Abstract
Background
Histone acetylation and DNA methylation are important mammalian epigenetic modifications that participate in the regulation of gene expression. Because dysregulation of histone deacetylase and DNA methyltransferases are hallmarks of malignancy, they have become promising therapeutic targets. In this study, we explored the anti-tumor activity of valproic acid (VPA), a histone deacetylase inhibitor (HDACi) and 5-Aza-2′-deoxycytidine (5-Aza), an inhibitor of DNA methyltransferases, on renal cell carcinoma (RCC) cell lines 786-O and 769-P.
Material/Methods
The cell proliferation was detected by xCELLigence RTCA DP Instrument, viability by CCK8 assay, cell apoptosis and cell cycle by flow cytometry, and cell migration by wound healing assay, Transwell assay and xCELLigence RTCA DP Instrument.
Results
We discovered that VPA and 5-Aza could individually induce decreased viability and have an inhibitory effect on the proliferation of 786-O and 769-P cells. This anti-growth effect was more pronounced when the cells were treated with both VPA and 5-Aza. The combination of VPA and 5-Aza also elicited more apoptosis and produced more cell cycle arrest in the G1 phase for both cell lines. On the other hand, treatment of RCC cells with VPA, 5-Aza, or a combination of both resulted in slow wound healing and impaired migration.
Conclusions
These findings clearly demonstrated that VPA combined with 5-Aza could significantly increase anti-RCC effects by inhibiting cellular proliferation, inducing apoptosis, promoting cell cycle arrest and prohibiting the migration of human RCC cells.
MeSH Keywords: Azacitidine; Carcinoma, Renal Cell; Cell Migration Inhibition; Cell Proliferation; Valproic Acid
Background
Renal cell carcinoma (RCC) is the most common malignant tumor of the urinary system and represents 80% of the total malignant tumors, accounting for 3% of all cancers in adults [1]. RCC is heterogeneous and comprises several subtypes that determine the corresponding clinical process and prognosis. Clear cell renal cell carcinoma (ccRCC), a representative subtype of RCC, accounts for approximately 80% of RCC [2,3]. ccRCC has a high risk of invasion and metastasis as more than 50% of ccRCC patients eventually develop metastatic disease, while less than 10% of ccRCC patients with metastatic disease survive for more than 5 years [4]. Until recently, surgery constituted the only appropriate strategy to treat RCC because the cancer cells display a poor sensitivity to radiotherapy and chemotherapy [5]. Furthermore, the biological behavior of RCC can change rapidly, and its pathogenesis is still largely unknown. Therefore, finding potent drugs to prevent RCC progression and metastasis is urgently needed.
Epigenetic alterations, such as histone acetylation and DNA methylation, play important roles in regulating the expression of some genes that are closely associated with the cell cycle and apoptosis [6]. As previously reported, histone hypo-acetylation is related to the development and progression of a number of cancers [7]. Thus, more attention has been given to the anti-tumor effects of histone deacetylase inhibitors (HDACis). Valproic acid (VPA, 2-propylvaleric acid) is a branched, short-chain fatty acid and is one of the HDACis that has been widely used as an antiepileptic drug [8]. Studies have shown that VPA exhibits anti-tumor effects in various malignancies, including glioblastoma, neuroblastoma, breast and ovarian cancers, colorectal cancer, prostate cancer, thyroid cancer, liver cancer, melanoma, etc. [9,10]. VPA can inhibit cancer cell proliferation as well as induces cell apoptosis. On the other hand, DNA methylation is another important epigenetic modification that occurs in the CpG islands near gene promoter regions. The cytosine residues of these CpG islands can be methylated by several DNA methyltransferases (DNMTs), leading to the transcriptional silencing of proximal genes [11]. 5-Aza-2′-deoxycytidine (5-Aza) is a nucleoside analog that acts as an inhibitor of DNMTs [12,13] and greatly represses DNMT functions during DNA replication through DNA demethylation or hemi-demethylation. Simultaneously, DNA demethylation can also upregulate gene expression in cis by relaxing chromatin structure [14].
Both VPA and 5-Aza have been used in clinical trials, but their functions in RCC are still not well defined. The aim of the study was to analyze the impact of VPA, 5-Aza and their combination on RCC.
Material and Methods
Cell lines and culture conditions
Two human renal cancer cell lines, 786-O and 796-P, were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The 2 cell lines were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (both from Gibco, USA) at 5% CO2 and 37°C in a humidified chamber. Cells were grown to confluence and harvested by trypsinization using a 0.25 mg/ml trypsin/EDTA solution (Invitrogen, USA) and resuspended in culture medium before use.
Drug acquisition and preservation
VPA was purchased from MERK (Germany). The powder was dissolved in purified water and stored as a 200 mmol/L stock. 5-Aza was purchased from Sigma-Aldrich (USA) and dissolved in DMSO to obtain a 1 mmol/L stock solution. Both drugs were stored at −20°C.
Cell proliferation assay
The proliferation of 786-O and 769-P cells after treatment with VPA, 5-Aza or combined treatment, was detected with the xCELLigence RTCA DP Instrument (ACEA Bioscience, Inc., San Diego, CA, USA). Cells were seeded in culture E-plates at a density of 5000 cells per well and incubated at 37°C and 5% CO2 overnight. After incubation, the supernatant was discarded and replaced with fresh culture medium. Different concentrations of VPA and 5-Aza were added to the cells. VPA was added at concentrations of 0, 1, 2, or 8 mmol/L to 786-O cells. 5-Aza was added at concentrations of 0, 1, 2, or 4 μmol/L to 786-O cells. A combination of VPA (2 mmol/L) and 5-Aza (4 μmol/L) was added to both 786-O cells and 769-P cells. The cell growth curves were automatically recorded with the xCELLigence System in real time. The cell index was followed for 72 h.
Cell viability assay
The viability of 786-O and 769-P cells was assessed using the CCK8 assay (Biosharp, China). Cells were seeded in 96-well plates at a density of 2×104 cells per well and incubated for 24 h. Next, the cells were treated with different concentrations (as described above) of VPA, 5-Aza, or their combination for 96 h. After the treatment, CCK8 (10 μl/well) was added to the wells and the cells were incubated at 37°C for 1 h. The absorbance at 450 nm was detected by a Multiwell plate reader (BioRad iMark, USA). Each treatment was performed in triplicate.
Cell cycle analysis
The effects of VPA and 5-Aza on the cell cycle were examined by flow cytometry. Briefly, 2.5×105 786-O and 769-P cells were treated with VPA and 5-Aza alone or in combination for 48 h. The cells were washed twice with 1× PBS, fixed with 70% ethanol for 30 min and stained with propidium iodide for 30 min at 4°C in the dark. The cell cycle distribution was analyzed by flow cytometry (Coulter, USA). Changes in the percentage of cells in different phases of the cell cycle were analyzed with ModFit LT software.
Apoptosis analysis
To quantify apoptosis, 786-O and 769-P cells were seeded in 6-well plates at a density of 2.5×105 cells per well and treated with VPA, 5-Aza, and their combination, as previously described. After 24/48 h, the cells were harvested and washed 3 times with 1× PBS. Next, the collected cells were stained with the annexin V-FITC and propidium iodide (PI) kit (Roche, Switzerland) according to the manufacturer’s instructions. Apoptosis was detected with an EPICS XL Flow Cytometer (Coulter, USA), and the data were analyzed with EXPO32 ADC Analysis software.
Wound healing assay
A total of 5×105 786-O and 769-P cells were seeded in a 6-well plate and cultured to 90% confluence. A 100-μl pipette tip was used to scratch the plate. Cells were treated with VPA, 5-Aza, or their combination. After 16 to 24 h of culture, the wounds were observed under the microscope and photographed.
Transwell assay
The migration ability of the cells was assessed using 8-μm, 24-well Transwell chambers (Milipore, Germany) according to the manufacturer’s instructions. Briefly, 786-O and 769-P cells (1×104) were seeded into the upper chamber in 200 μl of serum-free medium containing VPA, 5-Aza, or their combination. The lower compartment was filled with 0.5 mL 1640 medium supplemented with 10% FBS (as a chemoattractant). Following a 24-h incubation step, the cells that migrated to the lower surface of the filter were fixed and stained with 0.1% crystal violet for 10 min at room temperature. The migrated cells on the underside of the filter were counted and recorded to obtain images using light microscopy. Each experiment was performed in triplicate.
Migration assays
To monitor cell migration in real time, the xCELLigence Real-Time Cell Analyzer Dual Plate (RTCA-DP) instrument was used according to the manufacturer’s recommendations (ACEA Bioscience, Inc., San Diego, CA, USA). A 2×105 cells/ml cell suspension was prepared. The lower CIM-Plate chamber was loaded with 165 μl 1640 RPMI supplemented with 10% FBS containing VPA, 5-Aza, or their combination. The upper CIM-Plate chamber was loaded with 30 μl serum-free 1640 RPMI. Next, the CIM-Plate was incubated for 1 h at 37°C. After scanning the plate, the upper CIM-Plate chamber was loaded with 150 μl cell suspension, and VPA, 5-Aza, or their combination, were also added to the corresponding wells. The cell index values were monitored every 15 min for 18 h. The final values were calculated and plotted using RTCA software 1.2.1 (RTCA xCELLigence system).
Statistical analysis
The data were analyzed using Student’s t-test. All data are presented as the mean ±SD. Statistical analysis was performed using GraphPad Prism 6.0. For all tests, a P value of less than 0.05 was considered to be statistically significant.
Results
Combined treatment of VPA and 5-Aza inhibits the proliferation of 786-O and 769-P cells
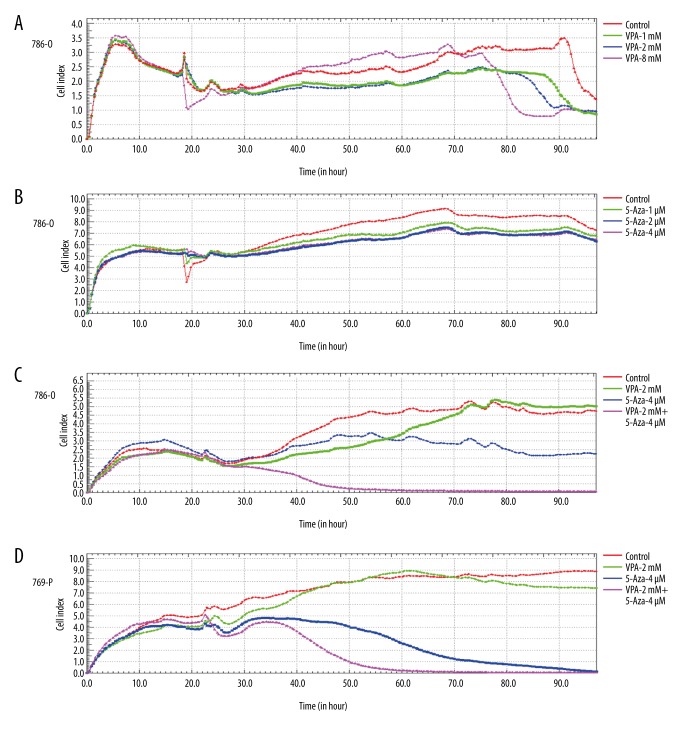
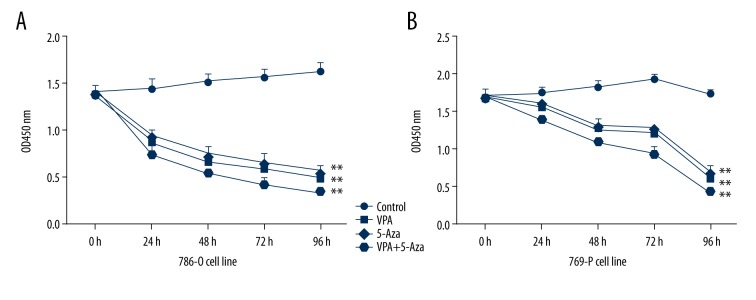
To determine the effects of VPA and 5-Aza on cells, a Real-Time Cell Analyzer (RTCA) was used to monitor the changes in the properties of 786-O and 769-P cells when exposed to the 2 drugs. We determined that VPA inhibited the proliferation of 786-O cells in a dose-dependent manner. Similar results were observed for 786-O cells treated with 5-Aza. Moreover, the combination of VPA and 5-Aza demonstrated a stronger inhibitory effect on 786-O and 769-P cells (Figure 1). In addition, the treated 786-O and 769-P cells were also examined with the CCK8 assay. We discovered that VPA or 5-Aza treatment can significantly decrease the viability of 786-O and 769-P cells. Furthermore, a combined treatment with VPA and 5-Aza exerts a significantly greater inhibitory effect on the viability of 786-O and 769-P cells (P<0.05, Figure 2).
Figure 1.
Inhibition of cell proliferation of 786-O and 769-P cells by VPA and 5-Aza. A total of 5×103 cells were seeded in E-plate 16. Following an overnight incubation, the cells were treated with VPA and 5-Aza, alone or in combination. Real-time proliferation monitoring of 786-O and 769-P cells was performed by measuring the cell index to evaluate the proliferation of 786-O and 769-P cells treated with VPA and 5-Aza, alone or in combination, using the xCELLigence RTCA-DP system. (A) 786-O cells were treated with different concentrations of VPA (1 mM, 2 mM, 8 mM); (B) 786-O cells were treated with different concentration of 5-Aza (1 μM, 2 μM, 4 μM); 786-O cells (C) and 769-P cells (D) were treated with VPA (2 mM), 5-Aza (4 μM) or with a combination of both drugs (VPA: 2 mM and 5-Aza: 4 μM).
Figure 2.
Inhibitory effect of VPA and 5-Aza on 786-O and 769-P cellular growth. 786-O cells (A) and 769-P cells (B) were treated with VPA, 5-Aza or a combination of both for 24 h, 48 h, 72 h, and 96 h. The cell viability was detected using a CCK8 assay. ** P<0.01, compared to the control group.
Treatment with VPA and 5-Aza forces cell cycle arrest in the G1 phase
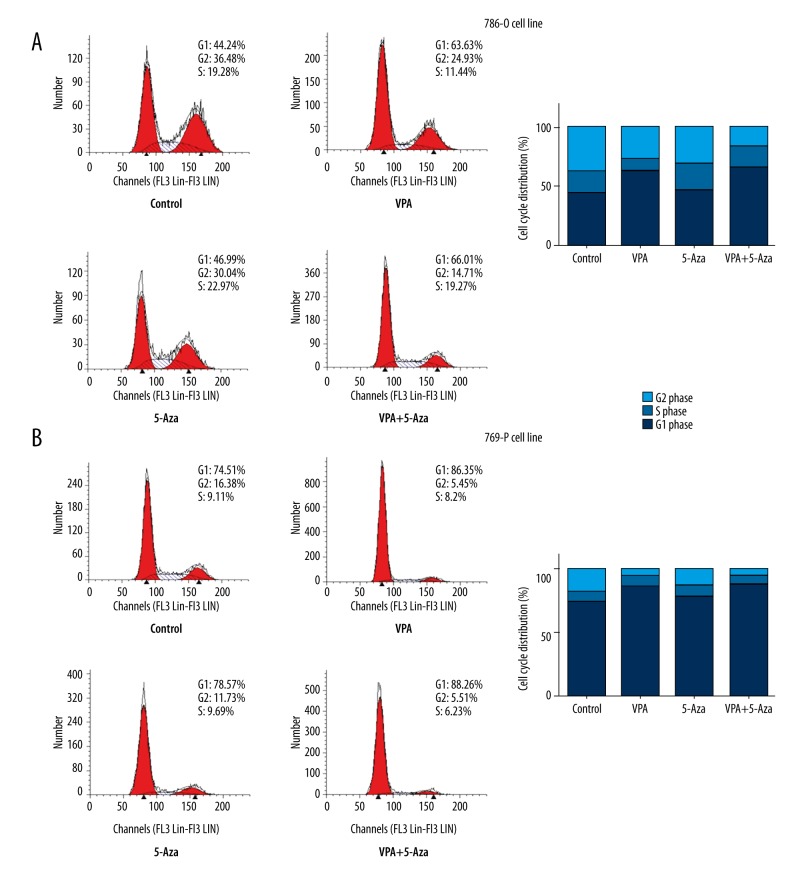
Flow cytometry was performed to evaluate the cell cycle distribution of 786-O and 769-P cells after a 48-h exposure to VPA and 5-Aza. The cell cycle distribution analysis showed that compared to the control group, VPA treatment alone caused more cell cycle arrest in the G1 phase (63.6% vs. 44.2% in 786-O cells, and 86.4% vs. 74.5% in 769-P cells), whereas 5-Aza treatment had little impact on the cell cycle distribution for both cell lines. When treatment with VPA and 5-Aza was combined, a higher proportion of G1 cells were detected for both 786-O and 769-P cells, although the increase was less than 5% compared to the VPA group (Figure 3).
Figure 3.
Effects of VPA, 5-Aza, and combined treatment with both drugs on the cell cycle. A cell cycle assay was performed after treatment with VPA, 5-Aza or a combination of both agents for 48 h. Treatment with VPA, 5-Aza, and with both drugs simultaneously resulted in G1 phase arrest in 786-O cells (A) and 769-P cells (B). Left, Flow cytometry results. Right, Histograms of the cell cycle.
Treatment with VPA, 5-Aza, and their combination induces apoptosis in 786-O and 769-P cells
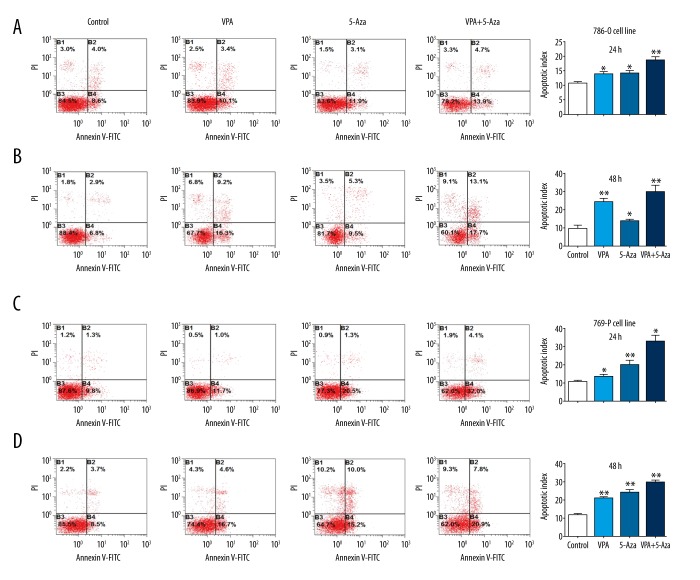
To assess the induction of apoptosis by VPA, 5-Aza, and their combination, 786-O and 769-P cells were treated with the drugs, and apoptosis was determined by flow cytometry. Treatment with VPA or 5-Aza alone induces apoptosis after 24 h or 48 h in both 786-O and 769-P cells. The percentage of apoptotic cells (B2+B4) before and after incubation with VPA was 12.6% vs. 13.5% at 24 h and 9.7% vs. 25.5% at 48 h for 786-O cells; the percentage of apoptotic cells before and after incubation with 5-Aza was 12.6% vs. 15% at 24 h and 9.7% vs. 14.8% at 48 h for 786-O cells; the percentage of apoptotic cells before and after the combination treatment was 12.6% vs 18.6% at 24 h and 9.7% vs. 30.8% at 48 h for 786-O cells (P<0.01, Figure 4A, 4B). Similar results were seen for the 769-P cells, which showed that the combined treatment of VPA and 5-Aza induced significantly more apoptosis at both 24 h and 48 h (P<0.01, Figure 4C, 4D).
Figure 4.
Effects of VPA, 5-Aza, and combined treatment with both drugs on apoptosis. Apoptosis experiments and analyses were performed 24 h or 48 h after treatment with VPA, 5-Aza, or a combination of both drugs. The 786-O cells were treated with VPA (2 mM), 5-Aza (4 μM), or the combination (VPA: 2 mM, 5-Aza: 4 μM) for 24 h (A) and 48 h (B). The 769-P cells were treated with VPA (2 mM), 5-Aza (4 μM) or the combination (VPA: 2 mM, 5-Aza: 4 μM) for 24 h (C) and 48 h (D); * P<0.05; ** P<0.01 compared to the control group.
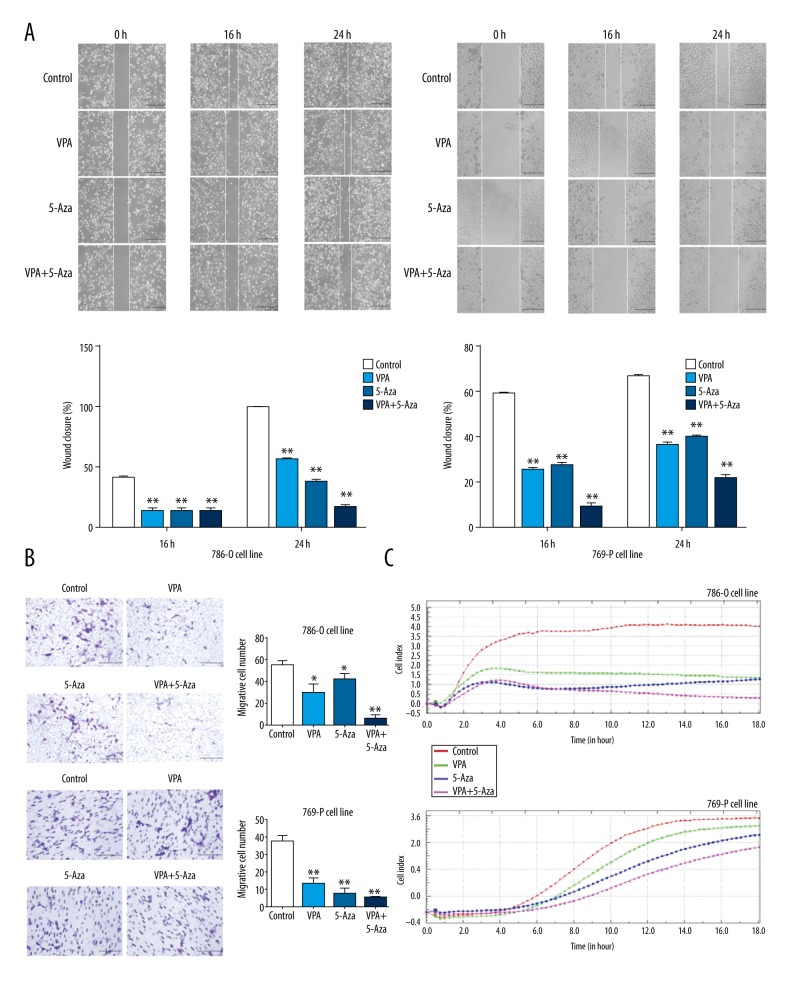
Treatment with VPA, 5-Aza, and their combination reduces cell migration capabilities
A wound healing assay demonstrated that the scratches in the VPA, 5-Aza, and their combined treatment groups healed more slowly after a 16-h incubation with the drugs (P<0.01). When the cells were treated with VPA, 5-Aza, or their combination for 24 h, the combination treatment group displayed a significantly slower rate of healing, while the scratches in the control group were quickly repopulated (P<0.01, Figure 5A. left: 786-O cells; right: 769-P cells). A Transwell assay suggested that the migratory ability of 786-O and 769-P cells was significantly decreased after treatment with VPA or 5-Aza, and the inhibitory effect of the combined treatment was more pronounced compared to the control group (P<0.01, Figure 5B. top: 786-O cells; bottom: 769-P cells). Inhibition of cell migration in the drug-treated 786-O and 769-P cells was also detected by the xCELLigence system, and the results were consistent with what was observed in the wound healing assay and Transwell assay (Figure 5C).
Figure 5.
Effects of VPA, 5-Aza, and combined treatment on cell migration. (A) Cells were seeded in 6-well plates and cultured to 90% confluence. A 100-μl pipette tip was used to scratch the plate. Cell migration was observed under the microscope after treatment with VPA, 5-Aza, or with both drugs at 0 h, 16 h, and 24 h; (B) Transwell assays were performed after treatment with VPA, 5-Aza, or with both agents for 24 h; (C) Real-time monitoring of the migration of 786-O and 769-P cells was performed by measuring the cell index to evaluate the proliferation of 786-O and 769-P cells treated with VPA, 5-Aza, or with both drugs using the xCELLigence RTCA-DP system. * P<0.05; ** P<0.01 compared to the control group.
Discussion
Renal cell carcinoma (RCC) is a common renal malignancy that results in significant human mortalities annually [15–17]. Surgical intervention constitutes the primary treatment for RCC. However, this treatment is not suitable for all patients since many develop metastasis or recurrent disease.
Despite the discovery of novel targeted therapies, most metastatic RCCs eventually result in death [18]. Therefore, new therapeutic approaches are urgently needed to increase patient survival [19]. Epigenetic changes, as important regulator of gene expression, play important roles in gene transcription and early events in tumorigenesis [20,21].
Histone acetyltransferases (HATs) and histone deacetylases (HDACs) are enzymes that control histone acetylation and deacetylation, which play important roles in modulation of chromatin structure and regulation of gene expression. Therefore, disruption of the balance between HATs and HDACs is known to be involved in cancer genesis and progression. Immunohistologic assessment and tissue microarray analysis have provided evidence that decreased histone acetylation is a common alteration in RCC and there is an inverse correlation between histone acetylation and pT-stage, distant metastasis, Fuhrman grading, and RCC progression [22,23]. Based on clinical data, it has been suggested that increasing the amount of acetylated histones by lowering HDAC might be a therapeutic option for RCC. Valproic acid (VPA), a branched-chain fatty acid, is one of the HDAC-Is, which has been found to efficiently induce tumor cell apoptosis, causing cell cycle arrest, impairing tumor invasion, and preventing metastasis in several cancer cells [24]. In RCC models, VPA led to distinct growth and invasion inhibition by blocking the interaction of RCC cells with endothelium and extracellular matrix or directly regulating proteins, such as CDK2, cyclin B, cyclin D3, p21, and Rb. [25,26]. In addition, studies have revealed that VPA can also sensitize RCC to chemotherapy [27] and increase treatment responses when combined with other chemotherapeutic drugs [28].
DNA methylation alterations are one of the most consistent epigenetic modifications occurring during carcinogenesis and are widely accepted as a feature of RCC [29,30]. As hypermethylation inactivates transcription of CpG dinucleotides in promoter regions of tumor suppressor genes leading to gene silencing, re-expression of epigenetically silenced tumor suppressor genes with DNA methyltransferase (DNMT) inhibitors is a rational strategy for the treatment of human neoplasms. 5-Aza is a DNMT inhibitor which is currently approved by the FDA for treatment of myelodysplastic syndromes. In RCC models, 5-Aza increased the expression of tumor suppressor genes like FBXW10, SMPD3, and Kank1 and thus suppress tumor cells growth and induce apoptosis [31,32]. 5-Aza treatment also can increase miR-200c, miR-492, and other miRNAs that have a suppressive role in epithelial-mesenchymal transition (EMT) and inhibit migration, invasion, and EMT in ccRCC cells [33,34]. Importantly, combining 5-Aza treatment with HDAC inhibitors results in a synergistic activation of silenced tumor suppressor genes, as well as synergistic antineoplastic effects on breast and lung carcinomas [35,36].
In this study, we determined the effects of 2 small-molecule compounds, VPA and 5-Aza, on 2 human kidney cancer cell lines, 786-O and 769-P. A proliferation assay demonstrated that treatment with VPA or 5-Aza at different concentrations could inhibit 786-O and 769-P cell growth in a time-dependent manner. The combination treatment of VPA and 5-Aza displayed a more pronounced inhibitory effect on 786-O and 769-P cells. A CCK8 assay indicated that both VPA and 5-Aza decreased the viability of 786-O and 769-P cells in a time-dependent manner. In both 786-O and 769-P cells, combined treatment with VPA and 5-Aza produced a synergistic inhibitory effect. Cell cycle analysis demonstrated that VPA and 5-Aza treatment of 786-O and 769-P cells resulted in cell cycle arrest in the G1 phase. Furthermore, an apoptosis assay showed that either VPA or 5-Aza treatment inhibited cell growth. Combining VPA with 5-Aza resulted in synergistically increased apoptosis. These results suggest that a combined treatment of VPA and 5-Aza produces a stronger anti-tumor effect on human ccRCC cells.
Metastasis is the main cause of morbidity and mortality in millions of patients with cancer. Undoubtedly, agents that can efficiently inhibit the growth and migration of cancer cells are candidates to suppress cancer progression and metastasis and thus are likely to reduce mortality. Previous reports have demonstrated that the anticancer activity of VPA is more effective on metastatic prostate cancer cells than non-metastatic cells [37]. In this study, we examined the effects of combined treatment with VPA and 5-Aza on the migratory capacity of 786-O and 769-P cells. A wound healing assay showed that VPA and 5-Aza inhibited wound healing. A Transwell assay suggested that VPA and 5-Aza can significantly decrease the cell migration rate. Migration was profoundly decreased after combined treatment with VPA and 5-Aza.
Conclusions
Our results demonstrate that the combined treatment of VPA and 5-Aza significantly enhanced the inhibition of human ccRCC cell proliferation and migration and induced apoptosis as well as cell cycle arrest. These findings suggest that the combined treatment of VPA and 5-Aza may have wide therapeutic and/or adjuvant therapeutic applications in RCC treatment and warrants further clinical testing.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the National Natural Sciences Foundation of China (No. 81472633, 81630069)
References
- 1.Lessi F, Mazzanti CM, Tomei S, et al. VHL and HIF-1α: Gene variations and prognosis in early-stage clear cell renal cell carcinoma. Med Oncol. 2014;31:840. doi: 10.1007/s12032-014-0840-8. [DOI] [PubMed] [Google Scholar]
- 2.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245–57. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Decastro GJ, McKiernan JM. Epidemiology, clinical staging, and presentation of renal cell carcinoma. Urol Clin North Am. 2008;35:581–92. doi: 10.1016/j.ucl.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Escudier B, Szczylik C, Porta C, Gore M. Treatment selection in metastatic renal cell carcinoma: expert consensus. Nat Rev Clin Oncol. 2012;9:327–37. doi: 10.1038/nrclinonc.2012.59. [DOI] [PubMed] [Google Scholar]
- 5.Leibovich BC, Blute ML. Surgical management of renal cell carcinoma. Semin Oncol. 2006;33:552–62. doi: 10.1053/j.seminoncol.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Fang JY, Lu YY. Effects of histone acetylation and DNA methylation on p21(WAF1) regulation. World J Gastroenterol. 2002;8:400–5. doi: 10.3748/wjg.v8.i3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi JH, Kwon HJ, Yoon BI, et al. Expression profile of histone deacetylase 1 in gastric cancer tissues. Jpn J Cancer Res. 2001;92:1300–4. doi: 10.1111/j.1349-7006.2001.tb02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chateauvieux S, Morceau F, Dicato M, Diederich M. Molecular and therapeutic potential and toxicity of valproic acid. J Biomed Biotechnol. 2010;2010 doi: 10.1155/2010/479364. pii: 479364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottlicher M, Minucci S, Zhu P, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–78. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duenas-Gonzalez A, Candelaria M, Perez-Plascencia C, et al. Valproic acid as epigenetic cancer drug: Preclinical, clinical and transcriptional effects on solid tumors. Cancer Treat Rev. 2008;34:206–22. doi: 10.1016/j.ctrv.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 12.Lindner DJ, Wu Y, Haney R, et al. Thrombospondin-1 expression in melanoma is blocked by methylation and targeted reversal by 5-Aza-deoxycytidine suppresses angiogenesis. Matrix Biol. 2013;32:123–32. doi: 10.1016/j.matbio.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu WG, Hileman T, Ke Y, et al. 5-aza-2′-deoxycytidine activates the p53/p21Waf1/Cip1 pathway to inhibit cell proliferation. J Biol Chem. 2004;279:15161–66. doi: 10.1074/jbc.M311703200. [DOI] [PubMed] [Google Scholar]
- 14.Momparler RL. Epigenetic therapy of cancer with 5-aza-2′-deoxycytidine (decitabine) Semin Oncol. 2005;32:443–51. doi: 10.1053/j.seminoncol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–31. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 16.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–90. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 17.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 18.Oudard S, Vano Y. The role of rechallenge with targeted therapies in metastatic renal-cell carcinoma. Curr Opin Urol. 2015;25:402–10. doi: 10.1097/MOU.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 19.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 20.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–92. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–28. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 22.Kanao K, Mikami S, Mizuno R, et al. Decreased acetylation of histone H3 in renal cell carcinoma: a potential target of histone deacetylase inhibitors. J Urol. 2008;180:113–36. doi: 10.1016/j.juro.2008.04.136. [DOI] [PubMed] [Google Scholar]
- 23.Mosashvilli D, Kahl P, Mertens C, et al. Global histone acetylation levels: Prognostic relevance in patients with renal cell carcinoma. Cancer Sci. 2010;101:2664–69. doi: 10.1111/j.1349-7006.2010.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaheta RA, Michaelis M, Driever PH, Cinatl J., Jr Evolving anticancer drug valproic acid: Insights into the mechanism and clinical studies. Med Res Rev. 2005;25:383–97. doi: 10.1002/med.20027. [DOI] [PubMed] [Google Scholar]
- 25.Jones J, Juengel E, Mickuckyte A, et al. Valproic acid blocks adhesion of renal cell carcinoma cells to endothelium and extracellular matrix. J Cell Mol Med. 2009;13:2342–52. doi: 10.1111/j.1582-4934.2008.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones J, Juengel E, Mickuckyte A, et al. The histone deacetylase inhibitor valproic acid alters growth properties of renal cell carcinoma in vitro and in vivo. J Cell Mol Med. 2009;13:2376–85. doi: 10.1111/j.1582-4934.2008.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juengel E, Nowaz S, Makarevi J, et al. HDAC-inhibition counteracts everolimus resistance in renal cell carcinoma in vitro by diminishing cdk2 and cyclin A. Mol Cancer. 2014;13:152. doi: 10.1186/1476-4598-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juengel E, Engler J, Mickuckyte A, et al. Effects of combined valproic acid and the epidermal growth factor/vascular endothelial growth factor receptor tyrosine kinase inhibitor AEE788 on renal cell carcinoma cell lines in vitro. BJU Int. 2010;105:549–57. doi: 10.1111/j.1464-410X.2009.08759.x. [DOI] [PubMed] [Google Scholar]
- 29.Arai E, Wakai-Ushijima S, Fujimoto H, et al. Genome-wide DNA methylation profiles in renal tumors of various histological subtypes and non-tumorous renal tissues. Pathobiology. 2011;78:1–9. doi: 10.1159/000322072. [DOI] [PubMed] [Google Scholar]
- 30.Morris MR, Ricketts CJ, Gentle D, et al. Genome-wide methylation analysis identifies epigenetically inactivated candidate tumour suppressor genes in renal cell carcinoma. Oncogene. 2011;30:1390–401. doi: 10.1038/onc.2010.525. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Li J, Gu J, et al. Abnormal methylation status of FBXW10 and SMPD3, and associations with clinical characteristics in clear cell renal cell carcinoma. Oncol Lett. 2015;10:3073–80. doi: 10.3892/ol.2015.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo FY, Xiao S, Liu ZH, et al. Kank1 reexpression induced by 5-Aza-2′-deoxycytidine suppresses nasopharyngeal carcinoma cell proliferation and promotes apoptosis. Int J Clin Exp Pathol. 2015;8:1658–65. [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang J, Yi BO, Ding S, et al. Demethylation drug 5-Aza-2′-deoxycytidine-induced upregulation of miR-200c inhibits the migration, invasion and epithelial-mesenchymal transition of clear cell renal cell carcinoma in vitro. Oncol Lett. 2016;11:3167–72. doi: 10.3892/ol.2016.4364. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Wu A, Wu K, Li M, et al. Upregulation of microRNA-492 induced by epigenetic drug treatment inhibits the malignant phenotype of clear cell renal cell carcinoma in vitro. Mol Med Rep. 2015;12:1413–20. doi: 10.3892/mmr.2015.3550. [DOI] [PubMed] [Google Scholar]
- 35.Boivin AJ, Momparler LF, Hurtubise A, Momparler RL. Antineoplastic action of 5-aza-2′-deoxycytidine and phenylbutyrate on human lung carcinoma cells. Anticancer Drugs. 2002;13:869–74. doi: 10.1097/00001813-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Nome RV, Bratland A, Harman G, et al. Cell cycle checkpoint signaling involved in histone deacetylase inhibition and radiation-induced cell death. Mol Cancer Ther. 2005;4:1231–38. doi: 10.1158/1535-7163.MCT-04-0304. [DOI] [PubMed] [Google Scholar]
- 37.Lee JE, Kim JH. Valproic acid inhibits the invasion of PC3 prostate cancer cells by upregulating the metastasis suppressor protein NDRG1. Genet Mol Biol. 2015;38:527–33. doi: 10.1590/S1415-475738420150028. [DOI] [PMC free article] [PubMed] [Google Scholar]