Abstract
Background
The aim of this study was to evaluate the role of remote ischemic postconditioning (RIPC) of the upper arm on protection from cardiac ischemia-reperfusion injury following primary percutaneous coronary intervention (PCI) in patients with acute ST-segment elevation myocardial infarction (STEMI).
Material/Methods
Eighty patients with STEMI were randomized into two groups: primary PCI (N=44) and primary PCI+RIPC (N=36). RIPC consisted of four cycles of 5 minutes of occlusion and five minutes of reperfusion by cuff inflation and deflation of the upper arm, commencing within one minute of the first PCI balloon dilatation. Peripheral venous blood samples were collected before PCI and at 0.5, 8, 24, 48, and 72 hours after PCI. Levels of creatine kinase-MB (CK-MB), serum creatinine (Cr), nitric oxide (NO), and stromal cell-derived factor-1α (SDF-1α) were measured. The rates of acute kidney injury (AKI) and the estimated glomerular filtration rate (eGFR) were calculated.
Results
Patients in the primary PCI+RIPC group, compared with the primary PCI group, had significantly lower peak CK-MB concentrations (P<0.01), a significantly increased left ventricular ejection fraction (LVEF) (P=0.01), a significantly lower rate of AKI (P<0.01) a significantly increased eGFR (P<0.01), and decreased area under the curve (AUC) of CK-MB, NO and SDF-1α.
Conclusions
RIPC of the upper arm following primary PCI in patients with acute STEMI might provide cardiac and renal protection from ischemia-reperfusion injury via the actions of SDF-1α, and NO.
MeSH Keywords: Ischemic Postconditioning, Myocardial Infarction, Myocardial Reperfusion, Percutaneous Coronary Intervention
Background
Worldwide, ischemic heart disease (IHD) causes a large health and economic burden [1]. The incidence rates for acute ST-segment elevation myocardial infarction (STEMI) have declined in developed countries over the past decade, but IHD in developing countries is increasing [2,3]. Rapid reperfusion of the ischemic myocardium using percutaneous coronary intervention (PCI) is the standard treatment for acute myocardial infarction (MI) to rescue the ischemic myocardium and reduce infarction size. Paradoxically, reperfusion itself may abrogate myocardial salvage and can induce further injury to the ischemic myocardium, a condition termed ‘ischemia-reperfusion injury,’ which accounts for up to 50% of the final size of a myocardial infarct [4]. Novel strategies for reducing myocardial ischemia-reperfusion injury have begun to be evaluated.
Remote ischemic postconditioning (RIPC), which includes a series of repetitive cycles of brief reperfusion, alternating with brief occlusion immediately after reperfusion, showed promising outcomes in preclinical and clinical studies [5,6]. However, postconditioning failed to show benefit and has shown non-beneficial effects in some experiments and clinical studies [7,8], including the large Third Danish Study of Optimal Acute Treatment of Patients With ST-Elevation Myocardial Infarction-Ischemic Postconditioning (DANAMI-3–iPOST) trial [9].
Remote ischemic conditioning (RIC) produced by brief episodes of ischemia-reperfusion of remote tissue or an organ can be used to precede or precondition [10], can be used during (percondition) [11,12], or to follow or postcondition [13] sustained coronary occlusion. RIPC appears to be promising in some clinical settings [14–18]. However, in a recently published clinical study performed by Verouhis et al. [19], in which up to seven cycles of lower-limb RIPC were used, with at least one cycle initiated prior to reperfusion, RIPC failed to reduce the size of the MI in patients with anterior STEMI. However, further clinical research is required.
The use of contrast agent for coronary angiography can lead to worsening of renal function, as contrast agent-related acute kidney injury (AKI), or contrast-induced nephropathy may occur [20]. Studies on patients receiving elective PCI have shown that RIC can reduce the incidence of contrast-induced nephropathy [21,22]. To our knowledge, there is no clinical trial about whether upper arm RIPC can reduce contrast-related AKI or contrast-induced nephropathy in patients with STEMI.
The exact mechanism of RIPC remains to be elucidated [23]. There have been some promising evidence-based cardioprotective factors identified, including stromal cell-derived factor-1α (SDF-1α) and nitric oxide (NO) [24]. Davidson et al. found that SDF-1α concentrations in RIPC rats were significantly elevated [25]. Whether RIPC can affect circulating SDF-1α concentrations in patients with STEMI has not been previously determined, but it is possible that RIPC in humans can affect serum SDF-1α levels in patients.
The aim of this study was to evaluate the role of RIPC of the upper arm on protection from cardiac ischemia-reperfusion injury following primary PCI in patients with acute STEMI.
Material and Methods
Study population
The study protocol complied with the Declaration of Helsinki (version, 1996) and was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (No. 2015027). All participants signed a written informed consent to participate in the study. Patient ages ranged from 18–80 years. All the patients presented within six hours of symptom onset. Inclusion criteria included patients aged 18 years or older, and chest pain for more than 30 minutes accompanied by at least 2-lead ST segment elevation >0.2 mV or new bundle branch block. Exclusion criteria included chronic kidney disease, estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2, patients who received hemodialysis, patients with coma or cardiogenic shock, pregnancy, patients who received trimethoprim, or glibenclamide, which can affect distant ischemic adaptation, patients with severe peripheral arterial disease (PAD), patients with a history of myocardial infarction (MI), or coronary artery bypass surgery (CABG). Figure 1 shows the clinical details of the patient groups.
Figure 1.

Study design: Patient flow chart.
Study design
This study was a single-center, prospective, randomized, controlled study. Eighty patients were diagnosed with STEMI from Jan 2015 to Jun 2016, and all patients underwent primary percutaneous coronary intervention (PCI). The patients were randomly divided into a conventional primary PCI group (N=44) or a primary PCI + remote ischemic postconditioning (RIPC) group (N=36). All patients were pre-treated with aspirin 300 mg, clopidogrel 600 mg, or ticagrelor 180 mg as an oral loading dose, and 70 IU/kg unfractionated heparin. Ioversol alcohol (Jiangsu Hengrui Pharmaceutical Co., Ltd.) was used in coronary angiography. Thrombectomy and inhibitors of glycoprotein IIb/IIIa were used as required. After primary emergency PCI, lifelong 100 mg aspirin daily was commenced, combined with an initial 12-month course of 75 mg clopidogrel daily or ticagrelor 90 mg twice daily.
Remote ischemic postconditioning (RIPC) protocol
All eligible patients were prepared with a limb cuff wrapped around the upper arm before arterial puncture. In the treatment group, the protocol was started within one minute of the first balloon dilatation. The upper arm was exposed to four cycles of ischemia-reperfusion, each with 5 minutes of cuff inflation at 200 mmHg, followed by 5 minutes of complete deflation.
Serum creatine kinase-MB (CK-MB)
Blood samples were collected before PCI and 0.5, 8, 24, 48, and 72 h after PCI. The serum samples were centrifuged at 3,000 rpm for 10 minutes. Stromal cell-derived factor-1α (SDF-1α), nitric oxide (NO), and serum creatine kinase-MB (CK-MB) concentrations were tested. Serum CK-MB was measured by use of a VITROS 350 dry biochemical analyzer. NO and SDF-1α were detected using the Shanghai Xitang enzyme-linked immunosorbent assay (ELISA) kit. GraphPad Prism 5.02 was used to measure the area under the curve (AUC).
Serum creatine (Cr) and acute kidney injury (AKI)
Blood was collected before PCI and at 24 and 72 hours after PCI, then centrifuged at 3,000 rpm for 10 min. The supernatant was used to measure serum creatine (Cr) using a VITROS 350 biochemical analyzer. The Modification of Diet in Renal Disease (MDRD) Study equation was used to estimate glomerular filtration rate (GFR). The incidence of acute kidney injury (AKI) was calculated based on preoperative serum Cr and the highest serum Cr was within 72 hours postoperatively. Contrast-associated AKI was defined as serum Cr levels that were increased by 25%, or the concentrations of serum Cr that increased 44 mmol/L compared with preoperative levels. ΔeGFR was calculated according to preoperative eGFR and minimum eGFR within 72 h after PCI.
Ultrasound cardiography (UCG)
The UCG was measured on the 7th day after PCI by a physician who was unaware of the treatment of each patient and using a GE Vivid 7 Color Ultrasound Imager. The left ventricle ejection fraction (LVEF) was measured by the double-plane Simpson method.
Statistical analysis
Data analysis were performed using SPSS version 21 (SPSS, Chicago, Ill, USA). Continuous variables were expressed as the mean ± standard deviation (SD), and the categorical variables were expressed as a percentage. The t-test was used for the continuous variables that conformed to a normal distribution. The Mann–Whitney U test was used for continuous variables that did not conform to a normal distribution. The Chi-square test was performed for categorical variables. P<0.05 was regarded as statistically significant.
Results
A total of 80 patients were enrolled in the study. There were 44 patients who underwent primary percutaneous coronary intervention (PCI) and 36 patients who underwent primary PCI + remote ischemic postconditioning (RIPC). The average ages of the two groups were 58.93±12.82 and 59.24±10.45 years, respectively. There were 29 men (81%) in the primary PCI+RIPC group and 40 males (91%) in the primary PCI group. There was no significant difference in age or sex ratio between the two groups. No patients died or suffered repeated myocardial infarction (MI) in either group. The clinical features of patients are summarized in Table 1.
Table 1.
Clinical characteristics of patients.
| PPCI+RIPostC | PPCI | P | |
|---|---|---|---|
| Number of cases | 36 | 44 | |
| Age | 58.93±12.82 | 59.24±10.45 | 0.91 |
| Gender | |||
| Man | 29 | 40 | 0.21 |
| Woman | 7 | 4 | |
| Body mass index, kg/m2 | 24.35±5.23 | 23.90±2.18 | 0.63 |
| Basic diseases | |||
| Hypertension (n) | 26 | 27 | 0.35 |
| Diabetes (n) | 7 | 8 | 0.99 |
| Dyslipidemia (n) | 7 | 3 | 0.10 |
| Smoking index, count year | 589±483 | 621±354 | 0.74 |
| Preoperative indicators | |||
| Heart rate, Number/min | 77±15 | 79±16 | 0.57 |
| Systolic pressure, mmHg | 124.14±21.85 | 128.58±26.01 | 0.41 |
| Diastolic pressure, mmHg | 75.39±15.36 | 80.42±18.66 | 0.19 |
| Serum creatinine, umol/L | 73.37±10.56 | 76.91±11.84 | 0.16 |
| Neutrophils, 109/L | 9.04±3.82 | 7.97±3.08 | 0.18 |
| Platelet count, 109/L | 219.67±47.67 | 213.08±48.36 | 0.54 |
| LDL-C, mmol/L | 2.8±0.8 | 3.1±1.2 | 0.19 |
| TG, mmol/L | 3.3±1.7 | 3.1±2.0 | 0.63 |
| Medication | |||
| Aspirin (n) | 36 | 44 | 1 |
| Clopidogrel/Ticagrelor (n) | 36 | 44 | 1 |
| Statins (n) | 36 | 44 | 1 |
| Beta blockers (n) | 20 | 35 | 0.999 |
| ACEI/ARB (n) | 12 | 15 | 0.999 |
Values are mean ±SD.
The PCI-related results between the two groups were similar (Table 2). Symptom once to balloon PCI time was 351±132 minutes in the primary PCI+RIPC group and 344±143 minutes in the primary PCI group (P=0.82). There was also a homogenous distribution of culprit vessel and multivessel disease between the two groups. Postoperative Thrombolysis In Myocardial Infarction (TIMI) flow grade was not significantly different between the two groups (P=0.96). In the primary PCI+RIPC group and primary PCI group, 31 and 40 patients received stent implantation, respectively (P=0.72). Primary PCI succeeded in both group without serious complications, although not all patients were stented. The contrast dose applied in the two groups were similar, 87.92±21.05 mL in the primary PCI+RIPC group, 92.50±21.02 mL in the primary PCI group (P=0.34).
Table 2.
PCI-related results.
| PPCI+RIPostC | PPCI | P | |
|---|---|---|---|
| Ischemic time, minutes | 351±132 | 344±143 | 0.82 |
| Vascular lesions (n) | |||
| Single branch | 28 | 37 | 0.57 |
| Multi-branch | 8 | 7 | |
| Culprit vessel (n) | |||
| LAD | 17 | 20 | 0.42 |
| RCA | 14 | 13 | |
| LCX | 5 | 11 | |
| Killip(n) | |||
| I | 34 | 42 | 0.98 |
| II | 1 | 1 | |
| III | 1 | 1 | |
| Postoperative TIMI | |||
| 3 | 29 | 37 | 0.96 |
| 2 | 5 | 6 | |
| 1 | 2 | 1 | |
| 0 | 0 | 0 | |
| Stent implantation (n) | |||
| Stent Implanted | 31 | 40 | 0.72 |
| No stent implanted | 5 | 4 | |
| Contrast dose, ml | 87.92±21.05 | 92.50±21.02 | 0.34 |
LAD – left anterior descending artery; LCX – left circumflex artery; RCA – right coronary artery.
Clinical results
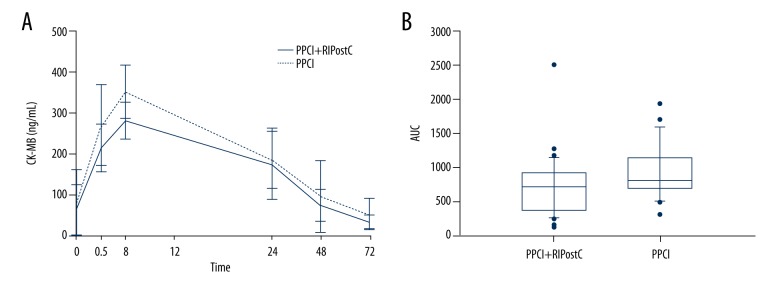
Preoperative creatine kinase-MB (CK-MB) levels were similar in both groups. Serum CK-MB levels at 0.5, 8, 24, 48, and 72 h after PCI in both groups are shown in Table 3. The levels of CK-MB in the primary PCI group at all time points were greater than those in the primary PCI+RIPC group. However, levels measured at 24 hours vs. 48 hours were not significantly different (P>0.05). The postoperative CK-MB peak in the primary PCI+RIPC group decreased significantly compared with the primary PCI group (280.60±45.83 ng/mL vs. 352.21±65.42 ng/mL) (P<0.01). CK-MB in both groups peaked at 8 hours after PCI. The curve of CK-MB levels at each time point is shown in Figure 2A.
Table 3.
Serum levels of CK-MB at different time before and after PCI.
| CK-MB, ng/ml | PPCI+RIPostC | PPCI | P |
|---|---|---|---|
| Preoperative | 60.16±63.67 | 77.15±81.44 | 0.31 |
| Postoprotive | |||
| 0.5 hour | 212.53±59.44 | 269.58±99.50 | <0.01** |
| 8 hour | 280.60±45.83 | 352.21±65.42 | <0.01** |
| 24 hour | 173.44±87.39 | 183.09±70.39 | 0.59 |
| 48 hour | 71.59±38.84 | 93.05±88.75 | 0.18 |
| 72 hour | 30.39±17.01 | 50.01±37.86 | <0.01** |
Values are mean ±SD.
P<0.01.
Figure 2.
Creatine kinase-MB (CK-MB) levels. The curve of CK-MB levels at each time point (A). The area under the curve (AUC) of CK-MB (B).
The area under the curve (AUC) of CK-MB was calculated by the trapezoidal method. The median AUC for the primary PCI+RIPC group was 717.25 (range, 364.63–921.98) and the median AUC for the primary PCI group was 807.00 (range, 693.30–1136.00). The infarct size in the primary PCI+RIPC group was significantly lower compared with that in the primary PCI group (U=327.5) (P=0.02) (Figure 2B).
Echocardiographic results
The postoperative left ventricular ejection fraction (LVEF) in the primary PCI+RIPC group was 54.50±9.73%, which was significantly greater than the 48.14±7.04% in the primary PCI group (P=0.01).
Kidney function and acute kidney injury (AKI)
A total of 22 patients experienced AKI after primary PCI, manifested as an increased serum creatinine and decreased glomerular filtration rate (GFR), with 18 cases (40.9%) in the primary PCI group and 4 cases (11.1%) in the primary PCI+RIPC group. The incidence of AKI in the primary PCI group was significantly greater compared with the primary PCI+RIPC group (P <0.01)
Preoperative eGFR in the primary PCI+RIPC group and the primary PCI groups were 102.26±18.33 ml/min/1.73 m2 and 100.34±12.86 ml/min/1.73 m2, respectively (P=0.60). Postoperative eGFR of the primary PCI+RIPC group was 98.72±13.34 ml/min/1.73 m2, which was significantly greater compared with the 78.13±12.40 ml/min/1.73 m2 in the primary PCI group.
The eGFRs were −3.54±14.45ml/min/1.73 m2 and −22.21±12.46 ml/min/1.73 m2 in the primary PCI+RIPC group and the primary PCI group, respectively (P<0.01). The eGFR tended to decrease after the PCI procedure in both groups but was more significantly decreased in the primary PCI group. The results of renal function in both groups are shown in Table 4.
Table 4.
Results of renal function.
| AKI | PPCI+RIPostC | PPCI | P |
|---|---|---|---|
| Cases | 4 | 18 | |
| Incidence, % | 11.10 | 40.90 | <0.01** |
| eGFR, ml/min/1.73 m2 | |||
| Preoperative | 102.26±18.33 | 100.34±12.86 | 0.60 |
| Postoperative | 98.72±13.34 | 78.13±12.40 | <0.01** |
| ΔeGFR | −3.54±14.45 | −22.21±12.46 | <0.01** |
ΔeGFR – eGFR difference before and after operation. Values are mean ±SD.
P<0.01.
Serum nitric oxide (NO)
Preoperative serum nitric oxide (NO) levels were similar in the primary PCI+RIPC group and the primary PCI groups, which were 1.50±0.29 mmol/mL and 1.32±0.66 mmol/mL, respectively (P=0.11). The serum levels of NO in the primary PCI+RIPC group at 0.5, 8, 24, and 72 hours after PCI were significantly higher than those in the primary PCI group (Table 5, Figure 3A).
Table 5.
Serum NO levels before and after PCI.
| NO, umol/ml | PPCI+RIPostC | PPCI | P |
|---|---|---|---|
| Preoperative | 1.50±0.29 | 1.32±0.66 | 0.11 |
| Postoprotive | |||
| 0.5 hour | 3.30±0.56 | 2.05±0.76 | <0.01** |
| 8 hour | 3.88±0.53 | 2.56±0.91 | <0.01** |
| 24 hour | 2.63±0.40 | 2.29±0.52 | <0.01** |
| 48 hour | 2.10±0.12 | 1.90±0.98 | 0.18 |
| 72 hour | 1.60±0.97 | 1.12±0.20 | <0.01** |
Values are mean ±SD.
P<0.01.
Figure 3.
Serum nitric oxide (NO) levels. Perioperative serum nitric oxide (NO) levels (A). The area under the curve (AUC) of NO levels (B).
The area under the curve (AUC) of NO levels was 5.826 (range, 3.923–13.172) in the primary PCI group, significantly higher than the 4.936 (range, 3.296–6.141) found in the primary PCI+RIPC group (P=0.048). The AUC of NO levels in the primary PCI+RIPC group was 5.826 (range, 3.923–13.172), significantly higher than the 4.936 (range, 3.296–6.141) found in the primary PCI group (P=0.048) (Figure 3B).
Serum stromal cell-derived factor-1α (SDF-1α)
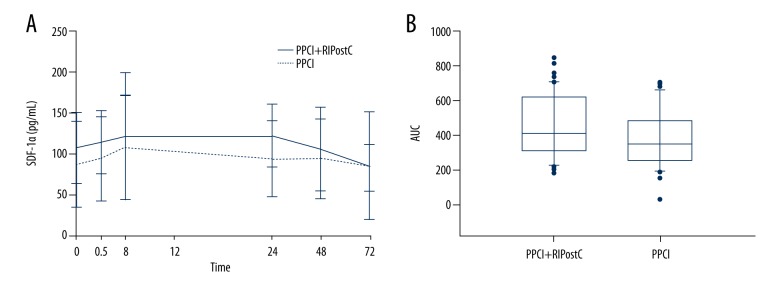
The serum levels of SDF-1α in the primary PCI+RIPC and primary PCI groups before PCI were 96.62±23.14 pg/mL and 88.34±32.99 pg/mL, respectively (P=0.19) (Table 6). The levels of serum SDF-1α in the primary PCI+RIPC group at 0.5, 24, and 48 hours after PCI were significantly higher than those in the primary PCI group. Postoperative blood levels of SDF-1α at different time points in the primary PCI+RIPC and primary PCI groups are shown in Figure 4A. The postoperative AUC of serum SDF-1α of the primary PCI+RIPC group was 410.800 (range, 314.200–629.000), which is significantly higher than the 351.000 (range, 257.600–478.900) found in the primary PCI group (P=0.044) (Figure 4B).
Table 6.
Serum levels of SDF-1α before and after PCI.
| SDF-1α, pg/ml | PPCI+RIPostC | PPCI | P |
|---|---|---|---|
| Preoperative | 96.62±23.14 | 88.34±32.99 | 0.19 |
| Postoprotive | |||
| 0.5 hour | 107.49±17.70 | 94.60±31.16 | 0.02* |
| 8 hour | 122.47±37.26 | 108.82±33.05 | 0.09 |
| 24 hour | 122.73±18.06 | 94.83±26.10 | <0.01** |
| 48 hour | 106.04±20.97 | 94.69±28.95 | 0.045* |
| 72 hour | 84.28±18.48 | 86.15±35.02 | 0.76 |
Values are mean ±SD.
P<0.05;
P<0.01.
Figure 4.
Stromal cell-derived factor-1α (SDF-1α) levels. Perioperative serum levels of SDF-1α (A). The area under the curve (AUC) of serum SDF-1α (B).
Discussion
The findings of this study showed that remote ischemic postconditioning (RIPC) of the upper arm following primary percutaneous coronary intervention (PCI) in patients with acute ST-segment elevation myocardial infarction (STEMI) could provide cardiac and renal protection from ischemia-reperfusion injury, and reduced creatine kinase-MB (CK-MB) release and preserved the left ventricular ejection fraction (LVEF), which might protect patients with STEMI from ischemia-reperfusion injury. Upper-arm RIPC was shown to decrease the rate of acute kidney injury (AKI) and attenuated the estimated glomerular filtration rate (eGFR) decline in patients with STEMI.
To the best of our knowledge, this is the first study to report the protective role of upper-arm RIPC on renal function in patients with STEMI undergoing primary PCI and is the first in which stromal cell-derived factor-1α (SDF-1α) was measured in patients with STEMI. RIPC on the upper arm can increase serum concentrations of nitric oxide (NO) and SDF-1α in patients with STEMI.
The culprit vessels in our study included the left anterior descending (LAD) artery, the circumflex coronary artery, and right coronary artery (RCA), while only the LAD artery was included in the study by Crimi et al. [18]. The peak CK-MB serum concentration in the primary PCI+RIPC group was significantly lower compared with the primary PCI group. The median CK-MB area under the curve (AUC) over 72 hours of primary PCI+RIPC was significantly less compared with that in conventional primary PCI.
The primary finding in this study was that RIPC could reduce the enzymatic changes, which may protect patients with STEMI from ischemia-reperfusion injury. The preserved LVEF in RIPC may be a result of reduced infarct size and edema or increase myocardial salvage in the acute phase of MI, though the exact mechanisms have yet to be elucidated. The humoral and neural signaling pathways, as well as the systemic inflammatory response, may work in combination. RIPC may share some factors in common with local ischemic conditioning. The reperfusion injury salvage kinase (RISK) pathway or the survivor activating factor enhancement (SAFE) pathway may be involved in the cardioprotection. However, due to the small number of patients included in this study, bias might not be excluded. Large multicenter controlled randomized clinical studies are required in the future.
The second main finding in this study was the detection of serum creatinine in patients, which showed the incidence of AKI. The incidence of AKI in the primary PCI group was significantly higher than that in the primary PCI+RIPC group, suggesting that upper-arm RIPC reduces the incidence of AKI in patients with STEMI. The eGFR in both groups tended to decrease,and decreased more significantly in the primary PCI group. AKI in patients with coronary artery disease (CAD), especially in patients with STEMI, may be associated with ischemia-reperfusion injury in the kidney [26]. The kidneys are target organs, just like the heart, for patients with STEMI undergoing PCI. RIPC in patients with STEMI may protect the kidneys and can reduce the incidence of AKI or contrast-induced nephropathy. Some of the findings of this study were consistent with a post hoc analysis by Crimi et al. [27], which reported that lower-limb RIPC was renal-protective in patients with baseline eGFR <77 mL/min/1.73 m2. Remote ischemic perconditioning was found to be protective against contrast-induced AKI by Yamanaka et al. [28] and Olafiranye et al. [29]. RIPC may share some features in common with remote ischemic perconditioning with respect to the mechanism of RIPC.
The serum levels of SDF-1α were measured in this study in patients with STEMI before PCI at 0.5 h, 1 h, 8 h, 24 h, 48 h, and 72 h after PCI. The results showed that the concentrations of SDF-1α and the AUC of the RIPC+primary PCI group were significantly higher compared with that of the primary PCI group. This is the first time that SDF-1α has been measured in patients with STEMI undergoing RIPC. The results suggest that SDF-1α may be one of the humoral factors by which RIPC protects in the target organ. SDF-1α, through binding to its receptor for CXCR4, has been previously shown to act on the ischemic area after myocardial infarction, promote cell repair, and reduce ischemia-reperfusion injury [30], and even improve left ventricular systolic function [31]. Studies have shown that SDF-1α-CXCR4 can promote the activation of PI3K, MEK1/2, and JAK via G1α protein activation, which in turn activates AKT, ErK1/2 and STATS, finally activating the SAFE and RISK signal transduction pathways, both of which are particularly important in mitigating ischemia-reperfusion injury [32] and may affect the mitochondrial ATP-sensitive potassium channel (mKATP) and mitochondrial permeability transition pore (MPTP), thereby reducing ischemia-reperfusion injury. Kamota et al. used mouse models of myocardial infarction through occlusion of the abdominal aorta to achieve RIPC, reporting that serum SDF-1α concentrations increased, contractile function improved, and myocardial apoptosis was reduced [33]. In the present study, the AUC of SDF-1α in the RIPC group was greater than that of the primary PCI group, which was consistent with the results of previously published animal studies.
The findings of the present study have, for the first time, detected nitric oxide (NO) in patients with STEMI who received primary PCI, with NO cumulative concentrations of RIPC and the AUC of NO at multiple time points being significantly higher, suggesting that RIPC in patients with STEMI may result in increased circulating NO concentrations. In a previously published animal study, RIPC was shown to be able to generate endogenous nitric oxide as an ischemic adaptation in an animal experiment [34]. Also, a previous study has shown that the protective effect of RIPC disappears when injected with NOS inhibitors [35]. The NIAMI study, a multicentre, double-blind, randomized, placebo-controlled trial designed to study the effect on myocardial infarct size of intravenous sodium nitrite over five minutes immediately prior to reperfusion by primary PCI in patients presenting with first acute STEMI showed that nitrates did not reduce the infarct size in patients with MI [36]. Therefore it is possible that the release of NO is only an important component of myocardial protection with RIPC.
This study had several limitations, including that it did not evaluate directly the clinical efficacy of the intervention, for example, the 30-day mortality, major adverse cardiac and cerebral event rate. The CK-MB level was the only surrogate endpoint. The degree of protection by RIPC with different culprit coronary arteries remains unclear. The effect of RIPC using upper-arm vs. lower-limb or the arm and leg together also need to be elucidated. The baseline renal function was normal or mildly impaired in patients enrolled in our study, with an eGFR >60 mL/min/1.73 m2, while patients with moderate or severe renal impairment or those requiring hemodialysis were excluded. However, patients with chronic renal insufficiency, especially those with eGFR <30 mL/min/1.73 m2, may be more likely to develop AKI; the incidence of contrast-induced nephropathy could be higher in clinical practice, and further research on RIPC is needed. Measurement of serum Cr is a suboptimal biomarker because it cannot rapidly reflect the degree of kidney injury [37]. More sensitive biomarkers such as urinary liver-type fatty acid-binding protein (L-FABP), neutrophil gelatinase-associated lipocalin, and cystatin-C are needed to evaluate renal function in future research studies. This study aimed at the preliminary observation of serum SDF-1α after RIPC in patients with STEMI. The relationship between RIPC and SDF-1α is not yet clear, and it is unknown how SDF-1α is produced and how its biological activity is preserved in the circulation. Therefore, the question of whether SDF-1α is a core humoral factor in RIPC remains unanswered. Finally, the findings of the study may not be sufficient to draw definitive conclusions due to the small sample size.
Conclusions
The findings of this small preliminary study showed that upper-arm remote ischemic postconditioning (RIPC) in patients with acute ST-segment elevation myocardial infarction (STEMI) followed by primary percutaneous coronary intervention (PCI) could reduce myocardial ischemia-reperfusion injury and protect renal function and that stromal cell-derived factor-1α (SDF-1α), and nitric oxide (NO) may be protective factors involved in RIPC.
Footnotes
Source of support: Departmental sources
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics – 2017 update: A report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39(2):119–77. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 4.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 5.Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: Comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–88. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 6.Staat P, Rioufol G, Piot C, et al. Postconditioning the human heart. Circulation. 2005;112:2143–48. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 7.Freixa X, Bellera N, Ortiz-Perez JT, et al. Ischaemic postconditioning revisited: lack of effects on infarct size following primary percutaneous coronary intervention. Eur Heart J. 2012;33:103–12. doi: 10.1093/eurheartj/ehr297. [DOI] [PubMed] [Google Scholar]
- 8.Hahn JY, Song YB, Kim EK, et al. Ischemic postconditioning during primary percutaneous coronary intervention: The effects of postconditioning on myocardial reperfusion in patients with ST-segment elevation myocardial infarction (POST) randomized trial. Circulation. 2013;128:1889–96. doi: 10.1161/CIRCULATIONAHA.113.001690. [DOI] [PubMed] [Google Scholar]
- 9.Engstrom T, Kelbaek H, Helqvist S, et al. Effect of ischemic postconditioning during primary percutaneous coronary intervention for patients with ST-segment elevation myocardial infarction: A randomized clinical trial. JAMA Cardiol. 2017;2:490–97. doi: 10.1001/jamacardio.2017.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Przyklenk K, Bauer B, Ovize M, et al. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–99. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- 11.Kerendi F, Kin H, Halkos ME, et al. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol. 2005;100:404–12. doi: 10.1007/s00395-005-0539-2. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt MR, Smerup M, Konstantinov IE, et al. Intermittent peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism: first demonstration of remote ischemic perconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H1883–90. doi: 10.1152/ajpheart.00617.2006. [DOI] [PubMed] [Google Scholar]
- 13.Andreka G, Vertesaljai M, Szantho G, et al. Remote ischaemic postconditioning protects the heart during acute myocardial infarction in pigs. Heart. 2007;93:749–52. doi: 10.1136/hrt.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Botker HE, Kharbanda R, Schmidt MR, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: A randomised trial. Lancet. 2010;375:727–34. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 15.White SK, Frohlich GM, Sado DM, et al. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2015;8:178–88. doi: 10.1016/j.jcin.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Yellon DM, Ackbarkhan AK, Balgobin V, et al. Remote ischemic conditioning reduces myocardial infarct size in STEMI patients treated by thrombolysis. J Am Coll Cardiol. 2015;65:2764–65. doi: 10.1016/j.jacc.2015.02.082. [DOI] [PubMed] [Google Scholar]
- 17.Hausenloy DJ, Kharbanda R, Rahbek Schmidt M, et al. Effect of remote ischaemic conditioning on clinical outcomes in patients presenting with an ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur Heart J. 2015;36:1846–48. [PubMed] [Google Scholar]
- 18.Crimi G, Pica S, Raineri C, et al. Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction: A randomized controlled trial. JACC Cardiovasc Interv. 2013;6:1055–63. doi: 10.1016/j.jcin.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Verouhis D, Sorensson P, Gourine A, et al. Effect of remote ischemic conditioning on infarct size in patients with anterior ST-elevation myocardial infarction. Am Heart J. 2016;181:66–73. doi: 10.1016/j.ahj.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 20.McCullough PA, Adam A, Becker CR, et al. Epidemiology and prognostic implications of contrast-induced nephropathy. Am J Cardiol. 2006;98:5K–13K. doi: 10.1016/j.amjcard.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Er F, Nia AM, Dopp H, et al. Ischemic preconditioning for prevention of contrast medium-induced nephropathy: Randomized pilot RenPro Trial (Renal Protection Trial) Circulation. 2012;126:296–303. doi: 10.1161/CIRCULATIONAHA.112.096370. [DOI] [PubMed] [Google Scholar]
- 22.Deftereos S, Giannopoulos G, Tzalamouras V, et al. Renoprotective effect of remote ischemic post-conditioning by intermittent balloon inflations in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2013;61:1949–55. doi: 10.1016/j.jacc.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 23.Heusch G, Botker HE, Przyklenk K, et al. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65:177–95. doi: 10.1016/j.jacc.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickard JM, Botker HE, Crimi G, et al. Remote ischemic conditioning: from experimental observation to clinical application: Report from the 8th Biennial Hatter Cardiovascular Institute Workshop. Basic Res Cardiol. 2015;110:453. doi: 10.1007/s00395-014-0453-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson SM, Selvaraj P, He D, et al. Remote ischaemic preconditioning involves signalling through the SDF-1alpha/CXCR4 signalling axis. Basic Res Cardiol. 2013;108:377. doi: 10.1007/s00395-013-0377-6. [DOI] [PubMed] [Google Scholar]
- 26.Tehrani S, Laing C, Yellon DM, Hausenloy DJ. Contrast-induced acute kidney injury following PCI. Eur J Clin Invest. 2013;43:483–90. doi: 10.1111/eci.12061. [DOI] [PubMed] [Google Scholar]
- 27.Crimi G, Ferlini M, Gallo F, et al. Remote ischemic postconditioning as a strategy to reduce acute kidney injury during primary PCI: A post-hoc analysis of a randomized trial. Int J Cardiol. 2014;177:500–2. doi: 10.1016/j.ijcard.2014.08.080. [DOI] [PubMed] [Google Scholar]
- 28.Yamanaka T, Kawai Y, Miyoshi T, et al. Remote ischemic preconditioning reduces contrast-induced acute kidney injury in patients with ST-elevation myocardial infarction: a randomized controlled trial. Int J Cardiol. 2015;178:136–41. doi: 10.1016/j.ijcard.2014.10.135. [DOI] [PubMed] [Google Scholar]
- 29.Olafiranye O, Ladejobi A, Wayne M, et al. Renal protection using remote ischemic peri-conditioning during inter-facility helicopter transport of patients with ST-segment elevation myocardial infarction: A retrospective study. J Interv Cardiol. 2016;29:603–11. doi: 10.1111/joic.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaruba MM, Franz WM. Role of the SDF-1-CXCR4 axis in stem cell-based therapies for ischemic cardiomyopathy. Expert Opin Biol Ther. 2010;10:321–35. doi: 10.1517/14712590903460286. [DOI] [PubMed] [Google Scholar]
- 31.Tang J, Wang J, Song H, et al. Adenovirus-mediated stromal cell-derived factor-1 alpha gene transfer improves cardiac structure and function after experimental myocardial infarction through angiogenic and antifibrotic actions. Mol Biol Rep. 2010;37:1957–69. doi: 10.1007/s11033-009-9642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: Underlying mechanisms and clinical application. Cardiovasc Res. 2008;79:377–86. doi: 10.1093/cvr/cvn114. [DOI] [PubMed] [Google Scholar]
- 33.Kubo M, Li TS, Kamota T, et al. Increased expression of CXCR4 and integrin alphaM in hypoxia-preconditioned cells contributes to improved cell retention and angiogenic potency. J Cell Physiol. 2009;220:508–14. doi: 10.1002/jcp.21803. [DOI] [PubMed] [Google Scholar]
- 34.Rassaf T, Totzeck M, Hendgen-Cotta UB, et al. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114:1601–10. doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]
- 35.Tang YH, Xu JJ, Li JX, Cheng XS. Remote postconditioning induced by brief pulmonary ischemia and reperfusion attenuates myocardial reperfusion injury in rabbits. Chin Med J (Engl) 2011;124:1683–88. [PubMed] [Google Scholar]
- 36.Siddiqi N, Neil C, Bruce M, et al. Intravenous sodium nitrite in acute ST-elevation myocardial infarction: A randomized controlled trial (NIAMI) Eur Heart J. 2014;35:1255–62. doi: 10.1093/eurheartj/ehu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Igarashi G, Iino K, Watanabe H, Ito H. Remote ischemic pre-conditioning alleviates contrast-induced acute kidney injury in patients with moderate chronic kidney disease. Circ J. 2013;77:3037–44. doi: 10.1253/circj.cj-13-0171. [DOI] [PubMed] [Google Scholar]