Abstract
FSGS describes a renal histologic lesion with diverse causes and pathogenicities that are linked by podocyte injury and depletion. Subclasses of FSGS include primary, genetic, and secondary forms, the latter comprising maladaptive, viral, and drug-induced FSGS. Despite sharing certain clinical and histologic features, these subclasses differ noticeably in management and prognosis. Without an accepted nongenetic biomarker that discriminates among these FSGS types, classification of patients is often challenging. This review summarizes the clinical and histologic features, including the onset and severity of proteinuria as well as the presence of nephrotic syndrome, that may aid in identifying the specific FSGS subtype. The FSGS lesion is characterized by segmental sclerosis and must be differentiated from nonspecific focal global glomerulosclerosis. No light microscopic features are pathognomonic for a particular FSGS subcategory. The characteristics of podocyte foot process effacement on electron microscopy, while helpful in discriminating between primary and maladaptive FSGS, may be of little utility in detecting genetic forms of FSGS. When FSGS cannot be classified by clinicopathologic assessment, genetic analysis should be offered. Next generation DNA sequencing enables cost-effective screening of multiple genes simultaneously, but determining the pathogenicity of a detected genetic variant may be challenging. A more systematic evaluation of patients, as suggested herein, will likely improve therapeutic outcomes and the design of future trials in FSGS.
Keywords: FSGS, Primary, Secondary, genetic renal disease, nephrotic syndrome
The lesion of FSGS represents a segmental increase in glomerular matrix with obliteration of the capillary lumina in at least one glomerulus in the entire kidney biopsy.1 This histologic lesion is caused by diverse etiologies and pathogenic mechanisms (Table 1), sharing the initiating and defining feature of podocyte alterations and depletion (podocytopathy). The lesion of FSGS can be broadly subdivided into primary (“idiopathic”), genetic, and secondary forms.
Table 1.
Causes of FSGS
| Primary | Circulating podocyte-toxic factor |
| Secondary: Maladaptive | Reduced number of functioning nephrons (e.g., unilateral renal agenesis, renal dysplasia, oligomeganephronia, glycogen storage disease, low birth weight) |
| Abnormal stress on an initially normal nephron population (e.g., morbid obesity, surgical reduction of renal mass [usually >75%], reflux nephropathy, high-protein diet, sickle cell disease, any advanced kidney disease with substantial loss of nephrons) | |
| Other causes: sleep apnea, cyanotic congenital heart disease, renal artery stenosis, malignant hypertension, cholesterol emboli | |
| Secondary: Viral | HIV (established), CMV (probably), parvovirus B19 (possibly), EBV (possibly), HCV (possibly), hemophagocytic syndrome (possibly) |
| Secondary: Drug induced | Direct-acting antiviral therapy (ledipasvir, sofosbuvir), mTOR inhibitors, calcineurin inhibitors, anthracyclines, heroin(adulterants), lithium, IFN, anabolic steroids |
| Genetic | Renal limited (Table 2) |
| Syndromic (Table 3) | |
| Unknown |
CMV, cytomegalovirus; EBV, Epstein-Barr virus; HCV, Hepatitis C virus; mTOR, mammalian target of rapamycin. IFN, interferon
Primary FSGS is presumably caused by a circulating factor, possibly a cytokine elaborated from extrarenal sources, which causes generalized injury to podocytes.2 Primary FSGS may respond to corticosteroids, immunomodulatory agents, plasmapheresis, or immunoadsorption3 and is prone to recur post-transplantation. Maladaptive forms of secondary FSGS ensue from a reduction in the number of functioning nephrons or from a normal nephron population subjected to abnormal stress, and should primarily be treated with renin-angiotensin-aldosteron system inhibition.4 Other forms of secondary FSGS include virus-associated FSGS5 and drug-induced FSGS,6 which typically improve on resolution of the infection or cessation of the drug. The genetic causes of FSGS may present as sporadic or familial disease, with autosomal dominant, autosomal recessive, X-linked, or mitochondrial (matrilineal) inheritance patterns. The age of onset of genetic FSGS is usually early childhood, but as additional mutations associated with FSGS are identified, adult-onset genetic FSGS assumes increasing significance. Genetic FSGS may be either limited to the kidney (Table 2) or part of a broader syndrome with extrarenal involvement (Table 3). Genetic FSGS is typically resistant to corticosteroids. Calcineurin inhibitors may be effective in few patients,7 possibly reflecting direct stabilization of the podocyte actin cytoskeleton rather than an immunosuppressive effect.8 A number of susceptibility genes may confer an increased risk of FSGS, the latter only overtly manifested after the imposition of genetic or environmental “second hits.”9 The best known of these are the G1 and G2 polymorphisms in the apo L1 (APOL1) gene in patients of African ancestry, which impart a greatly increased risk of adult-onset FSGS; these polymorphisms also impose a higher risk of other kidney diseases, in particular hypertensive nephropathy and HIV-associated nephropathy.10,11 The presence of one or two high-risk APOL1 alleles is associated with more rapid disease progression and worse prognosis, but preliminary data suggest that the APOL1 high-risk alleles do not influence the sensitivity to corticosteroids or immunosuppressive treatment.11,12 Many patients with FSGS cannot be readily classified, because an underlying cause or genetic mutation is not identified (the “unknown” forms of FSGS). At least some of these are likely genetic in origin.
Table 2.
Genetic causes of FSGS: Renal-limited FSGS
| Gene Locus Inheritance | Protein | Protein Function | Phenotype | Response to Therapy |
|---|---|---|---|---|
| Slit diaphragm–associated proteins | ||||
| NPHS1 | Nephrin | Essential component of the slit diaphragm | Finnish type congenital nephrotic syndrome, sporadic childhood FSGS, rarely adult-onset FSGS | Resistant to immunosuppression, reported patients with response to immunosuppression in heterozygous mutations or variants |
| 19q13.1 | ||||
| AR | ||||
| NPHS2 | Podocin | Transmembrane protein involved in recruitment of nephrin to the slit diaphragm | Familial or sporadic FSGS or DMS in early childhood–, adolescence- or adult-onset FSGS in particular in compound heterozygotes for one pathogenic NPHS2 mutation and p.R229Q polymorphism | Resistant to immunosuppression, reported patients with response to immunosuppression in heterozygous mutations or variants |
| 1q25.2 | ||||
| AR | ||||
| CD2AP | CD2-associated protein | Scaffolding molecule between slit diaphragm and actin cytoskeleton | Childhood-onset FSGS | Resistant to immunosuppression |
| 6p12 | ||||
| AD, rarely AR | ||||
| PLCE1 | Phospholipase Cε1 | Signaling protein, interacts with nephrin | Isolated DMS, sporadic and familial early childhood–onset FSGS | Reported patients with (partial) response to immunosuppression |
| 10q23.33 | ||||
| AR | ||||
| TRPC6 | Transient receptor potential cation channel 6 | Receptor-activated calcium channel localized at the foot process membrane, interacts with nephrin and podocin | Familial or sporadic adult-onset FSGS, childhood-onset FSGS has also been described | Reported patients with (partial) response to cyclosporin |
| 11q22.1 | ||||
| AD | ||||
| MAGI2 | Membrane-Associated Guanylate Kinase, WW, and PDZ domain–containing 2 | Scaffolding molecule between slit diaphragm and actin cytoskeleton | Familial and sporadic congenital nephrotic syndrome | Resistant to immunosuppression |
| 7q11.23-q21.11 | ||||
| AR | ||||
| Actin cytoskeleton and regulation | ||||
| ACTN4 | α–Actinin-4 | Member of the spectrin gene superfamily, cytoskeletal protein | Familial or sporadic adult-onset FSGS | Resistant to immunosuppression |
| 19q13 | ||||
| AD | ||||
| MYO1E | Nonmuscle myosin 1e | Involved in intracellular movement and membrane trafficking | Familial childhood-onset FSGS | Reported patients with (partial) response to cyclosporin |
| 15q22.2 | ||||
| AR | ||||
| ANLN | Anillin | F-actin binding protein, involved in slit diaphragm-cytoskeleton binding | Familial adult-onset FSGS | Resistant to immunosuppression |
| 7p15-p14 | ||||
| AD | ||||
| ARHGDIA | Rho GDP dissociation inhibitor α | Regulation of podocyte migratory phenotype and shape | Congenital or early childhood-onset nephrotic syndrome | Resistant to immunosuppression, may respond to RAC1 inhibitors (eplerenone) |
| 17q25.3 | ||||
| AR | ||||
| ARHGAP24 | RhoGTPase activating protein 24 | Regulation of podocyte migratory phenotype and shape | Adolescence-onset FSGS | Resistant to immunosuppression |
| 4q22.1 | ||||
| AD | ||||
| TTC21B | Tetratricopeptide repeat domain 21B | Intraflagellar transport-A component, regulation cytoskeleton adult podocytes | Adolescence- or adult-onset FSGS associated with atrophic tubules | Resistant to immunosuppression |
| 2q24.3 | ||||
| AR | ||||
| KANK 2 | Kidney ankyrin repeat-containing protein 2 | Regulation Rho GTPase activity in podocytes (cell migration and shape) | Familial early-onset SRNS | Resistant to immunosuppression |
| 19p13.2 | ||||
| AR | ||||
| Nuclear pore complex proteins | ||||
| NUP93 | Nucleoporine 93 kD | Component of the nuclear pore complex | Familial childhood-onset SRNS | Resistant to immunosuppression |
| 16q13 | ||||
| AR | ||||
| NUP205 | Nucleoporine 205 kD | Component of the nuclear pore complex | Familial childhood-onset SRNS | Resistant to immunosuppression |
| 7q33 | ||||
| AR | ||||
| XPO5 | Exportin 5 | Component of the nuclear pore complex | Familial childhood-onset SRNS | Resistant to immunosuppression |
| 6p21.1 | ||||
| AR | ||||
| NUP107 | Nucleoporine 107 kD | Component of the nuclear pore complex | Childhood-onset FSGS | Resistant to immunosuppression |
| 12q15 | ||||
| AR | ||||
| Cell membrane–associated proteins | ||||
| PTPRO | Protein tyrosine phosphatase, receptor type O | Member of the R3 subtype family of protein tyrosine phosphatases at the apical surface of polarized cells | Childhood-onset FSGS | Resistant to immunosuppression, reported patients with partial response to immunosuppression |
| 12p13-p12 | ||||
| AR | ||||
| EMP2 | Epithelial membrane protein 2 | Regulation of the amount of CAVEOLIN-1, EMP2 depletion causes decreased cell proliferation | Childhood-onset FSGS | Reported patients with response to steroids |
| 16p13.2 | ||||
| AR | ||||
| PODXL | Podocalyxin | Component of glycocalyx | Familial childhood- and adult-onset FSGS | Resistant to immunosuppression |
| 7q32.3 | ||||
| AD | ||||
| GBM protein | ||||
| LAMA5 | Laminin α-5 | Member of the α-subfamily of laminin chains, major component of basement membranes | Adult-onset FSGS | Likely resistant to immunosuppression |
| 20q13.2-q13.3 | ||||
| AD (?) | ||||
AR, autosomal recessive; DMS, diffuse mesangial sclerosis; AD, autosomal dominant; SRNS, steroid-resistant nephrotic syndrome.
Table 3.
Genetic causes of FSGS: Syndromic FSGS
| Gene, Locus, Inheritance | Protein | Protein Function | Syndromic Association | Response to Therapy | |
|---|---|---|---|---|---|
| MYH9 | Myosin heavy chain 9 | Protein with several important functions, including cytokinesis, cell motility, and maintenance of cell shape | Epstein–Fechtner syndrome (FSGS, sensorineural deafness, cataracts, macrothrombocytopenia, leukocyte inclusions) | Resistant to immunosuppression | |
| 22q12.3 | |||||
| AD | |||||
| KANK 1 | Kidney ankyrin repeat-containing protein 1 | Regulation Rho GTPase activity in podocytes (cell migration and shape) | Familial early-onset SRNS and intellectual disability | Resistant to immunosuppression | |
| 9p24.3 | |||||
| AR | |||||
| KANK 4 | Kidney ankyrin repeat-containing protein 4 | Regulation Rho GTPase activity in podocytes (cell migration and shape) | Familial early-onset FSGS, intellectual disability, facial dysmorphism, and atrial septal defect | Resistant to immunosuppression | |
| 1p31.3 | |||||
| AR | |||||
| ITGA3 | Integrin-α3 | Component of integrin-α3 β1 involved in podocyte-GBM interaction | NEP syndrome (early-onset FSGS, epidermolysis bullosa, and interstitial lung disease) | Resistant to immunosuppression | |
| 17q21.33 | |||||
| AR | |||||
| ITGB4 | Integrin-β4 | Component of integrin-α6 β4 involved in podocyte-GBM interaction | Early-onset FSGS, epidermolysis bullosa, and pyloric atresia | Resistant to immunosuppression | |
| 17q11 | |||||
| AR | |||||
| LAMB2 | Laminin-β2 | Extracellular matrix glycoprotein implicated in cell adhesion, differentiation, migration, signaling | Pierson Syndrome (microcoria, neuromuscular junction defects, early-onset DMS or FSGS), isolated congenital or childhood-onset SRNS | Resistant to immunosuppression | |
| 3p21 | |||||
| AR | |||||
| LMX1B | LIM homeobox transcription factor 1β | Nuclear transcription factor | Sporadic FSGS, Nail–Patella syndrome (hypoplastic or absent patella, dysplasia of elbows, dystrophic nails, frequently glaucoma) | Resistant to immunosuppression | |
| 9q34 | |||||
| AD | |||||
| PAX2 | Paired box 2 | Nuclear transcription factor | Renal coloboma syndrome (childhood-onset FSGS, optic nerve colobomas, and renal hypoplasia), isolated adult-onset FSGS | Resistant to immunosuppression | |
| 10q24 | |||||
| AD | |||||
| WT1 | Wilms tumor 1 | Nuclear transcription factor | Frasier syndrome (FSGS, gonadoblastoma, male pseudohermaphroditism), Denys–Drash syndrome (DMS, Wilms’ tumor, male pseudohermaphroditism), isolated congenital or childhood-onset DMS or FSGS | Reported patients with (partial) response to cyclosporin | |
| 11p13 | |||||
| AD | |||||
| NUP107 | Nucleoporine 107 kD | Component of the nuclear pore complex | Childhood-onset FSGS and microcephaly | Resistant to immunosuppression | |
| 12q15 | |||||
| AR | |||||
| SMARCAL1 | SMARCA-like protein | Protein with helicase and ATPase activities | Schimke immuno-osseous dysplasia (immunodeficiency, skeletal dysplasia, childhood-onset FSGS) | Resistant to immunosuppression | |
| 2q34–36 | |||||
| AR | |||||
| NXF5 | Nuclear RNA export factor 5 | Member of family of nuclear RNA export factor genes | Adult-onset FSGS and cardiac conduction disorders | Resistant to immunosuppression | |
| Xq22 | |||||
| X-linked | |||||
| COQ2 | Coenzyme Q2 4-hydroxybenzoate polyprenyl transferase | Enzyme involved in the biosynthesis of CoQ10 | Childhood-onset collapsing FSGS, encephalopathy | May respond to coenzyme Q10 | |
| 4q21.23 | |||||
| AR | |||||
| COQ6 | Coenzyme Q6 monooxygenase | Enzyme involved in the biosynthesis of CoQ10 | Early childhood-onset FSGS/DMS, sensorineural deafness | May respond to coenzyme Q10 | |
| 14q24.3 | |||||
| AR | |||||
| PDSS2 | Prenyl diphosphate synthase subunit 2 | Enzyme involved in the biosynthesis of CoQ10 | Congenital SRNS and encephalomyopathy, Leigh syndrome | May respond to coenzyme Q10 | |
| 6q21 | |||||
| AR | |||||
| ADCK4 | aarF domain containing kinase 4 | Enzyme involved in the biosynthesis of CoQ10 | Childhood and early adult–onset FSGS (neurologic manifestations in 20% of patients) | May respond to coenzyme Q10 | |
| 19q13.2 | |||||
| AR | |||||
| MTTL1 | Mitochondrially encoded tRNA leucine 1 | Mitochondrial tRNA | MELAS syndrome (mitochondrial encephalomyopathy, lactic acidosis, stroke-like episodes), isolated adult-onset FSGS | Resistant to immunosuppression | |
| mtDNA | |||||
| Maternal | |||||
| MTTL2 | Mitochondrially encoded tRNA leucine 2 | Mitochondrial tRNA | MELAS syndrome (mitochondrial encephalomyopathy, lactic acidosis, stroke-like episodes), isolated adult-onset FSGS | Resistant to immunosuppression | |
| mtDNA | |||||
| Maternal | |||||
| MTTY | Mitochondrially encoded tRNA tyrosine | Mitochondrial tRNA | MELAS syndrome (mitochondrial encephalomyopathy, lactic acidosis, stroke-like episodes), isolated adult-onset FSGS | Resistant to immunosuppression | |
| mtDNA | |||||
| Maternal | |||||
| EYA1 | Eyes absent homolog 1 | Transcriptional activator involved in kidney, branchial arches, eye and ear development | Adult-onset FSGS and Branchio-Oto-Renal syndrome (abnormalities in branchial, ear and renal development) | Resistant to immunosuppression | |
| 8q13.3 | |||||
| AD | |||||
| LMNA | Lamin A/C | Component of the inner nuclear membrane | Familial partial lipodystrophy with adult-onset FSGS | Resistant to immunosuppression | |
| 1q21.2-q21.3 | |||||
| AD | |||||
| SCARB2 | Scavenger receptor class B member 2 | Type 3 glycoprotein located primarily in limiting membranes of lysosomes and endosomes | Action myoclonus-renal failure syndrome (ataxia, myoclonus, collapsing FSGS) | Resistant to immunosuppression | |
| 4q13–21 | |||||
| AR | |||||
| COL4A3 | α3 type 4 collagen | Subunit of type 4 collagen (main component of GBM) | Alport syndrome, familial/sporadic FSGS | Resistant to immunosuppression | |
| 2q36–37 | |||||
| AR | |||||
| COL4A4 | α4 type 4 collagen | Subunit of type 4 collagen (main component of GBM) | Alport syndrome, familial/sporadic FSGS | Resistant to immunosuppression | |
| 2q35–37 | |||||
| AR | |||||
| COL4A5 | α5 type 4 collagen | Subunit of type 4 collagen (main component of GBM) | Alport syndrome, familial/sporadic FSGS | Resistant to immunosuppression | |
| Xq22 | |||||
| X-linked | |||||
| CUBN | Cubilin | Receptor for intrinsic factor-vitamin B12 complexes, role in reabsorption of albumin at renal tubular compartment | Childhood-onset SRNS and megaloblastic anemia, rarely associated with urinary tract malformation (Imerslund–Gräsbeck syndrome) | May respond to vitamin B12 | |
| 10p12.31 | |||||
| AR | |||||
| WDR73 | WD repeat domain 73 | Scaffold protein for the assembly of protein complexes | Childhood-onset SRNS and Galloway–Mowat syndrome (microcephaly and developmental delay) | Resistant to immunosuppression | |
| 15q22 | |||||
| AR | |||||
| ALG1 | Asparagine-linked glycosylation 1 | Catalyzes the first mannosylation step in the biosynthesis of lipid-linked oligosaccharides | Congenital nephrotic syndrome in setting of disorder of glycosylation type 1-k | Resistant to immunosuppression | |
| 16p13.3 | |||||
| AR | |||||
| PMM2 | Phosphomannomutase 2 | Synthesis of dolichol-P-oligosaccharides | Childhood-onset SRNS and deficient glycoprotein syndrome type 1 | Resistant to immunosuppression | |
| 16p13.3 | |||||
| AR | |||||
| CD151 | Tetraspanin CD151 | Member of the transmembrane 4 superfamily, cell-surface proteins | Childhood-onset FSGS and bullous skin lesions, sensorineural deafness, β-thalassemia | Resistant to immunosuppression | |
| 11p15.5 | |||||
| AR | |||||
| CRB2 | Crumbs Homolog 2 | Regulation of podocyte cell polarity, differentiation and protein trafficking of slit diaphragm components | Familial childhood-onset FSGS, congenital nephrotic syndrome and cerebral ventriculomegaly | Resistant to immunosuppression | |
| 9q33.4 | |||||
| AR | |||||
| INF2 | Inverted formin 2 | Member of the formin family, function in de- and polymerization of actin filaments | Familial or sporadic adolescence and adult-onset FSGS, association with Charcot–Marie–Tooth disease | Resistant to immunosuppression | |
| 14q32.33 | |||||
| AD | |||||
| SGPL1 | Sphingosine-1-phosphate lyase | Enzyme involved in sphingolipid catabolic pathway | Familial and sporadic congenital nephrotic syndrome or childhood-onset FSGS, association with adrenal insufficiency, ichthyosis, immunodeficiency, and neurologic defects | Resistant to immunosuppression | |
| 10q22 | |||||
| AR | |||||
| ZMPSTE24 | Zinc Metalloproteinase Ste 24 | Enzyme involved in degradation of prelamin A | Young adult–onset sporadic FSGS, associated with mandibuloacral dysplasia | Likely resistant to immunosuppression | |
| 1p34.2 | |||||
| AR | |||||
AD, autosomal dominant; AR, autosomal recessive; SRNS, steroid-resistant nephrotic syndrome; NEP, Nephrotic syndrome, epidermolysis bullosa and pulmonary disease; DMS, diffuse mesangial sclerosis; mtDNA, mitochondrial DNA; tRNA, transfer RNA; MELAS, mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes.
Despite the heterogeneous etiology and pathogenesis of FSGS, the clinical and pathologic presentations may be similar (Figure 1). In the absence of a serum or urine nongenetic biomarker that reliably discriminates primary from secondary and genetic forms, correctly classifying the type of FSGS is often challenging.13 Importantly, a misclassification may lead to inappropriate and potentially harmful therapy. As an example, primary FSGS can occur in a morbidly obese individual, and physicians may be reluctant to prescribe corticosteroids, because the lesion may be considered to be obesity-related FSGS. Conversely, an adult patient with a sporadic and unrecognized genetic form of FSGS and without a family history or clinical evidence of secondary FSGS may be exposed to a long course of steroids and/or calcineurin inhibitors in the belief that the underlying lesion is primary FSGS. This review discusses the clinical and pathologic characteristics of the FSGS lesion and offers clues in differentiating primary, maladaptive, and genetic forms, in particular in adult-onset FSGS. Viral- and drug-associated forms of FSGS are not the focus of this review, because they are often identified by viral serology and exposure context.
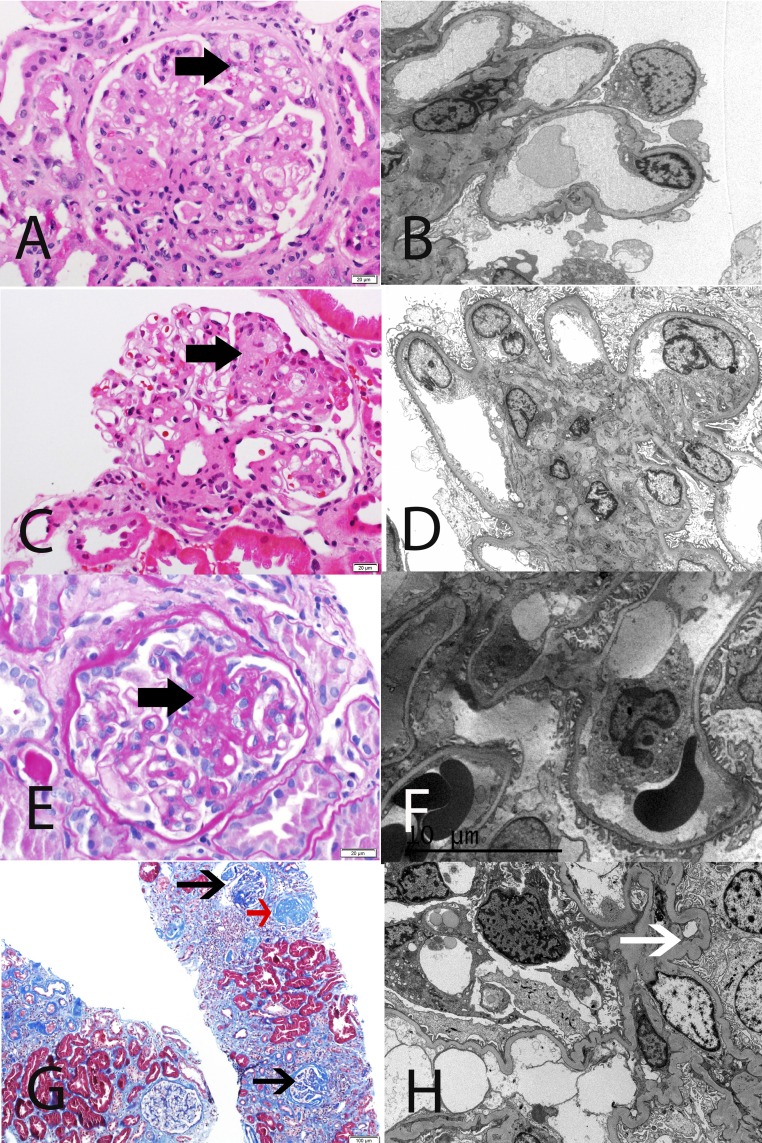
Figure 1.
Representative LM and EM findings in FSGS and FGGS. (A and B) Primary FSGS in a 61-year-old white man with serum creatinine 1.5 mg/dl, proteinuria 14.2 g/24 h, and serum albumin 2.5 mg/dl. LM shows segmental sclerosis, and EM highlights diffuse FPE. (C and D) Maladaptive FSGS in a 53-year-old white man with serum creatinine 1.9 mg/dl, proteinuria 5.8 g/24 h, and serum albumin 4.1 mg/dl. LM shows segmental sclerosis, and EM reveals well preserved foot processes. (E and F) Genetic FSGS in a 39-year-old white man with familial history of PLCE1 mutation, serum creatinine 1.3 mg/dl, proteinuria 2.0 g/24 h, and serum albumin 4 g/dl. LM shows segmental sclerosis, and EM features only minimal FPE. (G and H) FGGS in a 38-year-old black man with a long history of uncontrolled hypertension, serum creatinine 5.1 mg/dl, proteinuria 2.8 g/24 h, and serum albumin 4.3 g/dl. LM shows ischemic changes, as well as a globally sclerosed glomerulus (red arrow), and EM shows relatively well preserved foot processes. Note ischemic capillary loops (white arrow). (A, C, E) Thick black arrow points to segmental sclerosis, and (G) thin black arrow points to ischemic glomeruli. (A and C) Hematoxylin and eosin. (E) Periodic acid–Schiff. (G) Masson trichrome stain. Original magnification, ×40 in A, C, and E; ×2500 in B; ×3000 in D; ×3500 in F; ×10 in G; ×5000 in H.
What Is FSGS?
Glomerulosclerosis
The glomerular visceral epithelial cells (podocytes) are anchored to the underlying glomerular basement membrane (GBM) by their foot processes. This limited attachment to the GBM, along with their continuous exposure to the flow of the primary filtrate, render podocytes prone to detachment and loss in the urine. Podocytes are terminally differentiated, postmitotic cells unable to proliferate to compensate for lost cells. The ability of neighboring podocytes to hypertrophy and cover denuded areas of the GBM is limited. The stress caused by the process of hypertrophy and extending over bare areas of the GBM weakens the attachment to the GBM in the remaining podocytes that, in turn, detach. In this way, podocyte detachment and GBM denudation become self-perpetuating processes. The GBM tends to bulge outward in these bare areas, because the intraglomerular capillary hydrostatic pressure is no longer opposed by the podocytes, and synechial attachments with the parietal epithelial cells and Bowman’s capsule occur. After podocyte loss has reached a critical point, the capillary loop collapses, and extracellular matrix accumulates, thus creating the characteristic segmental obliteration of the glomerular capillary tuft. In an animal model, glomerular growth exceeding the capacity of podocytes to adapt and adequately cover the filtration surface also resulted in a FSGS lesion, without a detectable reduction in average podocyte number per glomerulus.14
Focal
The term “focal” denotes a heterogeneous involvement of the glomerular population in the renal cortex. However, the segmental sclerotic lesion has only a small volume (on average, 12% of the entire glomerular volume)15 and can thus be easily missed on a single section. Serial morphologic analysis in the various forms of FSGS shows that the sclerotic lesions involve the great majority of the glomeruli,15–17 revealing that FSGS is not as focal as the name implies.
Segmental
The segmental pattern (affecting only a portion of the glomerular tuft) characteristic of the lesion of FSGS must be distinguished from nonspecific focal global glomerulosclerosis (FGGS; affecting the entire glomerular tuft) observed in aging and hypertensive nephropathy (Figure 1). FGGS seen in the aging kidney usually occurs without FSGS. The likely driving process for age-related FGGS is arteriosclerosis and glomerular ischemia, resulting in podocyte stress and depletion.18 An alternative and more podocyte-centered viewpoint suggests that progressive podocyte loss with aging is the primary event, resulting in an ever-increasing hypertrophic stress on remaining podocytes.19 Whatever the initiating event, after a critical number of podocytes are lost, catastrophic podocyte detachment leads to glomerular tuft collapse and rapid obsolescence of the entire glomerulus. Global glomerulosclerosis exceeding the threshold expected for a given age20 is indicative of CKD as a consequence of hypertensive damage. This insight may be of particular clinical relevance in patients of African descent with hypertension, in whom FGGS and not FSGS was found to be the dominant lesion on kidney biopsy.21
Not Nonspecific Scarring
FSGS should also be differentiated from focal segmental scarring that develops in immune-mediated GN (e.g., IgA nephropathy, ANCA-associated GN, and lupus nephritis) as a result of postinflammatory scarring of necrotizing or proliferative lesions. In addition to nonspecific scarring, a significant proportion of segmental sclerotic lesions in IgA nephropathy may represent podocyte injury with mechanisms similar to those seen in FSGS.22
The Clinical and Pathologic Hallmarks of FSGS
Segmental versus Diffuse Foot Process Effacement
Podocytes play a cardinal role in glomerular barrier function because of their specialized architecture. Neighboring podocytes form meandering cell-cell contacts through the formation of foot processes that are arranged in an interdigitating pattern. The slit diaphragm between adjacent interdigitating podocyte foot processes consists of a complex membrane-like structure that acts as a sieve and represents the major barrier to protein leakage. Podocytes can be damaged by a spectrum of mechanisms that encompass nonmechanical insults (immunologic, toxic, viral), mechanical stress, and genetic mutations, which compromise specific cellular components. Whatever the type of stress, the podocyte initially responds with loss of the interdigitating foot process pattern, termed foot process effacement (FPE). FPE starts with sealing of the filtration slits between neighboring cells through replacement of the slit diaphragm with occluding junctions. It proceeds with retraction, shortening, and widening of the foot processes, ultimately resulting in a continuous and flattened cytoplasmic sheet covering the GBM. Whether FPE is merely a sign of derangement of a highly organized system or rather, a coordinated process to promote cell survival remains controversial.23 However, substantive evidence supports the latter view, i.e. that FPE may be a protective response, whereby podocytes attempt to secure adhesion to the GBM and thus escape detachment. Indeed, FPE is potentially reversible. When local conditions improve, podocytes resume their original shape, and the functionality of the filtration barrier steadily improves. In contrast, the persistence of stress of whatever cause may overwhelm the capacity of FPE to enable podocyte adherence to the GBM, thereby leading to irreversible podocyte detachment. The type and severity of the stress and the adequacy of the response vary considerably, and this likely underlies the observed variability in extent, distribution, and speed of development of FPE in the different forms of FSGS.
Maladaptive FSGS results from a mismatch between glomerular load and glomerular capacity in conditions associated with hyperfiltration, glomerular capillary hypertension, and glomerular hypertrophy.24 Hyperfiltration and glomerular capillary hypertension represent a major mechanical strain to the podocytes. Podocytes are extremely sensitive to shear stress generated by the increased filtrate flow through the filtration slits and over their apical surface.25,26 Glomerular hypertrophy challenges the podocytes to cover an increased filtration surface. However, the ability of the foot processes to display hypertrophic growth is limited. The podocytes may be unable to maintain a normal foot process pattern, leading to a further increase in local shear stress. When the rheologic stress becomes untenable, the process of FPE is set in motion to redistribute the mechanical forces and decrease the local shear stress. Although glomerular capillary hypertension affects all capillaries to comparable degrees, shear stress is unevenly distributed along the glomerular capillaries, decreasing toward the end of the capillary network.25 FPE as a response to increased fluid shear stress is, therefore, typically a segmental phenomenon, encountered only in the parts of the podocyte that are affected by the rheologic disturbances, whereas the other parts display an intact foot process pattern.25 This crucial new insight explains why FPE develops slowly and has a heterogeneous distribution in maladaptive FSGS (Figure 1). The mean percentage of the glomerular surface area affected by FPE was reported to be 40% in obesity-related FSGS27,28 and 25% in reflux nephropathy.27 In a mixed cohort of patients with secondary FSGS (that also included some patients with genetic FSGS), the median degree of FPE was 30%.29
In primary FSGS, however, a putative circulating factor capable of crossing the GBM barrier causes generalized podocyte dysfunction, ensuing in sudden and widespread FPE (Figure 1). In a series of adult and predominantly white patients with primary FSGS, median FPE was 100%.29 In two older series, the mean degrees of FPE in primary FSGS were 75%28 and 65%, with the greatest degrees in the collapsing (82%) and cellular (87%) forms.27 There was considerable overlap with the degree of FPE observed in secondary FSGS, possibly related to the inclusion of unrecognized genetic FSGS under the primary FSGS designation. A morphometric analysis of foot process width, which excluded patients with heredofamilial forms of FSGS, found broader foot processes in patients with primary FSGS compared with those with secondary FSGS.30 A foot process width >1500 nm adequately differentiated primary from secondary FSGS.30 In patients with primary FSGS who develop recurrent disease in the allograft, FPE was observed within minutes after reperfusion,31 further supporting the concept of sudden podocyte cytoskeletal dysregulation caused by an as yet elusive circulating factor.
The mechanism of virus-associated FSGS, epitomized by HIV-associated nephropathy, involves direct infection of the podocytes, resulting in dysregulation of the cellular phenotype and apoptosis.32–34 In accordance, diffuse FPE (referring to the entire population of glomeruli) (mean, 89%) was reported in patients with HIV-associated nephropathy,27 although in another cohort, only 57% of patients had FPE covering >80% of the glomerular capillary surface.35 Direct podocyte toxicity resulting in dysregulation of the cytoskeleton has also been described in drug-induced FSGS. As an example, biopsies of patients with collapsing FSGS caused by high-dose pamidronate featured extensive FPE (mean, 84%; range, 60%–100%) associated with loss of expression of the cytoskeletal protein synaptopodin.36
Genetic FSGS results from a mutation in genes encoding vital podocyte proteins, including those involved in slit diaphragm structure and function, actin cytoskeleton, or cell signaling apparatus (Tables 2 and 3). No systematic evaluation of FPE in genetic FSGS has yet been performed, but from a pathophysiologic point of view, a broad variability can be expected. FPE is initiated by the intrinsic dysfunction of the podocyte rather than by a response to extrinsic injury. For example, in genetic FSGS caused by NPHS1 mutations, the essential slit diaphragm component nephrin is absent, resulting in diffuse FPE with severe congenital nephrotic syndrome.37 In contrast with these immediate pathophysiologic effects attendant on the loss of an indispensable slit diaphragm protein, other genetic mutations or polymorphisms may merely render the podocyte more vulnerable to stress, and thus require modifier genes or additional metabolic, hypertensive, or other stress to develop the fully expressed phenotype.9 The plausibility of this scenario was supported by findings in patients with mutations in ACTN4, the gene encoding the actin binding protein α–actinin-4.38 The mutant protein may cause a cytoskeletal disorganization that decreases the resistance of the podocyte to mechanical stress, explaining the phenotype of adult-onset FSGS with biopsy characteristics suggestive of a maladaptive FSGS.38 The importance of environmental, genetic, or epigenetic modifiers is further illustrated by the development of distinct phenotypes in family members affected by the same mutation39 (Figure 2).
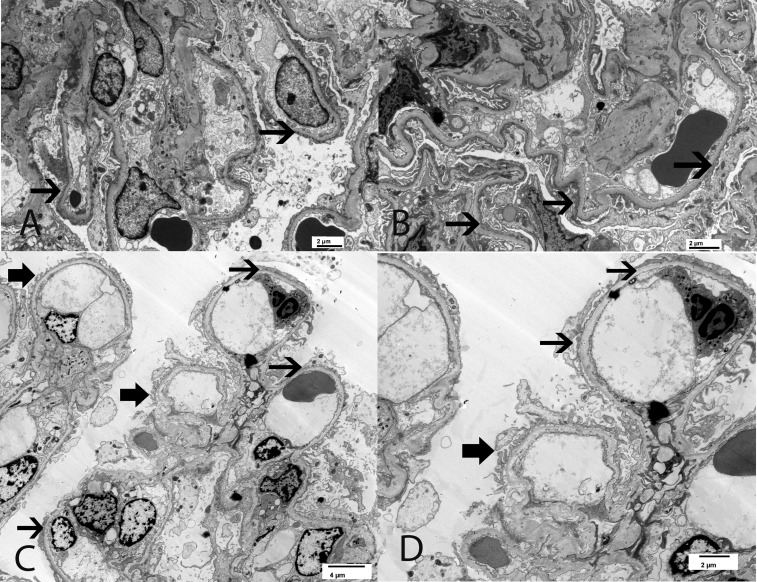
Figure 2.
EM findings in two siblings who are both compound heterozygotes for the NPHS2 mutation and the R286fs pathogenic variant along with a non-neutral polymorphism R229Q. (A and B) The 63-year-old brother with serum creatinine 1.1 mg/dl, proteinuria 6.6 g/24 h, and serum albumin 3.4 g/dl. EM shows diffuse FPE. (C and D) The 47-year-old sister with serum creatinine 0.9 mg/dl, proteinuria 2.5 g/24 h, and serum albumin 4 g/dl. EM shows segmental FPE. Thick black arrow points to preserved foot processes, and thin black arrow points to effaced foot processes. Original magnification, ×5000 in A and B; ×2900 in C; ×4800 in D.
Nephrotic Syndrome, Nephrotic-Range Proteinuria, and Subnephrotic Proteinuria
Proteinuria is the cardinal presenting clinical feature of FSGS. The distinction between nephrotic syndrome (defined as urinary protein excretion ≥3.5 g/24 h, serum albumin concentration of ≤3.5 g/dl, often but not necessarily accompanied by hyperlipidemia, lipiduria, and/or edema), nephrotic-range proteinuria (defined as urinary protein excretion ≥3.5 g/24 h in the absence of low serum albumin), and subnephrotic proteinuria (defined as urinary protein excretion >0.2 and <3.5 g/24 h)40 is helpful in the differential diagnostic evaluation of patients with an FSGS lesion.
Patients with primary FSGS typically present with abrupt-onset marked proteinuria (sometimes as much as 20 g/24 h or greater) and severe nephrotic syndrome. The prevalence of nephrotic syndrome in primary FSGS has been reported to vary from 54%28 to 58%41 to 70%42 to 90%.43 Such variability may be due to the inclusion of unrecognized sporadic genetic FSGS, because primary FSGS has historically been defined as FSGS with absence of conditions typically associated with secondary FSGS. However, when primary FSGS was defined as FSGS with absence of conditions typically associated with secondary FSGS and with presence of diffuse FPE, the prevalence of nephrotic syndrome was 100%.29
More consistently, patients with well-defined forms of maladaptive FSGS (such as obesity, vesicoureteral reflux, and renal mass reduction) present with subnephrotic-range to nephrotic-range proteinuria and rarely, if ever, develop nephrotic syndrome,28,29,43–45 despite often marked proteinuria well above 3.5 g/24 h.
Most patients with childhood-onset genetic FSGS have autosomal recessive mutations that almost always convey full penetrance and present with or progress to severe nephrotic syndrome.46 However, adult-onset genetic FSGS is generally inherited as autosomal dominant disease with variable penetrance, and it exhibits proteinuria of usually <5 g/24 h and more slowly progressive CKD. It should be noted, however, that there is a dearth of detailed information on the clinical presentation of adult-onset genetic FSGS, and it is often unclear whether patients have nephrotic-range proteinuria or nephrotic syndrome.47
Proposal for a Clinicopathologic Approach to a Lesion of FSGS
Clinical Evaluation
Detailed documentation of the clinical phenotype remains of unassailable importance, even when a kidney biopsy is the next diagnostic step. Medical, medication and family history, body mass index, birth weight, and viral serology should be documented. Clinical evidence of a syndromic presentation should also be sought (e.g., hearing loss, skin or eye abnormalities, cardiac dysfunction or anatomic disturbances, hepatosplenomegaly, etc.). Measurement of serum albumin concentration and quantitation of urinary proteins are the required first steps in patient stratification (Table 4). It is also important to determine the identity of the urinary proteins. Initially, a urinary protein-to-creatinine ratio can be compared with a urinary albumin-to-creatinine ratio. If <50% of total proteinuria is due to albumin, then the possibility of tubular proteinuria or presence of light chains should be considered.47,48
Table 4.
Differential diagnostic characteristics of primary FSGS, genetic FSGS, maladaptive FSGS, and FGGS
| Primary FSGS | Genetic FSGS | Maladaptive FSGS | FGGS | |
|---|---|---|---|---|
| Clinical | NS | NS common in childhood, less common in adults | Nephrotic- or subnephrotic-range proteinuria without NS | Variable proteinuria, usually subnephrotic |
| LM | FSGS | FSGS | FSGS | FGGS |
| Often no other damage (unless late in disease course) | FGGS common in adult-onset, uncommon in juvenile forms | Often perihilar | No FSGS | |
| Glomerulomegaly uncommon | Other signs of scarring | No glomerulomegaly | ||
| FGGS in many glomeruli | Ischemic glomerulia | |||
| Glomerulomegaly common | Associated with tubulointerstitial fibrosis, vascular sclerosis | |||
| EM | Diffuse FPE (>80%) | Variable (diffuse or segmental) FPE, characteristic features in some mutations | Segmental FPE | Minimal or no FPE in unaffected glomeruli |
NS, nephrotic syndrome.
Characterized by thickening and wrinkling of the GBM and distention of the Bowman space.
Pathologic Evaluation
Light Microscopy
The Columbia classification subdivides the lesion of FSGS irrespective of underlying etiology or pathogenesis by its appearance on LM into collapsing, tip lesion, cellular, perihilar lesion, and not otherwise specified variants.1,49 A detailed description of the histologic characteristics and prognostic implications of the different variants is beyond the scope of this review. Not otherwise specified is the most common variant, and it is equally distributed among primary and secondary forms.29,50 Perihilar lesions are more usual in maladaptive FSGS, although they can also occur in primary FSGS29,50,51 and genetic FSGS.38 Tip lesion, cellular, and collapsing variants usually share the presenting features of heavy proteinuria and the nephrotic syndrome, but they may also present with subnephrotic-range proteinuria. Tip lesions tend to occur more frequently in white patients, are more likely to respond to therapy, and have overall the best prognosis.49 Conversely, the collapsing variant affects predominantly patients from African heritage and is particularly malignant in its course. The clinical course and morphologic characteristics of the collapsing variant are different from the other FSGS variants, such that some believe that it is a different entity altogether. The collapsing pattern of injury occurs in patients with primary FSGS, but it is also the characteristic lesion seen in HIV-associated nephropathy, parvo B19 virus infection, and pamidronate toxicity. Individuals of African ancestry with high-risk APOL1 alleles are prone to this FSGS variant.
Glomerulomegaly is very common in FSGS caused by obesity, reflux nephropathy, or surgical- or disease-related reductions in nephron mass or in low-birth weight individuals,27,28,43,44,50 but it is also observed in 10%–30% of patients with presumed primary FSGS.28,50 A reliable determination of glomerulomegaly requires the measurement of maximal glomerular diameter on multiple sections in approximately 50 glomeruli. It is, therefore, neither a dependable nor a practical parameter to discriminate between primary and maladaptive FSGS.
Because primary FSGS presents with an abrupt onset of nephrotic syndrome, kidney biopsy is often done early in the course and generally shows a relatively well preserved parenchyma with few glomeruli featuring the characteristic FSGS lesion. In contrast, maladaptive FSGS often presents with progressive proteinuria, and the kidney biopsy is done later in the course. As such, varying degrees of parenchymal scarring are often associated with maladaptive FSGS. Advanced primary FSGS and maladaptive FSGS are indistinguishable on LM.
Taken together, none of the LM features are pathognomonic for a particular type of FSGS. Thus, the etiopathogenesis of FSGS cannot be reliably determined by LM alone.
Immunofluorescence Microscopy
Granular deposition of IgM and C3 with a distribution of the segmental sclerosis is frequently detected in FSGS and thought to represent nonspecific macromolecular trapping rather than specific deposition. More recently, it has been suggested that the exposure of glomerular neoepitopes by nonimmune injury may lead to secondary IgM deposition and complement activation.52 Whatever the underlying molecular mechanism, the staining for IgM and C3 is clearly a secondary phenomenon and does not help to differentiate between the various forms of FSGS. The main purpose of immunofluorescence studies in FSGS is to rule out nonspecific segmental sclerosis as a consequence of postinflammatory scarring in immune-mediated GN, such as in IgA nephropathy.
Electron Microscopy
The major ultrastructural finding on electron microscopy (EM) is FPE. The speed of development and extent of FPE are determined by the underlying mechanism of podocyte injury as detailed above. As a consequence, the FPE characteristics can be used to help discriminate between primary and secondary forms of FSGS (Table 4). Directly overlying the lesions of segmental sclerosis, there usually is complete FPE. It is, therefore, essential to select at least two relatively intact glomeruli to evaluate the degree of foot process integrity. Diffuse FPE is not necessarily a defining signature of primary FSGS, but it eliminates maladaptive mechanisms as the cause of the FSGS lesion. Features such as podocyte vacuolization and microvillous transformation are related to the degree of proteinuria, and as such, they are more likely to be present in primary FSGS than in secondary FSGS; however, they are nonspecific. A genetic form of FSGS must be considered when either diffuse or segmental FPE is observed.
Some mutations have distinctive features on EM that are of diagnostic relevance. Mutations in ACTN4, encoding for α–actinin-4, are characterized by segmental FPE and irregularly aggregated electron-dense material in the podocyte cytoplasm, most likely composed of actin and mutant α–actinin-4.38 Mutations in INF2, encoding a member of the formin family of actin-regulating proteins, feature segmental FPE and preserved foot processes that focally appear irregular and jagged, often with unusually prominent longitudinal actin bundles.53 EM can also help to uncover FSGS as a manifestation of the expanding spectrum of collagen 4 nephropathies by revealing typical (alternating thinning and thickening with lamellation of the lamina densa) or more subtle (abnormal uniform thinning) GBM abnormalities.54,55 EM can also identify other rare but important causes of secondary FSGS, such as Fabry disease and lecithin acyl cholesterol transferase deficiency.
Quality and Timing of the Biopsy
The probability of finding a segmental lesion critically depends on the number of glomeruli evaluated. In addition, the FSGS lesions preferentially affect the juxtamedullary cortex in the early stages of disease. Adequate sampling is thus essential in disclosing the lesion of FSGS and to avoid misdiagnosis as minimal change disease. Ideally, 12–15 serial sections each containing a minimum of eight glomeruli15 should be evaluated.
The timing of the biopsy with respect to the disease course is also important. The initial changes (podocyte FPE) are only detectable by EM, because the characteristic sclerotic lesion takes time to develop.56 It is, therefore, not uncommon that FSGS lesions are absent on the initial biopsy, and a diagnosis of minimal change disease is made, whereas a later biopsy reveals the true nature of the disease by showing clear FSGS lesions. This was typically illustrated by serial biopsy data in kidney transplant recipients with documented FSGS in the native kidneys.51 Biopsy at the moment of proteinuria recurrence showed only minimal change disease (widespread FPE), whereas subsequent biopsies revealed FSGS lesions, except in those patients who achieved complete remission under treatment regimens for recurrence.51 As FSGS progresses, more widespread and global glomerulosclerosis develops, and segmental and global sclerotic lesions can be concomitantly observed.1 A correction for the degree of FGGS anticipated from normal aging alone must always be applied.20 The timing of the renal biopsy also needs to be correlated with the use of immunosuppressive therapy, because a patient with primary FSGS recently treated with immunosuppression may be going into remission at the time of biopsy. In this scenario, EM examination will likely show segmental FPE as opposed to widespread FPE seen in untreated patients with primary FSGS.
Genetic Testing: Why, How, Whom, and When?
The vast majority of patients with monogenic forms of FSGS do not respond to corticosteroids (Tables 2 and 3) and have a very low risk of recurrence in the allograft. Establishing a genetic cause of disease thus avoids exposure to regimens used to treat primary FSGS and to prevent post-transplantation recurrence. Conversely, mutations with a potentially favorable response to cyclosporin, coenzyme Q10, or vitamin B12 may be identified. Genetic testing allows genetic counseling, preimplantation diagnosis and selection of living donors to ensure that they are not asymptomatic carriers. Finally, patients presenting with apparent secondary FSGS in the absence of defining disease characteristics may be unmasked as having atypical presentations of collagen 4 nephropathies, nephronophthisis,54,55 or even Fabry disease.
The likelihood of identifying a monogenic cause of FSGS or steroid-resistant nephrotic syndrome correlates inversely with the age of disease onset. Pathogenic mutations are identified in 60%–100% during the first year of life, 40%–60% of young children, 25%–40% of older children, and 10%–25% of adolescents.46,57–60 Not surprisingly, the yield is higher in patients with familial compared with sporadic cases and in those with syndromic compared with renal-limited disease cases. In patients with adult-onset FSGS, a genetic cause was established in only 8%–14%.54,57,61
On the basis of these observations, genetic screening has been recommended in early-onset disease resistant to immunosuppressive therapy, syndromic, and familial disease, but has not been recommended in sporadic adolescence or adult-onset FSGS that does not have clinical evidence of a genetic syndrome.57 However, this restrictive approach may no longer be valid in the face of a continuously expanding library of genes implicated in the development of FSGS (currently >50) and rapid advances in DNA sequencing technology. Conventional Sanger sequencing allows screening of only the few most frequently mutated genes according to a stepwise algorithm on the basis of age of onset, mode of inheritance, and clinicopathologic presentation, at a high cost and long processing time.46,57,59,61 However, next generation high-throughput sequencing enables the rapid analysis of vast amounts of DNA at a fraction of the cost. Whole-exome sequencing scans the entire subset of DNA that encodes proteins, known as the exome (constituting about 1% of the genome), in search of protein-altering mutations.62,63 An intermediate approach is targeted next generation sequencing of a large panel of genes known to be involved in FSGS.54,58,60,64,65 With the broad implementation of this technology, the proportion of genetic causes of FSGS is expected to grow substantially (Figure 3). As an example, the yield of mutations in a cohort of patients with adult-onset FSGS increased to 20%.54 However, the pathogenicity of some of the mutations is currently not established,54 immediately revealing the Achilles heel of this technology. The pathogenicity of a novel mutation or sequence variant can be predicted by computer models that consider the structure of the protein, but validation ultimately requires difficult and time-consuming functional assays of the gene and gene products.66 Some variants may not be directly pathogenic, but rather require the interaction with other mutations to render podocytes susceptible to injury,66 adding another layer of complexity to the interpretation of the genetic results.
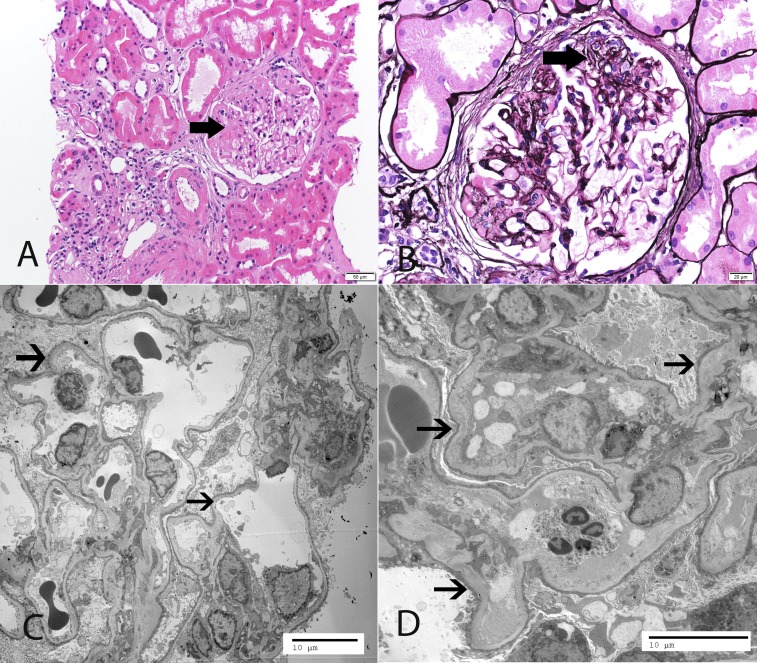
Figure 3.
Representative LM and EM findings. A 46-year-old white woman, in whom the initial biopsy at age 18 years old was reported as minimal change disease, presented with serum creatinine 1.5 mg/dl, proteinuria 5.8 g/24 h, and serum albumin 3.4 g/dl. The patient was resistant to corticosteroids and calcineurin inhibitors. Mutation analysis with a next generation sequencing panel showed two mutations in NPHS2 and a heterozygous variation in WDR73, which is known to cause the Galloway–Mowat syndrome. (A and B) LM shows segmental sclerosis (thick arrows point to segmental sclerosis). (C and D) EM reveals diffuse FPE (thin arrows point to effaced foot processes). (A) Hematoxylin and eosin. (B) Silver methenamine stain. Original magnification, ×20 in A; ×40 in B; ×1900 in C; ×2900 in D.
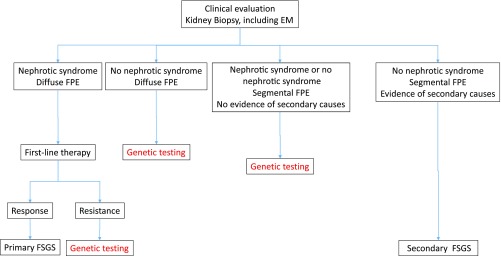
We propose that genetic testing be performed in all patients with adult-onset FSGS that cannot be readily categorized by clinicopathologic assessment (Figure 4). In genetic FSGS, response to immunosuppression is rare, and any such response is generally partial. Therefore, response to first-line corticosteroid treatment can be taken as a prima facie confirmation of the diagnosis of primary FSGS and precludes the need for genetic analysis. In addition, patients with conditions well known to be associated with secondary FSGS and a compatible clinicopathologic presentation should not be offered genetic analysis. Testing should be done by targeted sequencing of the most recent panel of genes associated with genetic podocytopathies (Tables 2 and 3). In patients of African descent, the G1 and G2 APOL1 alleles should be screened.
Figure 4.
Opinion-based approach to genetic testing in adult-onset FSGS. Note that viral- and drug-associated forms of FSGS are usually excluded by clinical and serologic evaluation.
Biomarkers
An in-depth discussion of the candidate biomarkers for primary FSGS is presented elsewhere,67 and it is beyond the scope of this review. To summarize briefly, currently, there are no reliable and clinically useful biomarkers that aid the diagnostic process of classifying FSGS lesions.
CONCLUSION
With the current state of knowledge, empirical treatment of FSGS with corticosteroids or immunosuppressive agents is no longer defensible: such treatment is often ineffective and may impose considerably toxicity. A central consideration in the management of patients with FSGS is the identification of those patients who would likely benefit from such therapies and the delineation of those patients for whom renin-angiotensin-aldosteron system blockade remains the prudent therapeutic approach.
FSGS should be differentiated from FGGS and nonspecific segmental scarring. When a typical FSGS lesion is identified on LM, careful interpretation of clinical and electron microscopic characteristics may point to one of the main subtypes. Patients with primary disease or high-penetrance mutations disruptive to podocyte function present with sudden onset of nephrotic syndrome and with diffuse FPE. Patients with maladaptive FSGS are characterized by slow development of subnephrotic- or nephrotic-range proteinuria without nephrotic syndrome and by segmental FPE. In contrast to these two phenotypes, there is a significant subset of patients lacking clear causative factors and exhibiting variable degrees of proteinuria and FPE. Many of these patients may have an undiagnosed genetic basis of FSGS.68 New sequencing technologies have dramatically increased the sensitivity for disease-associated variants and mutations compared with the traditional Sanger sequencing. However, many of those genetic abnormalities require additional hits, such as other susceptibility genes or disease-related stressors, before FSGS ensues. The emerging picture is that, in the majority of adults, the lesion of FSGS may be the final common pathway of a complex interplay of factors, each contributing only a small amount of the risk. Although the identification of all etiologic factors may be difficult or even impossible at this stage, it should be clear that such patients should not be treated with immunosuppression or specific measures to prevent post-transplant recurrence.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.D’Agati VD, Fogo AB, Bruijn JA, Jennette JC: Pathologic classification of focal segmental glomerulosclerosis: A working proposal. Am J Kidney Dis 43: 368–382, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Kachurina N, Chung CF, Benderoff E, Babayeva S, Bitzan M, Goodyer P, Kitzler T, Matar D, Cybulsky AV, Alachkar N, Torban E: Novel unbiased assay for circulating podocyte-toxic factors associated with recurrent focal segmental glomerulosclerosis. Am J Physiol Renal Physiol 310: F1148–F1156, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Allard L, Kwon T, Krid S, Bacchetta J, Garnier A, Novo R, Deschenes G, Salomon R, Roussey G, Allain-Launay E: Treatment by immunoadsorption for recurrent focal segmental glomerulosclerosis after paediatric kidney transplantation: A multicentre French cohort [published online ahead of print July 28, 2017]. Nephrol Dial Transplant 10.1093/ndt/gfx214 [DOI] [PubMed] [Google Scholar]
- 4.D’Agati VD: Podocyte growing pains in adaptive FSGS. J Am Soc Nephrol 28: 2825–2827, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandra P, Kopp JB: Viruses and collapsing glomerulopathy: A brief critical review. Clin Kidney J 6: 1–5, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izzedine H, Launay-Vacher V, Bourry E, Brocheriou I, Karie S, Deray G: Drug-induced glomerulopathies. Expert Opin Drug Saf 5: 95–106, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Büscher AK, Beck BB, Melk A, Hoefele J, Kranz B, Bamborschke D, Baig S, Lange-Sperandio B, Jungraithmayr T, Weber LT, Kemper MJ, Tönshoff B, Hoyer PF, Konrad M, Weber S; German Pediatric Nephrology Association (GPN) : Rapid response to Cyclosporin a and favorable renal outcome in nongenetic versus genetic steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol 11: 245–253, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P: The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu H, Artomov M, Brähler S, Stander MC, Shamsan G, Sampson MG, White JM, Kretzler M, Miner JH, Jain S, Winkler CA, Mitra RD, Kopp JB, Daly MJ, Shaw AS: A role for genetic susceptibility in sporadic focal segmental glomerulosclerosis. J Clin Invest 126: 1067–1078, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampson MG, Robertson CC, Martini S, Mariani LH, Lemley KV, Gillies CE, Otto EA, Kopp JB, Randolph A, Vega-Warner V, Eichinger F, Nair V, Gipson DS, Cattran DC, Johnstone DB, O’Toole JF, Bagnasco SM, Song PX, Barisoni L, Troost JP, Kretzler M, Sedor JR; Nephrotic Syndrome Study Network : Integrative genomics identifies novel associations with APOL1 risk genotypes in black NEPTUNE subjects. J Am Soc Nephrol 27: 814–823, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopp JB, Winkler CA, Zhao X, Radeva MK, Gassman JJ, D’Agati VD, Nast CC, Wei C, Reiser J, Guay-Woodford LM, Pollak MR, Hildebrandt F, Moxey-Mims M, Gipson DS, Trachtman H, Friedman AL, Kaskel FJ; FSGS-CT Study Consortium : Clinical features and histology of apolipoprotein L1-associated nephropathy in the FSGS clinical trial. J Am Soc Nephrol 26: 1443–1448, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayek SS, Koh KH, Grams ME, Wei C, Ko YA, Li J, Samelko B, Lee H, Dande RR, Lee HW, Hahm E, Peev V, Tracy M, Tardi NJ, Gupta V, Altintas MM, Garborcauskas G, Stojanovic N, Winkler CA, Lipkowitz MS, Tin A, Inker LA, Levey AS, Zeier M, Freedman BI, Kopp JB, Skorecki K, Coresh J, Quyyumi AA, Sever S, Reiser J: A tripartite complex of suPAR, APOL1 risk variants and αvβ3 integrin on podocytes mediates chronic kidney disease. Nat Med 23: 945–953, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zand L, Glassock RJ, De Vriese AS, Sethi S, Fervenza FC: What are we missing in the clinical trials of focal segmental glomerulosclerosis? Nephrol Dial Transplant 32[Suppl 1]: i14–i21, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Nishizono R, Kikuchi M, Wang SQ, Chowdhury M, Nair V, Hartman J, Fukuda A, Wickman L, Hodgin JB, Bitzer M, Naik A, Wiggins J, Kretzler M, Wiggins RC: FSGS as an adaptive response to growth-induced podocyte stress. J Am Soc Nephrol 28: 2931–2945, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuiano G, Comi N, Magri P, Sepe V, Balletta MM, Esposito C, Uccello F, Dal Canton A, Conte G: Serial morphometric analysis of sclerotic lesions in primary “focal” segmental glomerulosclerosis. J Am Soc Nephrol 7: 49–55, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Fogo A, Glick AD, Horn SL, Horn RG: Is focal segmental glomerulosclerosis really focal? Distribution of lesions in adults and children. Kidney Int 47: 1690–1696, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Remuzzi A, Mazerska M, Gephardt GN, Novick AC, Brenner BM, Remuzzi G: Three-dimensional analysis of glomerular morphology in patients with subtotal nephrectomy. Kidney Int 48: 155–162, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Hommos MS, Glassock RJ, Rule AD: Structural and functional changes in human kidneys with healthy aging. J Am Soc Nephrol 28: 2838–2844, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodgin JB, Bitzer M, Wickman L, Afshinnia F, Wang SQ, O’Connor C, Yang Y, Meadowbrooke C, Chowdhury M, Kikuchi M, Wiggins JE, Wiggins RC: Glomerular aging and focal global glomerulosclerosis: A podometric perspective. J Am Soc Nephrol 26: 3162–3178, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kremers WK, Denic A, Lieske JC, Alexander MP, Kaushik V, Elsherbiny HE, Chakkera HA, Poggio ED, Rule AD: Distinguishing age-related from disease-related glomerulosclerosis on kidney biopsy: The Aging Kidney Anatomy study. Nephrol Dial Transplant 30: 2034–2039, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fogo A, Breyer JA, Smith MC, Cleveland WH, Agodoa L, Kirk KA, Glassock R: Accuracy of the diagnosis of hypertensive nephrosclerosis in African Americans: A report from the African American Study of Kidney Disease (AASK) Trial. AASK Pilot Study Investigators. Kidney Int 51: 244–252, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Bellur SS, Lepeytre F, Vorobyeva O, Troyanov S, Cook HT, Roberts IS; International IgA Nephropathy Working Group : Evidence from the Oxford Classification cohort supports the clinical value of subclassification of focal segmental glomerulosclerosis in IgA nephropathy. Kidney Int 91: 235–243, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV: The podocyte’s response to stress: The enigma of foot process effacement. Am J Physiol Renal Physiol 304: F333–F347, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Kriz W, Lemley KV: A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J Am Soc Nephrol 26: 258–269, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kriz W, Lemley KV: Potential relevance of shear stress for slit diaphragm and podocyte function. Kidney Int 91: 1283–1286, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Friedrich C, Endlich N, Kriz W, Endlich K: Podocytes are sensitive to fluid shear stress in vitro. Am J Physiol Renal Physiol 291: F856–F865, 2006 [DOI] [PubMed] [Google Scholar]
- 27.D’Agati V: The many masks of focal segmental glomerulosclerosis. Kidney Int 46: 1223–1241, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD: Obesity-related glomerulopathy: An emerging epidemic. Kidney Int 59: 1498–1509, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Hommos MS, De Vriese AS, Alexander MP, Sethi S, Vaughan L, Zand L, Bharucha K, Lepori N, Rule AD, Fervenza FC: The incidence of primary vs secondary focal segmental glomerulosclerosis: A Clinicopathologic Study. Mayo Clin Proc 92: 1772–1781, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deegens JK, Dijkman HB, Borm GF, Steenbergen EJ, van den Berg JG, Weening JJ, Wetzels JF: Podocyte foot process effacement as a diagnostic tool in focal segmental glomerulosclerosis. Kidney Int 74: 1568–1576, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Chang JW, Pardo V, Sageshima J, Chen L, Tsai HL, Reiser J, Wei C, Ciancio G, Burke GW 3rd, Fornoni A: Podocyte foot process effacement in postreperfusion allograft biopsies correlates with early recurrence of proteinuria in focal segmental glomerulosclerosis. Transplantation 93: 1238–1244, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marras D, Bruggeman LA, Gao F, Tanji N, Mansukhani MM, Cara A, Ross MD, Gusella GL, Benson G, D’Agati VD, Hahn BH, Klotman ME, Klotman PE: Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nat Med 8: 522–526, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Winston JA, Bruggeman LA, Ross MD, Jacobson J, Ross L, D’Agati VD, Klotman PE, Klotman ME: Nephropathy and establishment of a renal reservoir of HIV type 1 during primary infection. N Engl J Med 344: 1979–1984, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Barisoni L, Kriz W, Mundel P, D’Agati V: The dysregulated podocyte phenotype: A novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 10: 51–61, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Laurinavicius A, Hurwitz S, Rennke HG: Collapsing glomerulopathy in HIV and non-HIV patients: A clinicopathological and follow-up study. Kidney Int 56: 2203–2213, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Markowitz GS, Appel GB, Fine PL, Fenves AZ, Loon NR, Jagannath S, Kuhn JA, Dratch AD, D’Agati VD: Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol 12: 1164–1172, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Ranganathan S: Pathology of podocytopathies causing nephrotic syndrome in children. Front Pediatr 4: 32, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henderson JM, Alexander MP, Pollak MR: Patients with ACTN4 mutations demonstrate distinctive features of glomerular injury. J Am Soc Nephrol 20: 961–968, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi HJ, Lee BH, Cho HY, Moon KC, Ha IS, Nagata M, Choi Y, Cheong HI: Familial focal segmental glomerulosclerosis associated with an ACTN4 mutation and paternal germline mosaicism. Am J Kidney Dis 51: 834–838, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Glassock RJ, Fervenza FC, Hebert L, Cameron JS: Nephrotic syndrome redux. Nephrol Dial Transplant 30: 12–17, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Chun MJ, Korbet SM, Schwartz MM, Lewis EJ: Focal segmental glomerulosclerosis in nephrotic adults: Presentation, prognosis, and response to therapy of the histologic variants. J Am Soc Nephrol 15: 2169–2177, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Thomas DB, Franceschini N, Hogan SL, Ten Holder S, Jennette CE, Falk RJ, Jennette JC: Clinical and pathologic characteristics of focal segmental glomerulosclerosis pathologic variants. Kidney Int 69: 920–926, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Praga M, Morales E, Herrero JC, Pérez Campos A, Domínguez-Gil B, Alegre R, Vara J, Martínez MA: Absence of hypoalbuminemia despite massive proteinuria in focal segmental glomerulosclerosis secondary to hyperfiltration. Am J Kidney Dis 33: 52–58, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Praga M, Hernández E, Morales E, Campos AP, Valero MA, Martínez MA, León M: Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Transplant 16: 1790–1798, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Praga M, Borstein B, Andres A, Arenas J, Oliet A, Montoyo C, Ruilope LM, Rodicio JL: Nephrotic proteinuria without hypoalbuminemia: Clinical characteristics and response to angiotensin-converting enzyme inhibition. Am J Kidney Dis 17: 330–338, 1991 [DOI] [PubMed] [Google Scholar]
- 46.Trautmann A, Bodria M, Ozaltin F, Gheisari A, Melk A, Azocar M, Anarat A, Caliskan S, Emma F, Gellermann J, Oh J, Baskin E, Ksiazek J, Remuzzi G, Erdogan O, Akman S, Dusek J, Davitaia T, Özkaya O, Papachristou F, Firszt-Adamczyk A, Urasinski T, Testa S, Krmar RT, Hyla-Klekot L, Pasini A, Özcakar ZB, Sallay P, Cakar N, Galanti M, Terzic J, Aoun B, Caldas Afonso A, Szymanik-Grzelak H, Lipska BS, Schnaidt S, Schaefer F; PodoNet Consortium : Spectrum of steroid-resistant and congenital nephrotic syndrome in children: The PodoNet registry cohort. Clin J Am Soc Nephrol 10: 592–600, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fervenza FC: A patient with nephrotic-range proteinuria and focal global glomerulosclerosis. Clin J Am Soc Nephrol 8: 1979–1987, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sethi S, Glassock RJ, Fervenza FC: Focal segmental glomerulosclerosis: Towards a better understanding for the practicing nephrologist. Nephrol Dial Transplant 30: 375–384, 2015 [DOI] [PubMed] [Google Scholar]
- 49.D’Agati VD, Alster JM, Jennette JC, Thomas DB, Pullman J, Savino DA, Cohen AH, Gipson DS, Gassman JJ, Radeva MK, Moxey-Mims MM, Friedman AL, Kaskel FJ, Trachtman H, Alpers CE, Fogo AB, Greene TH, Nast CC: Association of histologic variants in FSGS clinical trial with presenting features and outcomes. Clin J Am Soc Nephrol 8: 399–406, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sethi S, Zand L, Nasr SH, Glassock RJ, Fervenza FC: Focal and segmental glomerulosclerosis: Clinical and kidney biopsy correlations. Clin Kidney J 7: 531–537, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canaud G, Dion D, Zuber J, Gubler MC, Sberro R, Thervet E, Snanoudj R, Charbit M, Salomon R, Martinez F, Legendre C, Noel LH, Niaudet P: Recurrence of nephrotic syndrome after transplantation in a mixed population of children and adults: Course of glomerular lesions and value of the Columbia classification of histological variants of focal and segmental glomerulosclerosis (FSGS). Nephrol Dial Transplant 25: 1321–1328, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Strassheim D, Renner B, Panzer S, Fuquay R, Kulik L, Ljubanović D, Holers VM, Thurman JM: IgM contributes to glomerular injury in FSGS. J Am Soc Nephrol 24: 393–406, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown EJ, Schlöndorff JS, Becker DJ, Tsukaguchi H, Tonna SJ, Uscinski AL, Higgs HN, Henderson JM, Pollak MR: Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet 42: 72–76, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gast C, Pengelly RJ, Lyon M, Bunyan DJ, Seaby EG, Graham N, Venkat-Raman G, Ennis S: Collagen (COL4A) mutations are the most frequent mutations underlying adult focal segmental glomerulosclerosis. Nephrol Dial Transplant 31: 961–970, 2016 [DOI] [PubMed] [Google Scholar]
- 55.Deltas C, Savva I, Voskarides K, Papazachariou L, Pierides A: Carriers of autosomal recessive Alport syndrome with thin basement membrane nephropathy presenting as focal segmental glomerulosclerosis in later life. Nephron 130: 271–280, 2015 [DOI] [PubMed] [Google Scholar]
- 56.Howie AJ, Pankhurst T, Sarioglu S, Turhan N, Adu D: Evolution of nephrotic-associated focal segmental glomerulosclerosis and relation to the glomerular tip lesion. Kidney Int 67: 987–1001, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Santín S, Bullich G, Tazón-Vega B, García-Maset R, Giménez I, Silva I, Ruíz P, Ballarín J, Torra R, Ars E: Clinical utility of genetic testing in children and adults with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol 6: 1139–1148, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCarthy HJ, Bierzynska A, Wherlock M, Ognjanovic M, Kerecuk L, Hegde S, Feather S, Gilbert RD, Krischock L, Jones C, Sinha MD, Webb NJ, Christian M, Williams MM, Marks S, Koziell A, Welsh GI, Saleem MA; RADAR the UK SRNS Study Group : Simultaneous sequencing of 24 genes associated with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol 8: 637–648, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lipska BS, Iatropoulos P, Maranta R, Caridi G, Ozaltin F, Anarat A, Balat A, Gellermann J, Trautmann A, Erdogan O, Saeed B, Emre S, Bogdanovic R, Azocar M, Balasz-Chmielewska I, Benetti E, Caliskan S, Mir S, Melk A, Ertan P, Baskin E, Jardim H, Davitaia T, Wasilewska A, Drozdz D, Szczepanska M, Jankauskiene A, Higuita LM, Ardissino G, Ozkaya O, Kuzma-Mroczkowska E, Soylemezoglu O, Ranchin B, Medynska A, Tkaczyk M, Peco-Antic A, Akil I, Jarmolinski T, Firszt-Adamczyk A, Dusek J, Simonetti GD, Gok F, Gheissari A, Emma F, Krmar RT, Fischbach M, Printza N, Simkova E, Mele C, Ghiggeri GM, Schaefer F; PodoNet Consortium : Genetic screening in adolescents with steroid-resistant nephrotic syndrome. Kidney Int 84: 206–213, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Sadowski CE, Lovric S, Ashraf S, Pabst WL, Gee HY, Kohl S, Engelmann S, Vega-Warner V, Fang H, Halbritter J, Somers MJ, Tan W, Shril S, Fessi I, Lifton RP, Bockenhauer D, El-Desoky S, Kari JA, Zenker M, Kemper MJ, Mueller D, Fathy HM, Soliman NA, Hildebrandt F; SRNS Study Group : A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol 26: 1279–1289, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Büscher AK, Konrad M, Nagel M, Witzke O, Kribben A, Hoyer PF, Weber S: Mutations in podocyte genes are a rare cause of primary FSGS associated with ESRD in adult patients. Clin Nephrol 78: 47–53, 2012 [DOI] [PubMed] [Google Scholar]
- 62.Ogino D, Hashimoto T, Hattori M, Sugawara N, Akioka Y, Tamiya G, Makino S, Toyota K, Mitsui T, Hayasaka K: Analysis of the genes responsible for steroid-resistant nephrotic syndrome and/or focal segmental glomerulosclerosis in Japanese patients by whole-exome sequencing analysis. J Hum Genet 61: 137–141, 2016 [DOI] [PubMed] [Google Scholar]
- 63.Bierzynska A, McCarthy HJ, Soderquest K, Sen ES, Colby E, Ding WY, Nabhan MM, Kerecuk L, Hegde S, Hughes D, Marks S, Feather S, Jones C, Webb NJ, Ognjanovic M, Christian M, Gilbert RD, Sinha MD, Lord GM, Simpson M, Koziell AB, Welsh GI, Saleem MA: Genomic and clinical profiling of a national nephrotic syndrome cohort advocates a precision medicine approach to disease management. Kidney Int 91: 937–947, 2017 [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Dang X, He Q, Zhen Y, He X, Yi Z, Zhu K: Mutation spectrum of genes associated with steroid-resistant nephrotic syndrome in Chinese children. Gene 625: 15–20, 2017 [DOI] [PubMed] [Google Scholar]
- 65.Sen ES, Dean P, Yarram-Smith L, Bierzynska A, Woodward G, Buxton C, Dennis G, Welsh GI, Williams M, Saleem MA: Clinical genetic testing using a custom-designed steroid-resistant nephrotic syndrome gene panel: Analysis and recommendations. J Med Genet 54: 795–804, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weber S, Büscher AK, Hagmann H, Liebau MC, Heberle C, Ludwig M, Rath S, Alberer M, Beissert A, Zenker M, Hoyer PF, Konrad M, Klein HG, Hoefele J: Dealing with the incidental finding of secondary variants by the example of SRNS patients undergoing targeted next-generation sequencing. Pediatr Nephrol 31: 73–81, 2016 [DOI] [PubMed] [Google Scholar]
- 67.Wada T, Nangaku M: A circulating permeability factor in focal segmental glomerulosclerosis: The hunt continues. Clin Kidney J 8: 708–715, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lepori N, Zand L, Sethi S, Fernandez-Juarez G, Fervenza FC: Clinical and pathological phenotype of genetic causes of FSGS in adults. Clin Kidney J (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]