Abstract
Anal incontinence is a devastating condition that significantly reduces the quality of life. Our aim was to evaluate the effect of human adipose stem cell (hASC) injections in a rat model for anal sphincter injury, which is the main cause of anal incontinence in humans. Furthermore, we tested if the efficacy of hASCs could be improved by combining them with polyacrylamide hydrogel carrier, Bulkamid. Human ASCs derived from a female donor were culture expanded in DMEM/F12 supplemented with human platelet lysate. Female virgin Sprague‐Dawley rats were randomized into four groups (n = 14–15/group): hASCs in saline or Bulkamid (3 × 105/60 μl) and saline or Bulkamid without cells. Anorectal manometry (ARM) was performed before anal sphincter injury, at two (n = 58) and at four weeks after (n = 33). Additionally, the anal sphincter tissue was examined by micro‐computed tomography (μCT) and the histological parameters were compared between the groups. The median resting and peak pressure during spontaneous contraction measured by ARM were significantly higher in hASC treatment groups compared with the control groups without hASCs. There was no statistical difference in functional results between the hASC‐carrier groups (saline vs. Bulkamid). No difference was detected in the sphincter muscle continuation between the groups in the histology and μCT analysis. More inflammation was discovered in the group receiving saline with hASC. The hASC injection therapy with both saline and Bulkamid is a promising nonsurgical treatment for acute anal sphincter injury. Traditional histology combined with the 3D μCT image data lends greater confidence in assessing muscle healing and continuity. Stem Cells Translational Medicine 2018;7:295–304
Keywords: Adipose stem cells, Anal sphincter injury, Anal incontinence, Micro‐computed tomography, Polyacrylamide hydrogel Bulkamid, Mesenchymal stem cells, Tissue engineering
Significance Statement.
The increasing awareness of good quality of life sets demands for better and less invasive treatment methods of the pelvic floor disorders, for example, anal incontinence. Human adipose stem cells (hASCs) are readily available, easily obtained, have low immunogenicity and high multilineage differentiation and are, therefore, an ideal cell source. In this study, an animal model was used to develop a mini‐invasive injection treatment method using hASCs. The functional measurements showed significant improvement in hASC‐treatment groups compared with the controls. A biocompatible carrier polyacrylamide hydrogel Bulkamid was found to be a suitable carrier for the stem cells, and a novel method of micro‐computed tomography was found useful for targeting the histological slides.
Introduction
Anal incontinence (AI) is a devastating condition that significantly reduces the quality of life. Especially in women, the fecal incontinence symptoms cause depression, embarrassment and lifestyle changes that have a negative effect their everyday life 1. The primary management of AI is conservative treatment including dietary, medical, and psychological interventions, as well as physiotherapy. A common operation for persistent AI has been secondary sphincteroplasty which may not have the desired long‐term results, especially with denervated sphincters 2. Sacral nerve modulation and transcutaneous posterior tibial neuromodulation have increasingly been used for fecal incontinence. However, the cure rates vary depending on the outcome measure in use with long‐term results reaching up to 54% for sacral neuromodulation 3, 4. Surgical methods often carry a risk of complications and are both demanding and expensive.
AI is defined as an involuntary loss of flatus, liquid, or solid stool that is a social or hygienic problem 5. There are different estimates of the incidence of AI varying from 3% to over 40% in the adult population, depending on the definition and study population 6, 7, 8. In women, obstetric injury is one of the main causes of AI, although its etiology is often multifactorial 9.
There are several studies concerning stem cells in the treatment of anal sphincter defects. Rat models with muscle‐derived stem cells or myoblasts as the stem cell origin are the most common study designs 10, 11, 12, 13, 14, although bigger animals (rabbits, dogs) have been used as well 15, 16. Mesenchymal stem cells (MSC) derived from bone marrow have been used in a few studies and the results are promising 17, 18, 19. Additionally, a small clinical trial with human adipose stem cells (hASCs) has recently been published 20.
Human ASCs are easily obtained, and their features include low immunogenicity and high multilineage differentiation capability 21. The ASCs have shown to differentiate into muscle cells 22, 23. Human ASCs have also demonstrated paracrine function—immunomodulation, cytokine secretion, cytoprotection, and neovascularization 21, 24, 25. Therefore, ASCs are an attractive source of cell material for regenerative medicine and tissue engineering to rehabilitate impaired organ function. However, there is a need for optimal carrier material to provide a favorable environment for ASCs’ paracrine actions. Bulkamid is a nondegradable viscoelastic water‐based polymer and it is mainly used for injection therapy of female urinary incontinence 26, 27. It has also been used for AI 28. Other bulking agents, such as bovine collagen, have been tested but the results are transient 29, 30.
The purpose of our study was to develop a novel, mini‐invasive, and effective treatment method utilizing hASCs combined with either saline (0.9% NaCl) or polyacrylamide hydrogel (Bulkamid) to cure AI due to acute anal sphincter trauma. To facilitate clinical translation, cells of human origin were used and hASCs were isolated and expanded in clinically viable conditions using human platelet lysate as a culture medium supplement instead of fetal bovine serum. This is, to our knowledge, the first study to use Bulkamid as a stem cell carrier, to study in vivo the hASCs in an animal functional model and to use micro‐computed tomography (μCT) as a tool to view the treated anal sphincter area.
Materials and Methods
The animal study protocol was approved by the Regional State Administrative Agency (AVI/Ella no ESAVI/2828/04.10.07/2015). Adipose tissue sample was obtained under the approval of the Ethics Committee of the Pirkanmaa Hospital District (Tampere, Finland, R15161). The hASCs were isolated from adipose tissue sample from a female donor undergoing elective plastic surgery at Tampere University Hospital (Tampere, Finland) with the patient's written consent. The final study was designed after a pilot study of 24 Sprague‐Dawley rats (results not included in the analysis). In the pilot study, the rat anorectal manometry (ARM) technique, anal sphincter cutting and repairing, the injection technique, the suitable amount of the gel, and the amount of stem cells per injection were tested. The cell amounts of 5 × 105/100 µl and 5 × 106/100 µl were compared, and the lower cell count was chosen due to better cell viability before injection.
Treatment Protocol
Sixty (60) Sprague‐Dawley female virgin rats (Janvier Laboratoires), weight 220–300 g, age 14 weeks were randomly selected, then anesthetized with intraperitoneal injections of medetomidine 0.25 mg/kg and ketamine 32.5 mg/kg, and buprenorfin 0.05 mg/kg s.c. and carprofen 5 mg/kg s.c. were used for postoperative pain. After the anal manometry and the sphincter operation, the anesthesia was reversed using atipamezole 1 mg/kg.
The ARM was performed using Polygraf ID manometry system with ERCP manometry triple lumen catheters (Medtronic, Polygram NET, computer unit Windows XP, Minneapolis, MN, USA). The measurements were conducted preoperatively and at 2 and 4 weeks after the operation. (Fig. 1, Supporting Information Fig. S1). The duration of ARM procedure was approximately 30 minutes, during which resting anal sphincter pressures and peak pressures during spontaneous contraction were measured (resting pressure 3–7 times [mean 5.64] and contraction pressure 7–12 times [mean 10.4]). Mean values of these measurements were used in further analysis.
Figure 1.
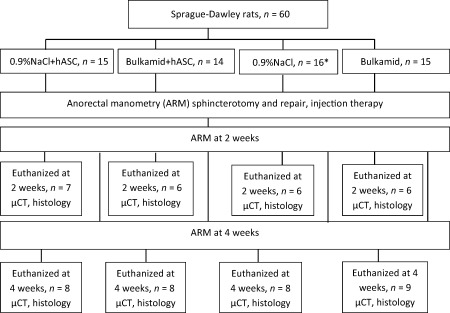
Flow chart of the study protocol. *2 rats died presumably due to anesthesia complication. Abbreviations: hASCs, human adipose stem cells; μCT, micro‐computed tomography; NaCl, sodium chloride.
The sphincter was cut from all animals to mimic an acute fourth grade anal sphincter tear (injury of the external and internal sphincter muscle and anal mucosa) and sewed back with 6‐0 poliglecaprone (Ethicon, Johnson & Johnson, Monocryl, Somerville, NJ, USA) continuous stiches utilizing magnifying loupes. First, the anal mucosa and internal sphincter were repaired, and then, the injections were delivered after which the perineal skin was closed.
The rats were divided into four groups for the injections: injection of 3 × 105 hASCs in saline (0.9% NaCl) solution (n = 15), hASCs in 2.5% polyacrylamide hydrogel (Bulkamid, Contura International A/S, Denmark) (n = 15). The control groups consisted of rats with only saline (n = 16, two died within the first postoperative day presumably because of anesthetic reaction and were excluded from the analysis) and polyacrylamide hydrogel injections (n = 14). There were two injections with a 25‐gauge needle on both ends of the external sphincter (degrees 30° and 330° on superimposed clock face, Supporting Information Fig. S3); the total injection volume of the four injections being 60 μl per animal for all Bulkamid and 0.9% NaCl‐groups.
The rats were again anesthetized for the anal manometry control with medetomidine and ketamine combination as described above. After the last anal manometry examination, the rats were euthanized using carbon monoxide. The anal manometry results were analyzed by a blinded researcher (HT) to exclude observer bias.
ASC Isolation, Cell Culture, Cell Viability, and Phenotype
The hASCs were isolated as previously described 31. The hASCs were expanded in DMEM/F12 (1:1) (Thermo Fisher Scientific Inc., Carlsbad, CA, USA, https://www.thermofisher.com) supplemented with 5% Pooled Human Platelet Lysate (Stemulate; Cook General Bio‐Technology, Indianapolis, IN, USA, http://cookregenterec.com), 1% antibiotics (100 U/ml penicillin and 0,1 mg/ml streptomycin; Thermo Fisher Scientific Inc.), and 1% l‐glutamine (Thermo Fisher Scientific Inc, GlutaMAX‐100, Indianapolis, IN, USA). The medium was changed twice a week, and the cells were divided upon reaching confluency. The cells were detached using TrypLE Select (Thermo Fisher Scientific Inc., Carlsbad, CA, USA). From 4 to 5 days prior to cell injections, the ASCs were labeled with 200 µg/ml magnetizable nanoparticles (PMP‐50; Kisker Biotech GmbH & Co., Steinfurt, Germany, https://kisker-biotech.com) for 48 hours for cell detection. For cell injections, 5 × 105 hASCs (passage between 4 to 7) were blended with 100 µl of 0.9% NaCl (Baxter Healthcare SA, Zurich, Switzerland, http://Baxter.com) or Bulkamid hydrogel (Contura International A/S, Soeborg, Denmark, https://bulkamid.com).
For the cell viability, 5 × 105 hACSs were blended with 100 µl Bulkamid, incubated at room temperature for 3 hours (the maximum delay between the cell preparation and the injections), and the cell viability was assessed with LIVE/DEAD Viability/Cytotoxicity kit for mammalian cells (Thermo Fisher Scientific Inc., Carlsbad, CA, USA). The hASCs in Bulkamid were stained with 1 µM Calcein AM and 0.8 µM Ethidium homodimer‐1 (EthD‐1) for 45 minutes. Fluorescence pictures were taken with Fluorescence Microscope (Olympus. IX51S18F‐2 and camera DP71, Japan).
The phenotype of the hASCs was assessed with fluorescence‐activated cell sorter (BD Biosciences, Franklin Lakes, NJ, USA, https://www.bdbiosciences.com. BD FACSAria Fusion Cell Sorter) at passage 7. Monoclonal antibodies against CD14‐phycoerythrincyanine (PE‐Cy7), CD19‐PE‐Cy7, CD45RO‐allophycocyanin (APC), CD54‐Fluorescein isothiocyanate (FITC), CD73‐phycoerythrin (PE), CD90‐APC (BD Biosciences, Franklin Lakes, NJ, USA), CD105‐PE (R&D Systems Inc., Minneapolis, MN, USA, http://www.rndsystems.com), CD34‐APC and HLA‐DR‐PE (Immunotools GmbH, Friesoythe, Germany, http://www.immunotools.de) were used. According to the International Society for Cellular Therapy standard criteria, cells positive for CD73, CD90 and CD105 but negative for CD14, CD34, CD45, and HLA‐DR are considered as MSCs 32, 33.
μCT Imaging
The analysis of the anal sphincter was performed after sacrificing the animals. The excised samples were imaged in a μCT instrument (Xradia MicroXCT‐400, Carl Zeiss, Pleasanton, CA, USA). To increase the contrast between the soft tissues, the samples were put through a staining regimen. The tissue samples were stored in 4% paraformaldehyde (PFA)‐solution (Sigma). The PFA was then changed to 70% ethanol solution, then to 95% ethanol, ≥99.5% ethanol, and 10 mg/ml iodine in ethanol. The samples were transferred into sample holders and were kept at room temperature for a minimum of 24 hours prior to imaging in order to avoid imaging artifacts resulting from thermal expansion of the tissue samples. The imaging parameters for all 58 samples were constant (Table 1). After imaging, the samples were transferred into a 70% ethanol solution for storage and following histological analyses. Tomographic three‐dimensional (3D) image reconstruction was performed with proprietary software installed in the instrument (Carl Zeiss, Xradia XMReconstructor, Pleasanton, CA, USA). The reconstructed image volume was imported into AVIZO image processing software (FEI Company, Hillsboro, OR, USA) for image processing and inspection. The anal sphincter complex was visualized through 3D volume rendering. It was used in combination with tomographic data exploration as freely selectable two‐dimensional (2D) views of the 3D dataset to confirm the histological assessment of muscle continuity. Additionally, it was used to visualize in 3D the entire sample instead of selected 2D sections as in histology and to monitor the presence of any detectable PMP‐50 labeled cells.
Table 1.
Technical details about the micro‐computed tomography imaging
| Voltage | 60 kV |
| Current | 166 µA |
| Source distance | 58 mm |
| Detector distance | 170 mm |
| Exposure time | 4 seconds |
| Imaging time | 2.5 hours |
| Pixel size | 17.0354 µm |
| Image volume dimensions | 1000 × 1000 × 1000 pixels |
Histology
The formalin fixed, paraffin embedded tissues were sectioned (4 µm) and stained with Hematoxylin & Eosin (HE) for morphological interpretation. Furthermore, picrosirius red staining was used to demonstrate collagen and Perls Prussian blue to demonstrate the presence of iron from tissue sections. Immunohistochemistry using anti‐human Vimentin 1:200 (Clone:BS13, BSH‐7100, Nordic Biosite, Täby, Sweden) and STEM121 1:500 (Clone: Stem121, Y40410, Takara Bio Inc., Shiga, Japan) antibodies was performed to detect hASCs from rat tissue sections. Smooth muscle actin antibody 1:200 (Clone BS66, BSH‐7459, Nordic Biosite, Täby, Sweden) were used to detect rat muscle tissue. Finally, CD68 antibody 1:200 (Clone: ED1, ab31630, ABCAM, Cambridge, United Kingdom) was used to detect rat macrophages and verify the origin (PMP‐50 containing hASCs or naturally iron containing rat macrophages) of positive Perls Prussian blue staining. All antibodies were produced in mouse and primary antibodies were detected using anti‐mouse horseradish peroxidase polymer. Rat and human paraffin control multi‐tissue sections were used as a negative and positive tissue controls for immunohistochemical analyses.
Olympus BX‐60 microscope (BSH 747) and an integrated color digital camera (Scion) were used for the evaluation of the slides. The slides were also imaged and digitalized with a 3D Histec Pannoramic MIDI instrument. The scoring of the inflammation was based on the inflammatory cell infiltration into the lesion site, edema, hemorrhage and necrosis of tissue 34. The number of inflammatory cells was evaluated manually from the low power field (×200) image frame using a “hot spot” selection. The inflammation was scored with following grades: 0 = no histological features of inflammation; 1 = diffuse inflammatory cell infiltration, <100 cells; 2 = diffuse inflammatory cell infiltration, 100–500 cells, mild edema and hemorrhage; 3 = inflammatory cell infiltration, >500 cells, edema and hemorrhage; 4 = inflammatory cell infiltration, >1,000 cells, edema, hemorrhage and necrosis. Fibrosis was evaluated separately with following grades: 0 = no fibrosis; 1 = mild fibrosis/collagen formation; 2 = strong fibrosis/collagen formation. The infiltration of the cells into the hydrogel was evaluated with samples containing Bulkamid.
Statistical Analysis
The statistical analysis was performed by using the IBM SPSS version 22 (IBM, Chicago, IL, USA). The four treatment groups were compared at baseline, at 2, and at 4 weeks. Statistical significance was tested by using chi‐square or Fisher's exact test with categorical and one‐way analysis of variance (ANOVA) with continuous variables. The groups and the time points within the groups were compared using ANOVA for repeated measures.
Results
In Vitro Results
Live/dead staining demonstrated that after 3 hours of incubating the hASCs in Bulkamid, the hASCs were mostly alive. However, there were also some dead cells in the mixture (Fig. 2). The hASC phenotype was assessed at passage 7 using flow cytometry. The hASCs expressed surface markers CD73 (99.9%), CD90 (99.9%), and CD105 (99.6%). Expression of CD14 (1.4%), CD45RO (0.6%), and HLA‐DR (0.3%) was very low, and expression of CD19 (6.0%), CD34 (8.1%), and CD54 (8.5%) was low. This confirms the mesenchymal origin of the hASCs.
Figure 2.
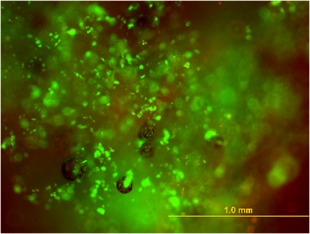
Live/dead staining of the human adipose stem cells in Bulkamid; dead cells (red), live cells (green). Scale bar 1.0 mm.
In Vivo Results
The baseline rat characteristics (weight, ARM measurements) of each group are presented in Table 2. The rats in the 0.9% NaCl and Bulkamid control groups were slightly, though statistically significantly, heavier than the hASC treatment groups’ rats. Otherwise, there was no difference in baseline characteristics between the groups.
Table 2.
The rat baseline characteristics, functional results from anorectal manometry and histology
| 0.9%NaCl + hASC | Bulkamid+hASC | 0.9%NaCl | Bulkamid | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean/median | SD/Q 1–Q 3 | Mean/median | SD/Q 1–Q 3 | Mean/median | SD/Q 1–Q 3 | Mean/median | SD/Q 1‐Q 3 | p value | |
| Weight (g) | 268.0/270.0 | 12.1/260.0–280.0 | 263.6/270.0 | 18.6/250.0–280.0 | 275.4/280.0 | 15.7/267.5–290.0 | 285.3/280.0 | 11.4/280.0–300.0 | .002 |
| PreopARM (n) | 15 | 14 | 14 | 15 | |||||
| Rest med | 9.1 | 3.4 | 8.4 | 2.8 | 7.8 | 2.4 | 8.2 | 2.3 | .640 |
| Peak contr | 97.5 | 25.2 | 88.5 | 29.6 | 81.3 | 22.1 | 85.0 | 23.3 | .349 |
| ARM 2 wk (n) | 15 | 14 | 14 | 15 | |||||
| Rest med | 9.3 | 2.6 | 9.6 | 2.1 | 4.9 | 3.2 | 5.2 | 1.6 | < .000 |
| Peak contr | 74.6 | 16.0 | 74.0 | 13.6 | 44.6 | 20.6 | 47.8 | 14.3 | < .000 |
| ARM 4 wk (n) | 8 | 8 | 8 | 9 | |||||
| Rest med | 10.1 | 3.0 | 9.2 | 2.3 | 5.4 | 2.5 | 6.1 | 3.4 | .005 |
| Peak contr | 79.5 | 16.4 | 76.2 | 21.3 | 52.4 | 27.2 | 46.6 | 26.7 | .014 |
| Inflammation | .003 | ||||||||
| gr 0 (%) | 0.0 | 21.4 | 28.6 | 33.3 | |||||
| gr 1 (%) | 20.0 | 28.6 | 50.0 | 46.7 | |||||
| gr 2 (%) | 40.0 | 42.9 | 21.4 | 20.0 | |||||
| gr 3 (%) | 13.3 | 7.1 | 0.0 | 0.0 | |||||
| gr 4 (%) | 26.7 | 0.0 | 0.0 | 0.0 | |||||
Abbreviations: ARM 2 wk, anorectal manometry at 2‐week time point; ARM 4 wk, anorectal manometry at 4‐week time point; hASC, human adipose stem cells; NaCl, sodium chloride; Peak contr, peak pressure during spontaneous contraction; Preop ARM, preoperative anorectal manometry; Q 1–Q 3, 25 and 75 percentiles; Rest med, median resting anal pressure; Weight, preoperative weight.
Anorectal Manometry Results
First, the four groups were compared based on the ARM results before injury and at 2 and 4 weeks. The measured variables were the median resting pressure and the peak pressure during spontaneous contraction of the anal sphincter complex. The median resting and the peak contraction pressures were higher in the hASC treatment groups at 2 and at 4 weeks (Table 2). Further analysis showed that the trend of the contraction pressure was significantly higher in the both hASC‐groups compared with the saline and Bulkamid control groups (Fig. 3). The difference between the groups remained statistically significant when adjusted for baseline measurement.
Figure 3.
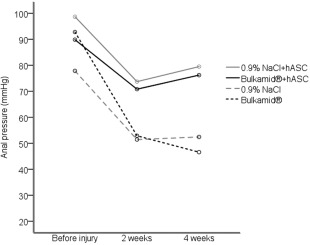
The trends of the four groups showed a significantly higher contraction pressures in both hASC treatment groups. Abbreviations: hASCs, human adipose stem cells; NaCl, sodium chloride.
Histology
In the histological analysis, no hASCs were recognized in the preparations neither at 2 nor 4 weeks according the Vimentin, STEM121, or Perls Prussian blue staining. This was confirmed with CD68 staining of rat macrophages. Rat endogenous macrophages containing iron stained positively with Perls Prussian blue and rat specific CD68 immunoperoxidase reaction located into the same cells (Fig. 4). Vimentin and STEM121 were positive in a cytoblock section prepared from the same cells that were injected into the rats (data not shown).
Figure 4.
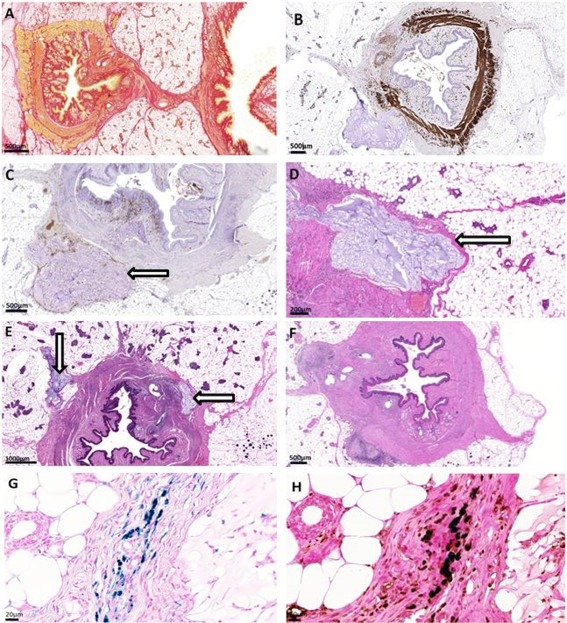
The histology stainings and examples of the inflammatory grading. (A): Picosirius red‐staining, 0.9% sodium chloride (NaCl) at 2 weeks; (B): Anti‐Desmin, Bulkamid at 4 weeks; (C): Immunohistochemistry staining CD68, Bulkamid+hASC at 2 weeks; (D): HE‐staining, Bulkamid+hASC at 2 weeks, inflammation grade 1; (E): HE‐staining, Bulkamid+hASC at 4 weeks, inflammation grade 2; (F): HE‐staining, 0.9%NaCl + hASC at 2 weeks, inflammation grade 4; (G): Perls Prussian blue‐staining for iron particles, Bulkamid+hASC at 2 weeks; (H): Combination of CD68 and Perls Prussian blue of the sample G showing that the iron particles localize at the rat endogenous macrophages. Arrow = Bulkamid+hASC‐injection. Scale bar Figures (A–F) 500 μm, Figures (G–H) 20 μm.
There was no statistical difference in sphincter muscle continuity, fibrosis, or collagen formation between the four groups. The Bulkamid‐hydrogel was well integrated in the tissue with minor foreign body reaction according to the HE staining. There was more inflammation in the hASC‐groups, especially in the 0.9% NaCl +hASC‐group (Table 2; Fig. 4, Supporting Information Fig. S2).
µCT Analysis
The µCT image datasets were used to confirm independently the continuity of the sphincter muscle shown in the histology. By viewing the image data in multiple orthogonal views, greater confidence could be attested to the histological assessment. (Fig. 5, Supporting Information video). There was total agreement between histology and μCT interpretation in 76% of the samples. There was minor disagreement in 11 samples and serious disagreement in muscle continuity in 3 samples (5%). This did not affect the statistical difference between the groups. Thus, the ability to conduct nondestructive histomorphometric analysis on samples provides valuable image data that can be used to perform robust 3D analyses when necessary. In some samples with Bulkamid, small regions with high x‐ray attenuation regions were observed that could indicate cells or remains of PMP‐50 particles (data not shown). However, it was not possible to confirm whether these regions indicate the presence of PMP‐50 particles or whether they were the result of local aggregation of iodine. As iron specific staining of the histological samples failed to detect PMP‐50 in any of the samples, the presence of PMP‐50 particles could not be confirmed.
Figure 5.
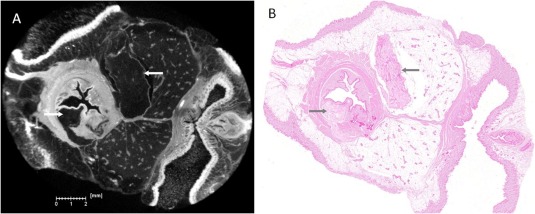
Comparison of the micro‐computed tomography (A) and histology (B) images. Arrow = injected Bukamid hydrogel.
Discussion
Our aim is to develop an effective, mini‐invasive treatment for AI, which is a highly distressing condition and all the more lacking an efficient treatment method. Studies about the existing treatment methods are heterogeneous and long‐term results are mostly missing. Tissue engineering and cell therapy have been considered to be compelling alternatives and hold a great deal of promise and excitement. The advantage of using ASCs compared with the other stem cell sources is that the tissue can easily be harvested and it is readily available in large quantities. ASCs can be easily expanded in vitro and have an extensive self‐renewal capacity 35, 36.
In our in vivo‐study, we found significant improvement in anal sphincter resting and contraction pressures in hASC treatment groups after acute sphincter injury compared with the control groups. Previous functional anal sphincter assessments have mostly been performed in vitro using anal sphincter muscle samples from euthanized animals to measure the contractility after electrical stimulation 10, 12, 17, 37. Salcedo et al. measured in vivo function of the anal sphincter after a sphincterotomy or pudendal nerve crush followed by either rat bone‐marrow derived MSC injection or saline 18. In their further investigations, the same research group found that both serial i.v. infusion and i.m. injections of MSCs after partial sphincter excision resulted in increased anal pressures 19. Pathi et al., on the other hand, did not find significant advantage in the iv‐administration of rat bone‐marrow derived MSCs compared with the PBS controls, but the local injections of MSCs were effective according to in vitro assessment 17. Recently Fitzwater et al. noticed that the administration of rat myogenic stem cells enhanced the contractile function of the sphincter without significant changes in histologic morphology, which addresses the paracrine processes in stem cell therapy 14. To simplify the cell isolation and proliferation process, Mazzanti et al. used freshly isolated minimally manipulated bone‐marrow derived mononuclear cells without expansion and found them to be as effective as in vitro expanded BM‐MSCs in the recovery of iatrogenic anal sphincter rupture 38. Muscle‐derived stem cell injections have been used in small pilot studies in women and the results are promising 39, 40, 41. Frudinger et al. treated 10 patients with autologous myogenic stem cells and found a significant improvement in the AI symptoms at 1‐ and 5‐year controls. However, there was no significant change in anal manometry measurements 39, 40. In a small pilot study, where hASCs therapy was used in combination with surgical sphincteroplasty in treatment of AI, there was no difference in AI symptoms between the hASC and saline injection group. However, the number of patients was limited and heterogeneous and the follow‐up time short. Despite these limitations, they discovered an increase in total muscle area in an endorectal sonography assessment in the hASC treatment group compared with the saline control group 20.
In our study, the anal pressures were comparable with the other animal studies although there are slight variations—possibly due to different techniques (balloon vs. double‐triple‐lumen‐catheter, electrical stimulation vs. spontaneous contraction) and different size of the anal canal in different species 13, 16, 19. Salcedo et al. stated that rat anal pressures tend to return to baseline after sphincterotomy at 4 weeks even without intervention, but this does not occur after pudendal nerve transection 42. In our results, both resting and contraction anal pressures were significantly lower in both control groups at 2 and 4 weeks compared with the hASC treatment groups. There was also a rising pressure trend within the stem cell groups that might have resulted in even higher pressures and better recovery in longer follow‐up.
We used ASCs from a single donor mainly because of the safety regulations. It is known that there is some heterogeneity between different donors; for example, age, sex, body mass index, and site of harvest are known to have an effect on the properties of the ASCs 43, 44, 45, 46, 47. Xenotransplantation has been used before to study development, physiology, and pathophysiology of human tissues in animal models, for example, enteric nervous system 48 and rodent models have been tested in treatment of stroke 49 and myocardial infarct 50. However, there are differences in human and animal physiology that have to be kept in mind when interpreting the results 51.
Different labeling systems of injected cells have been used in previous studies. Kang et al. found fluorescent dye PKH‐26 labeled rat myogenic stem cells in fluorescent microscopy of rat anal sphincters at one week after the injection 10. PKH‐26 label in animal autologous myogenic stem cells were also used by Kajbafzadeh (rabbit) and Oh (dog): both groups found labeled cells at 2 months and 3 months control, respectively 15, 16. Aghaee‐asfhar et al. used bromodeoxyuridine labeled human umbilical cord stem cells as well as rabbit bone marrow stem cells (BMSCs) in a rabbit model, the labeled cells were found in immunohistochemistry at 2 weeks control 52. On the other hand, Cruz et al. demonstrated that the green fluorescent protein (GFP) labeled rat BMSCs disappear in 10 days after i.v.‐administration 53. Salcedo et al. found no visible GFP‐labels in the anal sphincter after administration of i.m. or i.v. MSCs derived from rat bone marrow 18. We attempted to find the PMP‐50 labeled hASCs in the tissue samples using µCT, histology and immunohistochemistry. Although some potential signs of PMP‐50 were detected in µCT, we were not able to confirm the PMP‐50 labeled cells either in the µCT scan or in the histology analysis. Histological sections stained positive with Perls Prussian blue but rat macrophage specific CD68 antibody stained the same cells indicating that the signal comes from hASCs ingested by rat macrophages or just iron that macrophages contain naturally. In our pilot study, there were tracks of the injected cells in hydrogel with the higher cell amount (5 × 106 cells/100 μl) and with bigger injection volume 100 μl (Supporting Information Fig. S2). In the actual study, due to better cell viability and injection experience, we decided to use lower cell count and lower volume and were not able to verify the presence of the stem cells. The evanescence of the cells may be due to the rat immune defense destroying the human cells. This is supported by the lack of human specific STEM121 and Vimentin staining of the tissue sections.
There are also the safety considerations in cell therapy. Jacobs et al. found no evidence of myogenic stem cell migration to the liver or lung. However, they detected local ectopic foci of growth in two treated rat anal sphincters after 30 days; the small tumors were benign tumors with no nuclear abnormalities 54. In our study, normal histology was confirmed both by 3D µCT scanning of the whole sphincter sample area, as well as by selected histological sections. The μCT is a valuable approach to visualize the entire treated area and the injection sites in 3D to target the histological slides. The ability to increase imaging resolution without proportionally decreasing sample size opens possibilities for performing analyses without destructive sample preparation. The µCT protocol applied in this study showcased this methodology by complementing the traditional histological analyses with increased amount of available data, which resulted in greater confidence in the analysis. Additionally, in combination with appropriate concentrations of labeling agents, it may also be feasible to monitor the distribution of introduced cells.
This animal model, like any other animal model, is not directly comparable with birth injury in humans. The trauma in the rat model was caused by a direct clean‐cut wound whereas in obstetric trauma the sphincter is torn, and the tissue has suffered from prolonged hypoxia and denervation. In an obstetric trauma, there is also presence of tissue edema and blood, which makes suturing the rupture more challenging in real life. In our animal model, the rupture was easy to identify with no delay, and the operative technique was consistent with only one operator. The small scale of the rat anatomy made it difficult to distinguish the inner and outer sphincter from each other and that is why the injections were aimed at the external sphincter rather than submucosa or between the sphincters. In humans, the intersphincteric injection might be better. However, our model applies to acute injury and is perhaps not applicable for chronic conditions with fibrous anal sphincters often seen in AI patients. Fibrosis is a histological finding often seen in scar tissue and in our model the follow up time was too short to analyze the possible impact on scar tissue formation. There is evidence that cell therapy might be able to prevent vocal fold scaring in laryngeal microsurgery and fibrous keloid formation after skin incision in acute trauma model 55, 56.
The possible mechanisms of AI stem cell therapy are direct stem cell integration and muscle regeneration, the bulking effect of the cells and the carrier, the trophic effect caused by the stem cell growth factor secretion or immune modulation, and the decreased inflammation that improves tissue healing 33, 57. The bulking effect is probably not the mechanism according to our results, because there was no advantage in the bulking agent Bulkamid compared with the 0.9% NaCl‐controls, and also because there were good results with hASCs in 0.9% NaCl as well. For human AI, the bulking effect in the injection therapy is perhaps not even desirable 58. According to our results, lack of differences in muscle continuity between the groups refers to paracrine effect rather than to direct stem cell differentiation into muscle cells. The mechanism may be through cell fusion, endogenous activation of satellite cells or some inflammatory process 59. From the inflammatory perspective, Bulkamid seems to have an advantage over 0.9% NaCl as a carrier, although it is not clear what stage of inflammation is desirable for the healing process. Bisson et al. showed that certain amount of inflammation and acute injury is required in cell therapy: injection of myoblasts on the opposite site of cryoinjured anal sphincter did not restore the anal sphincter function, whereas the injection into the lesion itself did 60. On the other hand, Salcedo et al. showed that systemic injection also had a positive effect; however, this was not seen in the study of Pathi et al. 17, 19. One advantage of hydrogel over 0.9% NaCl is that the cell sedimentation and aggregation are mostly avoided with Bulkamid. Bovine collagen gel (Contigen) has also been used in clinical trials experimenting stem cell injection therapy for female urinary incontinence 61, 62.
It has been suggested that stem cell transplantation may facilitate endogenous repair even in patients with advanced age and comorbidities who have compromised repair function 13. Therefore, stem cell therapy would be especially useful in patients with compromised anal sphincter function. Bohl et al. have successfully implanted engineered biosphincters in rabbits with iatrogenic AI to restore anal sphincter function 63. Sphincter transplantation is however a major surgical operation. Stem cell injection therapy can be performed under local anesthesia in an outpatient setting with no major complications. According to our preliminary results, the ASC treatment is effective in treatment of an acute anal sphincter injury. In the future, animal models for chronic injury are needed to develop an effective and clinically relevant treatment method.
Conclusion
ASCs combined with either saline or synthetic nondegradable hydrogel is a novel and attractive, mini‐invasive treatment method for acute anal sphincter rupture and AI. Due to low inflammatory response and good tissue integration, Bulkamid hydrogel appears to be a suitable injection agent and carrier for the ASCs. The technique of anal sphincter injection therapy is simple in an animal setting and the use of human ASCs in rat AI model is feasible. The 3D μCT is a valuable addition to the traditional histology in analyzing soft tissue samples. Further studies with suitable chronic injury animal models with longer follow‐up periods are needed to confirm the functional restoration and to develop a truly effective treatment method for AI patients.
Author Contributions
K.K.: conception and design, administrative support, provision of study material, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; M.J. and N.N.G.: conception and design, provision of study material, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; H.T.: conception and design, data analysis and interpretation, final approval of manuscript; H. H.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript; K.N. and J.H.: conception and design, administrative support, data analysis and interpretation, manuscript writing, final approval of manuscript; S.M.: conception and design, financial support, administrative support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Supporting information
Supplement Figure 1
Supplement Figure 2R1
Supplement Figure 3R1
Supplement Online Video
Acknowledgments
We thank Tampere University Vetlab animal attendants for their assistance, Sari Kalliokoski from BioMediTech for the preparation of the anal sphincter samples, and Dr. Jukka Kallio for lending the magnifying loupes. The histological samples were prepared and evaluated by BioSiteHisto/Teppo Haapaniemi as an outsourced service. This study was financially supported by the Competitive State Research Financing of the Expert Responsibility area of Tampere University Hospital and TEKES (the Finnish Funding Agency for Innovation) Human Spare Parts project. The expense of the animal acquisition and maintenance, including drugs used in the operation; the study equipment (anal manometry); the stem cell GMP‐laboratory expense and the outsourced histology service was covered by a competitive research grant for associate professor Susanna Miettinen from Pirkanmaa Hospital District. Kirsi Kuismanen has received a 1‐month research grant for the study from Tampere University hospital/Pirkanmaa Hospital District.
Authored by a member of CBA
References
- 1. Mundet L, Ribas Y, Arco S et al. Quality of life differences in female and male patients with fecal incontinence. J Neurogastroenterol Motil 2015;22:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pescatori LC, Pescatori M. Sphincteroplasty for anal incontinence. Gastroenterol Rep (Oxf) 2014;2:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thin NN, Horrocks EJ, Hotouras A et al. Systematic review of the clinical effectiveness of neuromodulation in the treatment of faecal incontinence. Br J Surg 2013;100:1430–1447. [DOI] [PubMed] [Google Scholar]
- 4. Riemsma R, Hagen S, Kirschner‐Hermanns R et al. Can incontinence be cured? A systematic review of cure rates. BMC Med 2017;15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sultan AH, Monga A, Lee J et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female anorectal dysfunction. Int Urogynecol J 2017;28:5–31. [DOI] [PubMed] [Google Scholar]
- 6. Varma MG, Brown JS, Creasman JM et al. Fecal incontinence in females older than aged 40 years: Who is at risk?. Dis Colon Rectum 2006;49:841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jerez‐Roig J, Souza DL, Amaral FL et al. Prevalence of fecal incontinence (FI) and associated factors in institutionalized older adults. Arch Gerontol Geriatr 2015;60:425–430. [DOI] [PubMed] [Google Scholar]
- 8. Brown SR, Wadhawan H, Nelson RL. Surgery for faecal incontinence in adults. Cochrane Database Syst Rev 2013;7:CD001757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hayden DM, Weiss EG. Fecal incontinence: Etiology, evaluation, and treatment. Clin Colon Rectal Surg 2011;24:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kang SB, Lee HN, Lee JY et al. Sphincter contractility after muscle‐derived stem cells autograft into the cryoinjured anal sphincters of rats. Dis Colon Rectum 2008;51:1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Craig JB, Lane FL, Nistor G et al. Allogenic myoblast transplantation in the rat anal sphincter. Female Pelvic Med Reconstr Surg 2010;16:205–208. [DOI] [PubMed] [Google Scholar]
- 12. White AB, Keller PW, Acevedo JF et al. Effect of myogenic stem cells on contractile properties of the repaired and unrepaired transected external anal sphincter in an animal model. Obstet Gynecol 2010;115:815–823. [DOI] [PubMed] [Google Scholar]
- 13. Lane FL, Jacobs SA, Craig JB et al. In vivo recovery of the injured anal sphincter after repair and injection of myogenic stem cells: An experimental model. Dis Colon Rectum 2013;56:1290–1297. [DOI] [PubMed] [Google Scholar]
- 14. Fitzwater JL, Grande KB, Sailors JL et al. Effect of myogenic stem cells on the integrity and histomorphology of repaired transected external anal sphincter. Int Urogynecol J 2015;26:251–256. [DOI] [PubMed] [Google Scholar]
- 15. Kajbafzadeh AM, Elmi A, Talab SS et al. Functional external anal sphincter reconstruction for treatment of anal incontinence using muscle progenitor cell auto grafting. Dis Colon Rectum 2010;53:1415–1421. [DOI] [PubMed] [Google Scholar]
- 16. Oh HK, Lee HS, Lee JH et al. Functional and histological evidence for the targeted therapy using biocompatible polycaprolactone beads and autologous myoblasts in a dog model of fecal incontinence. Dis Colon Rectum 2015;58:517–525. [DOI] [PubMed] [Google Scholar]
- 17. Pathi SD, Acevedo JF, Keller PW et al. Recovery of the injured external anal sphincter after injection of local or intravenous mesenchymal stem cells. Obstet Gynecol 2012;119:134–144. [DOI] [PubMed] [Google Scholar]
- 18. Salcedo L, Mayorga M, Damaser M et al. Mesenchymal stem cells can improve anal pressures after anal sphincter injury. Stem Cell Res 2013;10:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salcedo L, Penn M, Damaser M et al. Functional outcome after anal sphincter injury and treatment with mesenchymal stem cells. Stem Cells Translational Medicine 2014;3:760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sarveazad A, Newstead GL, Mirzaei R et al. A new method for treating fecal incontinence by implanting stem cells derived from human adipose tissue: Preliminary findings of a randomized double‐blind clinical trial. Stem Cell Res Ther 2017;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev 2011;7:269–291. [DOI] [PubMed] [Google Scholar]
- 22. Zuk PA, Zhu M, Mizuno H et al. Multilineage cells from human adipose tissue: Implications for cell‐based therapies. Tissue Eng 2001;7:211–228. [DOI] [PubMed] [Google Scholar]
- 23. Liu Y, Yan X, Sun Z et al. Flk‐1+ adipose‐derived mesenchymal stem cells differentiate into skeletal muscle satellite cells and ameliorate muscular dystrophy in mdx mice. Stem Cells Dev 2007;16:695–706. [DOI] [PubMed] [Google Scholar]
- 24. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007;110:3499–3506. [DOI] [PubMed] [Google Scholar]
- 25. Gnecchi M, Zhang Z, Ni A et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 2008;103:1204–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Christensen LH, Nielsen JB, Mouritsen L et al. Tissue integration of polyacrylamide hydrogel: An experimental study of periurethral, perivesical, and mammary gland tissue in the pig. Dermatol Surg 34(Suppl 1):77. discussion S77. [DOI] [PubMed] [Google Scholar]
- 27. Pai A, Al‐Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (bulkamid((R))) in the management of stress and mixed urinary incontinence: Three year follow up outcomes. Cent European J Urol 2015;68:428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Altman D, Hjern F, Zetterstrom J. Transanal submucosal polyacrylamide gel injection treatment of anal incontinence: A randomized controlled trial. Acta Obstet Gynecol Scand 2016;95:528–533. [DOI] [PubMed] [Google Scholar]
- 29. Al‐Abed YA, Ayers J, Ayantunde A et al. Safety and efficacy of permacol injection in the treatment of fecal incontinence. Ann Coloproctol 2016;32:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maeda Y, Laurberg S, Norton C. Perianal injectable bulking agents as treatment for faecal incontinence in adults. Cochrane Database Syst Rev 2013;2:CD007959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lindroos B, Boucher S, Chase L et al. Serum‐free, xeno‐free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy 2009;11:958–972. [DOI] [PubMed] [Google Scholar]
- 32. Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. the international society for cellular therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 33. Galipeau J, Krampera M, Barrett J et al. International society for cellular therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy 2016;18:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nolte T, Brander‐Weber P, Dangler C et al. Nonproliferative and proliferative lesions of the gastrointestinal tract, pancreas and salivary glands of the rat and mouse. J Toxicol Pathol 2016;29:125S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lo Furno D, Mannino G, Cardile V et al. Potential therapeutic applications of adipose‐derived mesenchymal stem cells. Stem Cells Dev 2016;25:1615–1628. [DOI] [PubMed] [Google Scholar]
- 36. Zuk PA. The adipose‐derived stem cell: Looking back and looking ahead. Mol Biol Cell 2010;21:1783–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lorenzi B, Pessina F, Lorenzoni P et al. Treatment of experimental injury of anal sphincters with primary surgical repair and injection of bone marrow‐derived mesenchymal stem cells. Dis Colon Rectum 2008;51:411–420. [DOI] [PubMed] [Google Scholar]
- 38. Mazzanti B, Lorenzi B, Borghini A et al. Local injection of bone marrow progenitor cells for the treatment of anal sphincter injury: In‐vitro expanded versus minimally‐manipulated cells. Stem Cell Res Ther 2016;7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Frudinger A, Kolle D, Schwaiger W et al. Muscle‐derived cell injection to treat anal incontinence due to obstetric trauma: Pilot study with 1 year follow‐up. Gut 2010;59:55–61. [DOI] [PubMed] [Google Scholar]
- 40. Frudinger A, Pfeifer J, Paede J et al. Autologous skeletal‐muscle‐derived cell injection for anal incontinence due to obstetric trauma: A 5‐year follow‐up of an initial study of 10 patients. Colorectal Dis 2015;17:794–801. [DOI] [PubMed] [Google Scholar]
- 41. Romaniszyn M, Rozwadowska N, Malcher A et al. Implantation of autologous muscle‐derived stem cells in treatment of fecal incontinence: Results of an experimental pilot study. Tech Coloproctol 2015;19:685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Salcedo L, Damaser M, Butler R et al. Long‐term effects on pressure and electromyography in a rat model of anal sphincter injury. Dis Colon Rectum 2010;53:1209–1217. [DOI] [PubMed] [Google Scholar]
- 43. Choudhery MS, Badowski M, Muise A et al. Donor age negatively impacts adipose tissue‐derived mesenchymal stem cell expansion and differentiation. J Transl Med 2014;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aksu AE, Rubin JP, Dudas JR et al. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose‐derived stem cells. Ann Plast Surg 2008;60:306–322. [DOI] [PubMed] [Google Scholar]
- 45. Pachon‐Pena G, Serena C, Ejarque M et al. Obesity determines the immunophenotypic profile and functional characteristics of human mesenchymal stem cells from adipose tissue. Stem Cells Translational Medicine 2016;5:464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Serena C, Keiran N, Ceperuelo‐Mallafre V et al. Obesity and type 2 diabetes alters the immune properties of human adipose derived stem cells. Stem Cells 2016;34:2559–2573. [DOI] [PubMed] [Google Scholar]
- 47. Strong AL, Hunter RS, Jones RB et al. Obesity inhibits the osteogenic differentiation of human adipose‐derived stem cells. J Transl Med 2016;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nagy N, Marsiano N, Bruckner RS et al. Xenotransplantation of human intestine into mouse abdomen or subcutaneous tissue: Novel platforms for the study of the human enteric nervous system. Neurogastroenterol Motil 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen X, Li Y, Wang L et al. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology 2002;22:275–279. [DOI] [PubMed] [Google Scholar]
- 50. Matsuda T, Miyagawa S, Fukushima S et al. Human cardiac stem cells with reduced notch signaling show enhanced therapeutic potential in a rat acute infarction model. Circ J 2014;78:222–231. [DOI] [PubMed] [Google Scholar]
- 51. Boltze J, Nitzsche F, Jolkkonen J et al. Concise review: Increasing the validity of cerebrovascular disease models and experimental methods for translational stem cell research. Stem Cells 2017;35:1141–1153. [DOI] [PubMed] [Google Scholar]
- 52. Aghaee‐Afshar M, Rezazadehkermani M, Asadi A et al. Potential of human umbilical cord matrix and rabbit bone marrow‐derived mesenchymal stem cells in repair of surgically incised rabbit external anal sphincter. Dis Colon Rectum 2009;52:1753–1761. [DOI] [PubMed] [Google Scholar]
- 53. Cruz M, Dissaranan C, Cotleur A et al. Pelvic organ distribution of mesenchymal stem cells injected intravenously after simulated childbirth injury in female rats. Obstet Gynecol Int 2012;2012:612946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jacobs SA, Lane FL, Pham QA et al. Safety assessment of myogenic stem cell transplantation and resulting tumor formation. Female Pelvic Med Reconstr Surg 2013;19:362–368. [DOI] [PubMed] [Google Scholar]
- 55. Mattei A, Magalon J, Bertrand B et al. Cell therapy and vocal fold scarring. Eur Ann Otorhinolaryngol Head Neck Dis 2017;134:339–345. [DOI] [PubMed] [Google Scholar]
- 56. Liu J, Ren J, Su L et al. Human adipose tissue‐derived stem cells inhibit the activity of keloid fibroblasts and fibrosis in a keloid model by paracrine signaling. Burns 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 57. Lane FL, Jacobs S. Stem cells in gynecology. Am J Obstet Gynecol 2012;207:149–156. [DOI] [PubMed] [Google Scholar]
- 58. Norton C. Treating faecal incontinence with bulking‐agent injections. Lancet 2011;377:971–972. [DOI] [PubMed] [Google Scholar]
- 59. Feki A, Faltin DL, Lei T et al. Sphincter incontinence: Is regenerative medicine the best alternative to restore urinary or anal sphincter function?. Int J Biochem Cell Biol 2007;39:678–684. [DOI] [PubMed] [Google Scholar]
- 60. Bisson A, Freret M, Drouot L et al. Restoration of anal sphincter function after myoblast cell therapy in incontinent rats. Cell Transplant 2015;24:277–286. [DOI] [PubMed] [Google Scholar]
- 61. Mitterberger M, Marksteiner R, Margreiter E et al. Autologous myoblasts and fibroblasts for female stress incontinence: A 1‐year follow‐up in 123 patients. BJU Int 2007;100: 1081–1085. [DOI] [PubMed] [Google Scholar]
- 62. Kuismanen K, Sartoneva R, Haimi S et al. Autologous adipose stem cells in treatment of female stress urinary incontinence: Results of a pilot study. Stem Cells Translational Medicine 2014;3:936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bohl JL, Zakhem E, Bitar KN. Successful treatment of passive fecal incontinence in an animal model using engineered biosphincters: A 3‐month follow‐up study. Stem Cells Translational Medicine 2017;6:1795–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1
Supplement Figure 2R1
Supplement Figure 3R1
Supplement Online Video


