Abstract
RGD-α-amanitin and isoDGR-α-amanitin conjugates were synthesized by joining integrin ligands to α-amanitin via various linkers and spacers. The conjugates were evaluated for their ability to inhibit biotinylated vitronectin binding to the purified αVβ3 receptor, retaining good binding affinity, in the same nanomolar range as the free ligands. The antiproliferative activity of the conjugates was evaluated in three cell lines possessing different levels of αVβ3 integrin expression: human glioblastoma U87 (αVβ3+), human lung carcinoma A549 (αVβ3−) and breast adenocarcinoma MDA-MB-468 (αVβ3−). In the U87, in the MDA-MB-468, and partly in the A549 cancer cell lines, the cyclo[DKP-isoDGR]-α-amanitin conjugates bearing the lysosomally cleavable Val-Ala linker were found to be slightly more potent than α-amanitin. Apparently, for all these α-amanitin conjugates there is no correlation between the cytotoxicity and the expression of αVβ3 integrin. To determine whether the increased cytotoxicity of the cyclo[DKP-isoDGR]-α-amanitin conjugates is governed by an integrin-mediated binding and internalization process, competition experiments were carried out in which the conjugates were tested with U87 (αVβ3+, αVβ5+, αVβ6−, α5β1+) and MDA-MB-468 (αVβ3−, αVβ5+, αVβ6+, α5β1−) cells in the presence of excess cilengitide, with the aim of blocking integrins on the cell surface. Using the MDA-MB-468 cell line, a fivefold increase of the IC50 was observed for the conjugates in the presence of excess cilengitide, which is known to strongly bind not only αVβ3, but also αVβ5, αVβ6, and α5β1. These data indicate that in this case the cyclo[DKP-isoDGR]-α-amanitin conjugates are possibly internalized by a process mediated by integrins different from αVβ3 (e.g., αVβ5).
Keywords: antitumor agents, cancer, drug delivery, integrins, peptidomimetics
Introduction
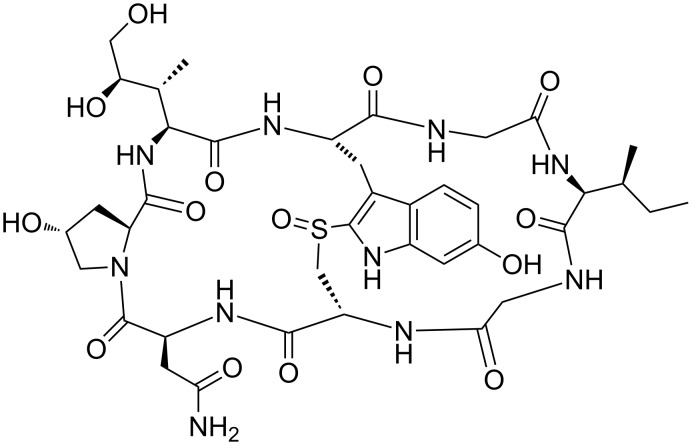
α-Amanitin is a bicyclic octapeptide toxin belonging to the amatoxin family, found in Amanita Phalloides (death cap mushroom), see Figure 1 [1]. Its mechanism of action consists in the inhibition of cellular transcription by an effective blocking of RNA polymerase II, which is present in the nuclei of eukaryotic cells and is responsible for the transcription of DNA to mRNA [1–2]. Despite this strong inhibitory activity, α-amanitin exhibits only a micromolar cytotoxicity and low cellular uptake in most mammalian cells, due to its strong polarity and poor membrane permeability [2]. One notable exception are human hepatocytes, where the transporting protein OATP1B3 internalizes amatoxins resulting in high liver toxicity [2–3].
Figure 1.
α-Amanitin.
This strong toxicity in the presence of endocytosis mediators allowing cell permeation, aroused interest towards the use of α-amanitin as a payload for targeted cancer therapy. In 1981, Davis and Preston reported the synthesis of the antibody–drug conjugate (ADC) α-amanitin-anti-Thy 1.2 IgG, which was 47-fold more toxic than the unconjugated α-amanitin in the murine T lymphoma S49.1 cell line [4]. In 2012, a new ADC containing α-amanitin and a chimerized anti-EpCAM (epithelial cell-adhesion molecule) monoclonal antibody was prepared by Moldenhauer and co-workers [5]. The cytotoxicity of this conjugate was tested in EpCAM-overexpressing cancer cell lines obtaining IC50 values from 2.5 × 10−10 to 2.0 × 10−12 M. Promising results were also observed in mice bearing BxPc-3 pancreatic xenograft tumors, with complete tumor regression in 90% of the cases after two injections of the α-amanitin-anti-EpCAM ADC at a dose of 100 μg/kg with respect to α-amanitin. In these two examples, the internalization of the monoclonal antibody and subsequent release of the toxin leads to the enhancement of α-amanitin activity on the targeted cells.
An alternative approach to the antibody targeted therapy is represented by small molecule–drug conjugates (SMDCs), where the small molecule – usually a peptide or peptidomimetic receptor ligand – avoids the drawbacks of ADCs such as high manufacturing costs, unfavorable pharmacokinetics (low tissue diffusion and low accumulation rate) and possible elicitation of immune response [6]. By conjugation to a specific cell-membrane-receptor ligand, the toxin can be delivered at the tumor site and internalized through receptor-mediated endocytosis. In 2013, Reshetnyak and co-workers conjugated α-amanitin to pHLIP (pH low insertion peptide) via linkers of different hydrophobicities [7]. The results indicated that pHLIP could deliver α-amanitin into cells and induce cell death in 48 h by a pH-mediated direct translocation across the membrane and cleavage of the disulfide linker in the cytoplasm. In another example, Perrin and co-workers conjugated the N-propargylasparagine of an amanitin analog to a cycloRGD integrin ligand (cyclo[RGDfK]) using a copper-catalyzed azide–alkyne cycloaddition [8]. The conjugates were tested in the U87 glioblastoma cell line, but only a slight enhancement in toxicity over α-amanitin was observed.
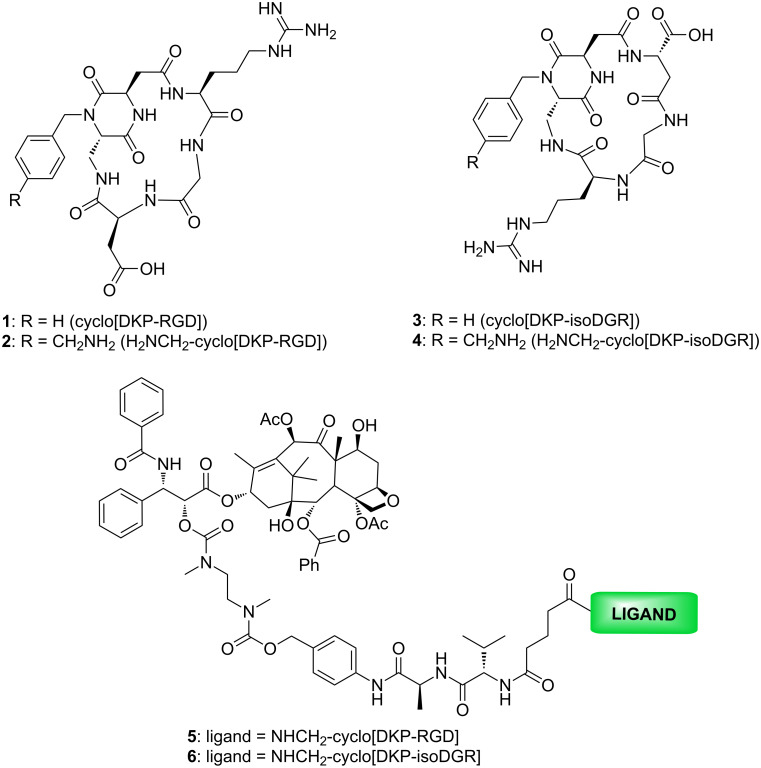
The transmembrane receptor αVβ3 integrin is widely expressed on the blood vessels of several human cancers (for example, breast cancer, glioblastoma, pancreatic tumor, prostate carcinoma) but not on the vasculature of healthy tissues [9–11], and therefore constitutes a suitable therapeutic target in the field of SMDCs. Integrin αVβ3 recognizes endogenous ligands by the tripeptide arginine-glycine-aspartate [12] (RGD) and also by the related sequence isoaspartate-glycine-arginine (isoDGR) [13–20]. Many synthetic peptides or peptidomimetics containing these sequences have been prepared and show low nanomolar IC50 values for integrin αVβ3 binding [21–27]. A number of cyclic RGD and isoDGR ligands containing a bifunctional diketopiperazine (DKP) scaffold have been developed by the Gennari and Piarulli groups in the last decade [24–27]. Among them, the cyclo[DKP-RGD] 1 [25] and cyclo[DKP-isoDGR] 3 [26] (Figure 2) showed a binding affinity for the purified receptor αVβ3 in the low nanomolar range and a good selectivity for this integrin in comparison with integrin αVβ5 (33–34 times, see Table 1).
Figure 2.
Structure of the ligands cyclo[DKP-RGD] (1), NH2CH2-cyclo[DKP-RGD] (2), cyclo[DKP-isoDGR] (3), NH2CH2-cyclo[DKP-isoDGR] (4) and the related SMDCs cyclo[DKP-RGD]-Val-Ala-PTX (5) and cyclo[DKP-isoDGR]-Val-Ala-PTX (6).
Table 1.
Inhibition of biotinylated vitronectin binding to αvβ3 and αvβ5 receptors.
| ligand | structure (name) | IC50 (nM)a αVβ3 | IC50 (nM)a αVβ5 |
| 1 | cyclo[DKP-RGD] | 4.5 ± 1.1 | 149 ± 25 |
| 3 | cyclo[DKP-isoDGR] | 9.2 ± 1.1 | 312 ± 21 |
aIC50 values were calculated as the concentration of compound required for 50% inhibition of biotinylated vitronectin binding as estimated by GraphPad Prism software. All values are the arithmetic mean ± the standard deviation (SD) of triplicate determinations.
Furthermore, these ligands were shown to inhibit the FAK (focal adhesion kinase) and Akt (protein kinase B) signaling cascade and the tumor cell infiltration process, performing as true integrin antagonists [27].
Ligands 1 and 3 were also functionalized with an aminomethyl group (-CH2NH2) as a handle for conjugation to cytotoxic drugs (Figure 2, ligands 2 and 4) [28–30]. Conjugates of the functionalized ligands 2 and 4 with paclitaxel (PTX) via a 2’-carbamate with a self-immolative spacer and the lysosomally cleavable Val-Ala linker [31] were synthesized (Figure 2, cyclo[DKP-RGD]-Val-Ala-PTX 5 and cyclo[DKP-isoDGR]-Val-Ala-PTX 6). Their tumor targeting ability was assessed in vitro in antiproliferative assays comparing an αVβ3 positive with an αVβ3 negative cell line [29–30]. The cyclo[DKP-isoDGR]-Val-Ala-PTX conjugate 6 displayed a remarkable targeting index (TI = 9.9), especially when compared to the strictly related cyclo[DKP-RGD]-Val-Ala-PTX conjugate 5 (TI = 2.4) [30].
Results and Discussion
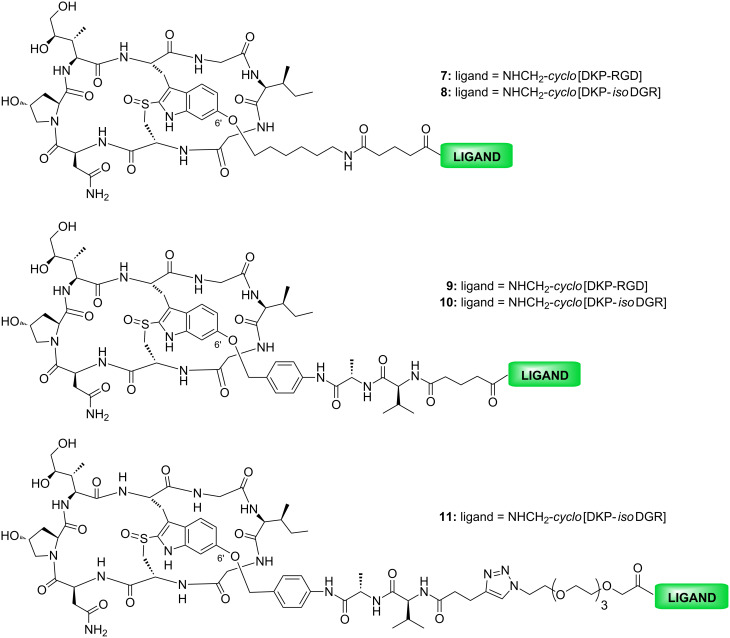
In the present paper, we report the synthesis and biological evaluation of two cyclo[DKP-RGD]-α-amanitin and three cyclo[DKP-isoDGR]-α-amanitin conjugates. In these conjugates, the integrin ligands are bound to α-amanitin via a 6’-ether with two different linkers: an “uncleavable” six carbon aliphatic chain (Figure 3, compounds 7 and 8) and a lysosomally cleavable Val-Ala linker bound to a self-immolative spacer (Figure 3, compounds 9, 10 and 11). Integrin receptor competitive binding assays and cell proliferation assays with an αVβ3 positive (U87) and two αVβ3 negative cell lines (A549 and MDA-MB-468) were performed for all the conjugates.
Figure 3.
Structure of the α-amanitin conjugates: cyclo[DKP-RGD]-uncleavable-α-amanitin (7), cyclo[DKP-isoDGR]-uncleavable-α-amanitin (8), cyclo[DKP-RGD]-Val-Ala-α-amanitin (9), cyclo[DKP-isoDGR]-Val-Ala-α-amanitin (10) and cyclo[DKP-isoDGR]-PEG-4-Val-Ala-α-amanitin (11).
Synthesis
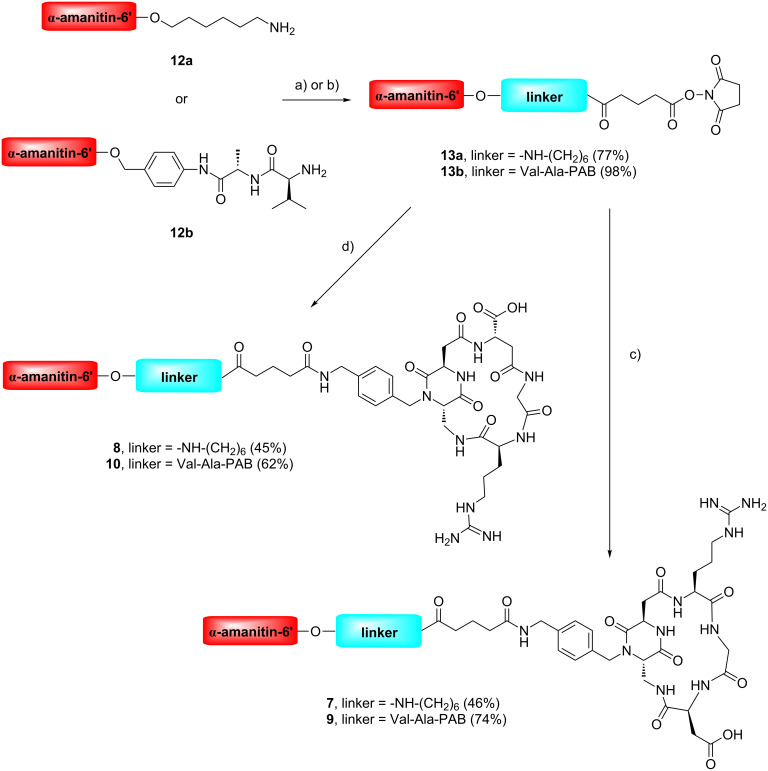
Cyclo[DKP-RGD]-α-amanitin and cyclo[DKP-isoDGR]-α-amanitin conjugates 7–11 were synthesized as described in Scheme 1 and Scheme 2, by joining the functionalized ligands H2NCH2-cyclo[DKP-RGD] (2) [18,29] and H2NCH2-cyclo[DKP-isoDGR] (4) [30] to α-amanitin via a 6’-ether with various linkers and spacers. Details are reported in Supporting Information File 1.
Scheme 1.
Synthesis of α-amanitin-cyclo[DKP-RGD] and α-amanitin-cyclo[DKP-isoDGR] conjugates 7–10. Reagents and conditions: a) 1. glutaric anhydride, DMAP, iPr2NEt, DMF, overnight, 2. DIC, N-hydroxysuccinimide, DMF, overnight; b) di-N-succinimidyl glutarate, iPr2NEt, rt, DMF, 6 h; c) H2NCH2-cyclo[DKP-RGD] (2), PBS/MeCN or PBS/DMF (pH 7.5), overnight; d) H2NCH2-cyclo[DKP-isoDGR] (4), PBS/DMF (pH 7.5), overnight. DMAP: 4-dimethylaminopyridine; DIC: diisopropylcarbodiimide; PBS: phosphate-buffered saline; PAB: p-aminobenzyl.
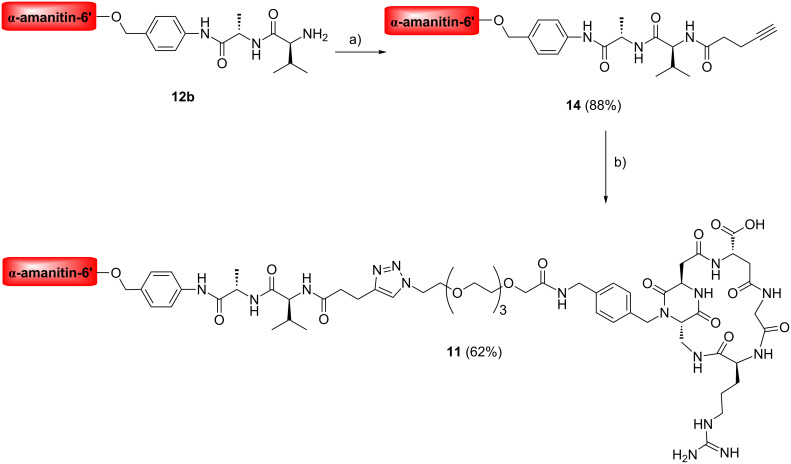
Scheme 2.
Synthesis of cyclo[DKP-isoDGR]-PEG-4-Val-Ala-α-amanitin conjugate 11. Reagents and conditions: a) 4-pentynoic acid N-hydroxysuccinimidyl ester, iPr2NEt, DMF, overnight; b) N3-PEG-4-cyclo[DKP-isoDGR], sodium ascorbate, CuSO4·5H2O, DMF/water, rt, overnight.
Integrin receptor competitive binding assays
Conjugates 7–11 were evaluated for their ability to inhibit biotinylated vitronectin binding to the purified αVβ3 receptor. The calculated half-maximal inhibitory concentrations (IC50) are listed in Table 2. Screening assays were performed by incubating the immobilized integrin receptor with solutions of the cyclo[DKP-RGD]-α-amanitin and cyclo[DKP-isoDGR]-α-amanitin conjugates 7–11 at different concentrations (10−12 to 10−5 M) in the presence of biotinylated vitronectin (1 µg mL−1) and measuring bound vitronectin.
Table 2.
Inhibition of biotinylated vitronectin binding to αvβ3 receptor.
| compound | structure (name) | IC50 (nM)a αVβ3 |
| 7 | cyclo[DKP-RGD]-uncleavable-α-amanitin | 11.6 ± 2.4 |
| 8 | cyclo[DKP-isoDGR]-uncleavable-α-amanitin | 6.8 ± 4.3 |
| 9 | cyclo[DKP-RGD]-Val-Ala-α-amanitin | 14.7 ± 6.6 |
| 10 | cyclo[DKP-isoDGR]-Val-Ala-α-amanitin | 6.4 ± 1.9 |
| 11 | cyclo[DKP-isoDGR]-PEG-4-Val-Ala-α-amanitin | 3.8 ± 0.3 |
aIC50 values were calculated as the concentration of compound required for 50% inhibition of biotinylated vitronectin binding as estimated by GraphPad Prism software. All values are the arithmetic mean ± the standard deviation (SD) of triplicate determinations.
It was found that the cyclo[DKP-RGD]-α-amanitin conjugates 7 and 9 and cyclo[DKP-isoDGR]-α-amanitin conjugates 8, 10 and 11 retain good binding affinity for αVβ3 integrin, in the same range as the free ligands (cf. Table 2 with Table 1). These results encouraged us to proceed with cell viability assays in αVβ3 positive and αVβ3 negative cell lines, to study the ability of the conjugates to selectively target αVβ3 expressing tumor cells.
Cell viability assays
The antiproliferative activity of the conjugates was evaluated in three cell lines expressing different levels of αVβ3 integrin. U87 cells (human glioblastoma) were selected as αVβ3 positive, while A549 cells (human lung carcinoma) and MDA-MB-468 (breast adenocarcinoma) [32] were used as αVβ3 negative. The expression of αVβ3 integrin on the cell membrane was assessed by flow cytometry (see Supporting Information File 1, Figure S1), and the results were in good agreement with the literature for U87 and A549 [33–35]. In the case of MDA-MB-468, while the presence of the β3 integrin subunit is still quite controversial in the literature [36–38], our FACS analysis could not detect any αVβ3 expression (see Supporting Information File 1).
The cell lines were treated with different concentrations of the free drug α-amanitin and conjugates 7–11 for 96 hours. The cell viability was evaluated with the CellTiterGlo 2.0 assay and the calculated IC50 are shown in Table 3.
Table 3.
Evaluation of anti-proliferative activity of α-amanitin and α-amanitin conjugates 7–11 in U-87, MDA-MB-468 and A549.
| entry | structure (name) | IC50 (nM)a | ||
| U87 (αVβ3+) | MDA-MB-468 (αVβ3−) | A549 (αVβ3−) | ||
| 1 | α-amanitin | 347 ± 132.5b | 185 ± 49.6b | 518 ± 305b |
| 2 | cyclo[DKP-RGD]-uncleavable-α-amanitin (7) | 2552 ± 37.6 | 1111 ± 228.4 | n.d.c |
| 3 | cyclo[DKP-isoDGR]-uncleavable-α-amanitin (8) | 3355 ± 19.1 | 2200 ± 96.2 | n.d.c |
| 4 | cyclo[DKP-RGD]-Val-Ala-α-amanitin (9) | 1446 ± 83.9 | 202 ± 10.3 | 2160 ± 23.3 |
| 5 | cyclo[DKP-isoDGR]-Val-Ala-α-amanitin (10) | 143 ± 33.8 | 59 ± 23.4 | 217 ± 98.3 |
| 6 | cyclo[DKP-isoDGR]-PEG-4-Val-Ala-α-amanitin (11) | 165 ± 4.0 | 66 ± 24.1 | 720 ± 98.1 |
aIC50 values were calculated as the concentration of compound required for 50% inhibition of cell viability. All cell lines were treated with different concentrations of α-amanitin and compounds 7–11 for 96 hours. The samples were measured in triplicate. bAverage values from three independent experiments. cn.d.: these data could not be determined.
The cyclo[DKP-isoDGR]-α-amanitin conjugate bearing the lysosomally cleavable Val-Ala linker 10 proved slightly more potent than α-amanitin in the U87 (αVβ3+) cell line, as well as in the A549 and MDA-MB-468 (αVβ3−) cell lines (2.4–3.1 times, cf. entry 1 with entry 5 in Table 3). The cyclo[DKP-isoDGR]-α-amanitin conjugate bearing the lysosomally cleavable Val-Ala linker and a PEG-4 spacer 11 proved slightly more potent than α-amanitin in both the U87 (αVβ3+) and MDA-MB-468 (αVβ3−) cell lines (2.1–2.8 times, cf. entry 1 with entry 6 in Table 3), while in A549 cell line (αVβ3−) it turned out to be less potent (1.4 times) than the free drug. Cyclo[DKP-RGD]-uncleavable-α-amanitin (7), cyclo[DKP-isoDGR]-uncleavable-α-amanitin (8) and cyclo[DKP-RGD]-Val-Ala-α-amanitin (9) proved less potent than α-amanitin in all cell lines (see Table 3, cf. entry 1 with entries 2–4). In general, one can conclude that the “uncleavable” compounds 7 and 8 are much less cytotoxic than free α-amanitin, while the lysosomally cleavable compounds 9–11 show variable results, with RGD compound 9 behaving worse than the free drug but better than the corresponding uncleavable conjugate 7. The isoDGR motif gives generally better results, with 10 and 11 behaving much better than the corresponding uncleavable conjugate 8 and slightly better than the free drug. Apparently, for all the α-amanitin conjugates there is no direct correlation between the cytotoxicity and the expression of αVβ3 integrin [39].
To determine whether the increased cytotoxicity of cyclo[DKP-isoDGR]-α-amanitin conjugates 10 and 11 is governed by an integrin-mediated binding and internalization process, competition experiments [40] were carried out in which conjugates 10 and 11 were tested on U87 (αVβ3+, αVβ5+, αVβ6−, α5β1+) [34–36] and on MDA-MB-468 (αVβ3−, αVβ5+, αVβ6+, α5β1−) [34–36] cells in the presence of 50-fold excess of cilengitide [23], with the aim of blocking integrins on the cell surface (Table 4, see also Supporting Information File 1, Biological assays). Using the U87 cell line, a modest IC50 increase of conjugate 11 from 91 nM (without cilengitide) to 143 nM (with excess cilengitide) was observed (Table 4, entry 2). Using the MDA-MB-468 cell line, a more pronounced IC50 increase was observed for both conjugates 10 (from 47 nM without cilengitide to 259 nM with excess cilengitide; Table 4, entry 1) and 11 (from 65 nM without cilengitide to 340 nM with excess cilengitide; Table 4, entry 2). From these results, no correlation emerges between the expression of integrin αVβ3 and cytotoxicity. However, it should be noted that these cell lines overexpress other integrins (α5β1 in U87, αVβ6 in MDA-MB-468, and αVβ5 in both) [34–36]. Thus, the increase of the IC50 of conjugates 10 and 11 (up to 5.5 times) may be possibly due to the block of other integrins with excess cilengitide, which is known to efficiently bind not only αVβ3 (IC50 = 0.6 nM) [23], but also αVβ5 (IC50 = 8.4 nM) [23], αVβ6 (IC50 = 82.8 nM) [41], and α5β1 (IC50 = 14.9 nM) [23].
Table 4.
Competition experiments of conjugates 10 and 11 in the presence of a 50-fold excess of cilengitide in U-87 and MDA-MB-468.
| entry | compound | IC50 (nM)a | |
| U87 (αVβ3+, αVβ5+, αVβ6−, α5β1+) | MDA-MB-468 (αVβ3−, αVβ5+, αVβ6+, α5β1−) | ||
| 1 | 10 | 107 ± 26.8 | 47 ± 21.1 |
| 10 + 50-fold excess of cilengitide | 106 ± 11.6 | 259 ± 55.2 | |
| 2 | 11 | 91 ± 30.6 | 65 ± 17.6 |
| 11 + 50-fold excess of cilengitide | 143 ± 59.3 | 340 ± 210.3 | |
aIC50 values were calculated as the concentration of compound required for 50% inhibition of cell viability. Both cell lines were treated with different concentrations of compounds 10 and 11 in the presence of a 50-fold excess of cilengitide during 96 hours. The samples were measured in triplicate.
Conclusion
In this paper, two cyclo[DKP-RGD]-α-amanitin and three cyclo[DKP-isoDGR]-α-amanitin conjugates were prepared in good yields following a straightforward synthetic route. Conjugates 7–11 retain good binding affinities for the purified αVβ3 receptor, in the same range as the respective free ligands. Cell proliferation assays were performed with three cell lines possessing different levels of integrin expression: human glioblastoma U87 (αVβ3+), human lung carcinoma A549 (αVβ3−) and breast adenocarcinoma MDA-MB-468 (αVβ3−). With all these cell lines the cyclo[DKP-isoDGR]-α-amanitin conjugate 10 proved slightly more potent than α-amanitin, whereas conjugate 11 showed enhanced potency compared to the free drug only on U87 and MDA-MB-468 cells.
Apparently, for all these α-amanitin conjugates there is no correlation between the cytotoxicity and the expression of αVβ3 integrin. To determine whether the slightly increased cytotoxicity of cyclo[DKP-isoDGR]-α-amanitin conjugates 10 and 11 is governed by an integrin-mediated binding and internalization process, competition experiments were carried out in which conjugates 10 and 11 were tested with U87 (αVβ3+, αVβ5+, αVβ6−, α5β1+) and MDA-MB-468 (αVβ3−, αVβ5+, αVβ6+, α5β1−) cells in the presence of 50-fold excess of cilengitide, with the aim of blocking integrins on the cell surface. Using the U87 cell line, a modest increase of the conjugate 11 IC50 was observed in the presence of cilengitide. Employing the MDA-MB-468 cell line, a more pronounced increase of IC50 was observed for both conjugates 10 and 11 in the presence of cilengitide. Therefore, it appears that blocking integrins with excess cilengitide, which is known to strongly bind not only αVβ3, but also αVβ5, αVβ6, and α5β1, results in an increase (up to 5.5 times) of the IC50 of conjugates 10 and 11 with the MDA-MB-468 cell line. These data suggest that cyclo[DKP-isoDGR]-α-amanitin conjugates 10 and 11 are possibly internalized by a process mediated by integrins different from αVβ3 (e.g., αVβ5), though the exact nature of this involvement is not clearly defined [42].
Finally, the IC50 values of the integrin ligand-α-amanitin conjugates are much worse (cytotoxicity increased three times compared to α-amanitin) than those exhibited by antibody α-amanitin conjugates for which the increase of cytotoxicity is 100–100000 times [5]. Therefore, despite the remarkable progresses that have been realized in recent years, integrin targeting SMDCs are still far from the clinic.
Experimental
Functionalized ligands H2NCH2-cyclo[DKP-RGD] (2) and H2NCH2-cyclo[DKP-isoDGR] (4) were prepared according to the literature [28–29]. 6’-Functionalized α-amanitin derivatives 12a and 12b were prepared according to references [43–44].
Supporting Information
Experimental details, characterization data and copies of spectra.
Acknowledgments
We thank the European Commission (Marie Skłodowska-Curie ITN MAGICBULLET 642004) for PhD fellowships (to L.B., P.L.R., B.K.) and financial support.
This article is part of the Thematic Series "Peptide–drug conjugates".
Contributor Information
Cesare Gennari, Email: cesare.gennari@unimi.it.
Umberto Piarulli, Email: umberto.piarulli@uninsubria.it.
References
- 1.Wieland T, Faulstich H. Experientia. 1991;47:1186–1193. doi: 10.1007/BF01918382. [DOI] [PubMed] [Google Scholar]
- 2.Anderl J, Echner H, Faulstich H. Beilstein J Org Chem. 2012;8:2072–2084. doi: 10.3762/bjoc.8.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Letschert K, Faulstich H, Keller D, Keppler D. Toxicol Sci. 2006;91:140–149. doi: 10.1093/toxsci/kfj141. [DOI] [PubMed] [Google Scholar]
- 4.Davis M T, Preston J F., III Science. 1981;213:1385–1388. doi: 10.1126/science.6115471. [DOI] [PubMed] [Google Scholar]
- 5.Moldenhauer G, Salnikov A V, Lüttgau S, Herr I, Anderl J, Faulstich H. J Natl Cancer Inst. 2012;104:622–634. doi: 10.1093/jnci/djs140. [DOI] [PubMed] [Google Scholar]
- 6.Krall N, Scheuermann J, Neri D. Angew Chem, Int Ed. 2013;52:1384–1402. doi: 10.1002/anie.201204631. [DOI] [PubMed] [Google Scholar]
- 7.Moshnikova A, Moshnikova V, Andreev O A, Reshetnyak Y K. Biochemistry. 2013;52:1171–1178. doi: 10.1021/bi301647y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao L, May J P, Blanc A, Dietrich D J, Loonchanta A, Matinkhoo K, Pryyma A, Perrin D M. ChemBioChem. 2015;16:1420–1425. doi: 10.1002/cbic.201500226. [DOI] [PubMed] [Google Scholar]
- 9.Desgrosellier J S, Cheresh D A. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danhier F, Le Breton A, Préat V. Mol Pharmaceutics. 2012;9:2961–2973. doi: 10.1021/mp3002733. [DOI] [PubMed] [Google Scholar]
- 11.De Franceschi N, Hamidi H, Alanko J, Sahgal P, Ivaska J. J Cell Sci. 2015;128:839–852. doi: 10.1242/jcs.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierschbacher M D, Ruoslahti E. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 13.Biochemical studies have shown that a spontaneous post-translational modification, occurring at the Asn-Gly-Arg (NGR) motif of the extracellular matrix protein fibronectin, leads to the isoAspGlyArg (isoDGR) sequence.
- 14.Curnis F, Longhi R, Crippa L, Cattaneo A, Dondossola E, Bachi A, Corti A. J Biol Chem. 2006;281:36466–36476. doi: 10.1074/jbc.M604812200. [DOI] [PubMed] [Google Scholar]
- 15.Curnis F, Sacchi A, Gasparri A, Longhi R, Bachi A, Doglioni C, Bordignon C, Traversari C, Rizzardi G-P, Corti A. Cancer Res. 2008;68:7073–7082. doi: 10.1158/0008-5472.CAN-08-1272. [DOI] [PubMed] [Google Scholar]
- 16.Corti A, Curnis F. J Cell Sci. 2011;124:515–522. doi: 10.1242/jcs.077172. [DOI] [PubMed] [Google Scholar]
- 17.Biochemical, spectroscopic and computational investigations showed that the isoDGR sequence can fit into the RGD-binding pocket of αVβ3 integrin, establishing the same electrostatic clamp as well as additional polar interactions, see ref. [18–20].
- 18.Spitaleri A, Mari S, Curnis F, Traversari C, Longhi R, Bordignon C, Corti A, Rizzardi G-P, Musco G. J Biol Chem. 2008;283:19757–19768. doi: 10.1074/jbc.M710273200. [DOI] [PubMed] [Google Scholar]
- 19.Curnis F, Cattaneo A, Longhi R, Sacchi A, Gasparri A M, Pastorino F, Di Matteo P, Traversari C, Bachi A, Ponzoni M, et al. J Biol Chem. 2010;285:9114–9123. doi: 10.1074/jbc.M109.044297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghitti M, Spitaleri A, Valentinis B, Mari S, Asperti C, Traversari C, Rizzardi G-P, Musco G. Angew Chem, Int Ed. 2012;51:7702–7705. doi: 10.1002/anie.201202032. [DOI] [PubMed] [Google Scholar]
- 21.Gottschalk K-E, Kessler H. Angew Chem, Int Ed. 2002;41:3767–3774. doi: 10.1002/1521-3773(20021018)41:20<3767::AID-ANIE3767>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 22.Auzzas L, Zanardi F, Battistini L, Burreddu P, Carta P, Rassu G, Curti C, Casiraghi G. Curr Med Chem. 2010;17:1255–1299. doi: 10.2174/092986710790936301. [DOI] [PubMed] [Google Scholar]
- 23.Kapp T G, Rechenmacher F, Neubauer S, Maltsev O V, Cavalcanti-Adam E A, Zarka R, Reuning U, Notni J, Wester H-J, Mas-Moruno C, et al. Sci Rep. 2017;7:No. 39805. doi: 10.1038/srep39805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.da Ressurreição A S M, Vidu A, Civera M, Belvisi L, Potenza D, Manzoni L, Ongeri S, Gennari C, Piarulli U. Chem – Eur J. 2009;15:12184–12188. doi: 10.1002/chem.200902398. [DOI] [PubMed] [Google Scholar]
- 25.Marchini M, Mingozzi M, Colombo R, Guzzetti I, Belvisi L, Vasile F, Potenza D, Piarulli U, Arosio D, Gennari C. Chem – Eur J. 2012;18:6195–6207. doi: 10.1002/chem.201200457. [DOI] [PubMed] [Google Scholar]
- 26.Mingozzi M, Dal Corso A, Marchini M, Guzzetti I, Civera M, Piarulli U, Arosio D, Belvisi L, Potenza D, Pignataro L, et al. Chem – Eur J. 2013;19:3563–3567. doi: 10.1002/chem.201204639. [DOI] [PubMed] [Google Scholar]
- 27.Panzeri S, Zanella S, Arosio D, Vahdati L, Dal Corso A, Pignataro L, Paolillo M, Schinelli S, Belvisi L, Gennari C, et al. Chem – Eur J. 2015;21:6265–6271. doi: 10.1002/chem.201406567. [DOI] [PubMed] [Google Scholar]
- 28.Colombo R, Mingozzi M, Belvisi L, Arosio D, Piarulli U, Carenini N, Perego P, Zaffaroni N, De Cesare M, Castiglioni V, et al. J Med Chem. 2012;55:10460–10474. doi: 10.1021/jm301058f. [DOI] [PubMed] [Google Scholar]
- 29.Dal Corso A, Caruso M, Belvisi L, Arosio D, Piarulli U, Albanese C, Gasparri F, Marsiglio A, Sola F, Troiani S, et al. Chem – Eur J. 2015;21:6921–6929. doi: 10.1002/chem.201500158. [DOI] [PubMed] [Google Scholar]
- 30.Zanella S, Angerani S, Pina A, López Rivas P, Giannini C, Panzeri S, Arosio D, Caruso M, Gasparri F, Fraietta I, et al. Chem – Eur J. 2017;23:7910–7914. doi: 10.1002/chem.201701844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dal Corso A, Pignataro L, Belvisi L, Gennari C. Curr Top Med Chem. 2016;16:314–329. doi: 10.2174/1568026615666150701114343. [DOI] [PubMed] [Google Scholar]
- 32.Kwon K C, Ko H K, Lee J, Lee E J, Kim K, Lee J. Small. 2016;12:4241–4253. doi: 10.1002/smll.201600917. [DOI] [PubMed] [Google Scholar]
- 33.Goodman S L, Grote H J, Wilm C. Biol Open. 2012;1:329–340. doi: 10.1242/bio.2012364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai S Y, Xu N, Chen C, Song Y-l, Hu J, Bai C-x. Clin Respir J. 2015;9:457–467. doi: 10.1111/crj.12163. [DOI] [PubMed] [Google Scholar]
- 35.Currier N V, Ackerman S E, Kintzing J R, Chen R, Interrante M F, Steiner A, Sato A K, Cochran J R. Mol Cancer Ther. 2016;15:1291–1300. doi: 10.1158/1535-7163.MCT-15-0881. [DOI] [PubMed] [Google Scholar]
- 36.Meyer, T.; Marshall J. F.; Hart, I. R. Br. J. Cancer 1998, 77, 530-536. doi:10.1038/bjc.1998.86 For reports on the lack of β3 integrin expression in MDA-MB-468, see also ref. [32–33].
- 37.Liu, Z.; Yan, Y.; Liu, S.; Wang, F.; Chen, X. Bioconjugate Chem. 2009, 20, 1016–1025, and ref. [38]. doi:10.1021/bc9000245
- 38.Lautenschlaeger T, Perry J, Peereboom D, Li B, Ibrahim A, Huebner A, Meng W, White J, Chakravarti A. Radiat Oncol. 2013;8:No. 246. doi: 10.1186/1748-717X-8-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sancey L, Garanger E, Foillard S, Schoehn G, Hurbin A, Albiges-Rizo C, Boturyn D, Souchier C, Grichine A, Dumy P, et al. Mol Ther. 2009;17:837–843. doi: 10.1038/mt.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cox N, Kintzing J R, Smith M, Grant G A, Cochran J R. Angew Chem, Int Ed. 2016;55:9894–9897. doi: 10.1002/anie.201603488. Angew. Chem. 2016, 128, 10048–10051. doi:10.1002/ange.201603488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Civera M, Arosio D, Bonato F, Manzoni L, Pignataro L, Zanella S, Gennari C, Piarulli U, Belvisi L. Cancers. 2017;9:No. 128. doi: 10.3390/cancers9100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Memmo L M, McKeown-Longo P. J Cell Sci. 1998;111:425–433. doi: 10.1242/jcs.111.4.425. [DOI] [PubMed] [Google Scholar]
- 43.Anderl J, Mueller C, Simon W, inventors. Amatoxin-conjugates with improved linkers. WO2012041504. WO Patent. 2012 Apr 5;
- 44.Anderl J, Hechler T, Mueller C, Pahl A, inventors. Amatoxin-antibody conjugates. WO 2016142049. WO Patent. 2016 Sep 15;
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental details, characterization data and copies of spectra.