Abstract
The importance of the kidney distal convoluted tubule (DCT) and cortical collecting duct (CCD) is highlighted by various water and electrolyte disorders that arise when the unique transport properties of these segments are disturbed. Despite this critical role, little is known about which proteins have a regulatory role in these cells and how these cells can be regulated by individual physiologic stimuli. By combining proteomics, bioinformatics, and cell biology approaches, we found that the E3 ubiquitin ligase CHIP is highly expressed throughout the collecting duct; is modulated in abundance by vasopressin; interacts with aquaporin-2 (AQP2), Hsp70, and Hsc70; and can directly ubiquitylate the water channel AQP2 in vitro. shRNA knockdown of CHIP in CCD cells increased AQP2 protein t1/2 and reduced AQP2 ubiquitylation, resulting in greater levels of AQP2 and phosphorylated AQP2. CHIP knockdown increased the plasma membrane abundance of AQP2 in these cells. Compared with wild-type controls, CHIP knockout mice or novel CRISPR/Cas9 mice without CHIP E3 ligase activity had greater AQP2 abundance and altered renal water handling, with decreased water intake and urine volume, alongside higher urine osmolality. We did not observe significant changes in other water- or sodium-transporting proteins in the gene-modified mice. In summary, these results suggest that CHIP regulates AQP2 and subsequently, renal water handling.
Keywords: CHIP, AQP2, Protein Quality Control, kidney, Proteomics, Water handling
The ability of the kidney to maintain body water balance and the systemic concentrations of various ions is critical for mammals to survive under varying fluid and dietary intakes. To allow these homeostatic processes to be performed simultaneously, the renal tubule has developed specialized epithelial cells that allow independent regulation of water and solute excretion. Two examples of these epithelial cells are the cells of the distal convoluted tubule (DCT) and principal cells of the cortical collecting duct (CCD).1
The unique functions of DCT and CCD cells are inferred, at least in part, by the selective expression and regulation of a variety of different membrane-associated transport proteins. For example, in DCT cells, there is expression of the divalent cation transporters TRPV5, NCX1, and TRMP6 that are essential for maintaining calcium and magnesium homeostasis and the thiazide-sensitive NaCl cotransporter NCC that plays a central role in regulated NaCl reabsorption.2 In contrast, CCD principal cells express a variety of aquaporin (AQP) water channels that help maintain body water balance.3
Despite differing transporter profiles, the functions of DCT and CCD principal cells are under tight regulation by similar hormones, such as aldosterone, angiotensin II (AngII), and vasopressin (AVP).2,4–6 Furthermore, transport proteins, such as the epithelial sodium channel, the chloride channel CLC-K2, and the potassium channel ROMK, are localized to both cell types.2,6–8 Therefore, critical to understanding how DCT and CCD cells maintain a unique transport profile but respond to similar hormones is knowledge of the regulatory proteins within each cell type that are responsible for both modulating cell-specific gene expression and also, determining hormone-selective regulatory responses within individual cells.
In this study, to identify novel and unique regulatory proteins important for modulation of DCT and CCD function, we determined the differential proteome of kidney DCT cells (mpkDCT) and CCD cells (mpkCCD), which are extensively characterized models of DCT cells and CCD principal cells.9–12 After extensive bioinformatics and confirmatory in vivo studies, we characterized the role of the CCD-enriched E3 ubiquitin ligase C terminus of Hsc70 Interacting Protein (CHIP) for modulating the function of AQP2 and subsequent renal water handling.
Results
mpkDCT and mpkCCD Differential Proteome Analyses
LC-MS/MS shotgun proteomics was used to investigate the differential proteome between mpkDCT and mpkCCD under basal, [deamino-Cys1,D-Arg8]vasopressin (dDAVP; a synthetic analog of AVP), and AngII conditions (Supplemental Figure 1). Of 4000 quantifiable proteins in each condition, 1101, 1294, and 1566 proteins passed the Benjamini–Hochberg FDR threshold of 1% under basal, dDAVP, and AngII conditions, respectively (http://interpretdb.au.dk/database/DCTCCDcomparison/Control.html, http://interpretdb.au.dk/database/DCTCCDcomparison/dDAVP.html, and http://interpretdb.au.dk/database/DCTCCDcomparison/AngII.html) (Figure 1A, Supplemental Table 1; Supplemental Tables 1–6 can be downloaded from http://interpretdb.au.dk/database/DCTCCDcomparison/download.html). Five hundred ninety-four proteins were present in all conditions, representing the shared and unchanged portion of the mpkDCT and mpkCCD proteome. Another 287 proteins of higher abundance were identified in mpkDCT cells, and 307 proteins were identified in mpkCCD cells (Supplemental Table 2); 1025 proteins were specific for mpkDCT (Supplemental Material discusses selection criteria), and 1211 proteins were specific for mpkCCD (Figure 1, B and C, Supplemental Table 3). Comparison of specific proteins with a mouse kinome database (http://kinase.com/kinbase) determined that ten and 22 kinases are selective for mpkDCT and mpkCCD cells, respectively (Figure 1, D and E); 24 and 29 proteins with known E3 ligase or E3 ligase adapter activity and six and seven proteins with deubiquitylase activity13,14 were identified in mpkDCT and mpkCCD cells, respectively (Figure 1, D and E). In addition several transcription factors were identified (Figure 1, D and E), but cross reference of these with matrix families for characteristic genes expressed in the DCT or CCD (Supplemental Table 4) indicated that only one matrix family, V$SIXF, seemed to be highly specific. The abundances of several proteins were confirmed using Western blotting of equal protein equivalents (Supplemental Figure 2) isolated from a different cohort of cells cultured under similar conditions (Supplemental Figures 3–5). Abundance ratios derived from semiquantitative immunoblotting or MS were highly similar, with Spearman correlations of 0.92, 0.57, and 0.93 under basal, dDAVP, and AngII conditions, respectively (Supplemental Figure 6).
Figure 1.
Quantitative proteomic and bioinformatics of mpkDCT and mpkCCD cells reveal cell- and treatment-specific kinases, E3 ligases, deubiquitylases (DUBs), transcription factors, and enriched functional annotations. (A) Volcano plots of quantification between mpkDCT and mpkCCD under three conditions. (A) BH-FDR 1% cutoff was used to select the differential proteome between these two cell types. (B) Number of mpkDCT-specific proteins under three conditions. (C) Number of mpkCCD-specific proteins under three conditions. (D) mpkDCT-specific kinases, E3 ligases, DUBs, and TFs under three conditions. (E) mpkCCD-specific kinases, E3 ligases, DUBs, and TFs under three conditions. (F) Heat maps of mpkDCT and mpkCCD cells illustrating highly enriched functional annotations under three conditions.
Gene Ontology and Functional Annotation Analyses
Panther gene ontology term molecular function analysis highlighted major processes highly enriched in mpkDCT and mpkCCD cells (Supplemental Figure 7). The Database for Annotation, Visualization and Integrated Discovery tool identified 11, 12, and 19 functional processes in basal, dDAVP, and AngII conditions that were highly enriched in at least one cell type (Figure 1F). For example, processes linked to PDZ domain proteins and N-linked glycosylation were enriched in mpkDCT cells, whereas protein biosynthesis was enriched in mpkCCD cells (Supplemental Table 5). Cluster analysis of 250 proteins with the largest standard deviations under three conditions showed six distinctive clusters (Supplemental Figure 8, Supplemental Table 6), indicating that dDAVP and AngII can have similar—or independent—effects on a subset of proteins in mpkDCT or mpkCCD cells.
Identification of DCT- and CCD-Selective Proteins In Vivo
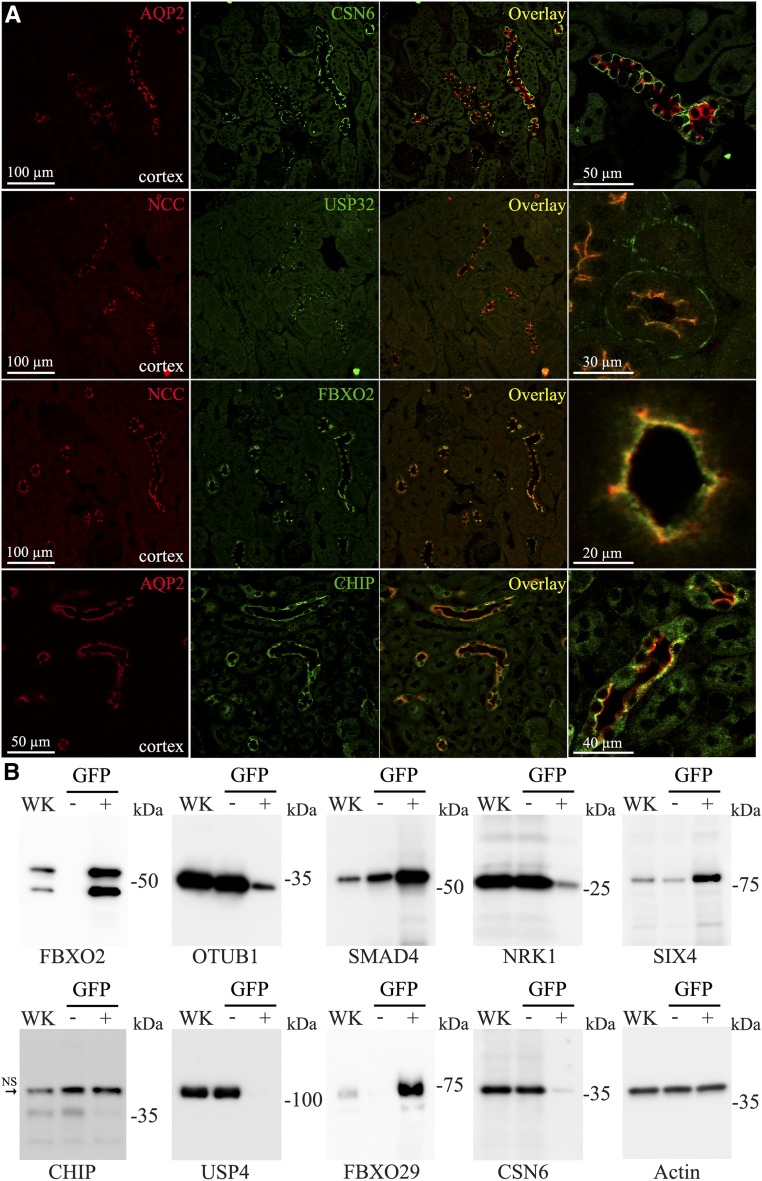
To confirm that our data could be translated to the in vivo situation, we initially performed immunohistochemistry (IHC) on mouse kidney tissue to localize selective proteins. Double labeling with antibodies against NCC or AQP2 was used to confirm localization to DCT or CCD cells, respectively. As observed in mpkCCD cells, COP9 Signalosome Subunit 6 was highly expressed in CD cells in vivo, where it was localized predominantly to the basolateral membrane of principal cells (Figure 2A). Similarly, compared with the DCT, the E3 ubiquitin ligase CHIP was highly expressed in the CD, where it partially colocalized with AQP2 (Figure 2A, Supplemental Figure 9). Matching the data from mpkDCT cells, the deubiquitylase USP32 was only identified in DCT cells, where it localized to both basolateral and apical membrane domains, with some degree of colocalization with NCC. Similarly, F-Box Protein 2 (FBXO2), was specific for DCT cells, where it colocalized with NCC at the apical plasma membrane. A similar distribution of FBXO2 (Supplemental Figure 10) and CHIP (Supplemental Figure 11) was observed in human kidney tissue. Because technical issues prevented analysis of the majority of commercial antibodies using formaldehyde-fixed, paraffin-embedded tissue or cryosections, we subjected purified DCT cells isolated by FACS from mice with selective green fluorescent protein expression in the DCT to Western blotting. The mpkDCT-enriched proteins FBXO2, SMAD4, SIX4, and FBXO29 were all abundant in isolated DCT cells (Figure 2B). In contrast, the mpkCCD-enriched proteins NRK1, USP4, COP9 Signalosome Subunit 6, and CHIP were not readily detected in isolated DCT cells.
Figure 2.
Confirmation of DCT or CCD enriched proteins in vivo. (A) Confocal images of mouse kidney sections immunolabeled for COP9 Signalosome Subunit 6 (CSN6), USP32, FBXO2, and CHIP alongside the DCT- or CCD-expressed proteins NCC or AQP2, respectively. (B) Immunoblotting of purified DCT cells (green fluorescent protein positive [GFP+]) illustrates that mpkDCT-enriched proteins FBXO2, SMAD4, SIX4, and FBXO29 are abundant in isolated DCT cells, whereas the mpkCCD-enriched proteins NRK1, USP4, CSN6, and CHIP are predominantly identified in the GFP− fraction (representing non-DCT cells).
Regulation of CHIP by AVP and Interaction of AQP2 and CHIP in mpkCCD Cells
The differential proteomics approach determined that the E3 ubiquitin ligase CHIP was highly enriched in cultured mpkCCD cells. Immunoblotting of homogenates isolated from different kidney zones (Supplemental Figure 12) confirmed the IHC results, namely that in vivo CHIP is expressed throughout the kidney. CHIP abundance was significantly decreased after long-term dDAVP exposure in mpkCCD cells (Supplemental Figure 13A) and mouse kidney (Supplemental Figure 13B).
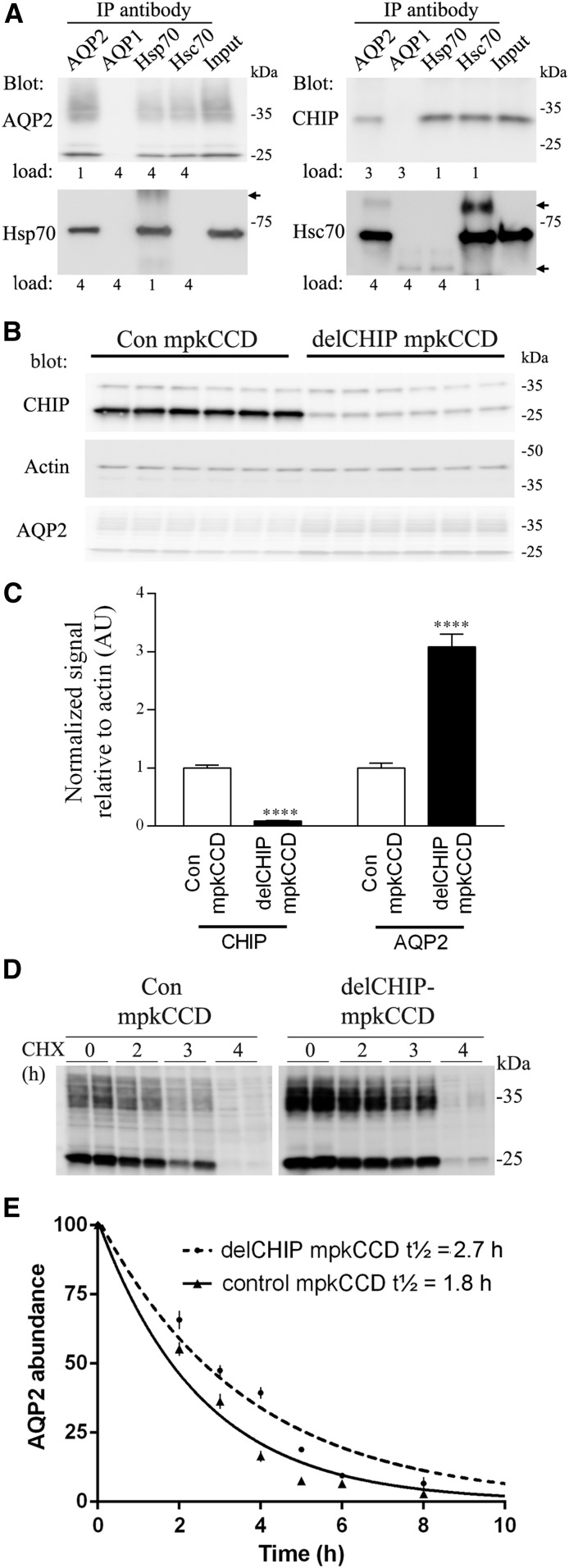
CHIP binds to the molecular chaperones Hsc70/Hsp7015 and can alter the turnover of bound clients. Because AQP2 interacts with Hsc70/Hsp7016,17 and CHIP is also expressed in CD principal cells, we hypothesized that CHIP regulates AQP2. In mpkCCD14 cell lysates, AQP2, CHIP, Hsc70, and Hsp70 could be detected in samples coimmunoprecipitated using an AQP2-specific antibody (Figure 3A). Similarly, we could identify AQP2 in samples immunoprecipitated using Hsc70 or Hsp70. Technical issues and antibody crossreactivity prevented clear identification of AQP2 in samples immunoisolated from mpkCCD cells using a CHIP antibody.
Figure 3.
CHIP interacts with AQP2 and modulates its abundance. (A) Coimmunoprecipitation of AQP2, Hsc70, Hsp70, and CHIP from mpkCCD14 cells. “Load” indicates the relative ratio of sample loaded on immunoblots. Arrows indicate nonspecific Igs. IP, immunoprecipitation. (B) Representative immunoblot showing increased AQP2 levels in A4delCHIP cells relative to control mpkCCD cells. (C) Summary of abundance data from three independent experiments. Asterisks indicate significance relative to control mpkCCD cells. ****P<0.001. (D) Representative AQP2 immunoblot from cells treated with cycloheximide (CHX) for various time periods shows that the increased AQP2 levels in A4delCHIP cells are due to reduced AQP2 degradation. Samples are on the same immunoblot with the same exposure time, and the break is for illustration purposes only. (E) Summarized CHX data fitted using nonlinear regression and a one-phase exponential decay equation. AQP2 abundance is normalized to the zero time point. Values are obtained from four independent experiments, with n=3–6 for each individual time point.
Stable Knockdown of CHIP in mpkCCD Cells Alters AQP2 Protein Abundance and t1/2
To examine the role of CHIP in regulation of AQP2, stable knockdown of CHIP in mpkCCD cells using various shRNA constructs was undertaken. Several independent cell populations (A1-A6delCHIP) showed significant reductions in CHIP mRNA by quantitative RT-PCR relative to scrambled shRNA control cells (Supplemental Figure 14A). Significant reductions in CHIP protein were detected in the A1-A6delCHIP lines by Western blotting, which correlated with increased AQP2 levels (Supplemental Figure 14B). One line (termed A4delCHIP) was used for subsequent studies (Supplemental Figure 14C). A consistent reduction in CHIP levels that corresponded with increased AQP2 levels was observed in total protein homogenates from A4delCHIP cells relative to control mpkCCD cells at various cell passage numbers (Figure 3, B and C). Cycloheximide studies (Figure 3D) showed that the increased AQP2 abundance was, at least in part, due to significantly increased AQP2 protein stability and reduced degradation, with the calculated AQP2 protein t1/2 in control mpkCCD cells 1.8 hours (95% confidence interval, 1.59 to 1.97) compared with 2.7 hours (95% confidence interval, 2.24 to 2.88) in A4delCHIP cells (Figure 3E).
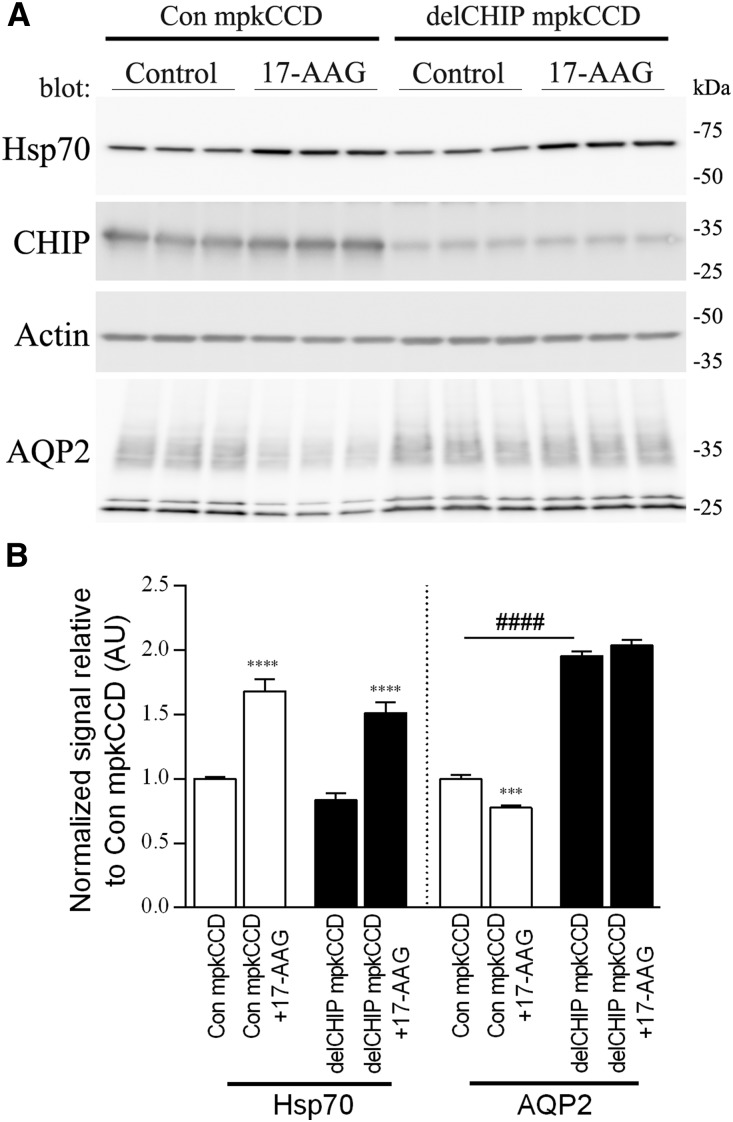
Inhibition of Hsp90 causes a shift from protein folding to protein degradation via CHIP.18 Supporting a role for CHIP-mediated AQP2 degradation, in control mpkCCD cells treated with the Hsp90 inhibitor 17-N-allylamino-17-demethoxygeldanamycin (17-AAG), Hsp70 levels were significantly increased (a key biomarker for the inhibition of Hsp90), and CHIP levels remained constant; however, AQP2 levels were significantly decreased (Figure 4). Despite significantly increased Hsp70 levels in 17-AAG–treated A4delCHIP cells, AQP2 levels remained constant. Furthermore, AQP2 levels were greatly reduced in control mpkCCD cells treated with the Hsp70 activator 2-[[3-Ethyl-5-(3-methyl-2(3H)-benzothiazolylidene)-4-oxo-2-thiazolidinylidene]methyl]-1-methyl-pyridinium chloride (Supplemental Figure 15), supporting a role of Hsp70 in modulating AQP2 expression.
Figure 4.
Inhibition of Hsp90 in control and delCHIP mpkCCD cells support a role of CHIP in AQP2 degradation. (A) Representative immunoblots from mpkCCD cells treated with the Hsp90 inhibitor 17-AAG. (B) Summary of 17-AAG data from three independent experiments. Asterisks indicate significance relative to control conditions within an individual cell line. ***P<0.001; ****P<0.001; ####P<0.001 (significance between cell lines).
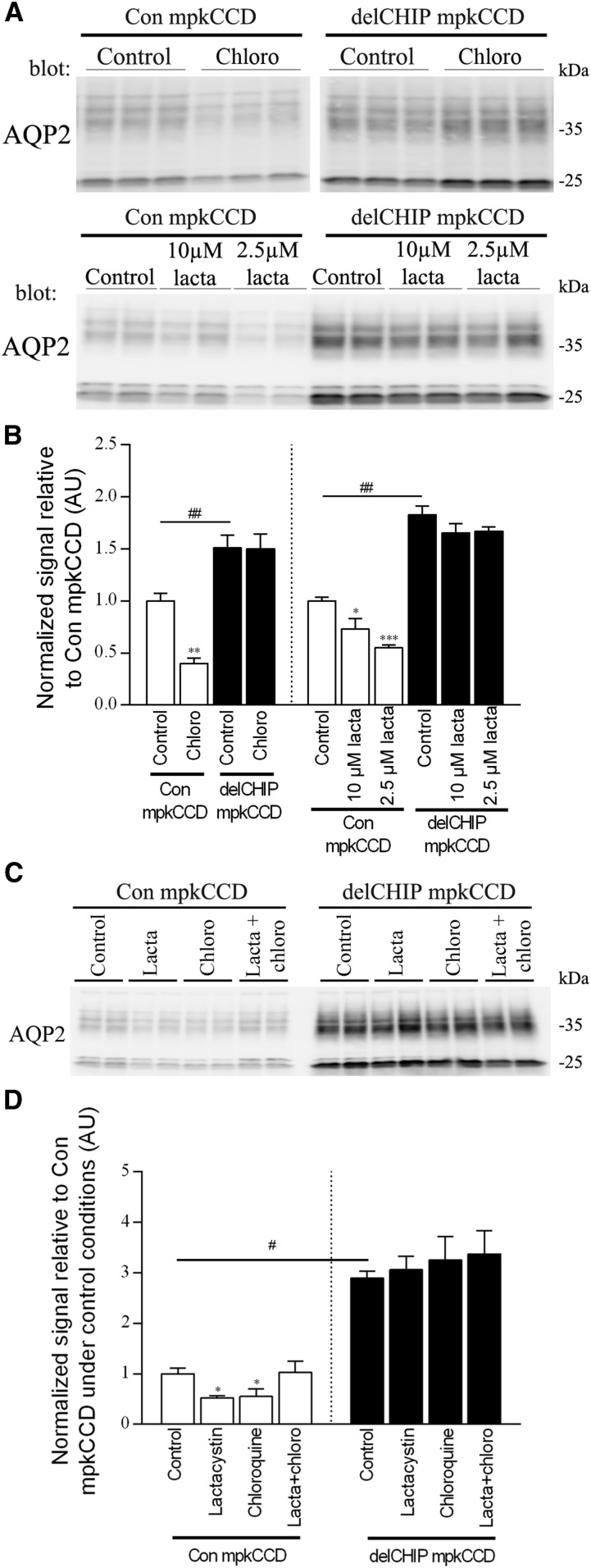
To examine the degradation pathway of AQP2 and the role of CHIP, the lysosomal inhibitor chloroquine and the proteasomal inhibitor lactacystin were used to inhibit the native degradation of AQP2 that occurs in mpkCCD cells after dDAVP removal. In control mpkCCD cells treated with chloroquine or lactacystin (Figure 5, A and B), AQP2 levels were significantly reduced relative to nontreated cells. However, when control mpkCCD cells were treated using chloroquine and lactacystin in combination, AQP2 levels were not significantly altered (Figure 5, C and D). In contrast, under similar conditions, chloroquine or lactacystin had no significant effect on AQP2 levels in A4delCHIP cells (Figure 5, C and D). These data indicate that AQP2 can be degraded by both lysosomal or proteasomal pathways and that CHIP plays a role in both processes.
Figure 5.
CHIP plays a role in both lysosomal and proteasomal degradation of AQP2. (A) Representative AQP2 immunoblot from cells treated with the lysosomal inhibitor chloroquine (chloro) or the proteasomal inhibitor lactacystin (lacta) for 20 hours. In con mpkCCD cells, AQP2 degradation can occur in the presence of lysosomal or proteasomal inhibition, but this response is absent in A4delCHIP cells. Samples are on the same immunoblot with the same exposure time, and the break is for illustration purposes only. (B) Summary of chloro and lacta data from three independent experiments per treatment. (C) Representative AQP2 immunoblot from cells treated with chloro, lacta, and chloro plus lacta combined. AQP2 levels were not significantly altered when control mpkCCD cells were treated using chloro and lacta in combination. Chloro or lacta alone had no significant effect on AQP2 levels in A4delCHIP cells. (D) Summary of chloro, lacta, and chloro plus lacta combined data from three independent experiments per treatment. Asterisks indicate significance relative to control conditions within an individual cell line; hashtags indicate significance between cell lines. *0.01<P<0.05; **0.001<P<0.01; ***P<0.001; #0.01<P<0.05; ##0.001<P<0.01.
Stable Knockdown of CHIP in mpkCCD Cells Alters AQP2 Membrane Targeting and Ubiquitylation
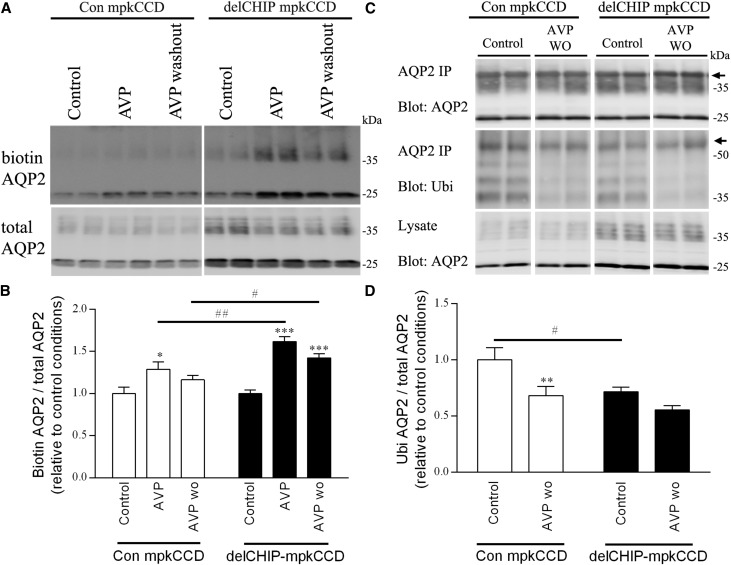
AQP2 is ubiquitylated, which modulates its plasma membrane accumulation and lysosomal/proteasomal degradation.19,20 A role for CHIP in these mechanisms was examined in A4delCHIP cells. Relative to control cells, apical plasma membrane AQP2 levels in A4delCHIP cells were significantly greater after dDAVP stimulation and remained higher 20 minutes after dDAVP removal (Figure 6, A and B). Under identical conditions, ubiquitylated AQP2 levels were significantly lower in untreated A4delCHIP cells but returned to similar levels as control cells 30 minutes after dDAVP washout (Figure 6, C and D).
Figure 6.
Stable knockdown of CHIP in mpkCCD cells alters AQP2 membrane targeting and ubiquitylation. (A) Representative immunoblots of apically biotinylated AQP2 in control or A4delCHIP cells under control, dDAVP (AVP), or dDAVP followed by 20 minutes washout (AVP washout) conditions. Samples are on the same immunoblot with the same exposure time, and the break is for illustration purposes only. (B) Summary of biotinylation data from five independent experiments. (C) Representative immunoblots of ubiquitylated AQP2 levels in control or A4delCHIP cells under control, dDAVP (AVP), or dDAVP followed by 20 minutes washout (AVP washout) conditions. Samples are on the same immunoblot with the same exposure time, and the break is for illustration purposes only. Arrows represent nonspecific IgG bands. (D) Summary of ubiquitylation data from three independent experiments. Asterisks indicate significance relative to control conditions within an individual cell line; hashtags indicate significance for a specific condition between cell lines. *0.01<P<0.05; **0.001<P<0.01; ***P<0.001; #0.01<P<0.05; ##0.001<P<0.01.
CHIP Modifies AQP2 In Vitro
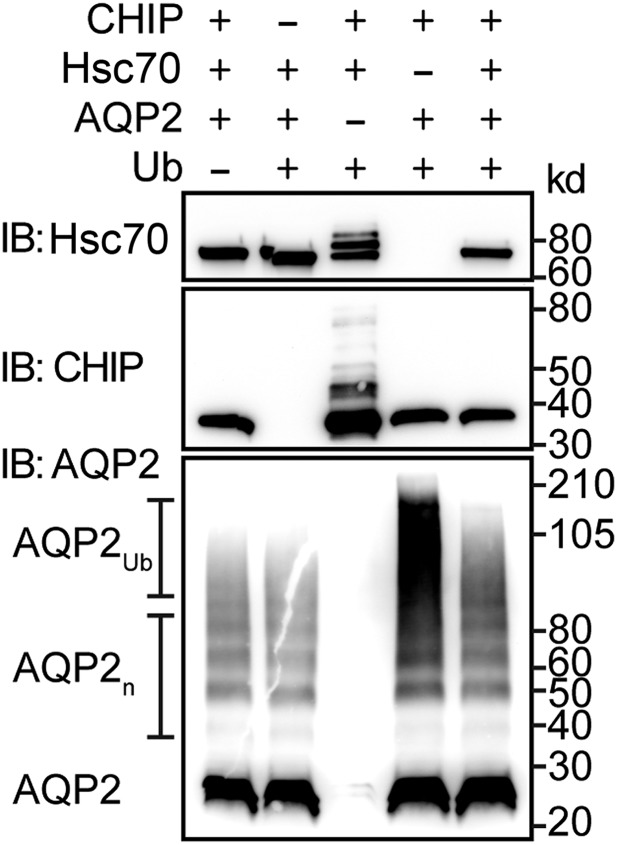
CHIP has ubiquitin ligase activity.21–24 To assess whether CHIP can directly ubiquitylate AQP2, we performed cellfree ubiquitylation assays. In the presence of ubiquitin, CHIP autoubiquitylation and CHIP-mediated ubiquitylation of Hsc70 were readily detectable (Figure 7).25 AQP2 readily forms stable oligomers that are still present after protein reduction (Figure 7); however, in the presence of CHIP and ubiquitin, AQP2 was modified, resulting in high molecular weight smearing typical of polyubiquitylated proteins. This high molecular weight shift did not occur in reactions lacking CHIP or ubiquitin. Interestingly, modification of AQP2 is attenuated in the presence of Hsc70, and the presence of AQP2 prevents both CHIP autoubiquitylation and CHIP-mediated ubiquitylation of Hsc70, suggesting that CHIP can ubiquitinate AQP2 in vitro; this interaction may be modified in the presence of Hsc70, altering protein triage.
Figure 7.
CHIP can ubiquitiylate AQP2 in vitro. Immunoblot analysis of in vitro ubiquitylation reactions in the presence or absence of the various recombinant proteins indicated, including ubiquitin (Ub). The target of antibody used in the immunoblot (IB), molecular mass markers (kilodaltons), and the presence of oligomeric AQP2 (AQP2n) and polyubiquitylated AQP2 (AQP2Ub) are specified.
Increased AQP2 Levels in CHIP Gene–Modified Mice
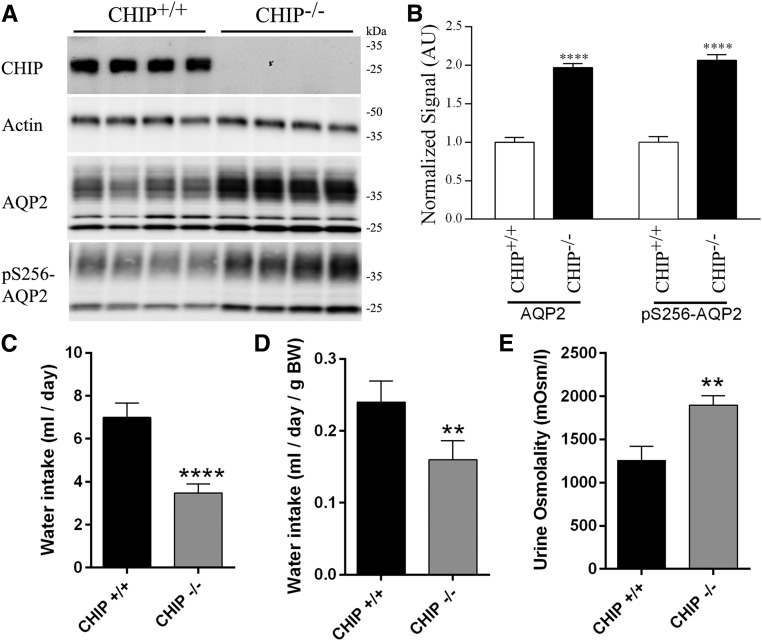
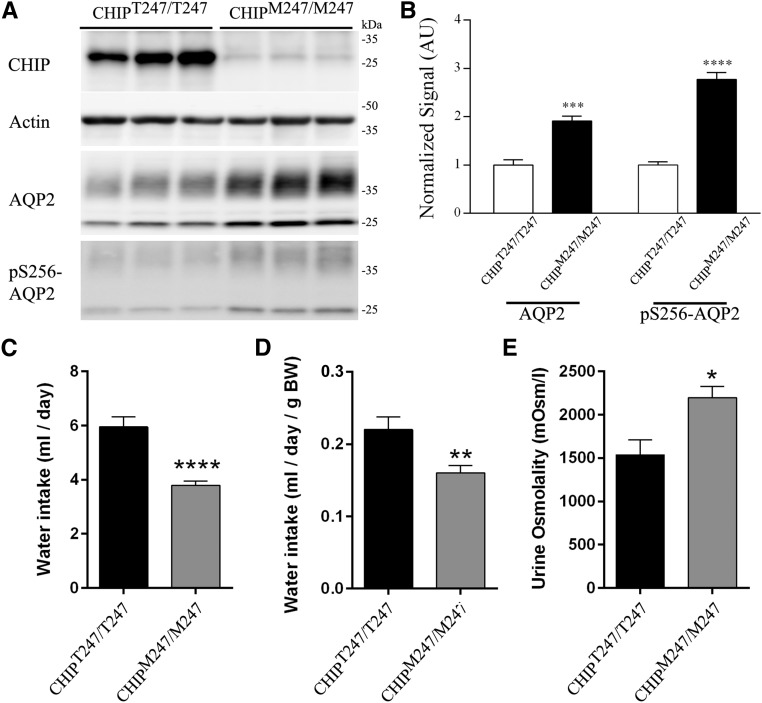
CHIP was undetectable in CHIP knockout mice (CHIP−/−) (Figure 8A). AQP2 and pS256-AQP2 (a phosphorylation site important for AQP2 trafficking26) levels in kidneys from CHIP−/− mice were significantly greater than those in controls (CHIP+/+) (Figure 8B). pS256-AQP2 levels were not increased in CHIP−/− mice when normalized to total AQP2 levels (Supplemental Figure 16). The increase in total AQP2 was qualitatively confirmed in all regions of the kidney using IHC (Supplemental Figure 17). Similarly, in kidneys from novel mice with a CHIP Thr247Met mutation that results in loss of CHIP ubiquitin ligase activity (CHIPM247/M247), AQP2 and pS256-AQP2 levels were significantly greater than those in control mice (CHIPT247/T247) (Figure 9, A and B). In this model, CHIP levels are also significantly lower (Figure 8, C and D), potentially reflecting the shorter t1/2 of the mutated CHIP protein.27,28 Because of technical difficulties, immunoprecipitating AQP2 from mouse kidney, and detecting ubiquitylated AQP2 with antiubiquitin antibodies, the levels of ubiquitylated AQP2 in vivo in the two mouse models could not be determined.
Figure 8.
Increased AQP2 levels correlate with altered renal water handling in control (CHIP+/+) and CHIP knockout (CHIP−/−) mice. (A) Representative immunoblots of AQP2 and pS256-AQP2 in CHIP+/+ and CHIP−/− mice kidneys. (B) Summary of data from CHIP+/+ and CHIP−/− mice, with ten individual mice per genotype. (C) Cumulative data for water intake over a 3-day period. (D) Cumulative data for water intake relative to mouse body weight (BW) over a 3-day period. (E) Cumulative data for urine osmolality over a 3-day period. Asterisks indicate significance between genotypes. **0.001<P<0.01; ****P<0.001.
Figure 9.
Increased AQP2 levels correlate with altered renal water handling in control (CHIPT247/T247) and CHIP gene–modified (CHIPM247/M247) mice. (A) Representative immunoblots of AQP2 and pS256-AQP2 in CHIPT247/T247 and CHIPM247/M247 mice kidneys. (B) Summary of data from CHIPT247/T247 and CHIPM247/M247 mice, with six individual mice per genotype. (C) Cumulative data for water intake over a 3-day period. (D) Cumulative data for water intake relative to mouse body weight (BW) over a 3-day period. (E) Cumulative data for urine osmolality over a 3-day period. Asterisks indicate significance between genotypes. *0.01<P<0.05; **0.001<P<0.01; ***P<0.001; ****P<0.001.
Altered Renal Water Handling in CHIP−/− and CHIPM247/M247 Mice
On 3 successive days, CHIP−/− mice had decreased fluid intake (Supplemental Figure 18A), with cumulative fluid intake approximately 2.5-fold less than that of controls (Figure 8, C and D). The osmolality of spontaneously voided urine from CHIP−/− mice was significantly higher on each recorded day (Supplemental Figure 18B), with average urine osmolality in CHIP−/− mice 1.5-fold greater than that of controls (Figure 8E). The higher urine osmolality in CHIP−/− mice relative to controls correlated with significantly greater urine sodium, potassium, chloride, and urea concentrations (Table 1). However, when urinary electrolyte concentrations were normalized to creatinine, no significant differences were observed between the genotypes (Table 1). The CHIPM247/M247 mice showed very similar baseline urine electrolyte profiles to the CHIP−/− mice (Table 1), with cumulative fluid intake in CHIPM247/M247 mice significantly lower and urine osmolality significantly higher than controls (Figure 9, Supplemental Figure 18, C and D). The baseline serum electrolyte profiles of CHIP−/−, CHIPM247/M247, and relevant control mice were unremarkable, with no significant differences in any of the measured parameters (Table 1).
Table 1.
Baseline urine and serum electrolyte profile of CHIP−/−and CHIPM247/M247 mice
| Parameter | CHIP+/+ | CHIP−/− | P Value | CHIPT247/T247 | CHIPM247/M247 | P Value |
|---|---|---|---|---|---|---|
| Plasma | ||||||
| Osmolality, mOsm/kg H2O | 374±8 | 370±4 | ns | 375±6 | 369±3 | ns |
| Na+, mmol/L | 147±0.5 | 146±0.5 | ns | 147±1 | 145±0.5 | ns |
| K+, mmol/L | 4.92±0.17 | 4.76±0.39 | ns | 4.85±0.26 | 4.65±0.29 | ns |
| Cl−, mmol/L | 114±1 | 114±0.5 | ns | 114±2 | 113±1 | ns |
| Urea, mmol/L | 10.6±0.6 | 10.4±0.4 | ns | 8.6±0.3 | 8.0±0.5 | ns |
| Urine | ||||||
| Na+, mmol/L | 70±11 | 97±5 | <0.05 | 67±7 | 93±8 | <0.05 |
| Na+-to-creatinine ratio | 24±2 | 28±3 | ns | 27±4 | 27±3 | ns |
| K+, mmol/L | 94±11 | 141±8 | <0.01 | 110±9 | 145±9 | <0.05 |
| K+-to-creatinine ratio | 34±4 | 38±3 | ns | 38±2 | 39±2 | ns |
| Cl−, mmol/L | 145±26 | 202±5 | <0.05 | 154±14 | 194±17 | ns |
| Cl−-to-creatinine ratio | 50±5 | 55±4 | ns | 56±6 | 54±5 | ns |
| Urea, mmol/L | 990±91 | 1349±112 | <0.05 | 960±60 | 1313±79 | <0.01 |
| Urea-to-creatinine ratio | 354±31 | 354±18 | ns | 347±24 | 351±19 | ns |
| Creatinine, mmol/L | 2.8±0.2 | 3.8±0.3 | <0.01 | 2.9±0.3 | 3.8±0.3 | <0.05 |
Na+, Sodium; K+, Potassium; Cl−, Chloride.
Expression of Sodium Transporters and AQPs
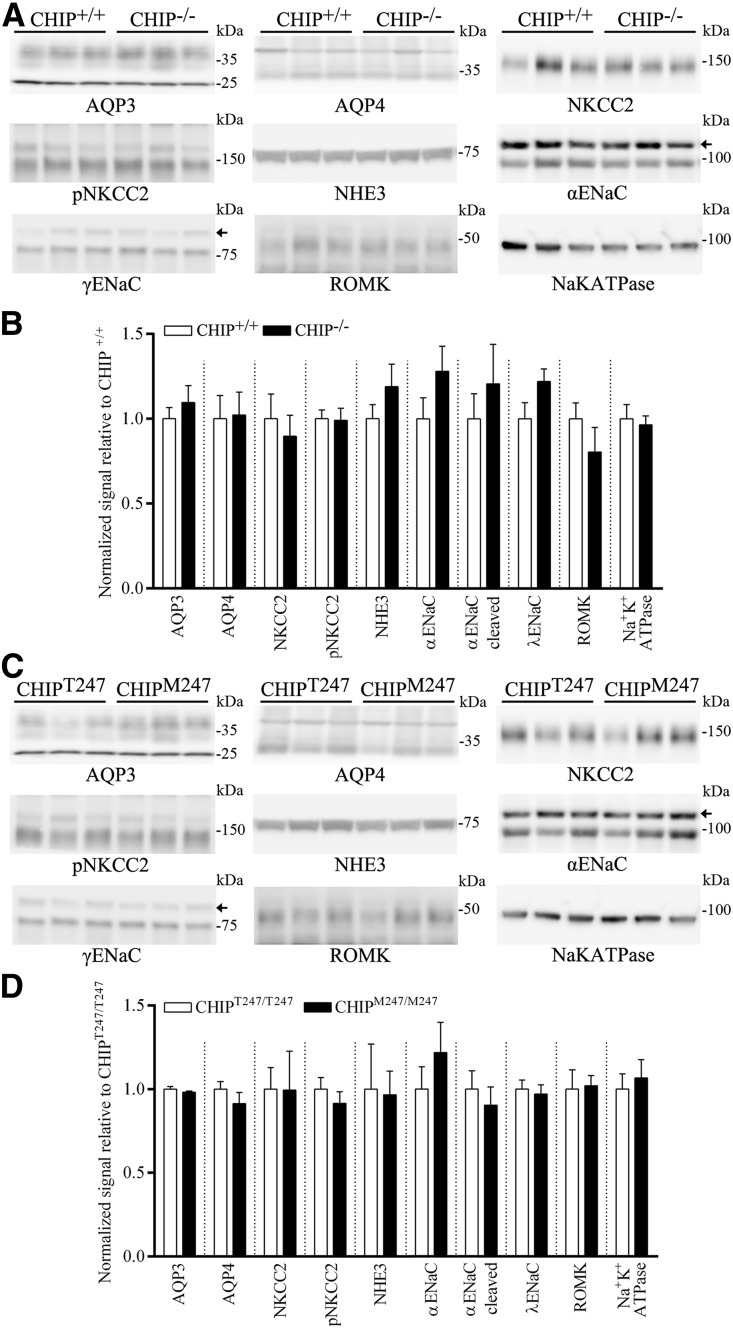
To examine whether the antidiuresis observed in the CHIP gene–modified mice could be explained by alterations in other transport proteins important for urinary concentration and/or proteins regulated by the AVP signaling pathway,29 we profiled the abundance of other major sodium transporters and AQPs in kidneys from CHIP−/−, CHIPM247/M247, and relevant control mice. No significant differences in abundance between the gene-modified mice and relevant controls were observed for any of the examined proteins (Figure 10).
Figure 10.
Abundances of other major sodium transporters and AQPs in kidneys from CHIP−/−, CHIPM247/M247, and relevant control mice. (A) Representative immunoblots of AQP3, AQP4, NKCC2, pNKCC2, NHE3, α-epithelial sodium channel (αENaC), γENaC, ROMK, and Na-K-ATPase (α1-subunit) in CHIP+/+ and CHIP−/− mice kidneys. Small arrows indicate nonspecific bands. (B) Summary of data from CHIP+/+ and CHIP−/− mice, with ten individual mice per genotype. (C) Representative immunoblots of AQP3, AQP4, NKCC2, pNKCC2, NHE3, αENaC, βENaC, ROMK, and Na-K-ATPase in CHIPT247/T247 and CHIPM247/M247 mice kidneys. (D) Summary of data from CHIPT247/T247 and CHIPM247/M247 mice, with six individual mice per genotype.
Discussion
Epithelial cells of the DCT and CCD both segregate and integrate the transport of water, solutes, and ions to maintain systemic levels of these substances and thus, body homeostasis. Despite this critical role, very little was known about which proteins play a regulatory role in DCT and CCD cells and how these cell types can be differentially regulated by similar physiologic stimuli. The major goal here was to use a systems biology approach to identify novel and unique regulatory proteins important for modulation of DCT and CCD function.
mpkCCD and mpkDCT cells are excellent models of the CCD and DCT.2,4–6 Our SILAC-based large-scale quantitative LC-MS/MS30 allowed exceptional quantitative comparisons of their proteomes and has generated a large unbiased database that will provide a solid foundation for detailed targeted investigations into the roles of various proteins for regulating various homeostatic mechanisms unique to DCT and CCD cells.
Our proteomics data allowed us to generate and test the novel hypothesis that the CCD-enriched cochaperone and E3 ubiquitin ligase CHIP would play a role in modulating the function of AQP2. It is well established that, in the endoplasmic reticulum, the chaperone proteins Hsp90 and Hsp70 play essential roles in maintaining the balance between protein folding and ubiquitin-dependent proteasomal degradation.31 If Hsp70-bound proteins are bound to the cochaperone Hop, this facilitates their association with the Hsp90 complex, promoting folding of the client protein. However, Hsp70-bound proteins associating with CHIP are targeted to endoplasmic reticulum–associated degradation via the ubiquitin proteasome system.32–34 The association of AQP2, CHIP, and Hsp70 in a protein complex plus the greater abundance of AQP2 in mpkCCD cells with stable knockdown of CHIP or in CHIP gene–modified mice suggest that AQP2 is subject to similar quality control (QC) mechanisms. Therefore, in the absence of CHIP, there is a shift in balance toward AQP2 protein folding rather than degradation. Support for the involvement of this pathway for regulation of AQP2 includes the continued degradation of AQP2 in mpkCCD cells during lysosomal inhibition (but not combined lysosomal/proteasomal inhibition), the increased AQP2 protein t1/2 in CHIP-deficient cells, and the fact that inhibition of Hsp90 in control but not CHIP-deficient mpkCCD cells decreased AQP2 abundance.
Coimmunoprecipitation indicated that, in addition to Hsp70, AQP2:CHIP may also form a complex with Hsc70. AQP2:Hsc70 complexes have been identified,16,17 and the role of this interaction has focused on AQP2 endocytosis. Because CHIP plays a role in plasma membrane QC (peripheral QC),35 it may also play a role in modulating AQP2 endocytosis (Figure 11), similar to, for example, unfolded CFTR.36 Such a mechanism is supported by (1) partial colocalization of CHIP and AQP2 in the apical plasma membrane domain of CD principal cells, (2) reduced AQP2 ubiquitylation in CHIP-deficient cells, (3) higher abundance of AQP2 in the apical plasma membrane in response to AVP in CHIP-deficient cells, and (4) greater abundance of AQP2 in the plasma membrane of CHIP-deficient cells after AVP removal. However, one caveat to this mechanism is that, in the presence of Hsc70, CHIP-mediated polyubiquitylation of AQP2 in vitro was reduced. This could indicate that an Hsc70:AQP2 complex limits CHIP-mediated ubiquitylation or that peripheral QC of AQP2 involves an additional as yet unidentified E3 ligase.35 A variety of other E3 ubiquitin ligases have been identified in CD cells that may be important for modulation of AQP2 function.37,38
Figure 11.
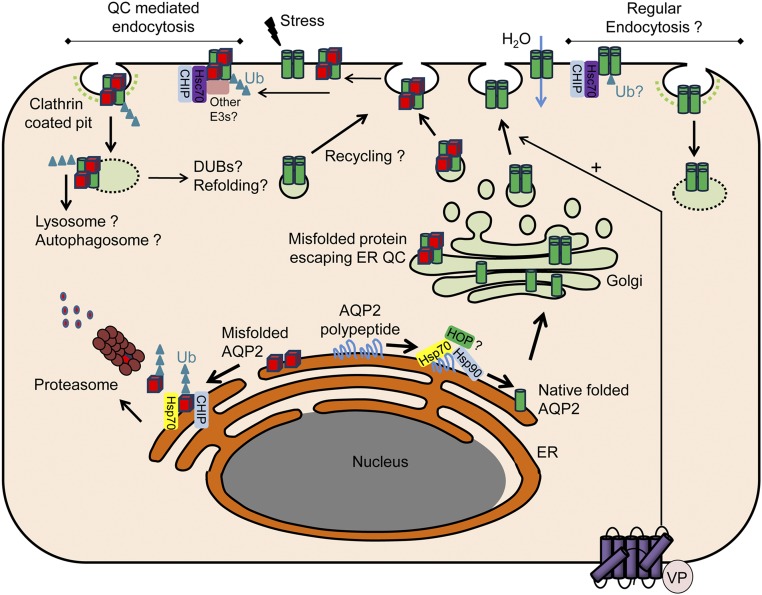
Potential role of CHIP in AQP2 QC and trafficking. In the endoplasmic reticulum (ER) under normal physiologic conditions, AQP2 polypeptide is bound by the chaperone protein Hsp70. The cochaperone Hop facilitates the association with the Hsp90 complex, promoting native folding of AQP2 that traffics to the Golgi apparatus, is organized into tetramers, and stored in transport vesicles. As part of the ER QC, Hsp70-bound AQP2 that is misfolded under normal conditions or after cellular stress associates with CHIP and is targeted to endoplasmic reticulum–associated degradation (ERAD) via the ubiquitin proteasome system. Other ERAD-associated E3 ligases are likely to be involved in ubiquitylation of AQP2. Despite ER QC, a proportion of AQP2 escapes to the Golgi apparatus and is subsequently stored in transport vesicles. Folded and misfolded AQP2 traffics to the plasma membrane (PM) after AVP (VP) simulation. Because of peripheral QC, ER-misfolded AQP2 or AQP2 misfolded within the PM due to environmental stresses are recognized and bound by Hsc70. Hsc70 associates with CHIP and other E3 ligases promoting AQP2 endocytosis and degradation. Alternatively, internalized AQP2 may be deubiquitylated, refolded, and recycled to the PM. In addition to QC-mediated endocytosis, regular AQP2 endocytosis may involve Hsc70/CHIP. A number of the collecting duct–specific E3 ligases and deubiquitylases (DUBs) identified may play important roles in these processes. Ub, ubiquitin.
The physiologic importance of CHIP for modulating AQP2 in vivo was shown in two gene-modified mice models, which had increased AQP2 levels, decreased fluid intake, and greater urine osmolality relative to controls. The animals do not seem to have “positive water balance,” because plasma electrolyte levels in the two models are not significantly different from controls. To some extent, the animals resemble a model of nephrogenic syndrome of inappropriate antidiuresis39 but seem to compensate by drinking less to maintain steady state and normal plasma osmolality/electrolytes. On the basis of these data, pharmaceutical inhibition of CHIP could be a novel approach to treat certain forms of nephrogenic diabetes insipidus and prevent the peripheral QC-mediated internalization of misfolded AQP2 mutants that may reach the plasma membrane but are functional.40 Such an approach to control the peripheral QC therapeutically was proposed for misfolded CFTR.41 Alternatively, activation of CHIP-mediated protein QC should substantially reduce the enhanced levels of AQP2 observed in various diseases with water retention (e.g., congestive heart failure or liver cirrhosis).40
Concise Methods
Extensive methods are included as Supplemental Material.
SILAC Labeling, High-pH Reversed Phase Fractionation, Nanoliquid Chromatography and Mass Spectrometry, Mass Spectrometry Data Analyses, and Bioinformatics
Extensive details are in Supplemental Material. The mass spectrometry data have been deposited to the ProteomeXchange Consortium via the PRIDE42 partner repository with the dataset identifier PXD005454.
Isolation of Enhanced Green Fluorescent Protein–Expressing Mouse DCT Cells
All animal protocols were performed in accordance with licenses for the use of experimental animals issued by the Danish Ministry of Justice. Extensive details are in Supplemental Material. Enhanced green fluorescent protein (EGFP) purity changed from 4% before sorting to 94% after sorting in the EGFP-positive sample and 0% in the EGFP-negative sample.
Antibodies
For Western blotting, the antibodies used, the supplier, and the predicted molecular weight of the target protein are detailed in Supplemental Table 7.
Fluorescent IHC and Confocal Microscopy Analyses
Mouse kidney tissue was processed and labeled as previously described.43
Generation of mpkCCD Cells with Stable CHIP Knockdown
Generation of mpkCCD cells with stable CHIP knockdown was performed as previously described44 using MISSION lentiviral transduction particles against CHIP (nos. 8527, 8528, 8530, 280520, and 280575) and corresponding control particles (SHC002V) from Sigma-Aldrich.
Real-Time Quantitative PCR
Real-time quantitative PCR was performed as previously described.44
mpkCCD14 Culture and Experimental Conditions for CHIP Analyses
mpkCCD14 cells were cultured on semipermeable supports as previously described.45 For acute dDAVP stimulation, cells were washed twice in pure media and reincubated in pure media for 3 hours before being restimulated with dDAVP (1 nM) from the basolateral side for 20 minutes at 37°C. In dDAVP washout experiments, cells were subsequently washed twice in pure media and reincubated for 30 minutes at 37°C before sample preparation. For 17-AAG studies, indicated cells were incubated with 2 μM 17-AAG for 16 hours before harvest. For Hsp70 activation studies, indicated cells were incubated with 5 μM 2-[[3-Ethyl-5-(3-methyl-2(3H)-benzothiazolylidene)-4-oxo-2-thiazolidinylidene]methyl]-1-methyl-pyridinium chloride (Sigma-Aldrich) for 16 hours in the presence of dDAVP before harvest. For proteasomal and lysosomal inhibition experiments, after 72 hours of dDAVP treatment, cells were washed twice in pure media and reincubated in pure media for 8 hours in the presence of 10 μM lactacystin, 150 μM chloroquine, or both as indicated. For cycloheximide chase studies, cells were incubated for various time points at 37°C and 5% CO2 in 50 μM cycloheximide.
Surface Biotinylation of mpkCCD14 Cells
Apical plasma membrane proteins were labeled with EZ-link hydrazide-biocytin (Pierce) as previously described.45
Immunoprecipitation Using mpkCCD14 Cells
Immunoprecipitation was performed as previously described.46
CHIP-Deficient Mice
Mice lacking CHIP expression (Stub1 targeted knockout, gene identification 56424) B6;129-Stub1tm1Cpat/Mmnc (RRID:MMRRC_037422-UNC) on a mixed background of 129S/SvEv and C57BL/6 were previously reported.47 For this line, knockout mice are delineated (CHIP−/−), and control animals are age- and sex-matched wild-type littermate controls (referred to as CHIP+/+).
Generation of CHIP (Stub1 Gene) T247M Mutant Mice
Extensive details of guide RNA cloning, guide RNA in vitro transcription, guide RNA activity test, in vitro cleavage assays, mouse production, genotyping, and off-target analysis are in Supplemental Material. For this line, knock-in mice are delineated (CHIPM247/M247), and control animals are age- and sex-matched wild-type littermate controls (referred to as CHIPT247/T247).
Water Intake and Urine Collection in CHIP−/− and CHIPM247/M247 Mice
All animal use was approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill under protocol 16–066. Extensive details are provided in Supplemental Material.
In Vitro Ubiquitylation Reactions
In vitro ubiquitylation reactions were performed as previously described.23
Statistical Analyses
For Western blotting and mouse physiologic recordings, data are expressed as mean±SEM. For two groups, data meeting the statistical assumptions of normality were assessed using an unpaired t test. Comparisons of more than two groups were performed using either a one-way ANOVA or a two-way repeated measures ANOVA followed by the Student–Newman–Keuls or the Tukey multiple comparison test, respectively. Significance was considered at P<0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to acknowledge the technical assistance of Inger-Merete Paulsen, Helle Høyer, Tina Drejer, Ahmed Abduljabar, and Christian Westberg. We also thank John Blake Belcher, Sabrina Madrigal, and Anna Elizabeth Robertson for their help with animal studies. The Animal Models Core directed by Dale Cowley at The University of North Carolina at Chapel Hill is thanked for assistance.
Q.W. is supported by European Union Horizon 2020 Marie Skłodowska-Curie Individual Fellowship project 705682, Danish Medical Research Council grant 6110-00118B, and Lundbeck Foundation grant R192-2015-804. M.L.A.K. is supported by Danish Medical Research Council grant 1333-00279. Funding is provided by National Institutes of Health grant R37 HL065619 (to C.P. and J.C.S.). Funding is also provided by the Novo Nordisk Foundation (R.A.F.), the Lundbeck Foundation (R.A.F.), and the Danish Medical Research Council (R.A.F.).
All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017050526/-/DCSupplemental.
References
- 1.Skorecki K, Chertow GM, Marsden PA, Taal MW, Yu ASL, Luyckx V: Brenner and Rector’s the Kidney, Philadelphia, Elsevier, 2015 [Google Scholar]
- 2.Subramanya AR, Ellison DH: Distal convoluted tubule. Clin J Am Soc Nephrol 9: 2147–2163, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kortenoeven ML, Fenton RA: Renal aquaporins and water balance disorders. Biochim Biophys Acta 1840: 1533–1549, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Kortenoeven ML, Pedersen NB, Rosenbaek LL, Fenton RA: Vasopressin regulation of sodium transport in the distal nephron and collecting duct. Am J Physiol Renal Physiol 309: F280–F299, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Giebisch G: Hormonal control of distal nephron function. Klin Wochenschr 63: 877–885, 1985 [DOI] [PubMed] [Google Scholar]
- 6.Pearce D, Soundararajan R, Trimpert C, Kashlan OB, Deen PM, Kohan DE: Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol 10: 135–146, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hennings JC, Andrini O, Picard N, Paulais M, Huebner AK, Cayuqueo IK, Bignon Y, Keck M, Cornière N, Böhm D, Jentsch TJ, Chambrey R, Teulon J, Hübner CA, Eladari D: The ClC-K2 chloride channel is critical for salt handling in the distal nephron. J Am Soc Nephrol 28: 209–217, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valles P, Wysocki J, Batlle D: Angiotensin II and renal tubular ion transport. Sci World J 5: 680–690, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu MJ, Miller RL, Uawithya P, Rinschen MM, Khositseth S, Braucht DW, Chou CL, Pisitkun T, Nelson RD, Knepper MA: Systems-level analysis of cell-specific AQP2 gene expression in renal collecting duct. Proc Natl Acad Sci U S A 106: 2441–2446, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loo CS, Chen CW, Wang PJ, Chen PY, Lin SY, Khoo KH, Fenton RA, Knepper MA, Yu MJ: Quantitative apical membrane proteomics reveals vasopressin-induced actin dynamics in collecting duct cells. Proc Natl Acad Sci U S A 110: 17119–17124, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajagopal M, Kathpalia PP, Widdicombe JH, Pao AC: Differential effects of extracellular ATP on chloride transport in cortical collecting duct cells. Am J Physiol Renal Physiol 303: F483–F491, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng L, Wu Q, Kortenoeven ML, Pisitkun T, Fenton RA: A systems level analysis of vasopressin-mediated signaling networks in kidney distal convoluted tubule cells. Sci Rep 5: 12829, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao T, Liu Z, Wang Y, Cheng H, Yang Q, Guo A, Ren J, Xue Y: UUCD: A family-based database of ubiquitin and ubiquitin-like conjugation. Nucleic Acids Res 41: D445–D451, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang HM, Chen H, Liu W, Liu H, Gong J, Wang H, Guo AY: AnimalTFDB: A comprehensive animal transcription factor database. Nucleic Acids Res 40: D144–D149, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonough H, Patterson C: CHIP: A link between the chaperone and proteasome systems. Cell Stress Chaperones 8: 303–308, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu HA, Sun TX, Matsuzaki T, Yi XH, Eswara J, Bouley R, McKee M, Brown D: Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J Biol Chem 282: 28721–28732, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Moeller HB, Praetorius J, Rützler MR, Fenton RA: Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci U S A 107: 424–429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kundrat L, Regan L: Balance between folding and degradation for Hsp90-dependent client proteins: A key role for CHIP. Biochemistry 49: 7428–7438, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamsteeg EJ, Hendriks G, Boone M, Konings IB, Oorschot V, van der Sluijs P, Klumperman J, Deen PM: Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc Natl Acad Sci U S A 103: 18344–18349, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nedvetsky PI, Tabor V, Tamma G, Beulshausen S, Skroblin P, Kirschner A, Mutig K, Boltzen M, Petrucci O, Vossenkämper A, Wiesner B, Bachmann S, Rosenthal W, Klussmann E: Reciprocal regulation of aquaporin-2 abundance and degradation by protein kinase A and p38-MAP kinase. J Am Soc Nephrol 21: 1645–1656, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, Patterson C: Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol 19: 4535–4545, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Höhfeld J, Patterson C: The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol 3: 93–96, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Jiang J, Ballinger CA, Wu Y, Dai Q, Cyr DM, Höhfeld J, Patterson C: CHIP is a U-box-dependent E3 ubiquitin ligase: Identification of Hsc70 as a target for ubiquitylation. J Biol Chem 276: 42938–42944, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Schisler JC, Rubel CE, Zhang C, Lockyer P, Cyr DM, Patterson C: CHIP protects against cardiac pressure overload through regulation of AMPK. J Clin Invest 123: 3588–3599, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi CH, Schisler JC, Rubel CE, Tan S, Song B, McDonough H, Xu L, Portbury AL, Mao CY, True C, Wang RH, Wang QZ, Sun SL, Seminara SB, Patterson C, Xu YM: Ataxia and hypogonadism caused by the loss of ubiquitin ligase activity of the U box protein CHIP. Hum Mol Genet 23: 1013–1024, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moeller HB, Olesen ET, Fenton RA: Regulation of the water channel aquaporin-2 by posttranslational modification. Am J Physiol Renal Physiol 300: F1062–F1073, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Hayer SN, Deconinck T, Bender B, Smets K, Züchner S, Reich S, Schöls L, Schüle R, De Jonghe P, Baets J, Synofzik M: STUB1/CHIP mutations cause Gordon Holmes syndrome as part of a widespread multisystemic neurodegeneration: Evidence from four novel mutations. Orphanet J Rare Dis 12: 31, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heimdal K, Sanchez-Guixé M, Aukrust I, Bollerslev J, Bruland O, Jablonski GE, Erichsen AK, Gude E, Koht JA, Erdal S, Fiskerstrand T, Haukanes BI, Boman H, Bjørkhaug L, Tallaksen CM, Knappskog PM, Johansson S: STUB1 mutations in autosomal recessive ataxias–evidence for mutation-specific clinical heterogeneity. Orphanet J Rare Dis 9: 146, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fenton RA, Knepper MA: Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev 87: 1083–1112, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Mann M: Functional and quantitative proteomics using SILAC. Nat Rev Mol Cell Biol 7: 952–958, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Edkins AL: CHIP: A co-chaperone for degradation by the proteasome. Subcell Biochem 78: 219–242, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Li P, Kurata Y, Maharani N, Mahati E, Higaki K, Hasegawa A, Shirayoshi Y, Yoshida A, Kondo T, Kurozawa Y, Yamamoto K, Ninomiya H, Hisatome I: E3 ligase CHIP and Hsc70 regulate Kv1.5 protein expression and function in mammalian cells. J Mol Cell Cardiol 86: 138–146, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Younger JM, Ren HY, Chen L, Fan CY, Fields A, Patterson C, Cyr DM: A foldable CFTRDeltaF508 biogenic intermediate accumulates upon inhibition of the Hsc70-CHIP E3 ubiquitin ligase. J Cell Biol 167: 1075–1085, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCaffrey K, Braakman I: Protein quality control at the endoplasmic reticulum. Essays Biochem 60: 227–235, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Apaja PM, Lukacs GL: Protein homeostasis at the plasma membrane. Physiology (Bethesda) 29: 265–277, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okiyoneda T, Barrière H, Bagdány M, Rabeh WM, Du K, Höhfeld J, Young JC, Lukacs GL: Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science 329: 805–810, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medvar B, Raghuram V, Pisitkun T, Sarkar A, Knepper MA: Comprehensive database of human E3 ubiquitin ligases: Application to aquaporin-2 regulation. Physiol Genomics 48: 502–512, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YJ, Lee JE, Choi HJ, Lim JS, Jung HJ, Baek MC, Frøkiær J, Nielsen S, Kwon TH: E3 ubiquitin-protein ligases in rat kidney collecting duct: Response to vasopressin stimulation and withdrawal. Am J Physiol Renal Physiol 301: F883–F896, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Feldman BJ, Rosenthal SM, Vargas GA, Fenwick RG, Huang EA, Matsuda-Abedini M, Lustig RH, Mathias RS, Portale AA, Miller WL, Gitelman SE: Nephrogenic syndrome of inappropriate antidiuresis. N Engl J Med 352: 1884–1890, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moeller HB, Rittig S, Fenton RA: Nephrogenic diabetes insipidus: Essential insights into the molecular background and potential therapies for treatment. Endocr Rev 34: 278–301, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loureiro CA, Matos AM, Dias-Alves Â, Pereira JF, Uliyakina I, Barros P, Amaral MD, Matos P: A molecular switch in the scaffold NHERF1 enables misfolded CFTR to evade the peripheral quality control checkpoint. Sci Signal 8: ra48, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Vizcaíno JA, Csordas A, del-Toro N, Dianes JA, Griss J, Lavidas I, Mayer G, Perez-Riverol Y, Reisinger F, Ternent T, Xu QW, Wang R, Hermjakob H: 2016 update of the PRIDE database and its related tools. Nucleic Acids Res 44: D447–D456, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moeller HB, Knepper MA, Fenton RA: Serine 269 phosphorylated aquaporin-2 is targeted to the apical membrane of collecting duct principal cells. Kidney Int 75: 295–303, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yde J, Keely S, Wu Q, Borg JF, Lajczak N, O’Dwyer A, Dalsgaard P, Fenton RA, Moeller HB: Characterization of AQPs in mouse, rat, and human colon and their selective regulation by bile acids. Front Nutr 3: 46, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moeller HB, Slengerik-Hansen J, Aroankins T, Assentoft M, MacAulay N, Moestrup SK, Bhalla V, Fenton RA: Regulation of the water channel aquaporin-2 via 14-3-3θ and -ζ. J Biol Chem 291: 2469–2484, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moeller HB, Aroankins TS, Slengerik-Hansen J, Pisitkun T, Fenton RA: Phosphorylation and ubiquitylation are opposing processes that regulate endocytosis of the water channel aquaporin-2. J Cell Sci 127: 3174–3183, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Dai Q, Zhang C, Wu Y, McDonough H, Whaley RA, Godfrey V, Li HH, Madamanchi N, Xu W, Neckers L, Cyr D, Patterson C: CHIP activates HSF1 and confers protection against apoptosis and cellular stress. EMBO J 22: 5446–5458, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.