimulation of TECs with BSA recapitulated this finding. Notably, the internalization of purified TEC exosomes by cultured macrophages increased if TECs were exposed to BSA. Macrophage internalization of exosomes from BSA-treated TECs led to an enhanced inflammatory response and macrophage migration, but CCL2 silencing in TECs prevented these effects. Using a GFP-CCL2 fusion mRNA construct, we observed direct transfer of CCL2 mRNA from TEC exosomes to macrophages. Mice subjected to tail vein injection of purified BSA-treated TEC exosomes developed tubular injury with renal inflammatory cell infiltration. However, injection of exosomes from BSA-treated CCL2-deficient TECs induced less severe kidney inflammation. Finally, in patients with IgA nephropathy, the increase of proteinuria correlated with augmented urinary excretion of exosomes with exaggerated expression of CCL2 mRNA. Moreover, the level of CCL2 mRNA in urinary exosomes correlated closely with levels of renal interstitial macrophage infiltration in these patients. Our studies demonstrate that the increasing release of exosomes that transfer CCL2 mRNA from TECs to macrophages constitutes a critical mechanism of albumin-induced tubulointerstitial inflammation.
Keywords: exosome, CCL2, mRNA, extracellular vesicle, albuminuria, macrophages

Albuminuria is a major manifestation of CKD. Evidence from both clinical and experimental studies suggest that albuminuria is not merely a simple marker of CKD progression, but is also a critical player in the development of the disease.1,2 Previous studies have suggested a key role of albuminuria in tubulointerstitial inflammation, but the mechanisms through which albumin enhances local immune responses in the kidney are still largely unknown. Tubular epithelial cells (TECs) are the major structural components of the kidney, which are directly exposed to filtered albumin and are also contiguous with the interstitium. We have recently demonstrated that albuminuria causes severe TEC injury and inflammation via the Nlrp3 inflammasome.2,3 Inflammatory chemokines or mediators released by TECs, like CCL2 (MCP-1), CCL5 (RANTES), or complement components could initiate accumulation of inflammatory cells in the renal interstitium.4 However, it is still unclear how an injury signal released by TECs in response to exposure to filtered albumin spreads to the interstitial space and induces tubulointerstitial inflammation. Understanding how albumin participates in the development of inflammation in the kidney should provide novel strategies for limiting the progression of CKD.
Beyond classic signaling through soluble cytokines and inflammatory mediators, extracellular vesicles have received considerable attention for their potential to deliver genetic information (e.g., mRNAs and regulatory microRNAs [miRNAs]).5 Recent studies have shown that cells can communicate with neighboring cells or with distant cells via extracellular vesicle-dependent information transfer.6 Exosomes in particular play a critical role transferring RNA between cells.7 Previous studies from our team and others have shown that exosomes and their packaged molecules can be novel biomarkers of CKD, reflecting the severity of tubulointerstitial fibrosis and the decline of renal function.8,9 These findings suggest a pathophysiologic role for exosomes in renal tissue damage and regeneration through the maintenance of internephron communication.10,11 Exosomes from injured tubular cells transfer TGFβ1 mRNA into fibroblasts, culminating in their proliferation, α-smooth muscle actin expression, F-actin–dependent cytoskeletal rearrangements, and type I collagen production.12 Tubular cells can also secrete miR-21 and deliver it via microvesicles into adjacent recipient tubules, promoting transition in tubular phenotype.13
Macrophages are key inflammatory cells involved in renal repair or fibrosis. It is increasingly recognized that molecules released by dying cells (damage-associated molecular patterns) can activate cellular pattern recognition receptors like Toll-like receptors,14 NOD-like receptors,3 and the Mincle receptor.15 However, the process through which inflammatory signals released from TECs initiate the infiltration of macrophages requires elucidation. In this study, we posited that exosomes could promote tubulointerstitial inflammation by transferring damage signals from TECs to interstitial macrophages after the exposure of TECs to filtered albumin. We found that albumin can trigger TECs to release exosomes loaded with CCL2 mRNA for subsequent transfer to macrophages and consequent induction of interstitial inflammation. Thus, our studies highlight a novel stepwise mechanism through which albuminuria propagates tubulointerstitial disease in the kidney.
Results
Exosome Packing with CCL2 mRNA Is Increased in an AKI Model
AKI was induced by LPS administration as previously reported.16 We intraperitoneally injected a single dose of LPS into adult BALB/c mice and analyzed kidney histology, kidney function, and urinary protein excretion. Serum creatinine and urine albumin-to-creatinine ratios were significantly increased in LPS-treated mice compared with controls. Histologically, the TEC injury, protein cast, and CD68+ macrophage infiltration were found in LPS-injected mice (Figure 1A). However, there were significant increases in mRNA expression of renal inflammatory cytokines (CCL2, IL1β, TNFα, IL6) (Figure 1B). Neutrophil gelatinase-associated lipocalin (NGAL), an important tubular epithelial injury marker, was similarly upregulated in the LPS-treated group (Figure 1C). To understand the change of exosome release in AKI, we isolated, characterized, and quantified exosomes from kidneys using transmission electron microscopy (TEM), Western blotting (using Alix, CD9, and CD63 as exosome markers), and ZetaView nanoparticle tracking analysis (NTA). Interestingly, exosome release from TECs was observed with TEM. EVs purified from kidney tissue showed the typical size and shape of exosomes (Figure 1D). We found that kidneys from LPS-treated mice exhibited an increase in exosome production compared with controls, as measured by exosome number, using NTA (3.8±1.15×1010 and 18±3.6×1010 particles/ml for control and LPS mice, respectively) and protein quantification of exosome markers (Figure 1, E and F). In addition, we demonstrated that injured kidneys secrete exosomes highly enriched with CCL2, TNFα, and IL6 mRNA compared with control kidneys (Figure 1G).
Figure 1.
Exosome packing with CCL2 mRNA is increased in an AKI model. (A) Histologic (PAS staining) and immunohistologic (CD68+ immunostaining) changes, serum levels of creatinine (Scr), and urine albumin-to-creatinine ratio (ACR) in the LPS-induced kidney injury model at 24 hours. (B) Inflammatory cytokine mRNA (CCL2, IL1β, TNFα, IL 6) expression in the kidney by real-time PCR. (C) Western blotting and real-time PCR analysis of NGAL expression in kidney. Data expressed as the mean±SD for groups of six mice or as independent experiments. (D) Representative electron micrograph of EVs released by renal TECs in kidney tissue and exosomes purified from the kidney. Scale bars, 200 and 100 nm. (E) Vesicle content of exosome markers of Alix, CD9, and CD63 confirmed by Western blotting. Quantification analysis showed increased exosome protein in LPS mice compared with controls. (F) Exosome size and number were quantified by NTA (n=3 for each group). (G) Inflammatory cytokine mRNA (CCL2, IL1β, TNFα, IL6) expression in kidney exosomes by real-time PCR. Original magnification, ×200. *P<0.05; **P<0.01; ***P<0.001 compared with the control (Ctrl) mice (n=5 for control mice, n=7 for LPS mice).
Exosome Packing with CCL2 mRNA Is Increased in a Chronic Kidney Injury Model
In order to explore TEC exosome release in a chronic kidney injury model, 5/6 nephrectomy rats were used. As expected, proteinuria and deteriorating renal function were detected in 5/6 nephrectomy rats (Figure 2A). At 16 weeks after surgery, significant TEC injury, protein cast, tubulointerstitial inflammation, and fibrosis were observed in the kidney as shown by periodic acid–Schiff (PAS) and Masson staining (Figure 2B). We detected significant increases in expression of inflammatory cytokine mRNAs (CCL2, IL1β) in the kidney (Figure 2C). Notably, the number of exosomes was increased in both the kidney and urine, as documented by Western blotting analysis of Alix, CD63, and CD9 (Figure 2, D and E). Further, NTA confirmed the particle size and the increased number of the exosomes in the chronic kidney injury model (4.15±1.77×1010 and 9.65±1.91×1010 particles/ml for control and 5/6 nephrectomy rats, respectively) (Figure 2F). Within the renal exosomes from the 5/6 nephrectomy animals, CCL2 mRNA was markedly enriched compared with control exosomes (Figure 2G).
Figure 2.
Exosome packing with CCL2 mRNA is increased in a chronic kidney injury model. (A and B) Histologic (PAS and Masson staining) changes, serum levels of creatinine (Scr), and proteinuria in the 5/6 nephrectomy rats model (SNx) at 16 weeks. (C) Inflammatory cytokine mRNA (CCL2, IL1β, TNFα, IL6) expression in the kidney by real-time PCR. (D) Kidney exosome was extracted from the same weight of kidney tissue and was confirmed by Western blot for Alix, CD9, and CD63 exosome markers. (E) Urine exosomes excreted was confirmed and quantified by Western blotting for Alix, CD9, and CD63 exosome markers. (F) Concentration and size distribution determined by NTA (n=3 for each group). (G) Inflammatory cytokine mRNA (CCL2, IL1β, TNFα, IL6) expression in kidney exosomes by real-time PCR. Original magnification, ×200. *P<0.05; **P<0.01; ***P<0.001 compared with sham-operated mice (n=8 for each group). Ctrl, control.
BSA Enhanced CCL2 mRNA Shuttling from TEC Exosomes to Macrophages
Exosomes are known to promote horizontal transfer of molecules to recipient cells. Given the importance of chemokine and cytokine signals in recruiting inflammatory cells into the tubulointerstitial compartment, we focused our analyses on inflammatory cytokine mRNA in TEC exosomes, profiling the mRNA expression of the eight most common chemokines and cytokines (CCL2, CCL5, CCL9, CXCL10, CXCL9, IL6, TNFα, and IL1β) in both TECs treated with BSA and their released exosomes. We found CCL2 and CCL5 were dramatically increased in BSA-treated TEC cells compared with controls. Further, CCL2 mRNA was extremely high in the secreted exosome fraction, which is consistent with the findings in vivo whereby CCL2 mRNA showed the most enrichment in kidney exosomes (Figure 3, A and B).
Figure 3.
CCL2 mRNA was most enriched in TEC exosomes compared with other inflammation-related chemokines and cytokines. TECs were cultured by BSA stimulation with serum-free medium and exosomes were isolated. Inflammation-related chemokines and cytokines mRNA (CCL2, CCL5, CCL9, CXCL10, CXCL9, IL6, TNFα, IL1β) was screened in TECs (A) and exosomes (B). **P<0.01; ***P<0.001 compared with PBS controls. The red boxes highlight the regulation of cellular and exosomal CCL2 mRNA in TECs.
To demonstrate the mRNA transfer by exosome, exosome was purified from Dio-stained TECs, which was a labeling approach with lower background compared with directly labeled extracellular vesicles.17 Purified exosome was washed in PBS and pelleted again before being added to RAW264.7. As shown in Figure 4A, there were significant increases of Dio-labeled exosomes internalized by macrophages when treated with BSA. The increasing internalization of BSA-exo (exosomes purified from TECs under BSA exposure) was associated with the upregulation of inflammatory markers (CCL2, IL1β, IL6) compared with controls. Interestingly, direct BSA treatment in macrophages did not upregulate these markers but did enhance TNFα expression (Figure 4B). Also, using transwell, we established coculture systems that mimic the milieu where TECs were exposed to urinary proteins on the apical surface and exosomes were released from the basolateral side to instigate interstitial inflammation.18 A schematic description showed the process of the experiment. Results demonstrated that increasing TEC exosomes were internalized by macrophage when BSA was added to the upper chamber of the transwell. Flow cytometry showed that more fluorescence-labeled exosomes were released and internalized by macrophages in the group of BSA-treated TECs (Figure 4C).
Figure 4.
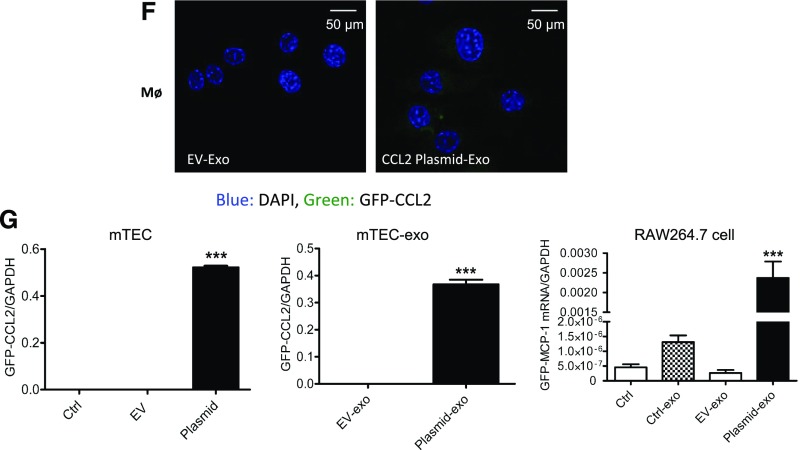
CCL2 mRNA transfers to macrophage cells via TEC exosome. (A) Exosomes purified from Dio fluorescence-labeled TECs was added to macrophage as depicted schematically. Representative data showing presence of Dio-labeled exosomes internalized in macrophages at 12 hours. (B) Inflammatory cytokine mRNA (CCL2, IL1β, TNFα, IL6) expression in RAW264.7 was detected by real-time PCR. **P<0.01; ***P<0.001 compared with cells without exosomes; ###P<0.001 compared with cells with control exosomes. (C) A transwell culture system was used to mimic the milieu where TECs were exposed to urinary proteins on the apical surface and exosomes were released from the basolateral side. Data shows that exosomes released from labeled TECs were internalized by macrophages. Flow cytometry showed an increased amount of fluorescence-labeled exosomes were released and uptaken by macrophages in the BSA-treated group (*P<0.05 compared with Ctrl). (D) CCL2 is efficiently knocked down by siRNA in TECs and TEC exosome at mRNA level. Knockdown of CCL2 prevents CCL2 upregulation in RAW264.7 macrophages after treatment with exosomes from siRNA/negative control (NC) transfected TECs. (E) Migration analysis of macrophages 24 hours after treatment with TEC exosomes. (D and E) *P<0.05; ***P<0.001 compared with NC/NC-exo; #P<0.05; ##P<0.01; ###P<0.001 compared with NC/NC-exo with BSA treatment. (F) TECs were transfected with GFP-tagged CCL2 plasmid. Fluorescence microscopy images showed RAW264.7 24 hours after treatment with TEC exosome. (G) Histogram showing GFP-CCL2 fusion mRNA levels detected by paired primers aligned to GFP and CCL2 in TECs, TEC exosomes, and RAW264.7 treated with TEC exosomes. ***P<0.001 compared with EV/EV-exo. BSA-exo, exosomes from TECs with BSA treatment; Ctrl-exo, exosomes from TECs with PBS treatment as controls; EV, empty vector; EV-exo, exosomes purified from TECs transfected with empty vector TECs; NC-exo/siRNA-exo, exosomes from TECs with NC nucleotides or CCL2 siRNA transfection; NC+BSA-exo, siRNA+BSA-exo are exosomes from TECs under NC/siRNA transfection plus BSA stimulation; plasmid exo, exosomes purified from TECs transfected with GFP-labeled CCL2 plasmid.
To demonstrate the functional transfer of CCL2 mRNA, exosomes were isolated from CCL2-silenced TECs and then incubated with macrophages. As shown in Figure 4D, CCL2 was knocked down efficiently in both TECs and the secreted exosomes. In this model, CCL2 mRNA was reduced correspondingly in the recipient macrophages (Figure 4D). Moreover, exosomes from CCL2-silenced TECs markedly reduced the migration of recipient macrophages, as demonstrated in a wound-healing assay (Figure 4E). Also, TEC cells were transfected with a GFP-tagged CCL2 plasmid (constructed by Shanghai Genechem Co., Ltd., China), after which exosomes were purified and applied to macrophages. Robust GFP fluorescence was detected in macrophages incubated with the exosomes from the plasmid-loaded TECs, whereas no detectable fluorescence was found in macrophages treated with exosomes from empty-vector TECs (Figure 4F). Direct transfer of CCL2 mRNA was demonstrated by detecting GFP-CCL2 fusion mRNA with paired primers covering the two genes (Figure 4G).
Exosomes from BSA-Treated TECs Caused Kidney Injury in Mice
To investigate the capacity of the TEC exosome in propagating albumin-induced kidney injury in vivo, exosomes released from BSA-treated TECs (BSA-exo) were injected via tail vein into mice. Mice was euthanized 12 and 72 hours after exosome injection, respectively. Kidney CCL2 mRNA was upregulated in the BSA-exo group compared with the control group at 12 hours, which might suggest the direct transfer of CCL2 mRNA from exogenous exosomes (P<0.01) (Figure 5A). However, no remarkable histologic change was observed by PAS staining (Figure 5B). At 72 hours after exosome injection, BSA-exo induced renal tubular injury and inflammatory cells accumulation compared with control exosomes (Figure 5C). Also, CCL2 mRNA was increased in kidney with marginal statistical significance (P=0.06). Western blotting revealed marked induction of renal NGAL expression in the BSA-exo recipients (Figure 5E). These data suggest that renal epithelium exposed to filtered albumin release CCL2-laden exosomes that contact resident or infiltrating macrophages to orchestrate significant tubulointerstitial inflammation and injury.
Figure 5.
Exosome from BSA-treated TECs caused kidney injury in mice. TECs were cultured and treated with BSA or PBS as controls and exosomes were produced for transfer to BALB/c mice (n=4 for each group). (A) CCL2 mRNA was detected by quantitative RT-PCR in mice kidney 12 hours after exosome injection. (B) Kidney histology by PAS staining showed no significant histologic change at 12 hours. (C) Representative data of histologic (PAS) and immunohistologic (CD68+ immunostaining) changes in mice injected with Ctrl-exo or BSA-exo at 72 hours. Note that tubular injury and inflammatory cell infiltration were present in BSA-exo mice compared with Ctrl-exo mice. (D) Quantitative PCR showed CCL2 mRNA in kidneys was increased in BSA-exo mice in comparison to Ctrl-exo group, with marginal statistical significance (P=0.06) at 72 hours. (E) NGAL expression was remarkably increased in BSA-exo mice compared with Ctrl-exo mice at 72 hours after exosome injection. *P<0.05; **P<0.01 compared with Ctrl-exo group. BSA-exo, exosomes from TECs with BSA treatment, Ctrl-exo, exosomes from control TECs with PBS.
CCL2 mRNA Participated in TEC Exosome-Mediated Renal Inflammation
To elucidate the specific role of CCL2 mRNA in TEC exosome-mediated renal inflammation, transfer of exosomes from CCL2 knockdown TECs was performed (n=3 for each group). TECs with or without CCL2 knockdown was treated with BSA and exosomes were purified and transferred into mice via the tail vein. Mice were euthanized 72 hours after exosome injection. As shown in Figure 6A, exosomes released by CCL2 knockdown TECs partly reduced the severity of renal inflammation and tubular injury, as demonstrated by PAS and immunohistochemistry staining of CD68 (Figure 6A). Flow cytometry confirmed that the number of infiltrating CD68+ macrophages was partly reduced in the kidney of mice received exosomes from CCL2 knockdown TECs when compared with those treated with control exosomes (P<0.05; Figure 6B). RT-PCR analysis of CCL2 and NGAL mRNA in kidneys showed a decline in the CCL2 knockdown group, although this did not reach statistical significance (Figure 6C). Western blotting showed that NGAL upregulation was partly reversed in mice with CCL2 knockdown exosomes (Figure 6D). The results suggest that CCL2 together with other functional cargo participated in TEC exosome-mediated renal inflammation.
Figure 6.
Transfer of exosomes from CCL2 knockdown TECs reduced renal inflammation. CCL2 mRNA was knocked down in TECs with siRNA or negative control (NC), followed by treatment with BSA (20 mg/ml) for 24 hours to produce exosomes for transferring into mice (n=3). Mice were euthanized on day 3. (A) Renal histologic (PAS staining) and CD68+ macrophages infiltration in the kidney. Note that transfer of exosomes from TECs with CCL2 knockdown (siRNA_TEC exo) partly reduced interstitial macrophage infiltration and tubular injury. (B) Flow cytometry confirmed that the number of infiltrating CD68+ macrophages was partly reduced in the kidney of mice receiving siRNA_TEC exo when compared with those treated with NC_TEC exo. (C) RT-PCR analysis showed CCL2 and Ngal mRNA tended to decline, although not with any statistical significance. (D) NGAL protein in kidneys of mice receiving siRNA_TEC exo was partly reduced (with no significance) compared with the NC_TEC exo group. *P<0.05 compared with the NC_TEC exo group. NC_TEC exo, mice receiving exosomes purified from TECs transfected with negative control nucleotides; siRNA_TEC exo, mice receiving exosomes purified from TECs transfected with CCL2 siRNA.
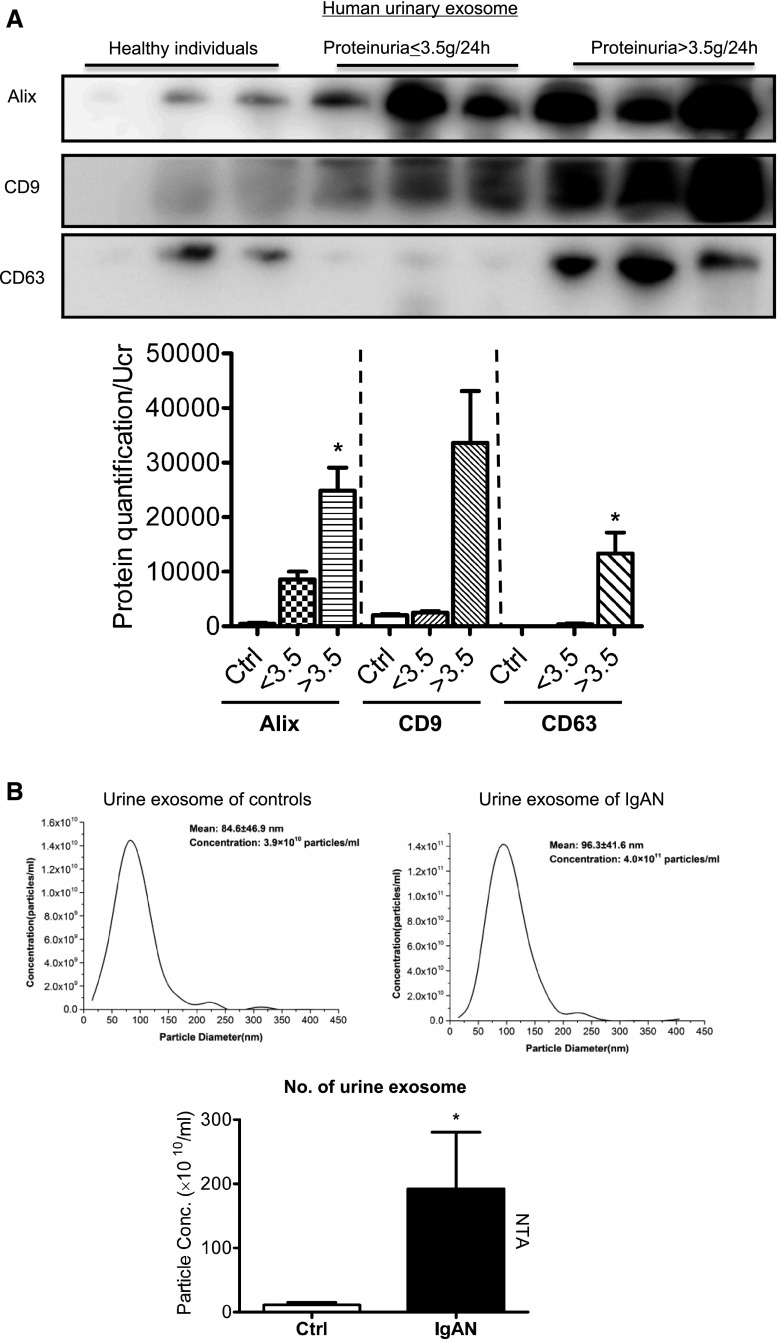
Exosomal CCL2 mRNA Correlated with Severity of Proteinuria and Tubulointerstitial Inflammation in Patients with IgA Nephropathy
To further demonstrate the role of exosomal CCL2 mRNA in patients with proteinuria, we analyzed the expression of exosomal CCL2 mRNA in patients with IgA nephropathy (IgAN). We isolated and characterized exosomes from the urine of patients with IgAN (n=36) and normal controls (n=16). The basic clinical characteristics of the participants were listed in Table 1. The average amount of proteinuria in IgAN was 3.442 g/24 h, whereas controls were negative for proteinuria. Exosome protein was reported to be normalized to urine creatinine or Tamm–Horsfall protein.19,20 Here, total urinary exosome protein was normalized to urine creatinine when quantification data were present. Urinary exosome excretion was significantly increased at higher levels of proteinuria, demonstrated by Western blotting analysis of exosome markers (Figure 7A). Further, urine exosome size and number were detected by NTA. Results confirmed the increased exosome number in IgAN compared with controls (P<0.05) (Figure 7B). Indeed, the number of urinary exosomes determined by exosome protein correlated closely with the extent of proteinuria (r=0.61, P<0.001) (Figure 7C). Moreover, the expression of CCL2 mRNA in urinary exosomes was significantly enhanced in patients with IgAN compared with normal controls, consistent with exosomal CCL2 mRNA promoting kidney injury (Figure 7D). Here again, expression of urinary exosomal CCL2 mRNA correlated closely with severity of macrophage infiltration, as determined by CD68 staining in kidney tissue (Figure 7E).
Table 1.
Characteristics of healthy controls and patients with IgAN
| Characteristics | Healthy Individuals (n=16) | Patients with IgAN (n=36) |
|---|---|---|
| Men/Women | 7/9 | 18/18 |
| Age, yr | 38.8±14.1 | 38.6±13.5 |
| BUN, mmol/L | 4.42±0.97 | 6.80±3.58a |
| Scr, μmol/L | 79.18±15.68 | 113.4±56.04a |
| UA, μmol/L | 307±73.7 | 349±93.5 |
| Proteinuria, g/24 h | — | 3.442±2.74 |
| eGFR | 89.25±16.31 | 70.74±28.85a |
Values are expressed as mean±SD (unpaired, two-tailed t test). Scr, Serum creatinine; UA, uric acid.
P<0.05, healthy individuals versus patients with IgAN.
Figure 7.
Exosome packing with CCL2 mRNA is increased and correlates with tubulointerstitial inflammation in IgA Nephropathy. Urinary exosomes were purified from different stages of IgAN (n=36) and healthy controls (n=16). IgAN patients were grouped according to the level of proteinuria (<3.5 g/24 h, ≥3.5 g/24 h). (A) Urinary exosomes were confirmed and quantified by Western blotting of exosome markers Alix, CD9, and CD63. Quantification of exosomes were normalized to urine creatinine (Ucr). *P<0.05 compared with controls. ##P<0.01 compared with the moderate proteinuria group (<3.5 g/24 h). (B) Urine exosome size and number were detected by NTA. (C) Correlation analysis showed that the number of exosomes (quantified by exosome protein concentration and normalized to urine creatinine in 13 patients with IgAN) released in urine correlated closely with levels of proteinuria (r=0.61; P<0.01). (D) Real-time PCR showed that CCL2 mRNA in urinary exosomes was upregulated in patients with IgAN compared with controls. ***P<0.001 compared with controls. (E) Representative image of CD68+ macrophage infiltration in the kidney of patients with IgAN, with moderate (<3.5 g/24 h) and severe proteinuria (≥3.5 g/24 h). Original magnification, X200. Correlation analysis of kidney CD68+ macrophage infiltration with urinary exosomal CCL2 (r= 0.37; P<0.05).
Discussion
The critical role of albuminuria in the progression of CKD has been recognized for many years, but the exact mechanism through which filtered albumin participates in renal injury is still largely unknown.21 We have recently demonstrated that albuminuria directly provokes tubulointerstitial inflammation.2,3 In this study, we further investigated the capacity of albumin to mediate tubulointerstitial inflammation by combining experimental models of acute and chronic kidney injury and in vitro cell culture studies. We found that transfer of exosomal CCL2 mRNA from TECs to macrophages after exposure of TECs to albumin is a critical initial step through which albuminuria directs tubulointerstitial inflammation. The correlations between urinary exosome CCL2 mRNA, proteinuria, and renal tubulointerstitial inflammation are also demonstrated in clinical samples from patients with IgAN. Our findings may facilitate the identification of novel therapeutic targets for the treatment of CKD.
Previous studies have suggested that exosome biogenesis and content are not only determined by their cellular source, but are also sensitive to cellular status and environmental inputs. Increased exosome release has been reported in hypoxia, acidic pH, heat shock, and oxidative stress.22 In this study, we found that exosome production was markedly increased in kidney and urine samples in both acute and chronic kidney injury models. Furthermore, there was increased production of exosomes in the supernatant when TECs were treated with BSA. This phenomenon was also demonstrated in patients with IgAN where the release of exosomes in urine correlated closely with the level of proteinuria. These data suggest albuminuria may constitute a critical element of the milieu that affects cellular exosome production, particularly in kidney disease. Exosomes are derived from the endosomal pathway, which involves the formation of multivesicular bodies via the endosomal sorting complex required for transport and the release mediated by a Rab27A/B-dependent pathway.23 However, the mechanism through which albuminuria increases exosome production has required further investigation.
When exosomes are released, they are specifically loaded with lipids, proteins, and RNAs. The repertoire of exosomes varies as a function of the parent cell and its physiologic state. In this study, we found that CCL2 mRNA was selectively enriched in the TEC exosome after BSA stimulation. More importantly, we found that CCL2 mRNA could be functionally delivered to the macrophage, culminating in macrophage activation and migration. By recruiting immune cells into injured tissues, chemokines play a critical role in regulating local inflammatory responses. In this study, we found that CCL2 mRNA was selectively enriched in TEC exosomes after BSA treatment to a greater extent than other chemokines and cytokines. Interestingly, previous studies showed that chemokine proteins IL8 (CXCL8) and fractalkine (CX3CL1) were found to be associated with extracellular vesicles, whereas CCL2, CCL3, CCL4, CCL5, and CCL20 were contained in exosomes from heat-stressed tumor cells.24 Consequently, exosomes may represent a novel vehicle through which chemokines can orchestrate inflammation. The pathogenic actions of TEC exosomes in kidney injury were also demonstrated in in vivo study. Kidney inflammation and tubular injury was observed in mice administered with BSA-treated TEC exosomes. Exosomes from CCL2 knockdown TECs with BSA treatment partly ameliorate renal inflammation. Because both groups of mice were transferred with exosomes from BSA-treated TECs, exosomes may also deliver other functional molecules that contribute to inflammatory response in macrophages. This may explain the partial reduction of inflammation in kidney. Thus, TEC exosome packing with CCL2 may represent a promising therapeutic target for kidney disease. Similarly, recent study by Shen et al.25 showed that C-C motif chemokine receptor-2–positive exosomes released by mesenchymal stem cells suppressed macrophage functions by acting as a decoy to suppress CCL2 activity. Further studies were needed to determine the role and therapeutic potential of targeting exosome CCL2 mRNA in kidney inflammation. Previous studies showed that miRNA was enriched in exosomes and may be a biomarker and active player for the development of kidney disease.9,13 The role of miRNA in addition to CCL2 in TEC exosomes in the development of interstitial inflammation need further investigation.
Despite several studies demonstrating that uptake of filtered albumin provokes interstitial inflammation, how danger signals spread from the TEC to the interstitial space with consequent macrophage infiltration has remained obscure.26 In our study, we demonstrated that TEC exosomes represent a novel signaling vehicle to exacerbate macrophage infiltration. A study by Macconi et al.27 showed that proximal tubular cells transport albumin to dendritic cells, where the protein was degraded into antigenic peptides via proteasomal cleavage. In turn, these peptides in the context of MHC complexes could activate CD8+ T cells during kidney inflammation. In our study, we found that direct exposure of macrophages to BSA provoked less inflammation than BSA-driven release of exosomes from TECs. This finding points to the crucial effect of TEC exosomes in activating macrophages rather than leakage of filtered albumin into the interstitial compartment.
Finally, the effect of albuminuria on exosome production and its relation to kidney injury was confirmed in clinical studies. These clinical data support our findings that albuminuria promotes upregulation of CCL2 mRNA in TEC exosomes with consequent exacerbation in interstitial inflammation. We further speculate that CCL2 mRNA may be an attractive candidate as a novel biomarker of CKD.
In summary, we have demonstrated that in the setting of proteinuric kidney disease, exosomes are released in greater numbers by TECs packed with CCL2 mRNA. The exosomal CCL2 mRNA can be delivered to interstitial macrophages, provoking their activation and autocrine recruitment of additional myeloid cells. Thus, results from this study suggest that exosomal CCL2 mRNA represents a novel vehicle to communicate danger signals from TECs to macrophages and thereby promote inflammatory kidney damage.
Concise Methods
Reagents
For Western blotting, the following reagents were used: goat anti-Ngal (1:1000, AF1857; R&D Systems), mouse anti–β-actin (1:4000, sc-47778; Santa Cruz Biotechnology), mouse anti-Alix (1:1000, sc-53540; Santa Cruz Biotechnology), mouse anti-CD63 (1:1000, ab193349; Abcam), rabbit anti-CD9 (1:1000, ab92726; Abcam). For immunohistochemistry, CD68 (1:100, ab-201340; Abcam) was used. For cell culture, albumin was purchased from Sigma (A8806). LPS was purchased from Sigma, and was from Escherichia coli O111:B4 and purified by phenol extraction (L2630; Sigma). Collagenase (17104–019; Gibco) and trypsin (25200–056; Gibco) were used for kidney digestion for exosome extraction. Dio (C1038; Beyotime, Shanghai, China) was used for cell labeling in in vitro study.
Exosome Purification and Characterization
Mouse tubular epithelial cells were immortal cells (a gift from Dr. Jeffrey B. Kopp, National Institutes of Health)28 that were cultured in DMEM-F12 (Hyclone, GE Healthcare Life Science) without serum when the medium was collected to purify exosomes. Supernatant fractions collected from cell cultures for 48–72 hours were subjected to exosome extraction. Urine specimen was collected from patients and healthy volunteers for exosome extraction, as described previously.9 For kidney exosome extraction, 100 mg of kidney cortex was collected and submitted to tissue digestion with collagenase and trypsin for 120 minutes at 37°C. Then the sample was submitted for exosome extraction.12 All samples were centrifuged at 2000×g for 20 minutes to eliminate the cells and debris and at 13,500×g for 20 minutes, followed by ultracentrifugation at 200,000×g for 120 minutes (Type 70 Ti rotor, Beckman Coulter Optima L-80 XP). The exosome pellet was washed in 20 ml of PBS and collected by ultracentrifugation at 200,000×g for 120 minutes.
The size distribution, morphology, and quantity were detected by electron microscopy and NTA. Protein concentration and surface markers (Alix, CD63, CD9) of isolated exosomes were detected by Western blotting. An equal proportion of exosome protein and weight of kidney or 25% of urinary exosome protein purified from 25 ml urine were used for Western blotting quantification.
Human Studies
Human urine samples were obtained from participants at Zhongda Hospital, from patients with histologically confirmed IgAN and healthy controls. All individuals provided informed consent for urine donation on the Zhongda Hospital approved ethical protocol. A whole-stream, early morning urine specimen was collected from patients and healthy volunteers. Urine samples was processed by centrifuge at 2000×g for 20 minutes within 2 hours after collection. The supernatant was collected and stored at −80°C before exosome purification.
Electron Microscopy
TEM was performed on the purified exosomes from kidney tissue or cell culture medium. The exosome pellet was mixed 1:1 with 4% paraformaldehyde and then applied to 200-mesh nickel grids. Samples were left to immobilization at room temperature for 20 minutes. The excess solution was soaked off by a filter paper, and the grid was stained with 2% uranyl acetate in water for 10 seconds, washed in distilled water for three times, and air-dried. For in situ kidney exosome detection by TEM, kidney tissue was fixed in 2.5% glutaraldehyde. After thorough washing in PBS, the samples were exposed to 1% osmium tetroxide, dehydrated through a series of graded ethanol. Tissues were then embedded and polymerized. Kidney tissue was cut into ultrathin sections of 50–70 nm and stained with uranyl acetate and lead citrate before analysis. Samples were examined using a Tecnai 10 transmission electron microscope (Fei, Acht, The Netherlands) at 80 kV.
ZetaView NTA
NTA was performed using the ZetaView PMX 110 (Particle Metrix, Meerbusch, Germany). Isolated exosome samples were appropriately diluted using 1× PBS buffer to measure the particle size and concentration. Measurement data from the ZetaView were analyzed using the corresponding software, ZetaView 8.04.02. Resuspension volumes and dilution factors were used to convert the yield from concentration to an absolute number of particles.
Exosome Uptake Studies
TECs were grown to 70%–80% confluence and cultured in serum-free medium for 24 hours. Then the cells were stained by Dio dye (5 μg/ml) for 30 minutes at 37°C and the free dye was washed away with PBS three times. TECs were then stimulated with BSA (20 mg/ml),2,3 as determined by our previous study, for 24 hours or PBS as a control. Exosomes released from Dio-stained TECs were purified and washed completely in 20 ml of PBS, collected by ultracentrifugation as described above. Exosomes released from per 1×107 TECs was added to RAW264.7 for 24 hours. Exosomes purified from TECs with PBS and Dio dye was used as a control to verify no dye contamination occurred.
Transwell Study
To demonstrate the process of TEC-exo communicating with macrophages in a proteinuric state, we established coculture systems with transwell that mimic the milieu where TECs are exposed to urinary proteins on the apical surface as described before.18 A total of 4×104 TECs were cultured 24 hours in the upper chamber of the transwell with 0.4-μm pores (Corning). Twelve hours after starvation with FBS free culture medium, TECs were labeled with Dio dye (5 μg/ml; Beyotime) in 37°C for 30 minutes. After staining, cells were washed completely for three times with PBS to remove free dyes. Then, TECs were grown to complete confluence and cocultured with RAW264.7, which was seeded in the lower compartment of the system. TECs were then stimulated with albumin (20 mg/ml; Sigma) in the apical side, and PBS buffer was used as a control. Exosome from Dio-labeled TECs were released on the basolateral side and was internalized by macrophages, which was observed using confocal microscopy and quantified by flow cytometry analysis.
Flow Cytometry Analysis
Total kidney tissues were digested by collagenase and trypsin into cell suspension, and fixed by IC Fixation Buffer (eBioscience) for 30 minutes. Then cells were stained with phycoerythrin-conjugated CD68 (137014; Biolegend). Isotype control antibodies were used as negative controls. Macrophages cocultured with TECs in transwell were harvested and analyzed directly. After being extensively washed, single cells were analyzed by FACSCaibur flow cytometer (BD Biosciences, San Jose, CA).
In Vivo Studies
AKI was established in LPS-treated mice as described previously.16 Male BALB/c mice (6–8 weeks old) were injected intraperitoneally with either 200 μg LPS (1 mg/ml in sterile PBS) in a total volume of 200 μl, or equal volumes of sterile PBS. Twenty four hours after injection, urinary protein excretion was measured, and kidneys were harvested and processed for PAS and CD68 immunostaining. The CKD model was established in rats by surgical 5/6 subtotal nephrectomy. Male rats were assigned to remnant kidney or sham-operated control groups. The animals were subjected to right nephrectomy and resection of the upper and lower one-third of the left kidney. A sham operation was performed on rats of control group. The rats were euthanized at week 16 and serum samples, 24-hour urine, and kidney tissues were harvested. To specifically examine the role of TEC exosome in kidney injury, 8-week-old male BALB/c mice were injected via the tail vein with exosomes generated in vitro by 8×106 TECs with BSA treatment or PBS as a control. Exosomes were harvested from cell medium by differential ultracentrifugation as described above. Mice were euthanized 12 or 72 hours after injection. Besides, exosomes from CCL2 knockdown TECs were harvested and transferred to mice to observe the role of CCL2 mRNA in kidney injury.
Quantitative Real-Time PCR and Small RNA Interference Studies
Frozen tissues or cell lines were analyzed for specific gene expression using quantitative real-time PCR. Briefly, total RNA was extracted from tissues or cells using the TRIzol (Takara), mRNA fraction of exosome was extracted using miRNeasy micro Kit (Qiagen). RNA was reverse-transcribed and using PrimeScript RT reagent kit (Takara). Quantitative real-time PCR was performed on a 7300 PCR System (Applied Biosystems). All the primers for quantitative real-time PCR are listed in Table 2. Relative expression was normalized to GAPDH levels. For siRNA-mediated knockdown of CCL2, hnRNP2AB1, TECs were transfected with 50 nM of siRNA against mouse CCL2 (sense 5′- CCGAUAGGCAGUCUGGAAATT-3′; antisense 5′- UUUCCAGACUGCCUAUCGGTT-3′). A scramble sense-control was used as negative control.
Table 2.
Primers for quantitative RT-PCR
| Primer | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| Mouse IL1β | TGCCACCTTTTGACAGTGATG | AAGGTCCACGGGAAAGACAC |
| Mouse TNFα | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC |
| Mouse IL6 | AAAGAGTTGTGCAATGGCAATTCT | AAGTGCATCATCGTTGTTCATACA |
| Mouse GAPDH | GCATGGCCTTCCGTGTTC | GATGTCATCATACTTGGCAGGTTT |
| Mouse CCL2 | CTTCTGGGCCTGCTGTTCA | CCAGCCTACTCATTGGGATCA |
| Mouse CCL5 | GTGCTCCAATCTTGCAGTCG | AGAGCAAGCAATGACAGGGA |
| Mouse CCL9 | TCGGTTTCCCAGCGGATTTT | TGGCTTACTGATGGAGGGGT |
| Mouse CXCL9 | AACGTTGTCCACCTCCCTTC | CACAGGCTTTGGCTAGTCGT |
| Mouse CXCL10 | CTGCCGTCATTTTCTGCCTC | TTCAAGCTTCCCTATGGCCC |
| GFP-CCL2 | CTTTTCCACAACCACCTCAAGC | TGAACAGCTCCTCGCCCTTG |
| Mouse NGAL | GCCCTGAGTGTCATGTGTCT | GAACTGATCGCTCCGGAAGT |
| Rat IL1β | GACTTCACCATGGAACCCGT | GGAGACTGCCCATTCTCGAC |
| Rat IL6 | CTGGTCTTCTGGAGTTCCGT | TGGTCCTTAGCCACTCCTTCT |
| Rat GAPDH | TCTCTGCTCCTCCCTGTTCT | GATGGTGATGGGTTTCCCGT |
| Rat TNFα | ACCATGAGCACGGAAAGCAT | ACCATGAGCACGGAAAGCAT |
| Rat CCL2 | GATCCCAATGAGTCGGCTGG | ACAGAAGTGCTTGAGGTGGTT |
| Human GAPDH | CTCTGCTCCTCCTGTTCGAC | GCGCCCAATACGACCAAATC |
| Human CCL2 | CCTTCATTCCCCAAGGGCTC | GGTTTGCTTGTCCAGGTGGT |
Assessment of Renal Injury
Kidney injury was observed by PAS staining. Infiltration of macrophages within the kidney was demonstrated by immunoperoxidase staining of periodate lysine paraformaldehyde-fixed, frozen, 6-mm-thick kidney sections. The primary monoclonal antibodies used were anti-CD68 antibody (1:100, ab-201340; Abcam).
Statistical Analyses
Data obtained from this study were presented as the mean±SD. Statistical analyses were performed using t test or one-way ANOVA, followed by Newman–Keuls post-test from Prism 5.0 GraphPad Software (San Diego, CA).
Disclosures
None.
Supplementary Material
Acknowledgments
This study is supported by the National Natural Scientific Foundation (grants 81470922, 31671194, 81720108007, and 81670696) and Clinic Research Center of Jiangsu Province (grant BL2014080).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J; Chronic Kidney Disease Prognosis Consortium : Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80: 93–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu D, Xu M, Ding LH, Lv LL, Liu H, Ma KL, Zhang AH, Crowley SD, Liu BC: Activation of the Nlrp3 inflammasome by mitochondrial reactive oxygen species: A novel mechanism of albumin-induced tubulointerstitial inflammation. Int J Biochem Cell Biol 57: 7–19, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu D, Wen Y, Tang TT, Lv LL, Tang RN, Liu H, Ma KL, Crowley SD, Liu BC: Megalin/cubulin-lysosome-mediated albumin reabsorption is involved in the tubular cell activation of NLRP3 inflammasome and tubulointerstitial tnflammation. J Biol Chem 290: 18018–18028, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baines RJ, Brunskill NJ: Tubular toxicity of proteinuria. Nat Rev Nephrol 7: 177–180, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Pitt JM, Kroemer G, Zitvogel L: Extracellular vesicles: Masters of intercellular communication and potential clinical interventions. J Clin Invest 126: 1139–1143, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tkach M, Théry C: Communication by extracellular vesicles: Where we are and where we need to go. Cell 164: 1226–1232, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO: Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Lv LL, Cao YH, Pan MM, Liu H, Tang RN, Ma KL, Chen PS, Liu BC: CD2AP mRNA in urinary exosome as biomarker of kidney disease. Clin Chim Acta 428: 26–31, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Lv LL, Cao YH, Ni HF, Xu M, Liu D, Liu H, Chen PS, Liu BC: MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am J Physiol Renal Physiol 305: F1220–F1227, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Bruno S, Porta S, Bussolati B: Extracellular vesicles in renal tissue damage and regeneration. Eur J Pharmacol 790: 83–91, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L: Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int 78: 838–848, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Borges FT, Melo SA, Özdemir BC, Kato N, Revuelta I, Miller CA, Gattone VH 2nd, LeBleu VS, Kalluri R: TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol 24: 385–392, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Xiong M, Fang L, Jiang L, Wen P, Dai C, Zhang CY, Yang J: miR-21-containing microvesicles from injured tubular epithelial cells promote tubular phenotype transition by targeting PTEN protein. Am J Pathol 183: 1183–1196, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Lin M, Yiu WH, Wu HJ, Chan LY, Leung JC, Au WS, Chan KW, Lai KN, Tang SC: Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol 23: 86–102, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lv LL, Tang PM, Li CJ, You YK, Li J, Huang XR, Ni J, Feng M, Liu BC, Lan HY: The pattern recognition receptor, Mincle, is essential for maintaining the M1 macrophage phenotype in acute renal inflammation. Kidney Int 91: 587–602, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P: Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest 113: 1390–1397, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grange C, Tapparo M, Bruno S, Chatterjee D, Quesenberry PJ, Tetta C, Camussi G: Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med 33: 1055–1063, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai KN, Leung JC, Chan LY, Guo H, Tang SC: Interaction between proximal tubular epithelial cells and infiltrating monocytes/T cells in the proteinuric state. Kidney Int 71: 526–538, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Llama P, Khositseth S, Gonzales PA, Star RA, Pisitkun T, Knepper MA: Tamm-Horsfall protein and urinary exosome isolation. Kidney Int 77: 736–742, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou H, Cheruvanky A, Hu X, Matsumoto T, Hiramatsu N, Cho ME, Berger A, Leelahavanichkul A, Doi K, Chawla LS, Illei GG, Kopp JB, Balow JE, Austin HA 3rd, Yuen PS, Star RA: Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int 74: 613–621, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer TW: Tubular injury in glomerular disease. Kidney Int 63: 774–787, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Higashijima Y, Sonoda H, Takahashi S, Kondo H, Shigemura K, Ikeda M: Excretion of urinary exosomal AQP2 in rats is regulated by vasopressin and urinary pH. Am J Physiol Renal Physiol 305: F1412–F1421, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Morrison EE, Bailey MA, Dear JW: Renal extracellular vesicles: From physiology to clinical application. J Physiol 594: 5735–5748, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O: Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4: 27066, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen B, Liu J, Zhang F, Wang Y, Qin Y, Zhou Z, Qiu J, and Fan Y: CCR2 positive exosome released by mesenchymal stem cells suppresses macrophage functions and alleviates ischemia/reperfusion-induced renal injury. Stem Cells Int 2016: 1240301, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theilig F: Spread of glomerular to tubulointerstitial disease with a focus on proteinuria. Ann Anat 192: 125–132, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Macconi D, Chiabrando C, Schiarea S, Aiello S, Cassis L, Gagliardini E, Noris M, Buelli S, Zoja C, Corna D, Mele C, Fanelli R, Remuzzi G, Benigni A: Proteasomal processing of albumin by renal dendritic cells generates antigenic peptides. J Am Soc Nephrol 20: 123–130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Q, Huang XR, Yu J, Yu X, Lan HY: Long noncoding RNA Arid2-IR is a novel therapeutic target for renal inflammation. Mol Ther 23: 1034–1043, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.