This issue of the Journal of the American Society of Nephrology contains three interrelated papers from the laboratory of Andrew McMahon, providing a comprehensive analysis of sequential human and mouse kidney development at the macroscopic, microscopic, cellular, and molecular level.1–3 Together, they represent a major contribution to the GenitoUrinary Development Molecular Anatomy Project (GUDMAP), a high-resolution interactive atlas of kidney development conceived in 2003 and funded by the National Institutes of Health.4 Throughout most of the 20th century, developmental nephrology was largely descriptive, and the etiology of congenital anomalies of the kidneys and urinary tract remained unexplained. The impetus for the formation of the GUDMAP consortium was provided by a late 20th century surge in the development of technologic innovations in cell biology, molecular genetics, and cell fate mapping. The rise of evolutionary developmental biology (“evo-devo”) was largely propelled by the discovery of homeobox genes. Many of these advances were on the basis of studies in the laboratory mouse, an organism with a high rate of reproduction, short gestation, genetic homogeneity, and 85% protein-coding homology with the human genome. However, 90 million years of evolution separate mouse and human from a common ancestor (Figure 1). To address this, the investigators of the three articles have meticulously delineated the conserved and divergent features of key stages of kidney development in a large sample of kidneys from both species.1–3 The results reveal deep conservation of nephron patterning between human and mouse, but marked divergence in gene expression patterns and progenitor cell types.
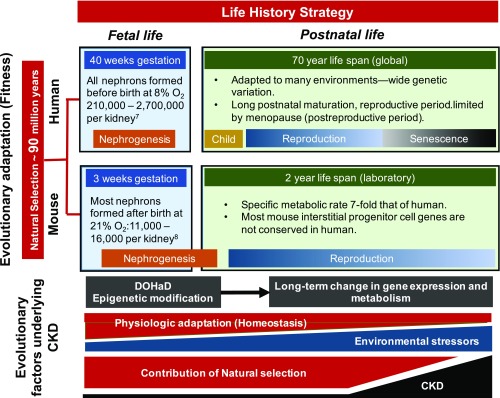
Figure 1.
Evolutionary factors play a central role in determining life history strategy, which spans fetal and postnatal life, and underlies differences between human and mouse kidney development as well as the course of CKD. Evolutionary divergence of mouse and human is estimated at 85-94 million years ago, the result of evolutionary adaptation driven by reproductive fitness (vertical axis). Differences in nephrogenesis between species are the product of natural selection over that interval, and reflect evolutionary adaptation to life history strategies spanning fetal and postnatal periods (horizontal axis). Human nephrogenesis is complete before the end of gestation, taking place in an 8% oxygen environment. Murine nephrogenesis begins in fetal life, but most nephrons are formed after birth in 21% ambient oxygen. In contrast to the mouse, the number of nephrons in the human kidney at birth is highly variable and subject to epigenetic modification of nephrogenesis in response to fetal environmental stimuli (developmental programming). A new discipline, the Developmental Origins of Health and Disease (DOHaD), is defining the role of this process in long-term gene expression, with reduced nephron number at birth now recognized as a risk factor for CKD in adulthood. Adapted to many environments with wide genetic variation, human life history strategy includes prolonged childhood followed by a reproductive and postreproductive period (menopause). In contrast, specific metabolic activity in the mouse is seven-fold that of human, a reflection of its small size (greater surface area/volume ratio) and greater metabolic demands. Mesenchymal progenitor cell types and transcriptional profiles are also markedly divergent in mouse and human nephrogenesis. In contrast to evolutionary adaptation, physiologic adaptation (such as nephron hypertrophy following reduced nephron number) is a short-term response that is less robust with advancing age, during which time accumulating environmental stressors also contribute to increasing CKD. Because natural selection is driven by reproductive fitness, its contribution to human evolution is largely limited in late adulthood (senescence), during which nephron number normally decreases by 50%, accounting for a sharp rise in CKD during this period.
The process of evolutionary adaptation is determined by natural selection, which is governed by reproductive fitness rather than longevity of the individual. As a consequence of evolutionary adaptation, each species develops a life history strategy (Figure 1). Although divergence of Homo sapiens and Pan troglodytes (chimpanzee) spans only 6.6 million years, a big brain, extended childhood, reproductive period truncated by menopause, and extended senescence are unique to humans. Compared with humans, the life span of mice is 35-fold shorter, size is 2500 times smaller, and its specific metabolic activity is seven-fold greater.5 There is high divergence in gene–phenotype relations and alternative splicing between human and mouse orthologs, and copy number variations are common among human and mouse genomes.6 Ontogeny of the kidney also differs significantly: in the human, all nephrons are formed before birth, with a 12-fold variation in nephron number, whereas in the laboratory mouse, most nephrons are formed postnatally, with less than two-fold variation in nephron number.7,8
There is mounting evidence for low nephron endowment as a significant risk factor for CKD.9 This has developed along with the new discipline of Developmental Origins of Health and Disease, which explores developmental programming during fetal and early postnatal life, affecting health throughout the life cycle (Figure 1).10 There is an evolutionary tradeoff between the robustness of developmental programs, such as those outlined in GUDMAP, and developmental plasticity revealed by wide variation in human nephron number at birth. Evolutionary adaptation is constrained by energy availability, which must be allocated to growth, maintenance, or reproduction. In response to environmental cues in early development (such as undernutrition, hypoxia, toxins, or infection), reduction in nephron number improves fitness by reducing energy consumption, thereby favoring survival through reproductive life. Fetal oxygen tension is only 8% compared with the postnatal environment (21%), and growth of in vitro cultured embryonic mouse kidneys is improved when exposed to 8% oxygen, but not in 1% oxygen.11 Exposure of pregnant mice to hypoxia results in kidney phenotypes similar to patients born with congenital anomalies of the kidneys and urinary tract, responses modulated by Hif1α, a transcription factor activated by hypoxia.11 Offspring of pregnant mice subjected to vitamin A deficiency or protein deficiency have reduced nephron number and phenotype similar to FGF7 knockout mice.12 Notably, decreased nephron number in fetuses subjected to these maternal nutrient restrictions is due to increasing variation in late nephron-branching morphogenesis (embryonic days 15.5–19.5).12 Factors underlying these effects remain to be elucidated, but epigenetic as well as genetic mechanisms are likely involved. For example, microRNAs activated by Dicer1 in the stromal compartment of the developing mouse kidney regulates nephrogenesis and ultimate nephron number.13
In contrast to evolutionary adaptation, physiologic adaptation provides a homeostatic response to a proximate stress or insult (Figure 1). In response to AKI, damaged tubular cells are replaced by cellular proliferation.14 However, after repeated ischemic insults, proximal tubular injury becomes irreversible with tubular atrophy and progression to CKD. Nephron hypertrophy following reduced nephron number is a homeostatic response that can be activated even in utero, as observed in cases of renal hypoplasia. As with AKI, if the stimulus is prolonged (progressive nephron loss), the tubule atrophies, the glomerulus becomes sclerotic, and the interstitium becomes hypoxic and fibrotic. In the context of physiologic adaptation, these have been regarded as maladaptive responses. However, either cellular proliferation or hypertrophy increases oxygen consumption, and maximal tubular growth is constrained by limits to tubular fluid flow and reabsorptive capacity. From an evolutionary perspective, by reducing energy allocation to failing nephrons, activation of cell death programs increases fitness by conserving energy for reproduction.15
How do these processes relate to kidney development? A number of studies have suggested that cellular mechanisms underlying these adaptive and maladaptive responses represent reactivation of developmental pathways.14 A likely explanation for such parallels is that the nephron microenvironment produced by injury induces cell signaling pathways that evolved in nephrogenesis. Evolution can be viewed as the unfolding of increasing complexity of cell–cell signaling in the progression from unicellular to multicellular organisms, paralleled by development from zygote to maturity.16 The recognition that cumulative episodes of AKI can lead to CKD, and that CKD is a risk factor for AKI, suggest a role for the proximal tubule as a key player in the progression of kidney disease.17 Packed with mitochondria, and accounting for the bulk of renal mass, this nephron segment is responsible for most of the oxygen consumed by the kidney, and is susceptible to hypoxic or oxidative injury. The glomerulotubular junction is particularly vulnerable to injury, which when prolonged, leads to cell death and the formation of atubular glomeruli.18 This process is activated in the final stages of virtually all causes of CKD, including genetic, obstructive, ischemic, metabolic, immunologic, or toxic etiologies.18 Formation of atubular glomeruli may represent an atavism that originated in a common ancestor with fish that adapted from a freshwater to saltwater environment over 435 million years ago. With increasing age in the sculpin (a marine fish), nephrons undergo progressive degeneration of the glomerulotubular junction, resulting in atubular glomeruli and aglomerular tubules.18 Reversion of a filtering nephron adapted to a freshwater environment to a secretory nephron adapted to a marine environment reduces the large energy required to reclaim filtered solutes; a process that appears maladaptive in the context of CKD, but not in the context of evolution.
The risk for CKD is <1% in children, and increases to 40% for those over 50 years of age, after which nephron number decreases by 50%.15,19,20 In postreproductive years, cumulative environmental stressors increase, whereas the efficiency of homeostatic responses and the contribution of natural selection decrease (Figure 1).15 Fetal nutritional restriction accelerates maturation after catch-up growth,21 whereas by conserving energy in the postreproductive period, calorie restriction in the aging rat increases longevity.22 To contextualize developmental mechanisms, CRISPR/Cas9 technology could be applied to nonmodel organisms to study environmental effects on gene expression.23 Consideration of developmental and evolutionary factors in the genesis and progression of kidney disease over the entire life cycle should lead to new therapeutic approaches. Advances in the prevention and treatment of CKD will depend upon the current and future additions to the GUDMAP database: a Rosetta Stone for nephrology.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related articles, “Conserved and Divergent Features of Human and Mouse Kidney Organogenesis,” “Conserved and Divergent Molecular and Anatomic Features of Human and Mouse Nephron Patterning,” and “Conserved and Divergent Features of Mesenchymal Progenitor Cell Types within the Cortical Nephrogenic Niche of the Human and Mouse Kidney,” on pages 785–805, 825–840, and 806–824, respectively.
References
- 1.Lindström NO, McMahon JA, Guo J, Tran T, Guo Q, Rutledge E, et al. : Conserved and divergent features of human and mouse kidney organogenesis. J Am Soc Nephrol 29: 785–805, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindström NO, Tran T, Guo J, Rutledge E, Parvez RK, Thornton ME, et al. : Conserved and divergent molecular and anatomic features of human and mouse nephron patterning. J Am Soc Nephrol 29: 825–840, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindström NO, Guo J, Kim AD, Tran T, Guo Q, De Sena Brandine G, et al. : Conserved and divergent features of mesenchymal progenitor cell types within the cortical nephrogenic niche of the human and mouse kidney. J Am Soc Nephrol 29: 806–824, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harding SD, Armit C, Armstrong J, Brennan J, Cheng Y, Haggarty B, et al. : The GUDMAP database--an online resource for genitourinary research. Development 138: 2845–2853, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perlman RL: Mouse models of human disease: An evolutionary perspective. Evol Med Public Health 2016: 170–176, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gharib WH, Robinson-Rechavi M: When orthologs diverge between human and mouse. Brief Bioinform 12: 436–441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE: Human nephron number: Implications for health and disease. Pediatr Nephrol 26: 1529–1533, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Zhong J, Perrien DS, Yang HC, Kon V, Fogo AB, Ichikawa I, et al. : Maturational regression of glomeruli determines the nephron population in normal mice. Pediatr Res 72: 241–248, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luyckx VA, Perico N, Somaschini M, Manfellotto D, Valensise H, Cetin I, et al. ; writing group of the Low Birth Weight and Nephron Number Working Group: A developmental approach to the prevention of hypertension and kidney disease: A report from the Low Birth Weight and Nephron Number Working Group. Lancet 390: 424–428, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wadhwa PD, Buss C, Entringer S, Swanson JM: Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med 27: 358–368, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkinson LJ, Neal CS, Singh RR, Sparrow DB, Kurniawan ND, Ju A, et al. : Renal developmental defects resulting from in utero hypoxia are associated with suppression of ureteric β-catenin signaling. Kidney Int 87: 975–983, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Sampogna RV, Schneider L, Al-Awqati Q: Developmental programming of branching morphogenesis in the kidney. J Am Soc Nephrol 26: 2414–2422, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagawa N, Xin C, Roach AM, Naiman N, Shankland SJ, Ligresti G, et al. : Dicer1 activity in the stromal compartment regulates nephron differentiation and vascular patterning during mammalian kidney organogenesis. Kidney Int 87: 1125–1140, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Little MH, Kairath P: Does renal repair recapitulate kidney development? J Am Soc Nephrol 28: 34–46, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chevalier RL: Evolutionary nephrology. Kidney Int Rep 2: 302–317, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torday JS: On the evolution of development. Trends Dev Biol 8: 17–37, 2014 [PMC free article] [PubMed] [Google Scholar]
- 17.Chevalier RL: The proximal tubule is the primary target of injury and progression of kidney disease: Role of the glomerulotubular junction. Am J Physiol Renal Physiol 311: F145–F161, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chevalier RL, Forbes MS: Generation and evolution of atubular glomeruli in the progression of renal disorders. J Am Soc Nephrol 19: 197–206, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Becherucci F, Roperto RM, Materassi M, Romagnani P: Chronic kidney disease in children. Clin Kidney J 9: 583–591, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMahon GM, Hwang SJ, Fox CS: Residual lifetime risk of chronic kidney disease. Nephrol Dial Transplant 32: 1705–1709, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Luyckx VA, Compston CA, Simmen T, Mueller TF: Accelerated senescence in kidneys of low-birth-weight rats after catch-up growth. Am J Physiol Renal Physiol 297: F1697–F1705, 2009 [DOI] [PubMed] [Google Scholar]
- 22.McKiernan SH, Tuen VC, Baldwin K, Wanagat J, Djamali A, Aiken JM: Adult-onset calorie restriction delays the accumulation of mitochondrial enzyme abnormalities in aging rat kidney tubular epithelial cells. Am J Physiol Renal Physiol 292: F1751–F1760, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Bono JM, Olesnicky EC, Matzkin LM: Connecting genotypes, phenotypes and fitness: Harnessing the power of CRISPR/Cas9 genome editing. Mol Ecol 24: 3810–3822, 2015 [DOI] [PubMed] [Google Scholar]