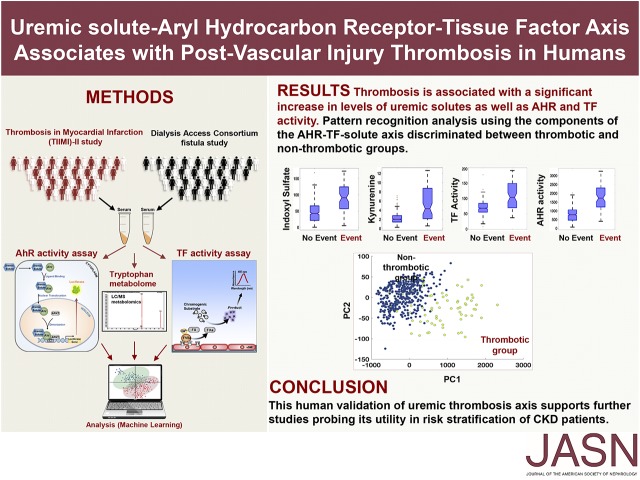
Abstract
Individuals with CKD are particularly predisposed to thrombosis after vascular injury. Using mouse models, we recently described indoxyl sulfate, a tryptophan metabolite retained in CKD and an activator of tissue factor (TF) through aryl hydrocarbon receptor (AHR) signaling, as an inducer of thrombosis across the CKD spectrum. However, the translation of findings from animal models to humans is often challenging. Here, we investigated the uremic solute–AHR–TF thrombosis axis in two human cohorts, using a targeted metabolomics approach to probe a set of tryptophan products and high-throughput assays to measure AHR and TF activity. Analysis of baseline serum samples was performed from 473 participants with advanced CKD from the Dialysis Access Consortium Clopidogrel Prevention of Early AV Fistula Thrombosis trial. Participants with subsequent arteriovenous thrombosis had significantly higher levels of indoxyl sulfate and kynurenine, another uremic solute, and greater activity of AHR and TF, than those without thrombosis. Pattern recognition analysis using the components of the thrombosis axis facilitated clustering of the thrombotic and nonthrombotic groups. We further validated these findings using 377 baseline samples from participants in the Thrombolysis in Myocardial Infarction II trial, many of whom had CKD stage 2–3. Mechanistic probing revealed that kynurenine enhances thrombosis after vascular injury in an animal model and regulates thrombosis in an AHR-dependent manner. This human validation of the solute-AHR-TF axis supports further studies probing its utility in risk stratification of patients with CKD and exploring its role in other diseases with heightened risk of thrombosis.
Keywords: uremic solute, thrombosis, Aryl hydrocarbon, tissue factor
Thrombosis is a frequent and serious complication of vascular interventions such as surgery or endovascular procedures.1 CKD increases the risk of vascular injury–associated thrombosis by 6.5–10-fold.2–7 Currently, serum creatinine concentration and CKD stage serve as generic indicators of thrombosis risk factor.8 Risk stratification using a broader set of mechanistically driven biomarkers is likely to improve thrombosis risk prediction and improve antithrombotic management of patients with CKD.
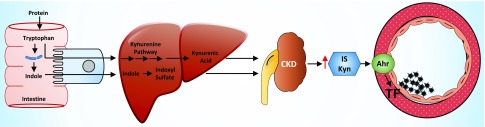
The role of retained solutes (uremic solutes) in enhancing thrombogenicity of the CKD milieu (uremia) has been demonstrated in ex vivo models and recently in mouse models.9–12 Particularly, indolic solutes, such as indoxyl sulfate (IS) produced by tryptophan metabolism and retained starting in the early stages of CKD, increase tissue factor (TF) expression in the vessel wall to trigger thrombosis10 through aryl hydrocarbon receptor (AHR) signaling, thereby defining the uremic solute–AHR–TF thrombosis pathway.11,13 Although these studies established a mechanistic basis for this pathway and its potential druggability,9,11 validation of these findings in humans has yet to be performed.
Here, we set out to comprehensively examine the uremic solute–AHR–TF axis in patients from two clinical trials that focused on thrombosis after different types of vascular injury. The Dialysis Access Consortium Clopidogrel Prevention of Early AV Fistula Thrombosis trial (DAC-Fistula)14 and the Thrombolysis in Myocardial Infarction (TIMI)–II study15 examined the thrombotic complications after hemodialysis arteriovenous fistula (AVF) creation and balloon angioplasty, respectively. Although these two types of injury models are distinct, their postinjury vascular beds share common characteristics including endothelial damage and exposure of vascular smooth muscle cells (vSMCs), both of which contribute to thrombosis.16–18 Using this mechanistic rationale, this study leverages a prevalidated liquid chromatography–mass spectrometry (LC/MS)–based targeted metabolomics analysis,19 and high-throughput AHR and TF activity assays in thrombosis-relevant cells11 to evaluate the abilities of sera obtained before enrollment to increase AHR signaling and TF activity. The DAC-Fistula trial randomized 877 patients with ESRD to clopidogrel or placebo with the primary outcome of AVF thrombosis within 6 weeks of its creation, which occurred in 137 (15%) participants.14,20 Given outcome of post–vascular injury thrombosis,21 the results obtained from analysis of the DAC-Fistula were validated in a subgroup of patients (n=377) from the TIMI-II trial who underwent percutaneous transluminal coronary angioplasty (PTCA) (n=1144) for ST-segment elevation myocardial infarction.15 Reinfarction or reocclusion of the coronary vessel in these patients was considered as a post-PTCA thrombotic “event” (9.8% of 377 participants). In this study, all of the sera samples were probed for a set of solutes, which are associated with tryptophan metabolism (such as IS) and retained in patients with CKD.22,23 This set included indole acetic acid (IA), L-kynurenine (Kyn) and ynurenic acid (KA), anthranilic acid (AA), and xanthurenic acid (XA).19
Results
Solute-AHR-TF Axis in AVF Thrombosis
A comprehensive analysis of components of the uremic solutes–AHR–TF axis (Figure 1, Supplemental Figure 1, A–C) in the samples from the DAC fistula trial was performed with the hypothesis that the components of this axis are likely to be higher in patients with AVF thrombosis. Because biosample collection was not initiated until approximately half of the trial participants were enrolled, baseline sera (before fistula creation) were available from only 473 participants. Because our focus was on identifying markers of thrombosis regardless of its time of occurrence, we included all AVF thrombosis events in this trial. The baseline characteristics of participants were similar between the thrombotic “event” and the nonthrombotic “no-event” groups (Table 1).
Figure 1.
Uremic thrombosis axis (uremic solutes-AHR-TF) in humans. Working hypothesis of the uremic thrombosis axis: Tryptophan degraded to indole by intestinal bacteria undergoes conversion to indolic solutes such as IS in the liver. Tryptophan absorbed through the portal circulation also undergoes metabolism in the liver to Kyn. IS and Kyn are retained in patients with CKD and their elevated levels activate the AHR pathway to increase TF levels in the vessel wall to enhance thrombosis. The uremic solutes–AHR–TF axis is a novel and CKD-specific thrombotic signaling pathway.
Table 1.
Baseline characteristics of DAC-Fistula trial participants
| Study/Baseline Characteristicsa | DAC-Fistula Trial (n=470) | ||
|---|---|---|---|
| Thrombosis Group (n=60) | Nonthrombosis Group (n=410) | P Value | |
| Age, yrb | 54.69 (15.85) | 52.35 (13.88) | 0.28 |
| Male | 33 (55.0%) | 278 (67.8%) | 0.06 |
| BMI, kg/m2b | 29.76 (8.20) | 30.02 (8.49) | 0.82 |
| Black | 32 (53.3%) | 202 (49.3%) | 0.58 |
| BP, mmHgb | |||
| Systolic | 138 (20.77) | 140 (21.28) | 0.48 |
| Diastolic | 77 (14.80) | 79 (13.30) | 0.38 |
| Diabetes mellitus | 30 (50.0%) | 208 (50.7%) | 1.00 |
| Cardiovascular diseases | 11 (18.3%) | 90 (21.9%) | 0.62 |
| Cerebrovascular diseases | 3 (5.0%) | 28 (6.8%) | 0.78 |
| Venous thromboembolism disease | 3 (5.0%) | 11 (2.7%) | 0.40 |
| Aspirin use | 47 (78.3%) | 337 (82.2%) | 0.48 |
| Clopidogrel use | 22 (36.7%) | 203 (49.5%) | 0.07 |
| Statin use | 19 (31.7%) | 108 (26.3%) | 0.44 |
| Hemoglobin, g/dlb | 11.70 (2.07) | 11.53 (1.90) | 0.56 |
| Serum albumin, g/dlb | 3.70 (0.64) | 3.73 (0.63) | 0.75 |
| Number of patients on HD before enrollment | 39 (65.0%) | 240 (58.5%) | 0.40 |
| Creatinine in patients on HD, mg/dlb | 8.32 (3.98) | 8.80 (3.73)c | 0.48 |
| Fistula location | |||
| Forearm | 38 (63.3%) | 212 (51.7%) | 0.09 |
| Upper arm | 22 (36.6%) | 198 (48.2%) | 0.09 |
BMI, body mass index; HD, hemodialysis.
This is the subgroup of DAC-Fistula trial participants for whom baseline blood samples were collected.
Mean (SD).
Creatinine data from only 238 subjects was available.
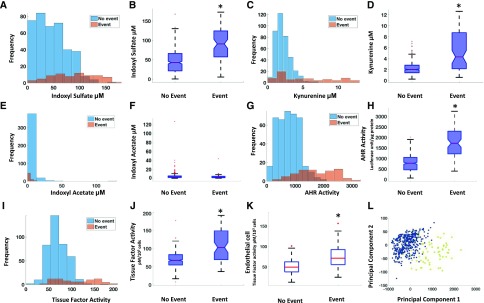
We first compared the distribution of the components of the solute-AHR-TF axis between event and no-event groups (Table 1). Among analyzed metabolites, IS, Kyn, and kynurenic acid showed a nonoverlapping distribution between both the groups and significantly higher levels (P<0.001) in the event group (Figure 2, A–D, Supplemental Figure 2A), whereas no differences were observed for other metabolites, including IA (Figure 2, E–F, Supplemental Figure 2, B and C). Similar differences in the distribution and higher levels of AHR activity were observed in the event group (Figure 2, G and H). Given the potential contribution of vSMCs and endothelial cells to AVF thrombosis, TF activities were analyzed in both these cell types. The results showed significantly higher TF activity in both of in the event group (Figure 2, I–K). Furthermore, significant correlations between IS-AHR (R2=0.59, P<0.001), Kyn-AHR (R2=0.51, P<0.001), and IS-TF (R2=0.24, P<0.05) were noted only in the event group.
Figure 2.
Increased levels of IS, Kyn, and AHR and TF activities in patients with AVF thrombosis in DAC-Fistula trial. (A, C, E, G, and I) Histograms of the metabolites as well as the functional activities of the proteins between the AVF thrombosis “event” (as defined in the text) and “no-event” groups are shown. The concentrations of metabolites are in micromolars and AHR and TF activities are in luciferase units/μg protein and pM/103 cells, respectively. (B, D, F, H, and J) Box plot visualization of the thrombosis “event” and “no-event” groups for the measured indicators in the DAC-Fistula trial. The lines in red in the boxes represent median levels of the measured indicator (metabolite or the level of activity). The lower and upper boundaries of the boxes represent the 25th and 75th percentiles, respectively. The lower and upper whiskers represent the minimum and maximum values, respectively. Note that statistically significant differences (P<0.001) were observed between the “event” and “no-event” groups for each case other than IA. (K) Box plot showing the endothelial cell TF activity for the “event” and “no-event” groups (P<0.001). (L) PCA on the components of the thrombosis axis was performed and the first two principal components capturing a major portion of the total variance of the original measurements are shown. The subjects belonging to the “event” group are shown in yellow and those in the “no-event” group are shown in blue. *P-value significant.
One of the central expectations of identifying a biomarker signature is to determine a potential set of molecules that associates with a process and differentiates patients on the basis of risk. We employed data clustering techniques that are part of the broad field of machine learning,24–26 to discern the discriminatory potential of the parameters of the solute-AHR-TF axis to segregate patients with events. For example, methods such as principal component analysis (PCA) (Figure 2L) allowed us to fully appreciate the cumulative effect of the components of the thrombosis axis by reducing such high-dimensional data to fewer dimensions without significant loss of information.25,27 The underlying hypothesis is that patients with thrombosis will be clustered together and away from the group with no thrombosis. PCA showed that the first two principal components plotted on a two dimensional (2D) map revealed a distinct separation between the “event” and “no-event” subgroups, and this clustering was also evident using several other dimensionality reduction methods (Supplemental Figure 2, D–F). Taken together, these results indicate that the combination of uremic metabolites, AHR, and TF can segregate the study participants with and without thrombosis, thus supporting their biomarker potential.
Validation of the Solute-AHR-TF Axis in the TIMI-II Study Cohort
We substantiated our findings of increased levels of solutes, AHR, and TF activities in patients with post–vascular injury thrombosis using another clinical cohort, the TIMI-II trial (Supplemental Figure 1D). Sera samples (n=377) were obtained from patients who underwent PTCA (n=1144). Of 377 patients, 66% had CKD stage 2 or 3 (Supplemental Figure 3A). Complications, such as postangioplasty protocol lesion thrombosis, nonprotocol lesion thrombosis, occlusion of infarct-related vessel, and occlusion of branch(es) of infarct-related vessels, were all grouped as thrombotic events (n=37). Baseline characteristics were similar in the participants with and without events (Table 2).
Table 2.
Baseline characteristics of TIMI-II trial participants included in this study
| Study/Baseline Characteristics | TIMI-II (n=377) | ||
|---|---|---|---|
| Thrombosis Group (n=35) | Control Group (n=342) | P Value | |
| Age, yra | 57.26 (9.85) | 55.26 (10.27) | 0.26 |
| Male | 28 (80.0%) | 274 (80.1%) | >0.99 |
| Black | 2 (5.7%) | 30 (8.8%) | 0.75 |
| Body mass index, kg/m2a | 27.97 (4.11) | 27.09 (4.77) | 0.84 |
| BP, mmHga | |||
| Systolic | 128 (19.00) | 128 (21.55) | 0.97 |
| Diastolic | 80 (12.28) | 80 (13.63) | 0.46 |
| Diabetes mellitus | 1 (2.8%) | 43 (12.5%) | 0.10 |
| Hypertension | 13 (37.1%) | 125 (36.6%) | >0.99 |
| Angina pectoris | 18 (51.4%) | 192 (56.1%) | 0.60 |
| Congestive heart failure | 1 (2.9%) | 5 (1.5%) | 0.45 |
| Previous myocardial infarction | 4 (11.4%) | 37 (10.8%) | 0.78 |
| Peripheral vascular disease | 1 (2.9%) | 10 (2.9%) | >0.99 |
| Serum creatinine, mg/dla | 1.14 (0.26)b | 1.12 (0.25)c | 0.66 |
| eGFR, (ml/min per 1.73 m2) CKD-EPI formulaa | 74.86 (17.60) | 74.73 (17.63) | 0.97 |
| Aspirin | 5 (14.3%) | 48 (14.0%) | >0.99 |
| Dipyridamole | 0 (0%) | 3 (0.88%) | >0.99 |
| Heparin | 1 (2.9%) | 6 (1.8%) | 0.49 |
| Other anticoagulants | 0 (0%) | 0 (0%) | >0.99 |
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration. The data are displayed as number (percentage of total group).
Mean (SD).
Creatinine values available in 31 subjects.
Creatinine values available in 325 subjects.
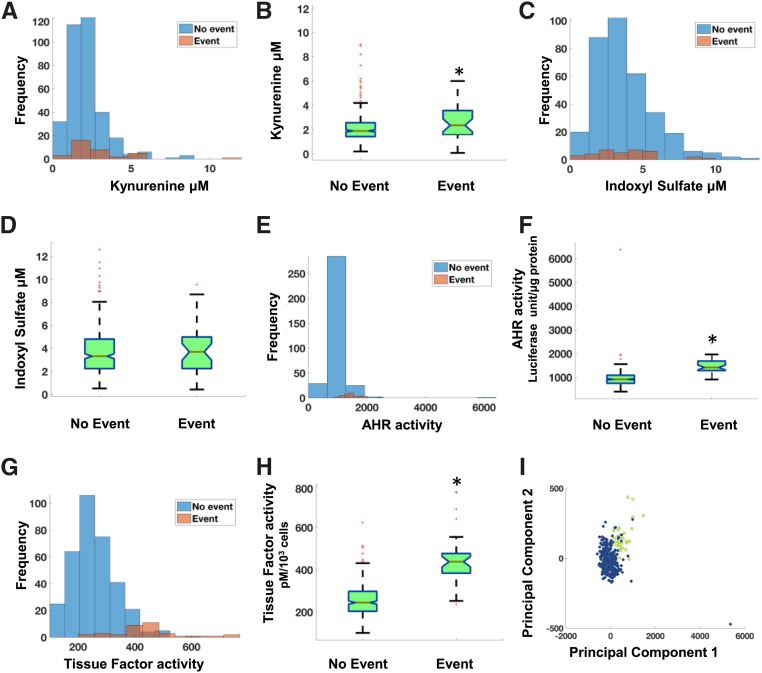
Among examined metabolites, Kyn and XA showed differences in the distribution pattern between event and no-event groups and significantly higher levels (Kyn P<0.001 and XA P=0.03) in the event group (Figure 3, A and B, Supplemental Figure 3A). Other metabolites including IS did not show any differences between the event and no-event groups (Figure 3, C and D, Supplemental Figure 3, B–E). Significantly higher AHR and TF activities were noted in the event group (Figure 3, E–H). Using levels of Kyn, AHR, and TF activities, the PCA performed on the TIMI-II data showed a distinct separation between the “event” and “no-event” patient groups (Figure 3I, Supplemental Figure 3, F–H), indicating the presence of a crossplatform signature (metabolite and cell activities) associated with the post–vascular interventional thrombosis.
Figure 3.
Increased levels of Kyn and AHR and TF activities in patients with postangioplasty thrombosis in TIMI-II trial. (A, C, E, and G) Histograms of the metabolites as well as the functional activities of the proteins show distinct differences between the thrombotic “event” and “no-event” groups. The concentrations of metabolites are in micromolars and AHR and TF activities are in luciferase units/μg protein and TF activity pM/103 cells, respectively. (B, D, F, and H) Box plot visualization of the thrombosis “event” and “no-event” groups for the measured indicators in the TIMI-II trial. Statistically significant differences (P<0.001) were observed between the “event” and “no-event” groups for each case. (I) First two principal components capturing a major portion of the total variance of the original data are shown. The patients belonging to the thrombotic “event” group are shown in yellow and ones in the “no-event” group are shown in blue. *P-value significant.
Kyn Mediates Thrombosis through AHR-TF Axis
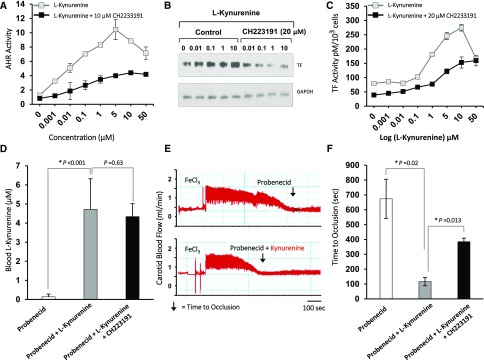
Although the association of Kyn with the blood levels of TF is known,22 its exact mechanism or relevance to the post–vascular injury thrombosis remains poorly understood. The data from both the cohorts showed significantly higher levels of Kyn in the event group; we therefore hypothesized that Kyn may enhance thrombosis after vascular injury. We first probed the mechanism of Kyn’s prothrombotic activity through the AHR-TF axis, considering earlier reports showing Kyn to be a ligand for AHR in other cell types28,29 and our own findings of AHR upregulating TF expression.11 We examined AHR signaling and TF in primary human aortic vSMCs in response to a wide range of Kyn concentrations. We observed a dose-dependent increase in AHR activity and TF expression and activity with increasing concentration of Kyn, all of which were suppressed by a specific AHR inhibitor (CH223191)30 (Figure 4, A–C). These data indicate that Kyn at the concentrations observed in patients with CKD activates AHR signaling to upregulate TF.
Figure 4.
Kyn regulates thrombosis through the AHR-TF axis. (A) Kyn activates AHR signaling in primary human aortic vSMCs. vSMCs stably expressing a xenobiotic responsive element promoter–luciferase reporter construct were treated with Kyn at levels corresponding to different CKD stages and an AHR antagonist CH2233191 (10 μM) for 24 hours. AHR activity was quantified by firefly luciferase units and normalized to protein content of the cells. An average of three independent experiments performed in duplicate is shown. Compared with control, P values for different Kyn concentrations were P=0.03 for 0.001 μM, P=0.001 for 0.01 μM, and P<0.001 for all of the other concentrations of Kyn. Compared with Kyn+CH2233191-treated samples, P values were <0.001 for all of the points. Error bars=SD. (B) AHR antagonist suppresses Kyn-induced TF expression. vSMCs treated with the indicated concentrations of Kyn with or without 20 μM CH223191 for 24 hours. The lysates were probed for TF. GAPDH served as a loading control. A representative of three independent experiments is shown. (C) Kyn induces procoagulant TF activity in an AHR-dependent manner. vSMCs were treated with indicated concentrations of Kyn with or without 20 μM CH223191 for 24 hours and TF activity was measured and normalized to total number of viable cells and expressed as pM/103 cells. An average of TF activity performed in three independent experiments done in duplicate is shown. Error bars=SD. Compared with the control (Kyn=0 μM), the P values were P=0.01 for Kyn of 1 μM and P<0.001 for Kyn concentrations of 10 and 100 μM. A significant reduction in TF activity was noted at all of the concentrations of Kyn from 0.001 to 10 μM with CH223191 (P=0.001 for Kyn from 0.001 to 5 μM and P=0.03 for Kyn 10 μM). (D) Generation of Kyn levels equivalent to levels found in patients with ESRD. Blood samples collected from animals exposed to a combination of probenecid and Kyn were analyzed using LC/MS. Probenecid-injected animals served as controls. An average of Kyn levels from five animals at the end of 4 days of exposure and before thrombosis assay is shown. The P value corresponds to a significant increase in serum Kyn levels in the probenecid+Kyn group compared with the probenecid group alone. Error bar=SD. (E) The pattern of carotid artery blood flow with occlusion due to thrombus is shown. An ultrasound probe measured carotid artery flow in five C57BL/6 animals and was noted to be 1.25–2 ml/min. The flow was monitored for 20 minutes after the application of 10% FeCl3 strip. The time till the blood flow dropped to baseline was considered as TtO and is depicted by an arrow. A representative carotid artery blood flow pattern is shown from five animals in each group. (F) Kyn enhances thrombogenicity in an AHR-dependent manner. The TtO was compared within two groups after 5 days of exposure to different agents. An average of TtO from five animals is shown. A significant reduction in TtO with Kyn was noted. Error bars=SD.
To evaluate the effect of Kyn on vascular injury–associated thrombosis in vivo, we generated a novel animal model wherein carotid artery thrombosis was examined in the milieu of elevated Kyn levels. Kyn levels were elevated in these animals by injecting Kyn and simultaneously blocking its excretion using probenecid, as done for other uremic solutes.9,31 This protocol and dosage yielded serum Kyn concentrations comparable to those observed in humans with ESRD32 (Figure 4D). The animals were subjected to the carotid artery ferric chloride (FeCL3) injury model, a standard model of post–vascular injury thrombosis.33 The drop in carotid artery blood flow to baseline (time to occlusion, TtO) as detected in real-time with an ultrasound probe served as an end point of the study (Figure 4E).9 There was a significant reduction in TtO in the Kyn group compared with the controls, and TtO improved significantly with CH223191. These data indicate that Kyn enhances postinjury thrombosis in an AHR-dependent manner (Figure 4F).
Discussion
Markers of thrombosis currently used in clinical practice do not include the disease-specific risk factors or the vessel wall mediators critical to thrombosis after vascular injury. We have performed a comprehensive analysis that elucidates the nodes of a thrombosis axis at the intersection of endogenous metabolites and vessel wall factors. These findings, along with previous observations in other models,11,13 underscore the importance of the uremic solute–AHR–TF axis as a potential marker of thrombosis in humans. We found higher blood concentrations of Kyn in the thrombosis groups from both the DAC-Fistula and TIMI-II trial cohorts, and higher blood levels of IS in the thrombosis group of the DAC-Fistula trial cohort. Importantly, we uncovered Kyn as regulator of TF through AHR. Further, we demonstrate higher AHR and TF activities in patients with thrombosis in both trials. Pattern recognition analysis highlighted the biomarker potential of this thrombosis axis.
Circulating metabolites are often associated with clinical events.34–36 However, the current work differentiates itself from such studies. First, in addition to metabolites, nodes of the signaling axis—the ligand (uremic solutes), the mediator (AHR signaling), and the effector molecule (TF)—were correlated to thrombosis. Second, the results from both the trials uncovered previously unknown association of Kyn through the AHR-TF axis to post–vascular injury thrombosis, which was further supported in a new animal model (Figure 4, D and E). In so doing, it also provided a mechanistic rationale to previous epidemiologic studies that linked Kyn to cardiovascular diseases in patients both with CKD and without CKD37–39 and to other reports of TF and AHR as mediators of atherothrombotic disease.40–42
Thrombosis after Vascular Surgery
Although the clopidogrel-treated group showed overall reduced AVF thrombosis compared with the placebo-treated group in the DAC-Fistula trial,14,20 12% of patients still experienced AVF thrombosis. Our subgroup analysis of clopidogrel-treated individuals with thrombosis revealed significantly higher levels of AHR and TF activities as well as uremic solutes compared with the nonthrombosis group (Supplemental Figure 4). This implies that even with an antiplatelet regimen, observed thrombotic events were perhaps triggered due to other signaling pathways. The uremic thrombosis axis represents such a pathway, which warrants further exploration in patients with CKD experiencing thrombosis despite being on antiplatelet therapy. The DAC-Fistula study did not show an increase in IA in the event group, whereas IS was significantly higher in patients with AVF thrombosis. This observation suggests that not all indolic solutes are the same and that IS and IA may have divergent roles.
The study of TF activity in endothelial cells has implications for other models of thrombosis. Venous thrombosis occurs on the dysfunctional endothelial monolayer.43 The increase in the endothelial cell TF activity in the thrombosis group in patients with advanced CKD/ESRD may suggest contribution of the uremic thrombosis axis to the pathogenesis of venous thromboembolism. This may also explain CKD/ESRD as a risk factor for venous thromboembolism (incidence in normal renal function 66 of 100,000 patients versus ESRD 527 of 100,000 patients).44,45
Postangioplasty Thrombosis
Although the TIMI-II study was conducted two decades ago, the findings from that trial are relevant currently because the nature of vascular injury and the postinterventional bed remain similar, with the current standard-of-care treatments such as PTCA followed by stenting.
Differences in the metabolomics patterns between two trials may point to specific mechanisms of solute elevation. In contrast to the DAC-Fistula trial, IS levels were not higher in the thrombotic group of the TIMI-II cohort (Figure 3D). Unlike the DAC-Fistula cohort (consisting of patients with CKD stage 5 and ESRD), the TIMI-II cohort had lower levels of IS, because it consisted of subjects with CKD stage 2 or 3 (66%) and none with ESRD. Although the level of IS corresponding to CKD stage 2–3 in vSMCs doubled TF levels in a previous study,11 this discrepancy between the cell-based model and human data warrants further investigation. Higher levels of Kyn were observed in the thrombotic group without any differences in renal function in the TIMI-II cohort (Table 2). This result implicates factors other than reduced excretion. In line with the complex multistep metabolism of Kyn, it is likely that the levels of Kyn may be influenced by factors including dietary composition, microbiome, and metabolic enzymes, etc.32,46,47 Furthermore, inflammation, oxidative stress, and endothelial dysfunction, all of which are increased in the setting of acute coronary syndrome, can also increase Kyn in blood.48
Enhanced TF levels in the vessel wall induced by solute-AHR signaling triggering the extrinsic coagulation pathway to generate thrombin in blood may have therapeutic implications. Although traditionally anticoagulants and antiplatelet agents are used for venous and arterial thrombosis, respectively,1 our current data support targeting either the CKD-specific thrombosis axis and/or the thrombin-based anticoagulants to suppress the extrinsic coagulation cascade downstream of TF activation, especially in patients with CKD, for effective antithrombosis after vascular intervention.
Pattern Recognition
Unsupervised clustering is a part of machine learning that is commonly used to cluster data that have several dimensions. Many of these methods also allow for dimensionality reduction which then facilitates data visualization,24–27 while retaining the same level of information but in an embedded form. When these reduced dimensions are visualized on a 2D plot, potential clustering/grouping of patients on the basis of their event status can be observed. For example, in our study, independent processing of the datasets using four pattern recognition techniques resulted in distinct clustering of the “event” group. These data suggest the solute-AHR-TF pathway as a candidate biomarker signature for thrombosis after vascular injury in patients with CKD. However, sufficiently powered prospective studies are warranted to validate the components of the uremic axis before being considered for clinical use.
Study Limitations
Because of limited availability of sera samples, only a subset of the TIMI-II cohort (11%) was analyzed. The TF activity was measured only in vSMC in the TIMI-II study due to inadequate quantity of samples. Both trials allowed analysis of a single sample for the parameters, which are known to fluctuate over time. To fully characterize the hyperthrombotic uremic milieu, future studies are required with access to larger numbers of samples obtained at multiple time points from cohorts with similar outcomes and preferably with longitudinal follow-up data.49 Although the stability of solutes was examined only over a 4-year period (Supplemental Figure 5), the detrimental effect of extended storage especially on TIMI-II samples cannot be ruled out. We did not analyze the free fractions of the solutes, which are considered to be pathogenic.23 This does not represent a major shortcoming, because studies have demonstrated a high degree of correlation of total solutes with the free fractions50 and with overall and cardiovascular mortality in patients at different CKD stages.51 Caution is warranted in interpreting eGFR values in both the studies. The creatinine assays for both the trials were not calibrated using isotope dilution mass spectrometry, an accurate method of creatinine assay standardization recommended for calculating eGFR equations and comparing creatinine values measured in different laboratories or at different times.52
Because thrombosis remains a common and a potentially fatal complication of vascular interventions, its precise risk estimation is imperative. Our study represents an integrative approach focused on probing the disease-specific risk mediators via mechanistic studies at biochemical and computational levels, such as machine learning–based analysis, in human cohorts. Such an approach will allow refinement of the thrombotic risk prediction, with a potential of enhancing the precision in the individualized antithrombotic management of a patient. This work supports further examination of the components of this thrombosis axis to stratify patients at risk for thrombosis. Although this study focused on patients with CKD, exploration of the solute-AHR-TF axis is warranted in other models of thrombosis such as venous thromboembolism and in various disorders characterized by hyperthrombotic phenotype.
Concise Methods
Acquisition of sera samples, assessment of the stability and preanalytic variability of metabolites and assays, cell lines used, and selection of optimum concentrations of sera in different assays are described in the supplemental information, i.e., in the legends to Supplemental Figures 5, 6, and 7, and in the Supplemental Methods.
Pattern Recognition
We leveraged both linear and nonlinear dimensionality reduction techniques to visualize high-dimensional data obtained from metabolomics and activity assays by projecting them onto a two-dimensional map while preserving the separation between “event” and “no-event” subgroups. This type of mapping takes advantage of the inherent structure of the high-dimensional data and attempts to embed the data into a subspace of lower dimensionality (typically 2D or three dimensional (3D) representations), making it convenient for one to visualize possible clustering of the subgroup data. Four different techniques were used—PCA, neighborhood components analysis, t-distributed stochastic neighbor embedding, and locality preserving projections. These techniques are described in more detail elsewhere.24–26
Generation of a Kyn-Specific Thrombosis Model
A group of C57BL/6 mice (Jackson Labs), 12–16-week-old females and males, were administered Kyn dissolved in DMSO and further diluted in PBS at 0.5 mg/kg intraperitoneally once a day, and the excretion was inhibited by probenecid (150 mg/kg administered intraperitoneally twice a day). Probenecid is an inhibitor of organic anion transporter 1 and 3, and suppresses the excretion of Kyn. Serum Kyn levels were measured on days 0 and 5, using LC/MS developed and validated for the measurement of Kyn in serum, after which the animals were subjected to FeCl3-mediated carotid injury, performed as we described previously.33 Briefly, the right carotid artery was exposed in animals under isoflurane anesthesia, and basal blood flow recorded using a 0.5PSB S-series flow probe connected to a TS420 perivascular transit-time flow meter (Transonic, Ithaca, NY). The probe was removed, and a piece of Whatman filter paper (1×10 mm) soaked in FeCl3 (Sigma-Aldrich, St. Louis, MO) was placed under the carotid artery for 1 minute. After washing with warm physiologic saline (0.9% NaCl), the probe was returned, and volume of blood flow was monitored for 20 minutes starting from the placement of the filter paper. Mean, maximum, and minimum carotid flow was recorded using Powerlab Chart5 version 5.3 software, in 1-second intervals. TtO was determined as the first measurement, 0.299 ml/min. Average basal flow volume corresponds to an average of measurements over the 30 seconds preceding placement of the filter paper.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors acknowledge Dr. David Salant (Boston University School of Medicine [BUSM]) for his valuable insights on this project and Dr. Nigel Mackman (University of North Carolina, Chapel Hill) for tissue factor activity assay. We acknowledge Dr. Nader Rahimi (BUSM) for providing Human umbilical vein endothelial cells (HUVEC)-telomerase reverse transcriptase (tert)-tert cell lines and Dr. David Sherr (BUSM) for establishing the aryl hydrocarbon receptor activity assay. We also thank the Metabolomics Core facilities of the Department of Medicine at Boston University School of Medicine.
This work was funded in part by R01HL132325, R01 CA175382, and the Evans Faculty Merit award to V.C.C.; the Hariri Research Award (#2016-10-009) from the Hariri Institute of Computing, Boston University, and the American Heart Association’s Scientist Development Grant (#17SDG33670323) to V.B.K.; National Heart, Lung, and Blood Institute R01 HL080442 to K. Ravid; the Sharon Anderson Research Fellowship grant award from the American Society of Nephrology and T32 training in renal biology T32DK007053-44 to K. Rijal; and T32 training grant in cardiovascular biology T32HL007224-40 to J.W. This work was also funded in part by the Affinity Research Collaborative on the Thrombosis and Hemostasis program from the Department of Medicine at Boston University School of Medicine.
V.B.K., M.E.B., J.M.F., K. Ravid, and V.C.C. conceptualized the idea and designed the study; M.S. and K. Rijal performed the tissue factor activity assay with Thrombolysis in Myocardial Infarction (TIMI)–II and Dialysis Access Consortium (DAC-Fistula) trial samples, respectively; F.A. and S.S. performed the aryl hydrocarbon receptor activity assay of TIMI-II and DAC trials, respectively; C.M.G. and L.M.D. provided insights into the TIMI-II and DAC-fistula studies, respectively, and assisted in data interpretation; V.B.K. and V.C.C. wrote the manuscript; M.E.B., C.M.G., L.M.D., J.M.F., and K. Ravid reviewed, edited, and contributed conceptually to the manuscript’s content; V.B.K. performed the analysis; M.E.B., F.A., M.S., and S.M. performed animal model and thrombosis assays.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017080929/-/DCSupplemental.
References
- 1.Mackman N: Triggers, targets and treatments for thrombosis. Nature 451: 914–918, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ocak G, van Stralen KJ, Rosendaal FR, Verduijn M, Ravani P, Palsson R, et al. : Mortality due to pulmonary embolism, myocardial infarction, and stroke among incident dialysis patients. J Thromb Haemost 10: 2484–2493, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, et al. : Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 16: 489–495, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, et al. : Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293: 2126–2130, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Chillon JM, Massy ZA, Stengel B: Neurological complications in chronic kidney disease patients. Nephrol Dial Transplant 31: 1606–1614, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Garimella PS, Hart PD, O’Hare A, DeLoach S, Herzog CA, Hirsch AT: Peripheral artery disease and CKD: A focus on peripheral artery disease as a critical component of CKD care. Am J Kidney Dis 60: 641–654, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Kimura T, Morimoto T, Kozuma K, Honda Y, Kume T, Aizawa T, et al. ; RESTART Investigators : Comparisons of baseline demographics, clinical presentation, and long-term outcome among patients with early, late, and very late stent thrombosis of sirolimus-eluting stents: Observations from the Registry of Stent Thrombosis for Review and Reevaluation (RESTART). Circulation 122: 52–61, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Casserly LF, Dember LM: Thrombosis in end-stage renal disease. Semin Dial 16: 245–256, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Shashar M, Belghasem ME, Matsuura S, Walker J, Richards S, Alousi F, et al. : Targeting STUB1-tissue factor axis normalizes hyperthrombotic uremic phenotype without increasing bleeding risk. Sci Transl Med 9, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chitalia VC, Shivanna S, Martorell J, Balcells M, Bosch I, Kolandaivelu K, et al. : Uremic serum and solutes increase post-vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor. Circulation 127: 365–376, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shivanna S, Kolandaivelu K, Shashar M, Belghasim M, Al-Rabadi L, Balcells M, et al. : The aryl hydrocarbon receptor is a critical regulator of tissue factor stability and an antithrombotic target in uremia. J Am Soc Nephrol 27: 189–201, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang K, Du C, Wang X, Li F, Xu Y, Wang S, et al. : Indoxyl sulfate induces platelet hyperactivity and contributes to chronic kidney disease-associated thrombosis in mice. Blood 129: 2667–2679, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Gondouin B, Cerini C, Dou L, Sallée M, Duval-Sabatier A, Pletinck A, et al. : Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int 84: 733–744, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, et al. ; Dialysis Access Consortium Study Group : Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: A randomized controlled trial. JAMA 299: 2164–2171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braunwald E: Thrombolysis in myocardial infarction (TIMI) phase II trial. N Engl J Med 321: 612, 1989 [DOI] [PubMed] [Google Scholar]
- 16.Roy-Chaudhury P, Sukhatme VP, Cheung AK: Hemodialysis vascular access dysfunction: A cellular and molecular viewpoint. J Am Soc Nephrol 17: 1112–1127, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Harnek J, Zoucas E, Carlemalm E, Cwikiel W: Differences in endothelial injury after balloon angioplasty, insertion of balloon-expanded stents or release of self-expanding stents: An electron microscopic experimental study. Cardiovasc Intervent Radiol 22: 56–61, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Srikanth S, Ambrose JA: Pathophysiology of coronary thrombus formation and adverse consequences of thrombus during PCI. Curr Cardiol Rev 8: 168–176, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang A, Rijal K, Ng SK, Ravid K, Chitalia V: A mass spectrometric method for quantification of tryptophan-derived uremic solutes in human serum. J Biol Methods 4: 75, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dember LM, Kaufman JS, Beck GJ, Dixon BS, Gassman JJ, Greene T, et al. ; DAC Study Group : Design of the Dialysis Access Consortium (DAC) clopidogrel prevention of early AV fistula thrombosis trial. Clin Trials 2: 413–422, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB; ISPOR-SMDM Modeling Good Research Practices Task Force : Model transparency and validation: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making 32: 733–743, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Pawlak K, Mysliwiec M, Pawlak D: Hypercoagulability is independently associated with kynurenine pathway activation in dialysed uraemic patients. Thromb Haemost 102: 49–55, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Vanholder R, Glorieux G: Introduction: Uremic toxicity - state of the art 2014. Semin Nephrol 34: 85–86, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Tenenbaum JB, de Silva V, Langford JC: A global geometric framework for nonlinear dimensionality reduction. Science 290: 2319–2323, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Roweis ST, Saul LK: Nonlinear dimensionality reduction by locally linear embedding. Science 290: 2323–2326, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Pang R, Lansdell BJ, Fairhall AL: Dimensionality reduction in neuroscience. Curr Biol 26: R656–R660, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinton GE, Salakhutdinov RR: Reducing the dimensionality of data with neural networks. Science 313: 504–507, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Stejskalova L, Dvorak Z, Pavek P: Endogenous and exogenous ligands of aryl hydrocarbon receptor: Current state of art. Curr Drug Metab 12: 198–212, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Kawasaki H, Chang HW, Tseng HC, Hsu SC, Yang SJ, Hung CH, et al. : A tryptophan metabolite, kynurenine, promotes mast cell activation through aryl hydrocarbon receptor. Allergy 69: 445–452, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Zhao B, Degroot DE, Hayashi A, He G, Denison MS: CH223191 is a ligand-selective antagonist of the Ah (Dioxin) receptor. Toxicol Sci 117: 393–403, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uwai Y, Honjo H, Iwamoto K: Interaction and transport of kynurenic acid via human organic anion transporters hOAT1 and hOAT3. Pharmacol Res 65: 254–260, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Saito K, Fujigaki S, Heyes MP, Shibata K, Takemura M, Fujii H, et al. : Mechanism of increases in L-kynurenine and quinolinic acid in renal insufficiency. Am J Physiol Renal Physiol 279: F565–F572, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Matsuura S, Mi R, Koupenova M, Eliades A, Patterson S, Toselli P, et al. : Lysyl oxidase is associated with increased thrombosis and platelet reactivity. Blood 127: 1493–1501, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng S, Larson MG, McCabe EL, Murabito JM, Rhee EP, Ho JE, et al. : Distinct metabolomic signatures are associated with longevity in humans. Nat Commun 6: 6791, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim CK, Bilgin A, Lovejoy DB, Tan V, Bustamante S, Taylor BV, et al. : Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci Rep 7: 41473, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu CC, Hsieh MY, Hung SC, Kuo KL, Tsai TH, Lai CL, et al. : Serum indoxyl sulfate associates with postangioplasty thrombosis of dialysis grafts. J Am Soc Nephrol 27: 1254–1264, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zuo H, Ueland PM, Ulvik A, Eussen SJ, Vollset SE, Nygård O, et al. : Plasma biomarkers of inflammation, the kynurenine pathway, and risks of all-cause, cancer, and cardiovascular disease mortality: The Hordaland Health study. Am J Epidemiol 183: 249–258, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedersen ER, Tuseth N, Eussen SJ, Ueland PM, Strand E, Svingen GF, et al. : Associations of plasma kynurenines with risk of acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol 35: 455–462, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Pawlak K, Tankiewicz J, Mysliwiec M, Pawlak D: Tissue factor/its pathway inhibitor system and kynurenines in chronic kidney disease patients on conservative treatment. Blood Coagul Fibrinolysis 20: 590–594, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Wu D, Nishimura N, Kuo V, Fiehn O, Shahbaz S, Van Winkle L, et al. : Activation of aryl hydrocarbon receptor induces vascular inflammation and promotes atherosclerosis in apolipoprotein E-/- mice. Arterioscler Thromb Vasc Biol 31: 1260–1267, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherr DH: Another important biological function for the aryl hydrocarbon receptor. Arterioscler Thromb Vasc Biol 31: 1247–1248, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Steffel J, Lüscher TF, Tanner FC: Tissue factor in cardiovascular diseases: Molecular mechanisms and clinical implications. Circulation 113: 722–731, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Lippi G, Franchini M: Pathogenesis of venous thromboembolism: When the cup runneth over. Semin Thromb Hemost 34: 747–761, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Kumar G, Sakhuja A, Taneja A, Majumdar T, Patel J, Whittle J, et al. ; Milwaukee Initiative in Critical Care Outcomes Research (MICCOR) Group of Investigators : Pulmonary embolism in patients with CKD and ESRD. Clin J Am Soc Nephrol 7: 1584–1590, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shashar M, Francis J, Chitalia V: Thrombosis in the uremic milieu--emerging role of “thrombolome”. Semin Dial 28: 198–205, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolodziej LR, Paleolog EM, Williams RO: Kynurenine metabolism in health and disease. Amino Acids 41: 1173–1183, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Kennedy PJ, Cryan JF, Dinan TG, Clarke G: Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 112[Pt B]: 399–412, 2017 [DOI] [PubMed] [Google Scholar]
- 48.Schefold JC, Zeden JP, Fotopoulou C, von Haehling S, Pschowski R, Hasper D, et al. : Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: A possible link between chronic inflammation and uraemic symptoms. Nephrol Dial Transplant 24: 1901–1908, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Cheung AK, Imrey PB, Alpers CE, Robbin ML, Radeva M, Larive B, et al. ; Hemodialysis Fistula Maturation Study Group : Intimal hyperplasia, stenosis, and arteriovenous fistula maturation failure in the hemodialysis fistula maturation study. J Am Soc Nephrol 28: 3005–3013, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liabeuf S, Drüeke TB, Massy ZA: Protein-bound uremic toxins: New insight from clinical studies. Toxins (Basel) 3: 911–919, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, et al. ; European Uremic Toxin Work Group (EUTox) : Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4: 1551–1558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller WG, Myers GL, Ashwood ER, Killeen AA, Wang E, Thienpont LM, et al. : Creatinine measurement: State of the art in accuracy and interlaboratory harmonization. Arch Pathol Lab Med 129: 297–304, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.