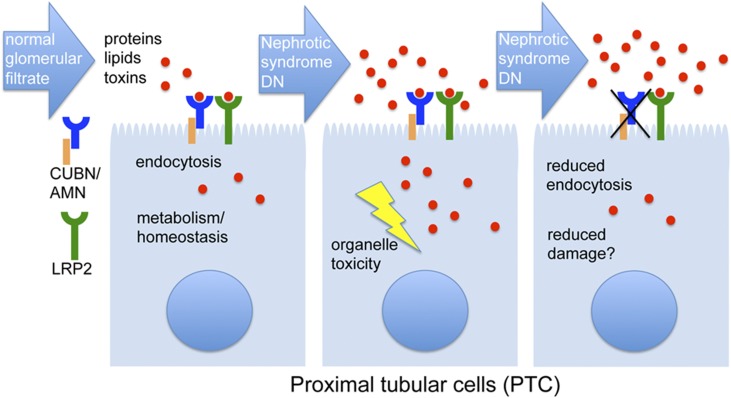
Constituting more than one half of renal mass, the proximal tubule is the main reabsorptive segment of the nephron. Proximal tubular cells (PTCs) take up almost all macromolecules filtered by the neighboring glomerulus, making use of a dense brush border and a dedicated endocytic apparatus. The energy to achieve this tremendous task is provided by a high number of mitochondria that mainly use fatty acids as metabolic fuels. The high energy demand makes PTCs very vulnerable to ischemia, metabolic dysfunction, or external toxins, such as drugs. In nephrotic syndrome or diabetic nephropathy, glomerular proteinuria imposes a large burden on the PTC (Figure 1), with the result that PTC damage becomes a main driver of disease progression.1 In addition to the protein overload, PTCs are also damaged by the molecules brought inside by the proteins, such as fatty acids or advanced glycation end products.
Figure 1.
The role of proximal tubular reabsorption in normal and proteinuric conditions. On the left, normal filtration and reabsorption of proteins, protein-bound lipids and toxins (such as drugs) is depicted. Protein and lipid catabolism in lysosomes and mitochondria is important to meet energy demands of PTC. In glomerular diseases, such as nephrotic syndrome or diabetic nephropathy (DN), PTC are exposed to protein, lipid and toxin overload (middle). Particularly, saturated fatty acids bound to albumin are known to cause cell damage through endoplasmic reticulum stress. Damage may be prevented by reducing PTC uptake, e.g. in patients with CUBN/AMN mutations (right).
As early as in 1982, Moorhead et al.2 proposed the hypothesis that lipid abnormalities contribute to the progression of kidney injury. Summarized under the term “lipotoxicity,” fatty acids, particularly those with saturated acyl chains, were since then repeatedly reported to damage the PTCs and their organelles. The most efficient carrier of fatty acids is albumin, which possesses about seven binding sites for fatty acids. The study of rare genetic diseases and animal models have suggested that albumin not only reaches the PTC when the glomerular filter is leaky but to some extent, also via regular filtration.3,4 This means that, even under normal conditions, the PTCs are “accessible” for toxins delivered by serum proteins. Albumin’s partners in crime are megalin (gene name: LRP2), cubilin (gene name: CUBN), and amnionless (gene name: AMN), which form a receptor complex mediating most of the protein uptake from the luminal side.
With all of this stress brought inside, why is it at all desirable for the PTCs to reabsorb so many proteins? It can first be argued that the proteins need to be recycled. However, it was recently shown that lack of megalin and cubilin does not change serum levels of albumin in nephrotic syndrome, suggesting that there is no significant albumin recycling.5 In how far this is true for other reabsorbed proteins remains to be determined. At least for albumin, it would mean that almost all endocytosed protein enters the lysosomal pathway for degradation. The resulting amino acids may be used for lysosomal mammalian target of rapamycin activation, which in turn, maintains PTC differentiation.6 The fatty acids released from albumin, however, can be used for β-oxidation in mitochondria to generate ATP. The two latter points nicely illustrate the coupling of endocytosis and metabolism, which is characteristic for PTC function.
So, what happens to PTC when the uptake is downregulated? This question is best answered from the human genetic perspective. LRP2, CUBN, and AMN are all genes with mutations that cause low molecular weight proteinuria and albuminuria in humans. LRP2 mutations lead to Donnai–Barrow syndrome (Online Mendelian Inheritance in Man: 222448), a multisystem developmental disorder causing facial dysmorphia, vision, and hearing problems among other symptoms. CUBN and AMN, however, are both causative genes for Imerslund–Gräsbeck syndrome (IGS; Online Mendelian Inheritance in Man: 261100). This disease is a rare autosomal recessive disorder initially described in Scandinavia that is characterized by a selective vitamin B(12) malabsorption, resulting in megaloblastic anemia, which is responsive to parenteral vitamin B(12) therapy (additional reading is in refs. 3 and 4). The phenotypic differences between Donnai–Barrow syndrome and IGS are explained by the fact that megalin can act independently of the other two proteins and has a broader tissue expression pattern.4 By contrast, cubilin and amnionless need each other for surface transport and function, and they are mainly expressed in the kidney and the small intestine.3,7
Genome-wide association studies have linked a variant in the CUBN gene with an increased risk for albuminuria.8 Moreover, Ovunc et al.9 identified two siblings in a consanguineous family with isolated proteinuria caused by a homozygous deletion of one base pair in exon 53 of the CUBN gene. The amount of proteinuria in these patients was variable, ranging from complete absence to 2 g/24 h. Similar patients with CUBN mutations have also been identified in our molecular diagnostic unit (C. Antignac, personal communication). What is important to point out is that neither the patients with IGS nor the patients with isolated proteinuria seem to show signs of renal failure, let alone to progress to ESRD. Although more patient follow-ups are needed, this would suggest that human kidneys continue to function, despite significant urinary protein loss.
With the advent of large reference genome databases (ExAC10 and gnomAD), it has been possible to not only improve the interpretations of specific candidate variants but also, determine allele frequencies in the normal population over the entire coding region of a gene of interest. Here, the following rule of thumb can be applied: the more essential that a gene is for survival, the fewer damaging variants (frameshift, stop gain, or essential splicing) can be found for this gene, even in the heterozygous state. Intolerance to functional variation can be quantified with the probability of being loss-of-function intolerant (pLI) metric.10 Low pLI scores are typically seen for redundant genes, genes that are not required for survival or reproduction, or genes with variants that may confer some selective advantage. Despite being disease genes, CUBN and AMN (but not LRP2) have very low pLI scores,10 suggesting that damaging variants are tolerated in these two genes in humans.
The mutation c.208–2A>G in intron 3 of AMN deserves particular attention. This mutation accounts for over 50% of the patients with IGS outside Scandinavia. It is found among diverse ethnicities and cultures, even Muslim and Jewish families. Studying the flanking sequences, it was possible to backdate the founder event to around 11,600 BC in the northern Mesopotamia region.11 There is only one human mutation that is thought to be older, and this is the Delta F508 CFTR mutation.11 This mutation is the most common cause of cystic fibrosis, and it is primarily found in European and European-derived populations.
With mutations as old as the pre-Neolithic era, one is, of course, invited to ponder over any selective advantages for the heterozygous carriers. Applying Darwinian logic to the origin of a human genetic disease that persists, we have to consider both genetic adaptations to an ancient environment and the more recent benefits of these disease alleles, despite their negative effects. The high frequency of the Delta F508 CFTR mutation has been hypothesized to arise from selective pressures generated by cholera outbreaks or the transmission of diarrhea-causing agents from domesticated animals, such as bovine, to humans.12 Also, the lactase polymorphism arose >5000 years ago as a consequence of domestication and spread rapidly due to strong positive selection.13 Although the CFTR mutation may limit fluid secretion in diarrhea,14 the lactase polymorphism is suggested to allow for lifelong lactose digestion.13
What might be the selective advantage of the AMN founder mutation? Recently, the suppression of PTC uptake by targeting LRP2 has been shown to be protective against kidney damage caused by nephrotoxic therapeutics, hemo- and myoglobin, and high-fat diet.4,15 Interestingly, in most mouse models, LRP2 inactivation was either incomplete or mosaic, implying that, also for humans, the renoprotective effect may be achieved by the loss of only one allele. Moreover, the CUBN gene has been shown to be expressed in a monoallelic fashion in the mouse kidney, which could be another evolutionary trick to maximize the effect in CUBN heterozygotes.7
Proving this hypothesis will not only require more vigorous phenotyping in animal models with CUBN or AMN deficiency but also, thinking about nephrotoxic agents present in prehistoric times. One thing, however, is possibly already safe to conclude from these evolutionary lessons: identifying drugs that downregulate PTC receptor complex activity might be a good strategy for renal diseases with PTC damage.
Disclosures
None.
Acknowledgments
I apologize for not having been able to cite all of the work relevant to this article due to space limitations. I am grateful to Corinne Antignac, Albert de la Chappelle, Gerd Walz, and Qais Al-Awqati for critical reading of the manuscript.
Funding in the laboratory of M.S. is supported by Fondation Bettencourt-Schüller, Cystinosis Research Foundation, ATIP-Avenir, and l’Agence Nationale de la Recherche NEPHROFLY grants.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Zoja C, Abbate M, Remuzzi G: Progression of renal injury toward interstitial inflammation and glomerular sclerosis is dependent on abnormal protein filtration. Nephrol Dial Transplant 30: 706–712, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Moorhead JF, Chan MK, El-Nahas M, Varghese Z: Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet 2: 1309–1311, 1982 [DOI] [PubMed] [Google Scholar]
- 3.Nielsen R, Christensen EI, Birn H: Megalin and cubilin in proximal tubule protein reabsorption: From experimental models to human disease. Kidney Int 89: 58–67, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Willnow TE, Christ A: Endocytic receptor LRP2/megalin-of holoprosencephaly and renal Fanconi syndrome. Pflugers Arch 469: 907–916, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Weyer K, Andersen PK, Schmidt K, Mollet G, Antignac C, Birn H, et al. : Abolishment of proximal tubule albumin endocytosis does not affect plasma albumin during nephrotic syndrome in mice [published online ahead of print October 13, 2017]. Kidney Int [DOI] [PubMed] [Google Scholar]
- 6.Gleixner EM, Canaud G, Hermle T, Guida MC, Kretz O, Helmstädter M, et al. : V-ATPase/mTOR signaling regulates megalin-mediated apical endocytosis. Cell Reports 8: 10–19, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Aseem O, Barth JL, Klatt SC, Smith BT, Argraves WS: Cubilin expression is monoallelic and epigenetically augmented via PPARs. BMC Genomics 14: 405, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Böger CA, Chen MH, Tin A, Olden M, Köttgen A, de Boer IH, et al. ; CKDGen Consortium: CUBN is a gene locus for albuminuria. J Am Soc Nephrol 22: 555–570, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ovunc B, Otto EA, Vega-Warner V, Saisawat P, Ashraf S, Ramaswami G, et al. : Exome sequencing reveals cubilin mutation as a single-gene cause of proteinuria. J Am Soc Nephrol 22: 1815–1820, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. ; Exome Aggregation Consortium: Analysis of protein-coding genetic variation in 60,706 humans. Nature 536: 285–291, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beech CM, Liyanarachchi S, Shah NP, Sturm AC, Sadiq MF, de la Chapelle A, et al. : Ancient founder mutation is responsible for Imerslund-Gräsbeck Syndrome among diverse ethnicities. Orphanet J Rare Dis 6: 74, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alfonso-Sánchez MA, Pérez-Miranda AM, García-Obregón S, Peña JA: An evolutionary approach to the high frequency of the Delta F508 CFTR mutation in European populations. Med Hypotheses 74: 989–992, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Bersaglieri T, Sabeti PC, Patterson N, Vanderploeg T, Schaffner SF, Drake JA, et al. : Genetic signatures of strong recent positive selection at the lactase gene. Am J Hum Genet 74: 1111–1120, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabriel SE, Brigman KN, Koller BH, Boucher RC, Stutts MJ: Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science 266: 107–109, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Kuwahara S, Hosojima M, Kaneko R, Aoki H, Nakano D, Sasagawa T, et al. : Megalin-mediated tubuloglomerular alterations in high-fat diet-induced kidney disease. J Am Soc Nephrol 27: 1996–2008, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]