Abstract
The amyloidoses are a complex group of disorders characterized by the deposition of proteinaceous amyloid fibrils in vital organs. The deposits are nonimmunogenic and may be composed of one of more than 35 proteins. We have developed a two-stage immunotherapeutic approach using peptides that recognize most, if not all, amyloid deposits to facilitate amyloid clearance. In the first embodiment, we have developed a bifunctional peptope to enhance and expand the utility of currently available antibodies. In the second, we have generated peptide-reactive antibodies that can be targeted to the amyloid deposits by peptides thereby providing alternative reagents for immunotherapy of amyloidosis. These technologies provide tools for treating the many forms of amyloid disease, restoring organ function and enhancing patient survival.
Keywords: : 11–1F4, amyloidosis, antibodies, immunotherapy, p5+14, peptides, peptope, pretargeting
Amyloidosis
The systemic deposition of amyloid is associated with a growing number of more than 35 disorders (Table 1) [1,2]. Amyloid deposits are composed principally of protein fibrils that share remarkably similar structural properties, regardless of the precursor protein from which they develop, in addition to hypersulfated heparan sulfate proteoglycans, such as perlecan. The most common forms of systemic amyloid disease – light chain associated (AL), transthyretin-associated (ATTR) and leukocyte chemotactic factor 2-associated (ALect2) amyloidoses – are orphan disorders with a combined incidence of approximately 4500 new cases per year in the USA [3]. In these patients, normally soluble serum proteins misfold and, in the appropriate microenvironment, self-aggregate to form amyloid fibrils which co-deposit in vital organs with heparan sulfate proteoglycans and other accessory proteins. Presently, there are no US FDA-approved treatments specifically designed to remove tissue amyloid deposits; however, several anti-amyloid passive immunotherapies are currently being evaluated in clinical trials [4].
Table 1. . Partial list of 36 currently recognized amyloid-related disorders.
| Amyloid type | Precursor | Organ distribution | Acquired (A)/hereditary (H)/organs | Syndrome |
|---|---|---|---|---|
| AL | Immunoglobulin light chain | Systemic or localized | A/All but CNS | Primary, myeloma-associated, MGUS |
| AH | Immunoglobulin heavy chain | Systemic or localized | A/All but CNS | Primary |
| Aβ2M | β2-microglobulin | Systemic | A/musculoskeletal | Hemodialysis |
| ATTR | Transthyretin variants | Systemic or localized | A/heart, tenosynovium, nerve | Germline mutation |
| Wild-type TTR | Transthyretin | Systemic | H/heart, eye, leptomen, nerve | Aging |
| AA | Serum amyloid protein A | Systemic | A/all but CNS | Chronic inflammation |
| AApoAI | Apolipoprotein AI | Systemic | H/heart, liver, etc. | Germline mutation |
| AApoAII | Apolipoprotein AII | Systemic | H/kidney | Germline mutation |
| AGel | Gelsolin | Systemic | H/PNS, cornea | Germline mutation |
| ALys | Lysozyme | Systemic | H/kidney | Germline mutation |
| ALect2 | Leukocyte chemotactic factor 2 | Systemic | A/kidney | Renal amyloid |
| AFib | Fibrinogen α variants | Systemic | H/kidney | Germline mutation |
| ACys | Cystatin variants | Systemic | H/PNS, skin | |
| ACal | (Pro) calcitonin | Localized | A/thyroid | Thyroid tumors |
| AMed | Lactadherin | Localized | A/senile aortic media | Aging |
| AIAPP | Islet amyloid polypeptide | Localized | A/islets of Langerhans | Type 2 diabetes |
| APro | Prolactin | Localized | A/pituitary | Aging pituitary |
| AIns | Insulin | Localized | A/injection site | Iatrogenic |
| APrP | Prion protein | Localized | A/H/brain | Spongiform encephalopathies |
| Aβ | Aβ precursor protein | Localized | A/H/brain | Alzheimer's disease and aging |
AL: Light chain-associated; ALect2: Leukocyte chemotactic factor 2-associated; ATTR: Transthyretin-associated.
The most prevalent systemic amyloid disorder, AL, is a degenerative plasma cell dyscrasia in which monoclonal light chain (LC) proteins in the circulation that serve as the precursors to the amyloid fibrils. The LC proteins, or components thereof, aggregate into well-organized fibrils that accumulate in visceral organs leading to dysfunction and often death [5]. Despite, advances in understanding of the disease etiology and nature of the pathology, the prognosis for these patients remains poor with a median survival of 3 years for most, and approximately 11 months if cardiac amyloidosis is the primary clinical manifestation [6]. The incidence of ATTR and ALect2 amyloidoses is considered to be significantly less than that of AL amyloidosis; however, increasing education and awareness has enhanced identification of patients with these forms of the disease, and patient numbers are increasing concomitantly [7–9]. Treatment approaches for patients with AL, ATTR, or ALect2 amyloidosis are diverse and specific for each [4]. However, since amyloid deposits share many structural commonalities, there is potential that a single agent could be developed and used to target and thereby treat all forms of amyloid-related disease effectively. This premise represents the underlying rationale for our development of biological agents – peptides – that bind all amyloid deposits to serve as clinical amyloid imaging agents and as the basis of pretargeting therapeutics.
Pretargeting immunotherapy
The principle of two-stage, pretargeting immunotherapy for amyloidosis is to develop a treatment paradigm wherein a pan-amyloid-binding agent coats the deposits after which an antibody can be administered that binds the agent and thereby opsonizes the amyloid. We have developed two strategies using our panel of polybasic peptides [10] to achieve this. The polybasic peptides, notably those designated p5 and p5+14 are the enabling technology for the pretargeting innovation described herein and have been shown to specifically bind many different forms of amyloid both in vitro and in vivo [10,11]. The peptides can interact with both the amyloid fibrils and hypersulfated heparan sulfate glycosaminoglycans, which are present in high concentrations in amyloid deposits, but not to the heparan sulfate ubiquitously expressed in healthy tissues [11]. We have demonstrated binding of these peptides with AL, ATTR and ALect2 amyloid (as well as others) in formalin-fixed human tissue sections in vitro [11] and evidenced specific amyloid reactivity in a mouse model of amyloidosis [12]. In the pretargeting application [13], we have exploited the specific amyloid reactivity of these polybasic peptides as tools to target opsonizing monoclonal antibodies (mAb) that can then recruit immune cells to mediate clearance of the deposits. This is the principle of pretargeting passive immunotherapy.
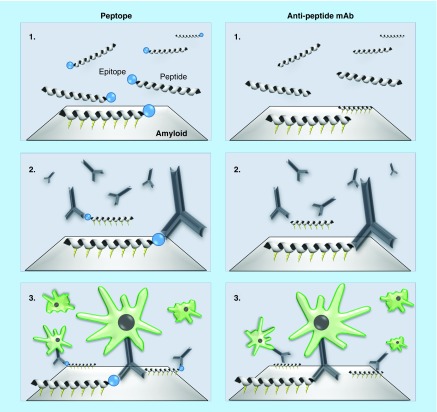
The pretargeting application [13] comprises 47 claims that describe methods for targeting an amyloid deposit with an amyloid-reactive peptide and then contacting the amyloid-reactive peptide with an antibody or functional fragment thereof that binds the amyloid-reactive peptide, wherein this reaction targets the amyloid deposit for clearance. This claim embodies the use of a bifunctional peptide (peptope) that comprises an amyloid binding motif and a high-affinity epitope binding sequence that reacts with a known mAb (Figure 1) [14]. Additionally, subsequent independent claims describe methods that use mAbs that directly bind targeted peptides that associate with amyloid (Figure 1). Because of the pan-amyloid reactivity of the peptides we have disclosed, claims are provided that cover methods for the treatment and clearance of deposits in all known amyloid-related disorders via opsonization and cell-mediated dissolution of the amyloid.
Figure 1. . Schematic illustration of the two approaches to two-stage, pretargeting immunotherapy.
The bifunctional peptope or peptide binds amyloid via electrostatic interactions (1). The peptides serve as targets for antibodies that opsonize the deposits (2) subsequently recruiting macrophages capable of removing the amyloid (3).
mAb: Monoclonal antibody.
Data taken from [Wall et al., University of Tennessee Medical Center, Unpublished Data].
Peptope
Given the complexity and costs associated with clinical development of novel therapeutic mAbs for each amyloid type, our initial approach was to develop a synthetic peptide for use with mAbs that have already been clinically validated. To this end, we generated a bifunctional peptope reagent, using the amyloid-reactive peptide p5+14 fused to an appropriate high-affinity linear epitope, that specifically binds a known mAb. We have initially developed the peptope technology for use with mAbs deemed safe for human use and shown capable of opsonizing amyloid deposits and recruiting macrophages that can phagocytose and clear tissue amyloid deposits. However, this approach could theoretically be used with any mAb for which a linear peptide epitope is known. Our principal partner for this technology is the 11–1F4 mAb, which is specified in several dependent claims. This mAb has proven efficacious in approximately two-thirds of AL amyloidosis patients but has not been shown effective in patients with ATTR, ALect2 and other forms of amyloidosis. Given our knowledge of the linear epitope reactive with this mAb, we have generated a peptope comprising peptide p5+14 and a 10-amino acid linear epitope sequence that binds 11–1F4 with subnanomolar affinity [15]. This peptope reacts with many types of amyloid in vitro, and in each case, the epitope remained accessible for subsequent 11–1F4 interactions. Intravenous pretreatment, with the peptope, of mice with systemic AA amyloidosis resulted in specific binding of the 11–1F4 mAb to the amyloid. The 11–1F4 mAb exhibits scant reactivity with murine AA amyloid in the absence of the peptope. These data indicate that both motifs of the peptope function effectively in the context of the entire molecule. Use of the peptope in combination with the 11–1F4 mAb in the clinical setting may increase the therapeutic success rate in patients with AL amyloidosis and, perhaps more importantly, may extend the value of this mAb for use in patients with many different forms of amyloid disease.
Peptide-reactive mAb
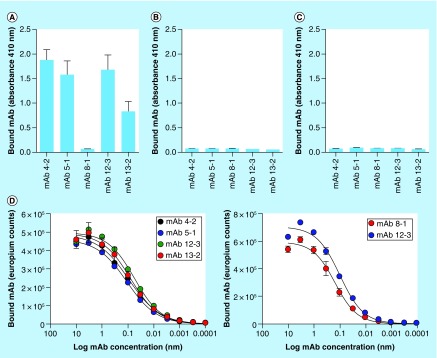
In our second approach, we have generated a series of five mAbs using, as immunogen, an amyloid-reactive peptide that includes three D-amino acids at the N-terminus – to enhance immunogenicity (Table 2). The clones we have generated are stable and produce IgG1κ mAbs that are readily purified using Protein A affinity chromatography. Characterization of the binding proclivities of each of the five mAbs was performed using synthetic peptides with modified primary structure to probe the nature of the epitopes (Table 2). The mAbs 4–2, 5–1, 12–13 and 13–12 all bound a motif within a charged heptad repeat region of the peptide. The peptides were rendered unreactive with these mAbs, however, when the lysine residues were replaced by arginine or glycine, when D-amino acids were used instead of L-variants, or when the spacing of the lysine residues was altered (Table 2). In contrast, reactivity of another clone, designated 8–1, was mapped to the three D-amino acids at the N terminus of the peptide and was dependent on dextro chirality (Table 2). Antibody binding specificity was further explored by using a capture assay, wherein each of the mAbs was adhered to the well of a microplate and a solution of detectably-labeled peptide, either p5+14 or negative control peptide variants, was added in the solution phase and the amount of captured peptide measured. In this assay, all mAbs except clone 8–1 bound peptide p5+14 (Figure 2A), and none of the mAb clones captured the negative control peptides p5R (Figure 2B) or p5G (Figure 2C). The affinity (expressed as the EC50) of all the mAbs for peptide was estimated to be approximately 0.1 nM for surface-adsorbed p5+14 (Figure 2D) or immunogen peptide (Figure 2E). This high-affinity interaction is suitable for generating stable peptide-mAb complexes on the amyloid surface that may, in turn, result in recruitment of cells of the immune system to facilitate dissolution of the amyloid. However, these studies do not demonstrate that the mAbs can bind to the peptide when it is associated with amyloid-like fibrils or amyloid extracts.
Table 2. . Summary of monoclonal antibody reactivity with peptides.
| Peptide | Primary sequence | Monoclonal antibody clone | ||||
|---|---|---|---|---|---|---|
| 4–2 | 5–1 | 8–1 | 12–13 | 13–12 | ||
| Imm. | [AQA]DYS KAQKA QAKQA KQAQK AQKAQ AKQAK Q | + | + | + | + | + |
| p5 | GGGYS KAQKA QAKQA KQAQK AQKAQ AKQAK Q | + | + | — | + | + |
| p5R | GGGYS RAQRA QARQA RQAQR AQRAQ ARQAR Q | — | — | — | — | — |
| p5G | GGGYS GAQGA QAGQA GQAQG AQGAQ AGQAG Q | — | — | — | — | — |
| p5(D) | [GGGYS KAQKA QAKQA KQAQK AQKAQ AKQAK Q]D | — | — | — | — | — |
| p5+14 | GGGYS KAQKA QAKQA KQAQK AQKAQ AKQAK QAQKA QKAQA KQAKQ | + | + | — | + | + |
| p19 | GGGYS KAQQA QAKQA QQAQK AQQAQ AKQAQ Q | — | — | — | — | — |
| p50 | AQAYS KAQKA QAKQA KQAQK AQKAQ AKQAK Q | + | + | — | + | + |
Imm.: Immunogen.
Data taken from [Wall et al., University of Tennessee Medical Center, Unpublished Data].
Figure 2. . Mapping the reactivity of a panel of antipeptide antibodies.
Antibodies were used in a capture assay with p5+14 (A), p5R (B) and p5G (C) as the substrate. With the exception of mAb 8–1, all mAb bound peptide p5+14 but not the other peptides. The affinity of mAb binding was shown to be approximately 0.1 nM in an ELISA using peptide p5+14 as the target (D) or the immunogen peptide (E).
mAb: Monoclonal antibody.
Data taken from [Wall et al., University of Tennessee Medical Center, Unpublished data].
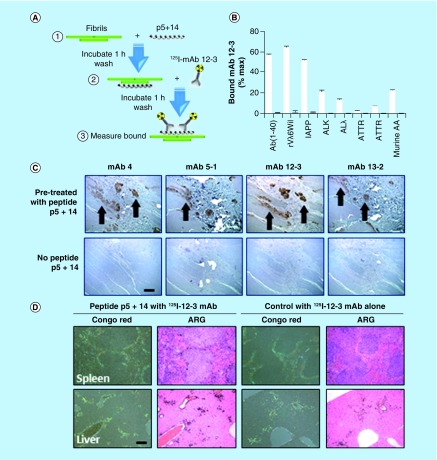
To address this, we performed pretargeting ‘pulldown’ assays (Figure 3A & B) using several targets: synthetic amyloid-like fibrils, composed of LC proteins (rVλ6Wil), Aβ peptide, or islet amyloid polypeptide (IAPP deposits as amyloid in patients with Type 2 diabetes), and human or murine amyloid extracts (AL, ATTR and AA respectively). Suspensions of these preparations were preincubated with peptide p5+14 (or a negative control peptide) before addition of radio-iodinated mAb 12–13, as a representative reagent (Figure 3A). In each case, iodine-125 (125I)–labeled mAb 12–13 bound the amyloid fibrils and extracts only when they had been pretreated with peptide p5+14 (Figure 3B, white bars) but not following addition of a control peptide (Figure 3B, gray bars). Similar pretargeting-dependent binding of mAb 12–13 was observed when human ATTR-laden tissue was incubated with biotinylated 12–13 mAb following addition of p5+14, which resulted in specific interaction of mAb with the ATTR deposits (Figure 3C). These findings support our hypothesis that mAb 12–13, an exemplar of the first generation of clones that we have developed, can be specifically targeted to amyloid deposits that have been previously decorated with an appropriate amyloid-reactive peptide. Antibody targeting was demonstrated, in vivo, when co-localization of 125I-labeled 12–13 mAb with AA amyloid (shown a green birefringent material in the Congo red-stained tissue) was observed in the liver and spleen of mice following pretreatment with peptide p5+14, but not control peptide administered intravenously (Figure 3D). These in vitro and in vivo data indicate that a peptide-reactive mAb, such as 12–13, can bind amyloid of many different types following pretreatment with a pan-amyloid reactive peptide, such as p5+14. Effective translation of this methodology into the clinic could lead to a universal, two-stage immunotherapy for patients with amyloidosis.
Figure 3. . Peptide pretargeting mediates specific binding of mAb to amyloid in vitro and in vivo.
(A) A pulldown assay, shown schematically, was used to demonstrate reactivity of mAb 12–13 with a panel of diverse synthetic amyloid-like fibrils, human amyloid extracts and mouse AA amyloid in the presence of peptide p5+14 (B, white bars) but not the control peptide (B, gray bars). (C) Binding of mAb 12–13, when biotinylated, specifically identified human transthyretin-associated amyloid in tissue sections only when the sample had been pretreated with peptide p5+14 (C, bar = 0.4 mm). (D) When injected into mice with systemic AA amyloidosis, radioiodinated monoclonal antibody 12–13 co-localized with amyloid in the liver and spleen when the mice had been treated 24 h earlier by intravenous injection of peptide p5+14, but not the control peptide. The presence of 125I-labeled 12–13 mAb was evidenced by black silver grains in the autoradiograph, which correlated with the presence of amyloid seen as green birefringence in consecutive tissue sections stained with Congo red (D, bar = 0.2 mm).
ALλ: λ Light chain-associated amyloid; ALκ: κ Light chain-associated amyloid; ARG: Autoradiograph: ATTR: Transthyretin-associated amyloid; mAb: Monoclonal antibody.
Data taken from [Wall et al., University of Tennessee Medical Center, Unpublished Data].
Current amyloid therapy & immunotherapy
Treatment regimina for patients with amyloidosis are diverse and are dictated by the type of amyloid protein deposited in the fibrils. Treatments generally focus on reducing the concentration of the amyloid fibril precursor protein in an attempt to halt or slow the growth of the deposits. Evidence suggests that reduction of serum free LC protein in the circulation can give rise to spontaneous dissolution of tissue amyloid deposits [16]; however, this result is erratic, and the resilience of many tissue amyloid deposits may hinder organ function recovery. When considering the disease paradigm, we have likened systemic AL amyloid deposits to metastatic disease in cancer: the deposits appear at distant and varied anatomic sites; are the major cause of morbidity and mortality, and; are resistant to treatments that effectively treat the primary ‘tumor’ (marrow-derived plasma cells). Anatomic distribution and the amyloid burden, like metastases, inform patient prognosis and survival. Further extending this analogy, we consider that detection and removal of tissue amyloid deposits can foster recovery of organ function, improve patient prognosis and prolong survival rates for patients with AL amyloidosis.
Over the last two decades, we and others have explored methods to achieve removal of tissue amyloid, principally by developing amyloid-reactive mAb as a means of directly opsonizing the amyloid deposits and thereby targeting them for removal by immune cell mechanisms – notably phagocytosis by macrophages [17,18]. Our initial therapeutic antibody, designated 11–1F4 (or CAEL-101), selectively binds AL amyloid fibrils; and not the circulating free LC [19–21]. When radiolabeled with iodine-124 (124I), murine 11–1F4 was shown, by PET/CT imaging, to bind organs in approximately 60% of AL amyloid patients (clinical trial # NCT00807872) [20]. A chimeric form of 11–1F4 has been generated and is currently being evaluated in a therapeutic Phase IA/B clinical trial at Columbia University Medical Center, NY (clinical trial # NCT02245867). In addition to the 11–1F4 mAb, our laboratory has also identified amyloid-reactive antibodies in commercial preparations of immunoglobulin intravenous [22]; however, this material was never evaluated in a clinical trial involving patients with systemic amyloidosis.
Two other amyloid-reactive mAbs are being evaluated in clinical trials, the humanized NEOD001 mAb and a humanized antiserum amyloid P component (SAP) IgG1 mAb. Antibody NEOD001, developed by Prothena (Prothena Biosciences Inc, South San Francisco, CA, USA; clinical trial # NCT02312206), binds AL amyloid fibrils [18] and is currently being evaluated in a Phase III trial in patients with AL amyloidosis [23,24]. Early data indicate that NEOD001 [25,26] has an excellent safety profile and induced a positive cardiac response in 57% of evaluable patients (n = 14) and stabilized cardiac function in the remaining 43%. Similar positive results were observed in patients with evaluable renal AL amyloidosis. Organ improvement was observed in patients as early as 4 months after initiation of NEOD001 treatment [26]. The second antibody binds SAP – a nonfibrillar, ubiquitous component of amyloid deposits [27,28]. Initial data from the Phase I human trial of this mAb (clinical trial # NCT01777243) suggest that amyloid load was reduced, notably in the liver, in approximately 30% of the 16 patients enrolled [29].
Each of these mAbs has been deemed safe for use in patients with amyloidosis, and each has demonstrated efficacy based on improvement of organ-associated biomarkers or reduction in amyloid load in a subset of patients. However, there is currently no evidence that any single mAb will provide comprehensive benefit, and mAbs have yet to be evaluated in patients with ATTR or ALect2 amyloidosis. As an alternative to generating a new mAb for each amyloid disease, we have developed a synthetic peptide-epitope construct (peptope), comprising a pan-amyloid-reactive peptide coupled to a high affinity epitope amino acid sequence [15], initially designed to be used in a pretargeting therapy paradigm with the 11–1F4 mAb. In this way, the pan-amyloid peptope could be used to ‘repurpose’ the 11–1F4 mAb for use in potentially all forms of amyloid disease. In another embodiment of this two-step technology, we have developed novel Abs that bind the amyloid-targeting peptides directly, when associated with amyloid deposits, and can, therefore, serve as additional pretargeted passive immunotherapeutics [13].
Future perspective
The systemic amyloidoses are complex, diverse and multi-faceted diseases. Current therapies, where available, rely on reducing or stabilizing the amyloid precursor proteins, of which there are more than 35. In this way, it is hoped that the amyloid deposits will not increase in mass. With the recent advent of passive immunotherapy, the goal now is to complement this approach with dissolution of tissue amyloid deposits, thereby restoring lost organ function. As our understanding of the disease process increases, a third general therapeutic paradigm may arise – namely preventing amyloid fibril growth by agents capable of blocking recruitment of additional precursor proteins. Such agents exist only in the laboratory; however, given the structural characteristics shared by all amyloid fibrils, the prospect of a pan-amyloid inhibitor is real. Of course, as with many progressive disorders, a major objective for effective clinical management and potential cure is early detection. Indeed, dissolution of tissue amyloid using immunotherapy would be most effective before signs of organ dysfunction become apparent. Additionally, it may abrogate the need for aggressive chemotherapy approaches, in the case of AL amyloidosis, if the amyloid load can be ‘kept in check’. It is likely that effective management of patients with amyloid will require the following: development of assays to predict those at risk before onset of symptoms; quantitative imaging strategies that can be used to assess organ involvement, disease burden and response to therapy – to optimize treatment dosing; and a multidimensional approach to prevent, stabilize and remove amyloid deposits. Only then can organ integrity be maintained and long-term survival of patients be assured.
Executive summary.
The amyloidoses are a rare, but often fatal, group of approximately 35 disorders characterized by the presence of amyloid deposits on vital organs and tissues.
Treatment of patients with amyloid-reactive monoclonal antibody (mAb), in clinical trials, has demonstrated beneficial outcomes based on improvement in levels of surrogate biomarkers of organ function as well as clearance of amyloid from organs, notably the liver.
We have developed a series of synthetic, polybasic peptides, such as peptide p5+14, that specifically bind many forms of amyloid, including light chain-associated, transthyretin-associated, leukocyte chemotactic factor 2-associated and serum amyloid protein A-associated deposits.
By coupling a well-characterized, high-affinity, linear peptide epitope to peptide p5+14, we have generated a bifunctional peptope reagent with pan-amyloid reactivity and the ability to recruit mAb.
By pretargeting amyloid deposits with the peptope, mAb, such as 11–1F4, can be recruited by many forms of amyloid, thereby opsonizing the amyloid and recruiting immune cells to mediate clearance.
Pretargeting amyloid using an appropriate peptope can enhance and expand the utility of well-characterized mAb that have been deemed safe in clinical trials and for which an epitope is known.
Antibodies that are capable of binding the pan-amyloid reactive peptides, when they are associated with amyloid deposits in vivo, represent a novel class of passive immunotherapeutics for amyloid removal.
The mAb 12–13 exhibits reactivity with numerous forms of amyloid in vitro and in vivo when the deposits are pretargeted or pretreated with the p5+14 peptide, offering the promise of generating a two-stage, pan-amyloid immunotherapy.
Removal of tissue amyloid from patients with systemic amyloidosis will result in improved organ function, prolonged patient survival and hopefully a cure for patients with these devastating diseases.
Footnotes
Financial & competing interests disclosure
JS Wall, JS Foster and SJ Kennel are inventors on PCT US 2016/0243230 and have received royalties associated with this technology. JS Wall and SJ Kennel are inventors of US patent 8,808,666, “Peptides that specifically target amyloid deposits”. JS Wall, EB Martin and SJ Kennel are founders and co-owners of Solex, Inc that sublicensed technology associated with US patent 8,808,666 from the University of Tennessee Research Foundation. All technologies generated by employees of UTMC are assigned to the University of Tennessee Research Foundation. This work was supported in part by PHS grant DK079984 from The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) at the NIH as well as funds from Prothena Biosciences Inc, South San Francisco, CA, USA, and by a New Grant Award from the University of Tennessee Health and Science Center. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641–2654. doi: 10.1016/S0140-6736(15)01274-X. [DOI] [PubMed] [Google Scholar]
- 2.Sipe JD, Benson MD, Buxbaum JN, et al. Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid. 2016;23(4):209–213. doi: 10.1080/13506129.2016.1257986. [DOI] [PubMed] [Google Scholar]
- 3.Amyloidosis foundation. www.amyloidosis.org/
- 4.Nuvolone M, Merlini G. Systemic amyloidosis: novel therapies and role of biomarkers. Nephrol. Dial. Transplant. 2016;32(5):770–780. doi: 10.1093/ndt/gfw305. [DOI] [PubMed] [Google Scholar]
- 5.Blancas-Mejia LM, Ramirez-Alvarado M. Systemic amyloidoses. Ann. Rev. Biochem. 2013;82:745–774. doi: 10.1146/annurev-biochem-072611-130030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dispenzieri A, Gertz MA, Buadi F. What do I need to know about immunoglobulin light chain (AL) amyloidosis? Blood Rev. 2012;26(4):137–154. doi: 10.1016/j.blre.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Jimenez-Zepeda VH, Leung N. ALECT2 amyloidosis: a new type of systemic amyloid highly prevalent in the Hispanic population. Rev. Invest. Clin. 2014;66(3):269–273. [PubMed] [Google Scholar]
- 8.Larsen CP, Beggs ML, Wilson JD, Lathrop SL. Prevalence and organ distribution of leukocyte chemotactic factor 2 amyloidosis (ALECT2) among decedents in New Mexico. Amyloid. 2016;23(2):119–123. doi: 10.3109/13506129.2016.1145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah KB, Mankad AK, Castano A, et al. Transthyretin cardiac amyloidosis in black americans. Circ. Heart Fail. 2016;9(6):e002558. doi: 10.1161/CIRCHEARTFAILURE.115.002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.2011. University of Tennessee Research Foundation: WO2011119608A1.
- 11.Wall JS, Martin EB, Richey T, et al. Preclinical validation of the heparin-reactive peptide p5+14 as a molecular imaging agent for visceral amyloidosis. Molecules. 2015;20(5):7657–7682. doi: 10.3390/molecules20057657. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes the preclinical data that supported the investigational new drug application for a PET/CT imaging study of 124I-p5+14.
- 12.Martin EB, Williams A, Richey T, et al. Comparative evaluation of p5+14 with SAP and peptide p5 by dual-energy SPECT imaging of mice with AA amyloidosis. Sci. Rep. 2016;6:22695. doi: 10.1038/srep22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.2016. University of Tennessee Research Foundation: WO2016032949 A1.
- 14.Wall JS, Williams A, Foster JS, et al. A bifunctional peptide, “peptope”, for pre-targeting antibody 7D8 to systemic amyloid deposits. Amyloid. 2017;24(sup1.):22–23. doi: 10.1080/13506129.2017.1295372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Nuallain B, Allen A, Ataman D, Weiss DT, Solomon A, Wall JS. Phage display and peptide mapping of an immunoglobulin light chain fibril-related conformational epitope. Biochem. 2007;46(45):13049–13058. doi: 10.1021/bi701255m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawkins PN. Serum amyloid P component scintigraphy for diagnosis and monitoring amyloidosis. Curr. Opin. Nephrol. Hypertens. 2002;11(6):649–655. doi: 10.1097/00041552-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Hrncic R, Wall J, Wolfenbarger DA, et al. Antibody-mediated resolution of light chain-associated amyloid deposits. Am J Pathol. 2000;157(4):1239–1246. doi: 10.1016/S0002-9440(10)64639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First description of a murine model of human light chain associated amyloidoma and its expedited dissolution by the amyloid-reactive monoclonal antibody (mAb) 11–1F4.
- 18.Wall JS, Kennel SJ, Williams A, et al. AL amyloid imaging and therapy with a monoclonal antibody to a cryptic epitope on amyloid fibrils. PloS ONE. 2012;7(12):e52686. doi: 10.1371/journal.pone.0052686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Nuallain B, Allen A, Kennel SJ, Weiss DT, Solomon A, Wall JS. Localization of a conformational epitope common to non-native and fibrillar immunoglobulin light chains. Biochem. 2007;46(5):1240–1247. doi: 10.1021/bi0616605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wall JS, Kennel SJ, Stuckey AC, et al. Radioimmunodetection of amyloid deposits in patients with AL amyloidosis. Blood. 2010;116(13):2241–2244. doi: 10.1182/blood-2010-03-273797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.1999. University of Tennessee Research Foundation: WO1999060024A1.
- 22.2008. University of Tennessee Research Foundation: WO2006113347A2.
- 23.2009. Elan Pharmaceuticals, Inc. J: WO2009086539A4.
- 24.2008. Elan Pharmaceuticals, Inc. and University of Tennessee Research Foundation: US7928203 B2.
- 25.Gertz MA, Landau H, Comenzo RL, et al. First-in-human Phase I/II study of NEOD001 in patients with light chain amyloidosis and persistent organ dysfunction. J. Clin. Oncol. 2016;34(10):1097–1103. doi: 10.1200/JCO.2015.63.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First report of a clinical trial evaluating passive immunotherapy, using mAb NEOD001, in patients with light chain associated amyloidosis.
- 26.Gertz MA, Landau HJ, Weiss BM. Organ response in patients with AL amyloidosis treated with NEOD001, an amyloid-directed monoclonal antibody. Am. J. Hematol. 2016;91(12):E506–E508. doi: 10.1002/ajh.24563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bodin K, Ellmerich S, Kahan MC, et al. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature. 2010;468(7320):93–97. doi: 10.1038/nature09494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.2008. Pentraxin Therapeutics Ltd.: WO2008EP58333.
- 29.Richards DB, Cookson LM, Berges AC, et al. Therapeutic clearance of amyloid by antibodies to serum amyloid P component. N. Engl. J. Med. 2015;373(12):1106–1114. doi: 10.1056/NEJMoa1504942. [DOI] [PubMed] [Google Scholar]; •• Clinical report of two-stage immunotherapy for systemic amyloidosis using anti-SAP mAb.