Summary
Regeneration Biology is the study of organisms with endogenous regenerative abilities, whereas Regenerative Medicine focuses on engineering solutions for human injuries that do not regenerate. While the two fields are fundamentally different in their approach, there is an obvious interface involving mammalian regeneration models. The fingertip is the only part of the human limb that is regeneration‐competent and the regenerating mouse digit tip has emerged as a model to study a clinically relevant regenerative response. In this article, we discuss how studies of digit tip regeneration have identified critical components of the regenerative response, and how an understanding of endogenous regeneration can lead to expanding the regenerative capabilities of nonregenerative amputation wounds. Such studies demonstrate that regeneration‐incompetent wounds can respond to treatment with individual morphogenetic agents by initiating a multi‐tissue response that culminates in structural regeneration. In addition, the healing process of nonregenerative wounds are found to cycle through nonresponsive, responsive and nonresponsive phases, and we call the responsive phase the Regeneration Window. We also find the responsiveness of mature healed amputation wounds can be reactivated by reinjury, thus nonregenerated wounds retain a potential for regeneration. We propose that regeneration‐incompetent injuries possess dormant regenerative potential that can be activated by targeted treatment with specific morphogenetic agents. We believe that future Regenerative Medicine‐based‐therapies should be designed to promote, not replace, regenerative responses. Stem Cells Translational Medicine 2018;7:262–270
Significance Statement.
The distal tip of the human and mouse digit is capable of endogenous regeneration after amputation and the identification of critical components of this response has led to treatments that expand the regenerative capabilities of nonregenerative amputation wounds. These studies begin to identify the regenerative potential of cells involved in nonregenerative healing, and how targeted treatment can lead to an organized multi‐tissue regeneration response. This study proposes that expanding the understanding of regenerative potential can lead to a future in which Regenerative Medicine‐based‐therapies target the promotion of regeneration, rather than the current strategy of simply replacing damaged structures.
Introduction
In thinking about regeneration, it is important to delineate Regeneration Biology from Regenerative Medicine. Regeneration Biology focuses on endogenous regenerative abilities which includes homeostatic turnover of tissues (Physiological Regeneration) as well as the response to injury regardless of whether repair is partial or complete (Reparative Regeneration). In reparative regeneration, the capacity to regenerate (Regenerative Ability) is an endogenous tissue or organ specific characteristic and a mechanistic understanding of the regeneration processes is proposed to impact nonregenerative responses. This proposal is justified because regenerative ability is an ancient characteristic that is robust in primitive organisms but limited among animals that have evolved more recently, such as mammals 1. This general phylogenetic relationship indicates that the inability to regenerate (Regenerative Failure) evolved from a regenerative precondition; regenerative failure results from a disruption of an endogenous regeneration process. Thus, cells involved in an injury response are inhibited from following a regeneration path, and since it is well established that the regeneration process involves a complex series of steps 2, much like embryonic development, a detailed understanding of the regeneration process can lead to the identification of strategies to stimulate regeneration at sites where regenerative failure is the norm. A number of recent studies have now demonstrated that regeneration can be stimulated in rodent models 3, 4, 5, 6, 7, 8, 9, 10, 11 and one general conclusion is that regenerative failure is linked to the microenvironment of the healing wound and not to an inability of cells to respond in a pro‐regenerative manner. Thus, cells involved in a nonregenerative injury evince a Regenerative Potential that underlies dormant pro‐regenerative pathways not activated during an injury response. These findings identify an interface in which regeneration biology can play a key role in the field of Regenerative Medicine.
Regenerative Medicine is a relatively new field dedicated to engineering solutions for Regenerative Failure in humans. Broadly speaking, the field encompasses all parts of the human body but uses two specific strategies: manipulation of stem cells (e.g., MSCs, iPSCs, etc.) and the engineering of extracellular templates (e.g., synthetic scaffolds, decellularized organs, etc.). These approaches have had an enormous impact on translational medicine, although not all positive. For example, clinical use of engineered trachea has been far from successful 12, and while stem cell based therapies are currently in clinical trials for various diseases (e.g., spinal cord injury, myocardial infarction, age‐related macular degeneration 13), it is acknowledged that scientific evidence supporting the efficacy of these therapies is lacking 14, 15. On the other hand, regenerative scaffolds have demonstrated clinical success. For instance, peripheral nerve allografts (Axogen, Avance Nerve Graft), and natural (Integra, NeuraGen), and synthetic (Polyganics, Neurolac) polymer‐based devices have been shown to promote peripheral nerve regeneration across injury gaps ranging from 20 mm to 3 cm 16, 17, 18, 19. Moreover, there are a spectrum of regenerative scaffolds used clinically for aiding skin regeneration after mechanical trauma, severe burns, and aging, with current therapies in wide use 20. The field of Regenerative Medicine is beginning to recognize that endogenous regenerative ability plays an important role in the therapeutic outcome 21, although an understanding of regenerative ability as well as regenerative potential is severely lacking.
The regeneration of the fingertip represents a clear example of human regenerative ability, and this contrasts the regenerative failure of amputations proximal to the fingertip. Fingertip regeneration was first reported in a case involving amputation of an infected adult fingertip treated with repeated dressing changes that resulted in a regenerative outcome documented by x‐ray imaging over the next 3 months 22. Fingertip regeneration following conservative treatment of amputations in children has also been documented in the clinical literature 23, 24. While the question of whether to treat fingertip amputations that present with exposed bone in a conservative manner remains controversial 25, there is clear evidence from regenerative models that closure of the amputation wound with mature skin is inhibitory for a regeneration response 26. Thus, in some ways, current clinical practices are contraindicated for a successful regenerative response.
The clinical literature demonstrates that the human fingertip has regenerative ability; however, it does not bring us closer to understanding the processes underlying this phenomenon. The importance of a mechanistic understanding of regeneration allows for targeted intervention to improve the response (e.g., a reduction in patient variability), and to begin to explore ways to stimulate regeneration where it does not normally occur. The mouse digit responds to amputation in a manner that parallels humans 27 and has become a premiere testing ground for studying regenerative ability as well as regenerative potential 28, 29, 30. In the remainder of this article, we will discuss what is currently known about the regenerative ability of the digit tip, and how we can exploit the regenerative potential of nonregenerative amputations of the digit and limb.
Part I: Endogenous Regeneration of the Mouse Digit Tip
The distal tip of the terminal phalanx (P3), the last bone in the mouse digit, regenerates after amputation (Fig. 1). Distal amputation removes 15%–20% of the P3 bone, but does not damage the bone marrow, fat pad, or proximal nail matrix. Digit tip regeneration is a complex process both resembling and differing from digit development, and occurs through sequential phases that include inflammation, histolysis, epidermal closure, blastema formation, and differentiation to restore amputated structures 31, 32. What distinguishes digit tip regeneration from other tissue‐specific regenerative responses, such as fracture repair and skeletal muscle injury, is that it is mediated by the formation of a blastema. The blastema, best characterized in salamander limb regeneration 2, is a transient structure comprised of proliferative undifferentiated cells that undergo pattern formation, morphogenesis, and differentiation to regenerate structures lost by amputation 33, 34. The mouse digit tip blastema is remarkably similar to the blastema formed in response to axolotl limb and zebrafish fin amputation, reviewed nicely in 35, 36. Blastema mediated regeneration is defined as an epimorphic response, and is considered a rare event in mammals including, in addition to digit tip regeneration, the regeneration of ear hole punch wounds in rabbits and some rodents and the annual regeneration of antlers in deer 37.
Figure 1.
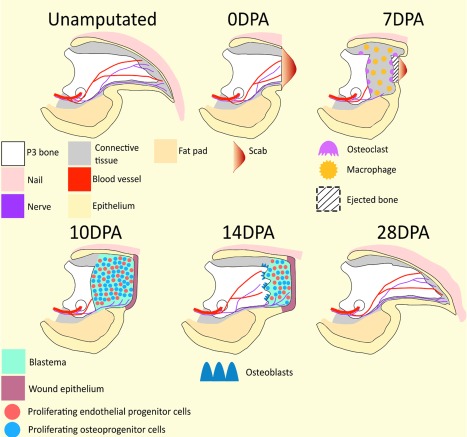
Digit tip regeneration. Unamputated: The mouse terminal phalanx (P3), is a triangular shaped cortical bone; wide at its base where it articulates with the second phalanx (P2; not pictured), gradually narrowing until it terminates as a pointed tip. Vasculature and nerves enter the P3 bone marrow via foramen referred to as os holes (shown as circles) located on either side of the ventral base of P3. The nail organ surrounds the entire digit except for the ventral surface where the ventral epidermis is an extension of the digital fat pad. Nerves and blood vessels are localized throughout the connective tissue located between the P3 bone and surrounding epidermis. 0 DPA: A scab forms in response to distal P3 amputation. Distal amputation removes 15%–20% of the P3 bone volume, but does not damage the bone marrow, fat pad, or proximal nail matrix (not shown). 7 DPA: Macrophages and other cells of the innate immune response (not shown) are scattered throughout the connective tissue and P3 bone marrow. Concurrently, large, multinucleated osteoclasts degrade the periosteal and endosteal surfaces of the P3 bone. Osteoclast activity erodes the P3 bone into two segments, thus exposing the P3 bone marrow. The remaining proximal bone stump will be reincorporated into the regenerated digit tip. 10 DPA: Epidermal migration proximal to the eroded bone functions to close the wound and eject the eroded bone. Wound closure is associated with subjacent blastema formation and the culmination of histolysis. The blastema is avascular, but is innervated. 14 DPA: Intramembranous bone redifferentiation and associated revascularization occurs proximal to distal within the wound environment. As new tissues are regenerated proximally, the distal blastema shrinks in size. 28 DPA: Digit regeneration is complete by 28 DPA, resulting in woven bone cosmetically larger than the unamputated digit. The regenerated digit tip is innervated, vascularized, and restores preamputation length. Distal is to the right. Abbreviation: DPA, days post amputation.
Inflammation
In response to digit tip amputation, a scab forms over injured tissues. At 3 days post amputation (DPA), F4/80+ macrophages and Ly6B.2+ neutrophils infiltrate the tissues adjacent to the amputation injury 38. Macrophage and neutrophil levels peak at 7 DPA, but are found in spatially distinct regions of the digit tip. Macrophages accumulate along the endosteum of the P3 bone and proximal dermis associated with the nail matrix while neutrophils localize to the P3 bone marrow and connective tissue surrounding the P3 bone 38. When the blastema forms at 10 DPA, neutrophils but not macrophages, are found throughout the blastema 38. From 14–21 DPA, macrophage and neutrophils return to preamputation levels.
Macrophages can be both permissive and inhibitory to regeneration. For instance, mice deficient in macrophages are capable of scar free wound healing suggesting that macrophages inhibit the regenerative response 39. However, macrophage depletion inhibits regeneration of the digit tip 38 as it does for the regeneration of zebrafish tail fins, axolotl limbs, and ear holes of the spiny mouse 40, 41, 42. During digit tip regeneration, macrophage depletion modifies early events in the regeneration process, including an inhibition of histolysis and wound closure, and this results in a failure of the blastema to form 38. The result indicates that macrophage recruitment during early wound healing stages is required to set the stage for blastema formation and the regeneration of the digit tip.
Histolysis
Histolysis is the second stage of digit tip regeneration and overlaps with the inflammatory stage. Histolysis is the enzymatic degradation of extracellular matrix resulting in the loss of organized tissues 43. While the histolytic response occurs in all tissues of the amputation wound, the best characterized response is the degradative response of bone because it can be monitored in vivo by microcomputed tomography imaging. At 5 DPA, large, multinucleated osteoclasts can be seen scattered across the P3 bone and it is thought that these osteoclasts arise from the fusion of monocytes recruited during inflammation. Osteoclast number peaks at 7 DPA, but are rapidly depleted by 10 DPA. During this 5‐day period, osteoclasts lining the endosteum and periosteum degrade through the P3 bone creating a secondary amputation and effectively splitting it into proximal and distal halves. The distal P3 bone is not reincorporated into the regenerate. Rather, the dorsal and ventral epidermis migrate to close the wound proximal to the distal P3 stump bone, effectively ejecting it along with the wound scab from the digit 31. The role that osteoclast‐driven bone degradation plays in regeneration is significant: initially 20% of the P3 bone volume is amputated; however, the histolytic response results in the additional degradation of 2.5 times more stump bone. It should be noted that 70% amputation of the digit identifies a nonregenerative proximal amputation 44, 45, suggesting that the histolytic phase is acting in a pro‐regenerative manner.
Why histolysis occurs remains in question, but there is precedence for it in other organisms such as the axolotl and the mammalian peripheral nerve 46, 47. There are a few things that we do know which helps to unravel this mystery. First, specifically inhibiting osteoclasts impairs the regeneration response, although it is not inhibited 32, 38. Second, osteoclasts and histolysis are regulated in part by dynamically changing oxygen tensions and the blastema itself is known to be transiently avascular and hypoxic 5, 48. Hyperbaric oxygen treatment to decrease hypoxia during the regeneration response extends the period of osteoclast activity and increases the amount of P3 bone degraded 48, 49. Conversely, facilitating wound closure with a Dermabond wound dressing enhances hypoxia, reduces osteoclast numbers, and decreases bone degradation 32. Third, blastema size positively correlates with the amount of histolysis; increased histolysis generates larger blastemas while decreasing histolysis creates smaller blastemas 32. These data support the hypothesis that tissue histolysis releases cells and factors embedded in mature tissues, contributing to a pro‐regenerative wound environment 50.
Wound Closure
Rapid wound re‐epithelization is a characteristic common to many regeneration models 47; however, in the digit tip response, wound closure correlates with the end of histolysis and is significantly delayed 31, 32. After amputation the injured epidermis does not migrate across the exposed P3 bone, but instead the epidermis retracts and establishes connections with the periosteum of the P3 bone stump, effectively sealing the amputation wound during the inflammation and histolytic phases. After bone degradation, epidermal migration through the region of degraded bone completes wound closure and forms a wound epithelium (WE) that caps the regenerating stump 31. The WE is a transient structure that is required for blastema formation and acts as a signaling center for mesenchymal blastema cells 8. Inhibiting WE formation or replacing it with mature skin inhibits regeneration in amphibians as well as mammals, including humans 24, 38, 51. In digit tip regeneration, the WE is a source for stromal cell‐derived factor 1 (SDF‐1), a known chemoattractant for blastema cells 9, and the WE is required for blastema formation 38. Additional studies are needed before we can fully appreciate the role that the WE plays in regulating the regeneration response, and given current clinical practices for the treatment of amputation wounds, it is an obvious and clinically relevant area of research to pursue.
Blastema
The completion of wound closure appears to function as a transitional switch that signals the end of the histolytic phase and the initiation of the blastema phase. The blastema is a transient aggregation of undifferentiated cells that forms between the proximal P3 bone stump and the distal WE. The mouse digit blastema is characterized as avascular, hypoxic, and highly proliferative 5, 31, 48. The cells of the blastema are heterogeneous and derived from multiple tissue sources, including the epidermis, bone, vasculature, and loose connective tissues 31, 52, 53. To date, studies show that blastema cells are lineage restricted 52, 53, that is, their fate during regeneration does not deviate from their tissue of origin; however, cell types known to be multipotent in other injury repair models 54 have yet to be studied. As mentioned above, the recruitment of blastema cells is mediated through the SDF‐1 signaling pathway, and many blastema cells express CXCR4 and CXCR7, known receptors for SDF‐1 9. As cells migrate to form the blastema, they organize themselves by producing an extracellular matrix (ECM) rich in Collagen III (Col3) and, as newly regenerated structures differentiate, the blastema ECM is degraded and replaced 55.
A hallmark characteristic of the blastema is its immense proliferative ability. Several signaling pathways have been shown to be critical to maintaining proliferation. Bone morphogenetic proteins (BMPs) have long been identified as potent proliferative molecules and a requirement for digit tip regeneration 4, 56. This is best demonstrated in loss‐of‐function studies in which amputated digits treated with noggin, a BMP‐inhibitor, do not regenerate 4. Paracrine signaling from surrounding tissues also influences blastema proliferation. Wnt signaling in the proximal nail bed enhances blastema proliferation by regulating FGF secretion from digital nerves 8. Evidence for mitogenic paracrine signaling from nerves is supported by denervation experiments that also reduce blastema proliferation. Recently, it has been suggested that mitogenic neurotrophic factors are not secreted from the axons, but by Schwann cells 10. Blastema signaling networks are highly regulated and exogenous growth factors that increase proliferation do not always enhance regeneration. For instance, the blastema is avascular and correlates with downregulation of pro‐angiogenic (Vegfa) and upregulation of anti‐angiogenic (Pedf) activity. Inducing precocious angiogenesis with exogenous VEGFA (or BMP9 which induces Vegfa expression) inhibits digit tip regeneration but can be rescued if digits are subsequently treated with PEDF 5.
Differentiation
The differentiation phase of regeneration is highly coordinated and progresses in a temporal sequence that initiates proximally and progresses to the distal tip. In this way, the differentiation of newly regenerated bone is graded, first building on the stump bone and progressively adding new bone as the digit tip elongates. The differentiation of regenerated bone from the blastema is rapid and new bone forms by direct ossification that involves the deposition of osteoid in a pericellular manner to form woven bone 57. In development, the digit tip is initially formed by endochondral ossification and its maturation involves an extended period of postnatal elongation involving a proximal growth plate and distal appositional ossification to form cortical bone 58. Thus, the regeneration response does not involve a slow and deliberate reiteration of digit formation, but instead adapted an alternative osteogenic mechanism to effect a similar outcome over a shorter time frame. While regenerated woven bone is highly porous and considered weaker than the original cortical bone, the regenerated P3 element is 50% larger than the original digit tip while maintaining a constant length 31. We suspect that this overshoot in regenerated bone volume evolved as a way to replace bone function with an inherently weaker structure. With time, regenerated bone increases in density but maintains a histologically distinct microarchitecture 31.
Expanding Regenerative Potential Based on Our Current Understanding of Regenerative Ability
So what lessons can we learn from the digit tip's innate Regenerative Ability? First, studying digit tip regeneration has identified cells, factors, and signaling pathways that are necessary for regeneration. All of these represent potential targets to remedy a failed regenerative response, and their deficiency in a nonregenerative wound environment would prompt the development of a pro‐regeneration strategy. Second, regeneration involves the recruitment and proliferation of stem/progenitor cells derived from local tissues that maintain spatial information required for an appropriate regeneration response. Stem‐cell based therapies which have pro‐regenerative effects without the direct integration of stem cells into injured tissue are likely functioning by activating the regenerative potential of local cell sources 21 in a manner that parallels endogenous regenerative ability. Third, regeneration is a dynamic process involving stepwise phases of overlapping and interdependent events, thus it is clear that manipulating one stage will likely affect later stages (Fig. 1). Therefore, in designing therapies, it is critical to consider how early treatments will alter later regeneration stages. Finally, the scaffold produced by regenerating cells is transiently modified during blastema formation, then remodeled to re‐establish the original scaffold 55. Such dynamic processing of the regenerative scaffold is inconsistent with strategies for engineered scaffold designs that are based on the architecture of the target structure. In the next section, we discuss digit and limb injuries that do not regenerate, and strategies for exploring their regenerative potential based on an understanding of regenerative ability.
Part II: Wound Repair and Induced Digit Regeneration
In mice, a strategy for enhancing regeneration of nonregenerative injuries was first established by studies showing that BMP signaling was required for P3 regeneration and that regeneration could be restored or stimulated by treatment to activate the BMP signaling pathway 4, 56. Similar loss of function and gain of function studies have also identified the nail organ/WNT signaling 8, 59, and Schwann cells/PDGF/Oncostatin M signaling 10 as essential for digit tip regeneration. Alternatively, angiogenesis during wound healing has been shown to be inhibitory for regeneration and the anti‐angiogenic factor PEDF counteracts this inhibition 5.
Induced Regeneration
A conceptually similar but significantly more challenging approach is to focus on a proximal amputation injury that is nonregenerative at all stages of development. It is generally recognized that developing or immature tissues display an enhanced level of regenerative ability when compared to adults. In humans, full thickness skin wounds undergo a defective healing response resulting in scar formation, whereas fetal skin wounds do not form scars but regenerate perfect replacement skin 60. Modulation of the transforming growth factor (TGF‐β) repertoire in adults to mimic that of fetal skin wounds results in scar free skin regeneration and thus indicates latent regenerative potential of adult skin that can be extrinsically activated 60. The embryonic chick limb is another example of regenerative potential; amputated early stage chick limbs fail to regenerate, yet targeted application of Fibroblast Growth Factors stimulates reprograming of local mesodermal cells and subsequent induced limb regeneration 61, 62, 63. These examples identify developing or immature tissues as possessing dormant regenerative potential that can be used to characterize defects in the injury response, and to identify agents that can stimulate regeneration.
Amputation of the nonregenerative neonatal mouse digit/limb has been used to demonstrate and characterize BMP induced regeneration 3, 4, 6, 11, 64. In amputated neonatal digits, BMP2 targeted treatment stimulates skeletal elongation by inducing the formation of a distal endochondral ossification center (EOC) at the amputation wound that functions as a morphogenetic center to organize the regeneration of new bone onto the stump 64. Targeted BMP treatment involves loading BMP onto a vehicle that is engrafted into the amputation wound after wound closure. This allows for transient release of BMP over a 2‐ to 3‐day period, and during that time the EOC is established. The actual regeneration of new bone is mediated by the EOC and is independent of additional BMP treatment. An important aspect of these studies is that the skeletal structure induced to regenerate is dictated by the amputation level and not by the BMP treatment itself, thus BMP is functioning as a morphogenetic agent to establish the EOC and not as a morphogen to dictate the structure that regenerates. These findings suggest the cells at differing amputation levels are regeneration responsive and retain a positional memory that can be triggered to determine the appropriate structure of the regenerate 64. This conclusion is supported by a number of different studies showing BMP induced regeneration is specific to distinct amputation levels in mice 3, 4, 6, 11, 64. Notably, in vitro studies using fibroblasts isolated from P3 (regeneration‐competent) or P2 (regeneration‐incompetent), digit regions suggest that these cells retain positional characteristics, but that positional memory alone does not limit or induce a regeneration response 65.
Regenerative Failure and Induced Regeneration in Adults
Amputation of the mouse digit at the level of the P2 bone has emerged as a standardized system to investigate regenerative failure 7, 11, 50, 64, 66, 67, 68, 69, 70, 71, 72, 73. P2 amputation removes all distal elements and traverses multiple tissue types, including the P2 diaphysis, bone marrow, dorsal elastic claw ligament, digital flexor tendon, and skin 74, and ultimately results in skeletal truncation covered by a fibrotic scar 50. While the outcome of amputation is skeletal truncation, the injury response is quite dynamic and initiates a skeletal repair response analogous to the proximal bone segment of long bone fracture repair 50. The well‐characterized cellular events of fracture repair are delineated into several stages, including inflammation, cartilaginous callus formation, boney callus formation, and eventual remodeling of the boney callus into a structure that resembles the preinjury bone 75, 76, 77, 78, 79. Likewise, P2 amputation initiates an inflammatory response that is followed by proliferation of local periosteal cells that differentiate into chondrocytes that form a cartilaginous callus external to the bone surface, that is, the peripheral callus. Vascular invasion and osteoblast recruitment reorganize the cartilaginous peripheral callus into a woven boney callus, and this is followed by remodeling of the boney callus into a lamellar structure that largely resembles the original bone stump 50. After remodeling, the truncated stump bone appears identical to the amputated P2 element, so there is the impression that the injury response is largely inert. However, the response is dynamic and consistent with the conclusion that the injury response involves an attempt at regeneration that ultimately fails 50. Because this injury response is dynamic, it establishes a model to test strategies for enhancing a regenerative response.
The transient formation of the peripheral cartilaginous callus by periosteal cells following amputation identifies a potential cell target for regenerative intervention. Periosteal cells are known to be critical for skeletal regeneration in fracture healing 79, 80, and are responsive to BMP2 81, 82, 83. Targeted BMP2 treatment during the periosteal response was found to induce the formation of a distal cartilaginous callus that paralleled EOC formation in neonatal models 11, 64. The distal callus is comprised of proliferating chondrocytes undergoing endochondral ossification and functions as a template for new bone formation, resulting in restoration of amputated skeletal length (Fig. 2) 11. These studies provide a proof of concept that targeted intervention of an injury response that fails to regenerate can be effectively induced to undergo a significant regenerative response. These findings also begin to characterize the regenerative potential of nonregenerative amputation injuries and show that latent regenerative ability can be activated by a single factor that is administered in a spatiotemporally targeted manner. Therefore, it is reasonable to conclude that the root cause of regenerative failure following amputation injury is not a lack of regeneration‐responsive cells, but rather a toxic wound environment that precludes a regeneration response.
Figure 2.
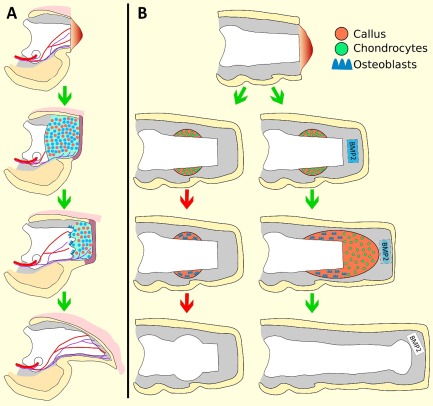
Schematic diagram of induced P2 regeneration. (A): The regeneration response following amputation of P3 is used as a regeneration competent model to identify factors required for blastema formation and a regeneration response. Refer to Figure 1 for full description. A number of factors, including BMP2, have been shown to be essential for the P3 regeneration response. (B): Amputation of P2 induces a dynamic wound repair response characterized by periosteal chondrogenic callus formation, followed by callus conversion to woven bone, and ultimately truncation of the bone at the original amputation plane. The wound healing response is likened to an attempt at regeneration that ultimately fails, indicated by the red arrows. BMP2 treatment to target the periosteal chondrogenic callus induces the formation of a distal chondrogenic callus that functions as a template for subsequent bone regeneration to restore the amputated skeletal length. The change in color gradient of the BMP2‐delivery‐vehicle reflects the exhaustion of BMP2. Distal is to the right.
The Regeneration Window
In neonatal mice, BMP2 treatment over a 2‐ to 3‐day period is sufficient to initiate a significant regenerative response. The use of a vehicle that effects transient BMP2 release has allowed an investigation into the temporal responsiveness of cells at the amputation wound. Such studies have identified a peak in responsiveness that correlates with the timing of wound closure and identified reduced responses of treatments at earlier and later time points 11. This illustrates a dynamic of the wound environment with respect to regenerative potential, and identifies a temporally specific Regeneration Window within which BMP2 is effective at activating a regeneration response. As the P2 amputation wound matures it becomes refractory to BMP2; however, we show that simple reinjury of the healed P2 stump reinitiates the injury response and recreates a wound environment that cycles through a BMP2 responsive window, complete with distal callus formation and subsequent skeletal regeneration (Fig. 3A) 11. In other words, mature nonregenerative amputation wounds respond to injury in a manner similar to the initial amputation, thus the process of nonregenerative wound healing does not alter the wound's regenerative potential. We note the clinical significance of this finding with regard to limb amputations; since BMP2 stimulates segment‐specific bone regeneration of adult limb (Fig. 3B) 64, we predict that previously healed amputation wounds in humans can be induced to regenerate.
Figure 3.
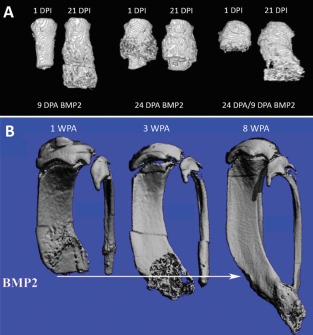
BMP2‐induced regeneration. (A): Micro‐computed tomography (MicroCT) three‐dimensional (3D) renderings of BMP2‐treated adult mouse P2 digits. BMP2 treatment during cartilaginous peripheral callus formation, at 9 DPA, induces robust skeletal regeneration, evident by 21 DPI. BMP2‐treatment after the cartilaginous peripheral callus conversion to boney tissue at 24 DPA does not induce skeletal regeneration. Reinjury of previously healed 24 DPA P2 digits stimulates a regeneration permissive environment in which BMP2 functions to induce regeneration. (B): Sequential MicroCT 3D renderings of the BMP2‐induced hind limb regeneration response in adult mice. Amputation plane shown as arrow. Hind limbs were treated with BMP2 at 2 WPA. BMP2‐induced regeneration is evident by 3 WPA, shown as the formation of woven bone distal to the amputation plane. By 8 WPA, distal skeletal fusion is shown, indicating the regeneration response is associated with the reestablishment of skeletal patterning. (A, B) Distal is to the bottom. (A) Reprinted from Dawson et al. 11. (B) Reprinted from Yu et al. 64. Abbreviations: DPA, days post amputation; DPI, days post implantation; WPA, weeks post amputation.
In its simplest form, and at a cellular level, the concept of a Regeneration Window can be viewed in the context of the availability of cells expressing appropriate receptors that can be activated by targeted treatment with a regeneration inducing factor. Indeed, targeted genetic or pharmacological knockdown of individual signaling pathways to inhibit the endogenous regeneration response supports this simplistic model 4, 8, 56. However, an alternative way to think about the regeneration window is that multiple cell types that function in a coordinated manner are required to effect a regeneration response. For example, BMP2 induced P2 regeneration in neonates involves the action of BMP2 as a mitogen for cells that become proliferating chondrocytes and establish the EOC 64. At the same time, BMP2 also induces expression of Sdf1a by cells of the wound epidermis and endothelial cells of the wound mesenchyme, and SDF1 functions to recruit CXCR4 expressing cells for the regeneration response 9. Thus, at a minimum, BMP2 is activating three different cell types to induce skeletal regeneration, and it is likely that all three are needed to induce the response. It is therefore important to recognize that the Regeneration Window identifies a multi‐tissue response that is coordinated by the morphogenetic action of the inducing agent, in this case BMP2. We anticipate that other key morphogenetic agents will be identified that induce regeneration of other complex tissues that lack regenerative ability but possess regenerative potential.
Conclusion
Endogenous epimorphic regenerative responses in mammals, such as the mouse digit tip and human fingertip, provide models in which all requirements for a successful regeneration response are intrinsically met, for example, angiogenesis, neurogenesis, inflammation, trophic factors, and regeneration‐competent cells. As we come to understand the regeneration response, it follows that the conceptual application of these requirements to regeneration‐incompetent injuries can effectively guide the design of therapeutic strategies for human regeneration. The example of the regeneration‐incompetent middle phalanx (P2) is a case in point. Investigating this amputation injury identified a responsive population of cells and targeted application of BMP2 successfully induced regeneration of the skeletal element. These studies lead to three important conclusions that can be generalized to other injury models. First, regeneration‐competent cells are present at traumatic injury wound sites, but they undergo dynamic changes and are only responsive during a restricted period of the healing process, the Regeneration Window. Second, since BMP2 can stimulate appropriately patterned regeneration responses from different amputation levels, it is acting as a morphogenetic agent to elicit patterned responses and not as a morphogen that instructs patterning. Third, regenerative failure is caused by a toxic wound environment that minimally lacks the signaling profile of a morphogenetic agent necessary to coordinate a multi‐tissue regenerative response. As we tease apart mammalian regeneration we are finding that partial regenerative responses can be stimulated from regeneration‐incompetent injuries, and continued studies are expected to enhance the diversity of responses. To date, the stimulation of partial regenerative responses has not involved the formation of a blastema, thus a long‐term goal will be to solve the puzzle of how to build a blastema, a structure that coordinates pattern formation, morphogenesis, and differentiation of a complete regenerative response.
Author Contributions
C.P.D. and L.A.D.: conception and design, data analysis and interpretation, manuscript writing; K.M.: conception and design, data analysis and interpretation, final approval of manuscript, financial support, administrative support.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
We would like to thank members of the Muneoka Laboratory for their thoughtful discussions. K. Muneoka is supported by Texas A&M University.
References
- 1. Sanchez Alvarado A. Regeneration in the metazoans: Why does it happen? Bioessays 2000;22:578–590. [DOI] [PubMed] [Google Scholar]
- 2. Tanaka EM. The molecular and cellular choreography of appendage regeneration. Cell 2016;165:1598–1608. [DOI] [PubMed] [Google Scholar]
- 3. Masaki H, Ide H. Regeneration potency of mouse limbs. Dev Growth Differ 2007;49:89–98. [DOI] [PubMed] [Google Scholar]
- 4. Yu L, Han M, Yan M et al. BMP signaling induces digit regeneration in neonatal mice. Development 2010;137:551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu L, Yan M, Simkin J et al. Angiogenesis is inhibitory for mammalian digit regeneration. Regeneration (Oxf) 2014;1:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ide H. Bone pattern formation in mouse limbs after amputation at the forearm level. Dev Dyn 2012;241:435–441. [DOI] [PubMed] [Google Scholar]
- 7. Mu X, Bellayr I, Pan H et al. Regeneration of soft tissues is promoted by MMP1 treatment after digit amputation in mice. PLoS One 2013;8:e59105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takeo M, Chou WC, Sun Q et al. Wnt activation in nail epithelium couples nail growth to digit regeneration. Nature 2013;499:228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee J, Marrero L, Yu L et al. SDF‐1α/CXCR4 signaling mediates digit tip regeneration promoted by BMP‐2. Dev Biol 2013;382:98–109. [DOI] [PubMed] [Google Scholar]
- 10. Johnston AP, Yuzwa SA, Carr MJ et al. Dedifferentiated Schwann cell precursors secreting paracrine factors are required for regeneration of the mammalian digit tip. Cell Stem Cell 2016;19:433–448. [DOI] [PubMed] [Google Scholar]
- 11. Dawson LA, Yu L, Yan M et al. The periosteal requirement and temporal dynamics of BMP2‐induced middle phalanx regeneration in the adult mouse. Regeneration 2017;4:140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cyranoski D. Surgeon commits misconduct. Nature 2015;521:406–407. [DOI] [PubMed] [Google Scholar]
- 13. Trounson A, DeWitt ND. Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol 2016;17:194–200. [DOI] [PubMed] [Google Scholar]
- 14. Marks PW, Witten CM, Califf RM. Clarifying stem‐cell therapy's benefits and risks. N Engl J Med 2017;376:1007–1009. [DOI] [PubMed] [Google Scholar]
- 15. Daley GQ. Polar extremes in the clinical use of stem cells. N Engl J Med 2017;376:1075–1077. [DOI] [PubMed] [Google Scholar]
- 16. Tian L, Prabhakaran MP, Ramakrishna S. Strategies for regeneration of components of nervous system: Scaffolds, cells and biomolecules. Regen Biomater 2015;2:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karabekmez FE, Duymaz A, Moran SL. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand (N Y) 2009;4:245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lohmeyer JA, Siemers F, Machens HG et al. The clinical use of artificial nerve conduits for digital nerve repair: A prospective cohort study and literature review. J Reconstr Microsurg 2009;25:55–61. [DOI] [PubMed] [Google Scholar]
- 19. Bertleff MJ, Meek MF, Nicolai JP. A prospective clinical evaluation of biodegradable neurolac nerve guides for sensory nerve repair in the hand. J Hand Surg Am 2005;30:513–518. [DOI] [PubMed] [Google Scholar]
- 20. Zhong SP, Zhang YZ, Lim CT. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2010;2:510–525. [DOI] [PubMed] [Google Scholar]
- 21. Caplan AI. Mesenchymal stem cells: Time to change the name! Stem Cells Translational Medicine 2017;6:1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McKim LH. Regeneration of the distal phalanx. Can Med Assoc J 1932;26:549–550. [PMC free article] [PubMed] [Google Scholar]
- 23. Douglas BS. Conservative management of guillotine amputation of the finger in children. Aust Paediatr J 1972;8:86–89. [DOI] [PubMed] [Google Scholar]
- 24. Illingworth CM. Trapped fingers and amputated finger tips in children. J Pediatr Surg 1974;9:853–858. [DOI] [PubMed] [Google Scholar]
- 25. Bickel KD, Dosanjh A. Fingertip reconstruction. J Hand Surg Am 2008;33:1417–1419. [DOI] [PubMed] [Google Scholar]
- 26. Mescher AL. Effects on adult newt limb regeneration of partial and complete skin flaps over the amputation surface. J Exp Zool 1976;195:117–128. [DOI] [PubMed] [Google Scholar]
- 27. Borgens RB. Mice regrow the tips of their foretoes. Science 1982;217:747–750. [DOI] [PubMed] [Google Scholar]
- 28. Zhao W, Neufeld DA. Bone regrowth in young mice stimulated by nail organ. J Exp Zool 1995;271:155–159. [DOI] [PubMed] [Google Scholar]
- 29. Said S, Parke W, Neufeld DA. Vascular supplies differ in regenerating and nonregenerating amputated rodent digits. Anat Rec A Discov Mol Cell Evol Biol 2004;278:443–449. [DOI] [PubMed] [Google Scholar]
- 30. Singer M, Weckesser EC, Géraudie J et al. Open finger tip healing and replacement after distal amputation in rhesus monkey with comparison to limb regeneration in lower vertebrates. Anat Embryol (Berl) 1987;177:29–36. [DOI] [PubMed] [Google Scholar]
- 31. Fernando WA, Leininger E, Simkin J et al. Wound healing and blastema formation in regenerating digit tips of adult mice. Dev Biol 2011;350:301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simkin J, Sammarco MC, Dawson LA et al. Epidermal closure regulates histolysis during mammalian (Mus) digit regeneration. Regeneration (Oxf) 2015;2:106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Han M, Yang X, Taylor G et al. Limb regeneration in higher vertebrates: Developing a roadmap. Anat Rec B New Anat 2005;287:14–24. [DOI] [PubMed] [Google Scholar]
- 34. Muneoka K, Allan CH, Yang X et al. Mammalian regeneration and regenerative medicine. Birth Defects Res C Embryo Today 2008;84:265–280. [DOI] [PubMed] [Google Scholar]
- 35. McCusker C, Bryant SV, Gardiner DM. The axolotl limb blastema: Cellular and molecular mechanisms driving blastema formation and limb regeneration in tetrapods. Regeneration (Oxf) 2015;2:54–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gemberling M, Bailey TJ, Hyde DR et al. The zebrafish as a model for complex tissue regeneration. Trends Genet 2013;29:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kierdorf U, Kierdorf H, Szuwart T. Deer antler regeneration: Cells, concepts, and controversies. J Morphol 2007;268:726–738. [DOI] [PubMed] [Google Scholar]
- 38. Simkin J, Sammarco MC, Marrero L et al. Macrophages are required to coordinate mouse digit tip regeneration. Development 2017;144:3907–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martin P, D'Souza D, Martin J et al. Wound healing in the PU.1 null mouse–tissue repair is not dependent on inflammatory cells. Curr Biol 2003;13:1122–1128. [DOI] [PubMed] [Google Scholar]
- 40. Petrie TA, Strand NS, Yang CT et al. Macrophages modulate adult zebrafish tail fin regeneration. Development 2014;141:2581–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci USA 2013;110:9415–9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Simkin J, Gawriluk TR, Gensel JC et al. Macrophages are necessary for epimorphic regeneration in African spiny mice. Elife 2017;6:e24623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stocum DL. Regenerative Biology and Medicine. 2nd ed San Diego, CA: Elsevier, 2011. [Google Scholar]
- 44. Neufeld DA, Zhao W. Phalangeal regrowth in rodents: Postamputational bone regrowth depends upon the level of amputation. Prog Clin Biol Res 1993;383A:243–252. [PubMed] [Google Scholar]
- 45. Chamberlain CS, Jeffery JJ, Leiferman EM et al. Level‐specific amputations and resulting regenerative outcomes in the mouse distal phalanx. Wound Repair Regen 2017;25:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Conforti L, Gilley J, Coleman MP. Wallerian degeneration: An emerging axon death pathway linking injury and disease. Nat Rev Neurosci 2014;15:394–409. [DOI] [PubMed] [Google Scholar]
- 47. Maden M. Axolotl/newt. Methods Mol Biol 2008;461:467–480. [DOI] [PubMed] [Google Scholar]
- 48. Sammarco MC, Simkin J, Fassler D et al. Endogenous bone regeneration is dependent upon a dynamic oxygen event. J Bone Miner Res 2014;29:2336–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sammarco MC, Simkin J, Cammack AJ et al. Hyperbaric oxygen promotes proximal bone regeneration and organized collagen composition during digit regeneration. PLoS One 2015;10:e0140156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dawson LA, Simkin J, Sauque M et al. Analogous cellular contribution and healing mechanisms following digit amputation and phalangeal fracture in mice. Regeneration (Oxf) 2016;3:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thornton CS. The effect of apical cap removal on limb regeneration in Amblystoma larvae. J Exp Zool 1957;134:357–381. [DOI] [PubMed] [Google Scholar]
- 52. Lehoczky JA, Robert B, Tabin CJ. Mouse digit tip regeneration is mediated by fate‐restricted progenitor cells. Proc Natl Acad Sci USA 2011;108:20609–20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rinkevich Y, Lindau P, Ueno H et al. Germ‐layer and lineage‐restricted stem/progenitors regenerate the mouse digit tip. Nature 2011;476:409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pang YW, Feng J, Daltoe F et al. Perivascular stem cells at the tip of mouse incisors regulate tissue regeneration. J Bone Miner Res 2016;31:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Marrero L, Simkin J, Sammarco M et al. Fibroblast reticular cells engineer a blastema extracellular network during digit tip regeneration in mice. Regeneration (Oxf) 2017;4:69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Han M, Yang X, Farrington JE et al. Digit regeneration is regulated by Msx1 and BMP4 in fetal mice. Development 2003;130:5123–5132. [DOI] [PubMed] [Google Scholar]
- 57. Simkin J, Sammarco MC, Dawson LA et al. The mammalian blastema: Regeneration at our fingertips. Regeneration (Oxf) 2015;2:93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Han M, Yang X, Lee J et al. Development and regeneration of the neonatal digit tip in mice. Dev Biol 2008;315:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lehoczky JA, Tabin CJ. Lgr6 marks nail stem cells and is required for digit tip regeneration. Proc Natl Acad Sci USA 2015;112:13249–13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ferguson MW, O'Kane S. Scar‐free healing: From embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci 2004;359:839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Taylor GP, Anderson R, Reginelli AD et al. FGF‐2 induces regeneration of the chick limb bud. Dev Biol 1994;163:282–284. [DOI] [PubMed] [Google Scholar]
- 62. Kostakopoulou K, Vogel A, Brickell P et al. 'Regeneration' of wing bud stumps of chick embryos and reactivation of Msx‐1 and Shh expression in response to FGF‐4 and ridge signals. Mech Dev 1996;55:119–131. [DOI] [PubMed] [Google Scholar]
- 63. Kostakopoulou K, Vargesson N, Clarke JD et al. Local origin of cells in FGF‐4 ‐ induced outgrowth of amputated chick wing bud stumps. Int J Dev Biol 1997;41:747–750. [PubMed] [Google Scholar]
- 64. Yu L, Han M, Yan M et al. BMP2 induces segment‐specific skeletal regeneration from digit and limb amputations by establishing a new endochondral ossification center. Dev Biol 2012;372:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wu Y, Wang K, Karapetyan A et al. Connective tissue fibroblast properties are position‐dependent during mouse digit tip regeneration. PLoS One 2013;8:e54764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schotte EO, Smith CB. Wound healing processes in amputated mouse digits. Biol Bull 1959;117:546–561. [Google Scholar]
- 67. Schotte OE, Smith CB. Effects of ACTH and of cortisone upon amputational wound healing processes in mice digits. J Exp Zool 1961;146:209–229. [DOI] [PubMed] [Google Scholar]
- 68. Neufeld DA. Bone healing after amputation of mouse digits and newt limbs: Implications for induced regeneration in mammals. Anat Rec 1985;211:156–165. [DOI] [PubMed] [Google Scholar]
- 69. Neufeld DA. Epidermis, basement membrane, and connective‐tissue healing after amputation of mouse digits: Implications for mammalian appendage regeneration. Anat Rec 1989;223:425–432. [DOI] [PubMed] [Google Scholar]
- 70. Agrawal V, Johnson SA, Reing J et al. Epimorphic regeneration approach to tissue replacement in adult mammals. Proc Natl Acad Sci USA 2010;107:3351–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Agrawal V, Kelly J, Tottey S et al. An isolated cryptic peptide influences osteogenesis and bone remodeling in an adult mammalian model of digit amputation. Tissue Eng Part A 2011;17:3033–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Agrawal V, Tottey S, Johnson SA et al. Recruitment of progenitor cells by an extracellular matrix cryptic peptide in a mouse model of digit amputation. Tissue Eng Part A 2011;17:2435–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Miura S, Takahashi Y, Satoh A et al. Skeletal callus formation is a nerve‐independent regenerative response to limb amputation in mice and Xenopus . Regeneration (Oxf) 2015;2:202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wong J, Bennett W, Ferguson MW et al. Microscopic and histological examination of the mouse hindpaw digit and flexor tendon arrangement with 3D reconstruction. J Anat 2006;209:533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Einhorn TA. The science of fracture healing. J Orthop Trauma 2005;19:S4–S6. [DOI] [PubMed] [Google Scholar]
- 76. Shapiro F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur Cell Mater 2008;15:53–76. [DOI] [PubMed] [Google Scholar]
- 77. Gerstenfeld LC, Cullinane DM, Barnes GL et al. Fracture healing as a post‐natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J Cell Biochem 2003;88:873–884. [DOI] [PubMed] [Google Scholar]
- 78. Schindeler A, McDonald MM, Bokko P et al. Bone remodeling during fracture repair: The cellular picture. Semin Cell Dev Biol 2008;19:459–466. [DOI] [PubMed] [Google Scholar]
- 79. Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res 2009;24:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tsuji K, Bandyopadhyay A, Harfe BD et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet 2006;38:1424–1429. [DOI] [PubMed] [Google Scholar]
- 81. Yu YY, Lieu S, Lu C et al. Bone morphogenetic protein 2 stimulates endochondral ossification by regulating periosteal cell fate during bone repair. Bone 2010;47:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Minear S, Leucht P, Miller S et al. rBMP represses Wnt signaling and influences skeletal progenitor cell fate specification during bone repair. J Bone Miner Res 2010;25:1196–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang Q, Huang C, Xue M et al. Expression of endogenous BMP‐2 in periosteal progenitor cells is essential for bone healing. Bone 2011;48:524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]


