Abstract
Merkel cell carcinoma (MCC) is a rare but often deadly skin cancer that is typically caused by the Merkel cell polyomavirus (MCPyV). Polyomavirus T-antigen oncoproteins are persistently expressed in virus-positive MCCs (˜80% of cases), while remarkably high numbers of tumor-associated neoantigens are detected in virus-negative MCCs, suggesting that both MCC subsets may be immunogenic. Here we review mechanisms by which these immunogenic tumors evade multiple levels of host immunity. Additionally, we summarize the exciting potential of diverse immune-based approaches to treat MCC. In particular, agents blocking the PD-1 axis have yielded strikingly high response rates in MCC as compared with other solid tumors, highlighting the potential for immune-mediated treatment of this disease.
Keywords: : exhaustion, immune evasion, immune therapy, Merkel cell carcinoma, Merkel cell polyomavirus, PD-1, PD-L1
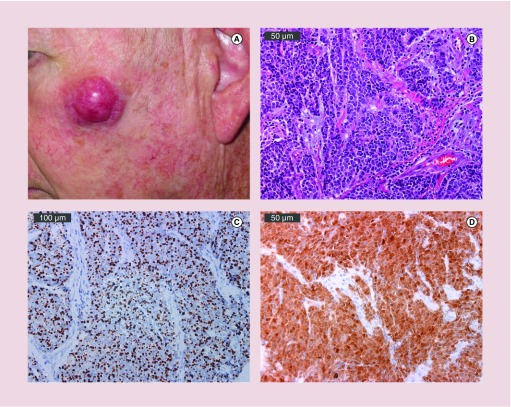
Merkel cell carcinoma (MCC) is a rare and often lethal skin cancer with an incidence of approximately 2000 new cases per year in the USA [1]. While infrequent, the reported incidence of MCC has tripled in the last 30 years [2,3]. This increased incidence is partially attributable to the identification of cytokeratin-20 (Figure 1C) as an immunohistochemical marker of MCC in 1992, which has greatly enhanced the detection of MCC [4]. Additionally, a rising prevalence of known risk factors for MCC including immune suppression, age over 50 and extensive prior sun exposure likely contribute to the increased number of reported MCC cases [5]. Clinically, MCCs present as painless, red or purple nodules (Figure 1A) and are commonly misdiagnosed as benign cysts or as another malignant neoplasm [2,5]. The vast majority of cases arise in Caucasians, predominantly in males and in sun-exposed areas, suggesting that UV-induced skin damage is a major contributing factor in the development MCC [5]. While the single most common site of presentation is on the head and neck, accounting for nearly half of cases, MCC can arise on non-sun exposed regions including on the skin of the buttocks as well as rarely on the oral and genital mucosae [2]. Our understanding of the etiology of MCC has expanded dramatically over the past several decades, most notably with the discovery of the Merkel cell polyomavirus (MCPyV), which is causative in approximately 80% of MCC cases [6].
Figure 1. . Clinical and pathologic presentation of Merkel cell carcinoma.
(A) A 2.5 cm primary MCC on sun exposed skin of the left cheek. (B) Hematoxylin & eosin magnification of MCPyV-positive MCC tumor. Bar indicates 50 μm. (C) Cytokeratin-20 immunohistochemical staining of an MCPyV-positive MCC demonstrates characteristic perinuclear dot-like expression. Bar indicates 100 μm. (D) Viral oncoprotein expression limited to tumor (not adjacent stroma). MCPyV LT antigen expression detected using CM2B4 antibody. Bar indicates 50 μm. Photos courtesy of Chris Lewis.
The majority of MCCs are associated with the Merkel cell polyomavirus (MCPyV)
MCC occurs more frequently in patients with immunodeficiency, including AIDS, suggesting that MCC may have an infectious etiology similar to Kaposi's sarcoma and EBV-induced Burkitt's lymphoma [7–10]. This was confirmed in 2008 when MCPyV was discovered in eight of ten MCC tumors using Digital Transcriptome Subtraction, a high-throughput cDNA sequencing platform that aligned MCC tumor transcripts against reference human sequences [6]. MCPyV was found to be clonally integrated in these tumors, suggesting that viral integration is a critical and early event in MCC development [6]. Viral integration occurs throughout the genome without apparent specificity [11] and therefore likely does not require perturbation of specific host cell genes to mediate oncogenesis. Furthermore, integration is probably a rare biological event as it prevents viral transmission and renders the MCC tumor cell a dead-end host for MCPyV [12].
MCPyV infection is widely prevalent and appears to be asymptomatic, with the exception of rare occurrences of MCC [13,14]. Seropositivity against the viral capsid protein VP1 as well as viral DNA from cutaneous swabs indicate that 40–88% of healthy adults have been infected, with primary exposure often occurring during childhood [13,15–18]. Viral DNA has also been detected in the respiratory tract, saliva, urine and the gastrointestinal system, suggesting possible fecal–oral transmission [14]. Fascinatingly, MCPyV is currently the only human polyomavirus known to be oncogenic, despite numerous studies investigating the carcinogenic potential of the 12 other human polyomaviruses [19].
MCPyV biology
MCPyV is a small (˜5 kb), double stranded DNA virus that consists of both early and late gene regions [20]. The early region contains the T (tumor) antigen locus that contains multiple, alternatively spliced transcripts encoding four unique gene products (large T [LT], small T [sT], the 57 kT and ALTO) while the late region encodes three viral capsid proteins (VP1, VP2 and VP3) [20]. Current evidence suggests that LT and sT are the major oncoproteins mediating MCPyV-driven tumorigenesis as knockdown of these T antigens results in cell cycle arrest and death in MCPyV-positive MCC cell lines [21–23].
MCPyV LT promotes oncogenesis partially through the highly conserved LXCXE motif, which binds to Rb [24]. Rb normally sequesters the transcription factor E2F, however, LT binding to Rb releases E2F resulting in increased expression of cyclin E and CDK2. This promotes entry into the S-phase of the cell cycle and subsequent cellular proliferation [20]. While it appears that the LXCXE motif is critical for MCPyV-driven oncogenesis, mutation of LT resulting in C-terminal truncation is another crucial event in MCC tumor development. This hallmark truncation event within MCCs eliminates uncontrolled viral replication, as is seen in other virally-driven cancers, thereby preventing initiation of DNA damage response and cell death [12,20].
While MCPyV sT shares the first 78 N-terminal residues with MCPyV LT, expression of sT alone mediates in vitro transformation of rodent fibroblasts independent of LT expression and can induce hyperplasia and transformation in transgenic mice [25–28]. MCPyV sT alters cap-dependent translation through inhibition of 4E-BP1 and can prevent degradation of MCPyV LT as well as other key oncoproteins including cyclin E, c-Myc, c-Jun, Notch, mTOR, MCL-2 and NF-κB2 through suppression of the E3 ubiquitin ligase, SCFFbw7 [29]. Detailed summaries of the currently known functions of LT and sT are presented in several recent reviews [20,30]. Importantly, these viral oncoproteins are persistently expressed in MCC tumors (Figure 1D) and are absent in normal tissues, thereby providing ideal targets for immune therapy.
Immune response against MCC
Immune suppression leads to a dramatically increased risk of developing MCC [5,7,8,31]. While 90% of MCC patients do not have clinically apparent immune dysfunction, patients on immunosuppressive regimens following organ transplantation or with compromised cell-mediated immunity (such as those with chronic lymphocytic leukemia and HIV/AIDs) are 10–30-fold more likely to develop MCC and suffer a higher MCC-specific mortality rate than the general population [5,31–34]. This suggests that impaired cellular immunity predisposes individuals to not only developing MCC, but also to poorly controlling their disease.
Additionally, MCCs can regress following withdrawal of immune suppressive treatment [35,36] and spontaneous regression of MCCs is associated with T cell and foamy macrophage infiltration suggesting that regression may be immune-cell mediated [37,38]. While rare, spontaneous regression in MCC is much more common (1.3 per 1000 cases) than in other malignancies (1 in 60,000–100,000 cases) [38]. Furthermore, a subset of advanced stage MCC patients present with unknown primary tumors (no primary skin lesions are detectable) likely as the result of immune-mediated clearance of the primary lesion and these patients have markedly improved overall and disease-specific survival [39].
Humoral response
The immune response against MCC encompasses both the humoral and cellular arms of adaptive immunity. While MCPyV infection is almost ubiquitous, MCC patients have significantly higher capsid protein antibody titers and higher MCPyV DNA levels on their skin than healthy controls, suggesting that these individuals have reduced viral control [15,18,40]. Humoral recognition of MCPyV T antigen oncoproteins on the other hand is restricted to MCC patients. Among MCC patients, approximately 40% are seropositive for the oncoproteins at the time of diagnosis while these antibodies are detected in <1% of healthy controls [16]. MCPyV oncoproteins are not expressed within MCPyV virions, however, viral integration in the setting of MCC results in persistent intracellular expression of LT and sT, potentially explaining why the presence of oncoprotein antibodies is restricted to MCC patients [41]. Oncoprotein antibody titers have been found to fluctuate with tumor burden and a clinical test monitoring oncoprotein antibody titers is now being used as a tool to monitor disease progression (www.merkelcell.org/sero) [42].
T cell response
The production of oncoprotein-specific antibodies implies the presence of a MCPyV-specific CD4 response. In an effort to identify MCPyV-specific T cells, Iyer et al. described an initial set of 24 epitopes within the persistently expressed region of the T-antigen oncoproteins [43]. Five of the 24 were recognized specifically by CD4 T cells and subsequently two additional CD4 epitopes have been reported [43–45]. Therefore, 7 MCPyV-specific CD4 epitopes have been described, however, further phenotypic and functional analysis is required in order to understand the role of these cells in the context of MCC.
The importance of the CD8 T cell response against MCC is highlighted by the finding that robust intratumoral (not peritumoral) infiltration of CD8+ TILs is associated with a striking 100% survival in a study of 146 patients [46]. Additional studies have also indicated that MCC TILs, including CD3+, CD8+ T cells, are associated with improved overall and disease-specific survival [47,48]. Furthermore, expression of genes encoding granzyme A, B, H and K, CCL19, lymphocyte activation genes (SLAMF1 and NKG2D) and CD8α are associated with favorable prognoses, independent of stage [46]. To date, 17 MCPyV-specific CD8 epitopes have been identified, for which 14 HLA-I tetramers have been generated, enabling functional and phenotypic analysis [43,45,49,50]. Importantly, while robust CD8 responses have been associated with improved outcome in MCC, only 4–18% of MCC patients present with significant CD8 infiltration, suggesting that most MCCs block intratumoral CD8 infiltration as a means of evading immune detection [46,51].
MCC tumor evasion mechanisms
Over the past few years, studies have reported several immunological barriers that often occur within the MCC tumor microenvironment.
MHC-I downregulation
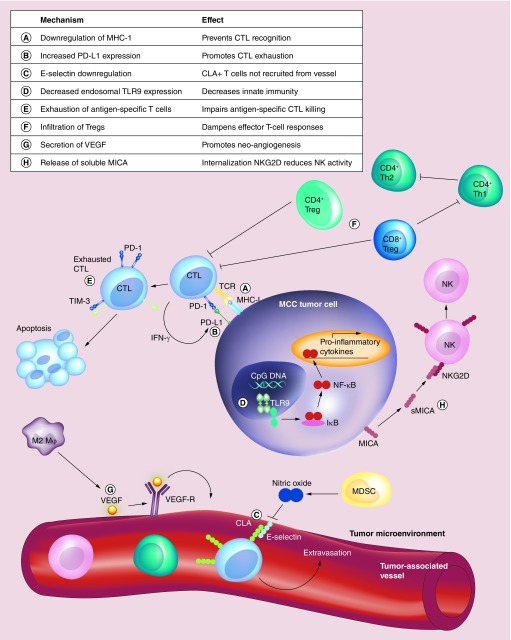
In order for a tumor to be immunologically detected by CD8 T cells, tumor-associated antigens must be presented in the context of MHC-I molecules. However, immunohistochemical evaluation of 114 MCC tumors indicated that 84% downregulated expression of MHC-I, with 51% being markedly downregulated (Figure 2: process ‘A’) [52]. Furthermore, mRNA expression levels of MHC-I closely correlated with expression levels of antigen processing machinery, including proteins involved in the antigen processing complex TAP. This suggests that multiple components involved in antigen processing and presentation are downregulated in MCC and may impair T-cell recognition of MCC tumors [52]. Importantly, treatment of MCC cell lines with type-I interferons, etoposide (a standard MCC chemotherapeutic) and radiation, can all induce MHC-I upregulation in vitro [52]. Notably, in vitro treatment of MCC cells lines with type-I interferons also reduced expression of MCPyV LT, which may further promote tumor destruction [53]. Downregulation of MHC-I can also be reversed in vivo and will be discussed subsequently in the context of intralesional IFN treatment.
Figure 2. . Schematic of documented and putative mechanisms of immune evasion in Merkel cell carcinoma.
The letters in the key above (A-H) indicate critical mechanisms implicated in immune evasion for Merkel cell carcinoma, which are detailed in the text.
Programmed cell death ligand-1 (PD-L1)
PD-L1 is a member of the B7 immunoglobulin superfamily [54] and is a ligand for the programmed death-1 (PD-1) receptor expressed primarily on T lymphocytes [55]. PD-L1 binding to PD-1 limits T cell expansion, promotes functional exhaustion of T cells by inhibiting IL-2 and IFN-γ production and decreases survival [56,57]. This mechanism is thought to play an important physiological role in facilitating tolerance and suppressing autoimmunity, however, evidence suggests that cancers and viruses (including HBV, HPV, EBV, HTLV-1) can induce PD-L1/PD-1 expression to promote local immune suppression [56,58]. Expression of PD-L1 within the tumor microenvironment in gastric carcinoma, RCC, and esophageal cancer is associated with poor prognosis [59–61]. Conversely, in melanoma and MCC, PD-L1 expression is associated with improved overall survival [58]. An evaluation of 67 MCC specimens from 49 MCC patients found that 49% of tumor cells and 55% of tumor-infiltrating lymphocytes (TILs) expressed membranous PD-L1 (Figure 2: process ‘B’) [58]. All of these PD-L1 expressing tumors had TILs while TILs were detected in only 47% of PD-L1 negative tumors [58]. Similarly, in another study PD-L1 protein and mRNA expression correlated with the presence of intratumoral CD8 T cells [49]. Therefore, while increased PD-L1 expression may be preventing a complete antitumor response, detection of intratumoral PD-L1 indicates some degree of immune activity against MCC and suggests that PD-1 blockade may be a promising therapeutic approach for this disease [58].
Downregulation of E-selectin
While MHC-I downregulation and PD-L1 expression may reduce activation of tumor-specific T cells, another mechanism of immune evasion is to prevent recruitment of T cells into the tumor microenvironment. Cutaneous lymphocyte antigen is expressed on skin-homing T cells and is critical for T-cell extravasation from the vasculature into the tissue [62]. Cutaneous lymphocyte antigen binds to E-selectin and/or P-selectin expressed by endothelial cells. In squamous cell carcinomas, E-selectin downregulation is mediated through nitric oxide (NO) signaling that is released by tumor-associated myeloid derived suppressor cells [63]. Nitration of proteins is a marker of NO production and evaluation of nitrotyrosine expression in MCCs indicated that increased levels of nitrotyrosine was associated with decreased E-selectin expression and CD8 T cell infiltration, suggesting that a similar mechanism is being employed within MCC tumors (Figure 2: process ‘C’) [64]. Notably, elevated expression of E-selectin was correlated with improved survival in MCC patients, implying that T-cell extravasation into the tumor microenvironment is critical for optimal immune function against MCC [64].
Decreased expression of TLR9
While most of the described mechanisms have related to adaptive immune responses to MCC, innate immune signaling can also elicit antitumor effects. Toll-like receptor 9 (TLR9) is expressed within the endosomal compartment and activates the NF-κB pathway in response to viral and bacterial CpG-DNA motifs thereby promoting a proinflammatory response [65]. MCPyV-LT and -sT have been shown to inhibit TLR9 expression in an epithelial and MCC cell line in vitro which may reduce inflammatory responses (Figure 2: process ‘D’) [66]. Several other oncogenic viruses (including HPV, EBV and HBV) have also been shown to alter TLR9 expression, suggesting that this is a common strategy to limit immune activation [67].
CD8 T cell exhaustion
T cells can become dysfunctional or exhausted within a few weeks after infection if the infectious agent persists and is not cleared by the host [68]. This has been extensively described in the setting of persistent viral infections or more recently in cancer [68]. The obligate expression of viral T-antigens in MCC, therefore may similarly induce a state of exhaustion in virus-specific T cells. Exhausted T cells have distinct transcriptional programs, impaired proliferative capacity, decreased cytokine production and reduced cytotoxicity [68]. A hallmark of exhausted T cells is the increased expression of various inhibitory receptors including PD-1, TIM-3, Lag-3 and 2B4 [68]. MCPyV-specific T cells isolated from MCC tumors and peripheral blood have been shown to express elevated levels of the inhibitory markers PD-1 and TIM-3 relative to control CMV- or EBV-specific cells (Figure 2: process ‘E’) [49]. Additionally, MCPyV-specific CD8 T cells often have a limited ability to secrete the effector cytokine IFN-γ following antigenic stimulation and TILs within MCC tumors have markedly lower expression of the early activation marker CD69 relative to T cells isolated from normal skin, supporting the notion that these cells are dysfunctional and exhausted [43,69,70].
CD4 T cell polarization
In several cancer types, intratumoral infiltration of Th1 CD4 T cells is strongly associated with good clinical outcomes while infiltration of other CD4 subtypes (Th2 and Th17) is associated with mixed outcomes [71]. Th1 cells produce large amounts of IFN-γ, which facilitate priming and expansion of CD8 T cells [72]. Th1 CD4s also serve to recruit NK and type-I macrophages (proinflammatory) to the tumor site, thereby orchestrating robust antitumor immunity [72]. In the setting of MCC, secretion of Th1 and Th2 type cytokines by bulk intratumoral CD4 T cells was observed from one MCC patient [43]. Whether a significant bias toward a particular subtype occurs in MCPyV-specific CD4s or in additional MCC tumors has not been investigated. Importantly, several therapeutic approaches (discussed below) that promote a Th1 type response have shown clinical promise in treating MCC suggesting that a Th1 response may be beneficial in MCC.
T regulatory cells
T regulatory cells (Tregs), typically identified through expression of CD25 and FOXP3, play a crucial role in mediating peripheral tolerance to self-antigens under normal conditions. However, in the setting of cancer they are generally thought to be tumor promoting [73,74]. It has been shown that high percentages of CD25+FOXP3+ T cells infiltrate MCC tumors relative to normal skin (Figure 2: process ‘F’) [70]. Notably, among FOXP3+ T cells, a discrete population of CD8+FOXP3+ T cells was observed in MCC tumors [70]. These CD8 Tregs are associated with disease progression in several other cancers including malignant melanoma, prostate, ovarian and colorectal [70]. These cells preferentially target Th1 CD4 cells while sparing Th2 cells which may contribute to a tumor-promoting polarization within the tumor microenvironment [75]. While Dowlatshahi et al. reported that intratumoral FOXP3 expression was not correlated with survival in MCC, a study by Sihto et al. indicated that increased FOXP3 expression was associated with improved survival [48]. Therefore, it is unclear whether Treg function is a decisive factor in immune evasion in MCC.
Infiltration of M2 macrophages
M2 macrophages are typically induced by type II cytokines (IL-4, IL-10 and IL-13) and have reduced antigen presentation capacity, promote angiogenesis through secretion of VEGF, facilitate tissue remodeling and ultimately tumor progression [76]. Evaluation of immune cell infiltrates in 21 MCC tumors, found that nearly all of the macrophages present within MCC stained positive for CD163, a marker often used to identify M2 macrophages. Immunohistochemical analysis of MCC tumor samples from 29 patients indicated that VEGF-A, VEGF-C as well as the VEGF-receptor-2 (VEGF-R2) are highly expressed (>75%) within MCC tumors (Figure 2: process ‘G’), suggesting that angiogenesis via VEGF-VEGF-R ligation may be occurring in this disease [77]. Importantly, CD163 expression alone is likely insufficient to fully identify M2 macrophages [78], therefore a more detailed analysis including additional markers could more definitively characterize macrophage phenotypes in this disease.
Inhibition of NK cell killing
NK cells can induce cytotoxicity against certain tumor types without prior stimulation and high levels of infiltrating NK cells have been correlated with favorable outcomes in patients with several types of solid tumors [79]. NK cells are regulated by a complex balance of inhibitory and stimulatory signals [80]. Inhibitory killer Ig-like receptors expressed on NK cells bind MHC-I molecules and prevent NK-mediated killing of normal tissues [80]. Stimulation occurs primarily through MHC class I-related chain -A and -B (MICA/MICB) binding of NKG2A and NKG2D expressed on NK cells [80]. Cancer cells have been shown to evade NK cell activation by cleaving surface MICA/B into a soluble form, which transiently activates NK cells nonspecifically, but ultimately causes inhibition by inducing downregulation of NKG2D (Figure 2: process ‘H’) [80]. The presence of soluble MICA in patient sera has been associated with poor outcome in some cancer types [80] but has not been reported in MCC. Infiltration of NK cells intratumorally has been reported in MCC [48] and the development of tumors despite significant downregulation of MHC-I implies that mechanisms of NK cell evasion are being employed within MCC tumors.
Other candidate mechanisms of immune evasion
Numerous additional mechanisms of evasion have been reported in other cancers that have yet to be investigated in MCC. These include elevated expression of the antiphagocytic signals CD200 and CD47 which have been associated with tumor progression and poor clinical outcome in several solid tumor types [81]. Secretion of immunosuppressive cytokines such as TGFβ, IL-10 and Fas-L within the tumor microenvironment can promote the expansion of Tregs and decrease the activation of cytotoxic T cells and NK cells [74]. Tumor cell secretion of indoleamine 2,3-dioxygenase (IDO) and galectins can impair antitumor T cell responses [74]. Additionally, other cellular infiltrates, including myeloid derived suppressor cells, can promote tumor growth through numerous mechanisms including increased angiogenesis and disruption of antigen presentation [82]. Notably, several of these mechanisms are targetable for therapeutic purposes and therefore may merit further investigation in MCC [74,81,82].
Virus-negative MCCs & UV-induced neoantigens
While the study of MCC has primarily focused upon the immunobiology of tumors caused by MCPyV, several studies have recently investigated the approximately 20% of MCCs that do not contain the virus. The prognosis and overall survival of these two subsets of MCC patients has been debated. Two studies have indicated that patients with virus-negative MCC experience decreased survival as compared with patients with virus-positive MCC [83,84]. Conversely, several others have reported no significant survival difference between the two groups [85–87]. Importantly, genetic analysis indicates that these two subsets are etiologically distinct [88–90]. Specifically, several recent studies have shown that MCPyV-negative MCCs have a very high mutation burden (median 1,121 somatic single nucleotide variants per exome). These are dominated by C > T transitions, characteristic of UV-induced DNA damage [88–90]. This UV-induced signature was not observed in MCPyV-positive tumors and the mutation burden was 19-fold lower (median 12.5 somatic single nucleotide variants per exome) indicating that these tumor types arise through distinct mechanisms [88–90]. High mutational burdens seen in melanoma, colorectal and several types of lung cancer have been associated with a higher prevalence of tumor-associated neoantigens, greater immunogenicity and improved response to immune-based therapies [91]. Strikingly, on average, MCPyV-negative tumors were found to contain more tumor neoantigens than either melanomas or non-small-cell lung cancers suggesting that these virus-negative MCCs have the potential to be highly immunogenic [88]. Furthermore, among virus-negative tumors, a subset expressed PD-L1 and these PD-L1-positive tumors harbor a higher mutational burden as compared with PD-L1-negative tumors, which may reflect immune recognition within these tumors [89]. Notably there were also varying grades of TIL within virus-negative tumors and an increased TIL infiltration correlated with improved survival, as has been described for MCC more generally [46,48,89]. These findings indicate that among virus-negative MCCs, tumors with higher mutational burdens have increased immune recognition which may indicate that this subset may be particularly responsive to checkpoint inhibitors as has been similarly described in other cancers [92,93].
Treatment of MCC
The standard initial management of MCC typically involves surgical excision and radiation therapy for local and regional disease while patients presenting with distant disease are primarily managed with systemic therapy [94]. Although virtually all patients are initially rendered free of detectable disease, roughly half of these patients will recur [95,96]. Once distant metastatic disease arises, cytotoxic chemotherapy leads to a response in >50% of cases, but the median time to progression is only 3 months and durable responses are exceedingly rare [97]. There is thus an urgent need for improved therapies.
Local immune therapies
The delivery of targeted therapies specifically into a tumor has proven efficacious for several immune-based agents and can significantly reduce toxicity that is observed with systemic treatment [98].
Single fraction radiation
Radiation therapy has been shown to increase antigen presentation and to diversify the T-cell receptor repertoire of intratumoral CD8 T cells [99,100]. Preclinical models using targeted single-fraction radiation therapy (SFRT) indicate that SFRT enhances antitumor immunity more effectively than fractionated radiation [101]. SFRT has been reported for treatment of bone metastases in other cancers and because of the known immunogenicity of MCC, SFRT has been used for palliative treatment of MCC patients who either developed chemotherapy-resistant disease or who were unable to receive fractionated radiation for logistical reasons [102]. This approach has yielded a remarkable 94% objective response rate of irradiated lesions in 26 patients who received SFRT to 93 tumors [102]. Complete responses were reported in 45% of tumors with no progression of 77% of tumors with a median follow-up time of 277 days [102]. Importantly, this approach is limited to ‘in field’ lesion control and therefore may not be appropriate for all tumors. However, SFRT may be combined with other immune-stimulating agents as a means of lowering tumor burden and increasing antigenicity to enhance systemic immune responses [99,102].
Intralesional IL-12 DNA electroporation
IL-12 is a Th1 promoting cytokine that can facilitate antitumor immune responses by inducing IFN-γ secretion and increasing proliferation and effector function of NK cells and T cells [103]. Furthermore, IL-12 can induce increased expression of MHC-I, MHC-II and ICAM-1 on human melanoma cells thereby enhancing antigen presentation and T-cell recruitment [104]. However, systemic administration is extremely toxic and can lead to temporary immune suppression and even death [103]. Localized delivery of plasmid-IL-12 using electroporation has significantly reduced toxicity and has shown promising results in melanoma [103]. Subsequently, a Phase II clinical trial using electroporation of intratumoral IL-12 DNA for MCC has fully enrolled and has had promising results in some patients (NCT01440816) [105].
Intralesional TLR4 agonist (GLA) injection
Activation of toll-like receptor signaling can lead to secretion of proinflammatory cytokines and type-I IFNs promoting adaptive and innate immune responses [106]. Glucopyranosyl Lipid-A is a recently generated synthetic TLR-4 agonist that is administered within a stable emulsion (GLA-SE) and specifically induces Th1 responses while minimizing Th2 responses [106]. A Phase I/II clinical trial for treating MCC patients has completed enrollment and has been efficacious in some patients (NCT02035657) [107].
Intralesional IFN-β
The majority of MCCs downregulate MHC-I expression thereby evading cellular immune responses, however, several case reports have described MHC-I upregulation on MCC tumors following either intralesional IFN-β injections or local radiation therapy [52,69]. The use of IFN-β injections clinically has also induced lesion shrinkage, which may be due to enhanced T-cell recognition of these tumors [52,69,108]. Notably, a woman in Japan with multiple MCC metastases on the right arm was treated with IFN-β injections and experienced a complete response that continued for more than 8 years, indicating the potential efficacy of this approach [108].
Topical dinitrochlorobenzene
Dinitrochlorobenzene forms stable protein conjugates that can stimulate T cells to secrete Th1 type cytokines and induce contact sensitization [109]. One patient with multiple local and regional MCC metastases experienced a complete response following 4 weeks of topical application of dinitrochlorobenzene [110]. Adjuvant radiation of the whole scalp was performed following regression and the patient had remained in remission for more than a year at the time of the report [110].
Systemic immune therapies
While several local immune therapies have shown clinical promise, the treatment options for patients with advanced distant disease remain severely limited, therefore systemic immune approaches are being intensively investigated.
Anti-4-1BB (CD137)
The TNF-family receptor, 4-1BB, is expressed on activated T cells and antibodies binding this receptor increase NF-κB activity resulting in cytokine production, leukocyte proliferation and antitumor efficacy in preclinical models [111]. MCPyV-specific T cells express elevated 4-1BB on their surface relative to other virus-specific cells suggesting that these cells may be particularly responsive to 4-1BB agonism [49]. A Phase-I trial in solid tumors (including MCC) and B-cell non-Hodgkin's lymphoma using a 4-1BB agonist (PF-05082566) has completed enrollment. The drug was well tolerated and had promising antitumor activity in an MCC patient [112].
Allogeneic NK cell therapy
NK cells can be dysfunctional or suppressed in the setting of cancer, which may be augmented by the infusion of allogeneic (nonself) NK cells [79]. Unlike autologous NK cells, allogeneic NK cells are not inhibited by host MHC-I expression and can therefore overcome NK cell suppression [79]. One such cell line is NK-92, a continuously growing, IL-2 dependent line that is highly cytotoxic against several tumor cell types in vitro and in vivo [113]. Not only are NK-92 cells allogeneic, they also lack expression of most inhibitory receptors, thereby enhancing their cytolytic function [113]. A Phase II clinical trial is currently recruiting MCC patients in order to evaluate the safety and efficacy of this approach (NCT02465957).
IL-2 fusion protein targeting tumor stroma
Tenascin C is expressed on reactive stromal cells in many solid tumor types predominantly around vascular structures [114]. The use of a monoclonal antibody (F16) targeting tenascin C fused to IL-2, enables targeted delivery of IL-2 to reactive tumor vasculature which may help mediate intratumoral immune activation [115]. Administration of F16-IL2 has been well tolerated and has shown clinical efficacy in trials of certain solid tumors. A Phase II trial using F16-IL2 in combination with a chemotherapeutic agent, paclitaxel, is currently enrolling for metastatic MCC patients in Europe (NCT02054884) under the auspices of IMMOMEC (www.immomec.eu).
Ipilimumab (anti-CTLA-4)
Over the last decade, the use of monoclonal antibodies targeting checkpoint inhibitors CTLA-4, PD-1 and PD-L1 has revolutionized clinical oncology. These agents have proven remarkably efficacious in treating a range of liquid and solid tumors [116–121]. Ipilimumab, an IgG1 monoclonal antibody targeting cytotoxic T-lymphocyte associated antigen 4 (CTLA-4), was the first checkpoint inhibitor to be approved by the US FDA for treating advanced melanoma [116]. Ipilimumab therapy augments the T-cell response through inhibition of T-regulatory cells and enhanced T-cell priming thereby expanding the T-cell repertoire [57,99,122]. Treatment of several cancer types with ipilimumab has shown promising results and a randomized clinical trial utilizing ipilimumab in the adjuvant setting for the treatment of MCC is currently enrolling in Europe (NCT02196961).
Nivolumab (anti-PD-1)
In melanoma, agents targeting the PD-1/PD-L1 axis have tended to show higher response rates than those targeting CTLA-4 [123]. PD-1 axis blockade, like ipilimumab, enhances T-cell function, though through a distinct mechanism. Instead of priming new responses, PD-1 blockade facilitates the expansion of pre-existing quiescent T cells [57,99]. A trial using nivolumab, an anti-PD-1 human IgG4 monoclonal antibody, was better tolerated than ipilimumab and reported a 28% response rate in melanoma patients. Responses were durable, with patients continuing to benefit even after drug discontinuation [117,124]. A Phase I/II clinical trial utilizing nivolumab is open for patients with virus-associated tumors including MCC (NCT02488759).
Pembrolizumab (anti-PD-1)
Pembrolizumab (MK3475), a humanized IgG4 antibody, has been most well studied in the context of melanoma but has shown promising clinical results in several tumor types [116]. In a recent Phase II study of pembrolizumab for a variety of advanced solid tumors (NCT01295827), the most dramatic response was observed in the single MCC patient who experienced a complete response that was ongoing at the time of last follow-up, reflecting 100+ weeks of durable response [125]. Results for a clinical trial using pembrolizumab for first-line treatment of advanced MCC (NCT02267603) have been striking and indicate some of the highest response rates observed in solid tumors to date [126]. 26 patients received at least one dose of pembrolizumab, 25 of which were evaluable. Ten experienced partial responses and four achieved complete regression indicating a remarkable 56% response rate. Importantly, responses were observed among patients with both virus-positive and negative tumors with objective response rates of 62% and 44% respectively. Two grade 4 adverse events were reported including myocarditis and transaminase elevation. Both patients were taken off therapy and improved following treatment with steroids. Interestingly, both of these patients had tumor responses that were still ongoing months after cessation of therapy [126].
Avelumab (anti-PD-L1)
The PD-1 axis can also be inhibited through antagonism of PD-L1, the ligand for PD-1 expressed primarily on cells of the monocyte lineage [54]. Avelumab (MSB0010718C) is a human monoclonal IgG1 antibody that binds to PD-L1 [127]. Binding of PD-L1 instead of PD-1 may reduce toxicity as anti-PD-1 blockade prevents PD-1 interaction with both PD-L1 and PD-L2 [128]. PD-L2 is expressed on normal parenchymal cells in the lung and kidneys and prevents autoimmunity against these tissues [128]. Indeed, anti-PD-1 agents such as nivolumab have induced adverse reactions in these tissues including severe pneumonitis [128]. Avelumab, however, retains PD-1/PD-L2 signaling, thereby preserving these potentially important mechanisms for avoiding autoimmunity. A Phase II clinical trial of avelumab has recently completed enrollment of 88 MCC patients who were refractory to chemotherapy (NCT02155647).
Autologous T cell therapy
Autologous T cell therapy involves isolating tumor-specific or tumor-infiltrating T cells from a patient, expanding them in culture and infusing them back into the patient. This approach has shown efficacy in treating several cancers including other virally-induced malignancies and melanoma [129–131]. In 2013, an MCC patient received three infusions of MCPyV-specific CD8s in combination with subcutaneous administration of IL-2 and HLA-I upregulating agents (single-dose radiation and IFN-β injections) [69]. This patient experienced mixed tumor responses but did not develop a recurrence for 535 days, significantly longer than median time (200 days) to next metastasis experienced by historical controls [69]. It appears that this immune therapy may have conferred benefit, in part because functional infused T cells persisted for >200 days and preferentially accumulated within tumor tissue [69]. A Phase I/II trial utilizing avelumab (anti-PD-L1) and HLA-I upregulation with or without the infusion of autologous T cells is currently enrolling (NCT02584829) and should shed light on whether PD-1 blockade could improve the efficacy of autologous T-cell therapy.
Conclusion & future perspective
Remarkable early responses have been observed among MCC patients treated with PD-1 axis blockade as well as other immune-based therapies. However, approximately 50% of patients with advanced MCC are either not candidates for immune checkpoint blockade or will require the addition of other therapies to achieve meaningful clinical benefit. Studies of potential predictive biomarkers will be important to identify patient subsets that are either particularly likely or unlikely to respond to a given therapy. One potential biomarker for response to PD-1 axis blockade is the expression of PD-L1 within tumors. In melanoma, PD-L1 expression within the tumor closely correlates with clinical response in several studies, however, some patients with PD-L1-negative tumors can also respond [132]. Notably, Tumeh et al. also reported that the presence of pre-existing CD8 T cells at both the invasive tumor margin as well as within the tumor, was associated with PD-L1 expression and response to PD-1 axis blockade [133]. Preliminary evidence suggests that expression of PD-L1 and CD8 T-cell infiltration prior to therapy is not predictive of checkpoint response in MCC, however, this study was limited to 25 evaluable patients and therefore further investigation is warranted [126]. Additionally, a recent study indicated that among malignant melanoma patients, those with higher neoantigen load, and expression of cytolytic markers responded better to CTLA-4 blockade [134]. Consequently, virus-negative MCCs with higher mutational burdens may respond better to checkpoint inhibitors than those with lower mutational burdens.
While identifying predictive biomarkers is of great significance, for patients that are refractory to monotherapeutic approaches, numerous immune-combination therapies have been reported for other cancers that may also be beneficial in MCC [135]. In melanoma, the combination of nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4) has shown markedly increased response rates and longer progression-free survival than monotherapy [136,137]. This is likely because these agents act through nonredundant mechanisms [57]. A preclinical model described by Twyman-Saint Victor et al. also reported that the triple therapy of radiation, anti-CTLA-4 and anti-PD-L1 yielded superior response rates as compared with dual checkpoint blockade without radiation, suggesting that triple therapy such as this may also be beneficial in human subjects [99].
Executive summary.
Merkel cell carcinoma (MCC) is a rare but deadly skin cancer caused by the Merkel cell polyomavirus in approximately 80% of cases.
Viral oncoproteins provide nonself epitopes for immune cell recognition in virus-positive MCCs.
Virus-negative MCCs have a 2.7-fold higher number of tumor associated neoantigens on average than melanoma suggesting that this subset of MCC can also be immunogenic.
Markers of immunity are observed in virus-positive and -negative MCCs.
Antibodies against MCPyV capsid and oncoproteins can be detected.
CD8 and CD4 T-cell epitopes within MCPyV have been identified.
Virus-positive and negative MCC tumors can express PD-L1, which is often associated with intratumoral T cells.
MCCs have developed diverse mechanisms to dampen and evade the immune system.
MCC tumors have several intrinsic evasion mechanisms including downregulation of MHC-I, E-selectin and TLR9 expression.
Effector T-cell dysfunction and immunosuppressive cell types are detected within MCCs.
Traditional therapies have limited efficacy for advanced MCC.
Although responses to cytotoxic chemotherapy are frequent, they are rarely durable.
Immune-based therapies in clinical trials have had promising results for treating MCC.
Localized therapies have shown promising local and distant control in some cases.
The use of systemic pembrolizumab (anti-PD-1) has led to a high response rate as compared with other solid tumors.
Footnotes
Financial & competing interests disclosure
This study is supported by grants from the National Institutes of Health (K24 CA139052, R01-CA162522 and NIH T32 ES007032). P Nghiem serves as a consultant for EMD Serono and receives research support from Bristol-Myers Squibb. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Albores-Saavedra J, Batich K, Chable-Montero F, Sagy N, Schwartz AM, Henson DE. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J. Cutan. Pathol. 2010;37(1):20–27. doi: 10.1111/j.1600-0560.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 2.Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J. Am. Acad. Dermatol. 2003;49(5):832–841. doi: 10.1016/s0190-9622(03)02108-x. [DOI] [PubMed] [Google Scholar]
- 3.Hodgson NC. Merkel cell carcinoma: changing incidence trends. J. Surg. Oncol. 2005;89(1):1–4. doi: 10.1002/jso.20167. [DOI] [PubMed] [Google Scholar]
- 4.Moll R, Lowe A, Laufer J, Franke WW. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am. J. Pathol. 1992;140(2):427–447. [PMC free article] [PubMed] [Google Scholar]
- 5.Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J. Am. Acad. Dermatol. 2008;58(3):375–381. doi: 10.1016/j.jaad.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Seminal paper describing the discovery and integration of MCPyV into the majority of Merkel cell carcinoma (MCC) tumors.
- 7.Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet. 2002;359(9305):497–498. doi: 10.1016/S0140-6736(02)07668-7. [DOI] [PubMed] [Google Scholar]
- 8.Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J. Invest. Dermatol. 2013;133(3):642–646. doi: 10.1038/jid.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266(5192):1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 10.Bibas M, Antinori A. EBV and HIV-related lymphoma. Mediterr. J. Hematol. Infect. Dis. 2009;1(2):e2009032. doi: 10.4084/MJHID.2009.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martel-Jantin C, Filippone C, Cassar O, et al. Genetic variability and integration of Merkel cell polyomavirus in Merkel cell carcinoma. Virology. 2012;426(2):134–142. doi: 10.1016/j.virol.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Shuda M, Feng H, Kwun HJ, et al. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc. Natl Acad. Sci. USA. 2008;105(42):16272–16277. doi: 10.1073/pnas.0806526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schowalter RM, Pastrana DV, Pumphrey KA, Moyer AL, Buck CB. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7(6):509–515. doi: 10.1016/j.chom.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118–130. doi: 10.1016/j.virol.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pastrana DV, Tolstov YL, Becker JC, Moore PS, Chang Y, Buck CB. Quantitation of human seroresponsiveness to Merkel cell polyomavirus. PLoS Pathog. 2009;5(9):e1000578. doi: 10.1371/journal.ppat.1000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paulson KG, Carter JJ, Johnson LG, et al. Antibodies to merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in merkel cell carcinoma patients. Cancer Res. 2010;70(21):8388–8397. doi: 10.1158/0008-5472.CAN-10-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen T, Hedman L, Mattila PS, et al. Serological evidence of Merkel cell polyomavirus primary infections in childhood. J. Clin. Virol. 2011;50(2):125–129. doi: 10.1016/j.jcv.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Foulongne V, Kluger N, Dereure O, et al. Merkel cell polyomavirus in cutaneous swabs. Emerg. Infect. Dis. 2010;16(4):685–687. doi: 10.3201/eid1604.091278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moens U, Rasheed K, Abdulsalam I, Sveinbjornsson B. The role of Merkel cell polyomavirus and other human polyomaviruses in emerging hallmarks of cancer. Viruses. 2015;7(4):1871–1901. doi: 10.3390/v7041871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wendzicki JA, Moore PS, Chang Y. Large T and small T antigens of Merkel cell polyomavirus. Curr. Opin. Virol. 2015;11:38–43. doi: 10.1016/j.coviro.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Recent review nicely summarizing MCPyV oncoprotein biology.
- 21.Houben R, Shuda M, Weinkam R, et al. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J. Virol. 2010;84(14):7064–7072. doi: 10.1128/JVI.02400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shuda M, Chang Y, Moore PS. Merkel cell polyomavirus-positive Merkel cell carcinoma requires viral small T-antigen for cell proliferation. J. Invest. Dermatol. 2014;134(5):1479–1481. doi: 10.1038/jid.2013.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrama D, Hesbacher S, Angermeyer S, et al. Serine 220 phosphorylation of the Merkel cell polyomavirus large T antigen crucially supports growth of Merkel cell carcinoma cells. Int. J. Cancer. 2015;138(5):1153–1162. doi: 10.1002/ijc.29862. [DOI] [PubMed] [Google Scholar]
- 24.Houben R, Adam C, Baeurle A, et al. An intact retinoblastoma protein-binding site in Merkel cell polyomavirus large T antigen is required for promoting growth of Merkel cell carcinoma cells. Int. J. Cancer. 2012;130(4):847–856. doi: 10.1002/ijc.26076. [DOI] [PubMed] [Google Scholar]
- 25.Shuda M, Kwun HJ, Feng H, Chang Y, Moore PS. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J. Clin. Invest. 2011;121(9):3623–3634. doi: 10.1172/JCI46323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verhaegen ME, Mangelberger D, Harms PW, et al. Merkel cell polyomavirus small T antigen is oncogenic in transgenic mice. J. Invest. Dermatol. 2015;135(5):1415–1424. doi: 10.1038/jid.2014.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shuda M, Guastafierro A, Geng X, et al. Merkel cell polyomavirus small T antigen induces cancer and embryonic Merkel cell proliferation in a transgenic mouse model. PLoS ONE. 2015;10(11):e0142329. doi: 10.1371/journal.pone.0142329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spurgeon ME, Cheng J, Bronson RT, Lambert PF, Decaprio JA. Tumorigenic activity of merkel cell polyomavirus T antigens expressed in the stratified epithelium of mice. Cancer Res. 2015;75(6):1068–1079. doi: 10.1158/0008-5472.CAN-14-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwun HJ, Shuda M, Feng H, Camacho CJ, Moore PS, Chang Y. Merkel cell polyomavirus small T antigen controls viral replication and oncoprotein expression by targeting the cellular ubiquitin ligase SCFFbw7. Cell Host Microbe. 2013;14(2):125–135. doi: 10.1016/j.chom.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Church CD, Nghiem P. How does the Merkel polyomavirus lead to a lethal cancer? Many answers, many questions, and a new mouse model. J. Invest. Dermatol. 2015;135(5):1221–1224. doi: 10.1038/jid.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buell JF, Trofe J, Hanaway MJ, et al. Immunosuppression and Merkel cell cancer. Transplant. Proc. 2002;34(5):1780–1781. doi: 10.1016/s0041-1345(02)03065-8. [DOI] [PubMed] [Google Scholar]
- 32.Penn I, First MR. Merkel's cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68(11):1717–1721. doi: 10.1097/00007890-199912150-00015. [DOI] [PubMed] [Google Scholar]
- 33.Brewer JD, Shanafelt TD, Otley CC, et al. Chronic lymphocytic leukemia is associated with decreased survival of patients with malignant melanoma and Merkel cell carcinoma in a SEER population-based study. J. Clin. Oncol. 2012;30(8):843–849. doi: 10.1200/JCO.2011.34.9605. [DOI] [PubMed] [Google Scholar]
- 34.Tadmor T, Aviv A, Polliack A. Merkel cell carcinoma, chronic lymphocytic leukemia and other lymphoproliferative disorders: an old bond with possible new viral ties. Ann. Oncol. 2011;22(2):250–256. doi: 10.1093/annonc/mdq308. [DOI] [PubMed] [Google Scholar]
- 35.Muirhead R, Ritchie DM. Partial regression of Merkel cell carcinoma in response to withdrawal of azathioprine in an immunosuppression-induced case of metastatic Merkel cell carcinoma. Clin. Oncol. (R. Coll. Radiol.) 2007;19(1):96. doi: 10.1016/j.clon.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Friedlaender MM, Rubinger D, Rosenbaum E, Amir G, Siguencia E. Temporary regression of Merkel cell carcinoma metastases after cessation of cyclosporine. Transplantation. 2002;73(11):1849–1850. doi: 10.1097/00007890-200206150-00028. [DOI] [PubMed] [Google Scholar]
- 37.Sugamata A, Goya K, Yoshizawa N. A case of complete spontaneous regression of extremely advanced Merkel cell carcinoma. J. Surg. Case Rep. 2011;2011(10):7. doi: 10.1093/jscr/2011.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int. J. Surg. Case Rep. 2015;7C:104–108. doi: 10.1016/j.ijscr.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foote M, Veness M, Zarate D, Poulsen M. Merkel cell carcinoma: the prognostic implications of an occult primary in stage IIIB (nodal) disease. J. Am. Acad. Dermatol. 2012;67(3):395–399. doi: 10.1016/j.jaad.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Carter JJ, Paulson KG, Wipf GC, et al. Association of Merkel cell polyomavirus-specific antibodies with Merkel cell carcinoma. J. Natl Cancer Inst. 2009;101(21):1510–1522. doi: 10.1093/jnci/djp332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura T, Sato Y, Watanabe D, et al. Nuclear localization of Merkel cell polyomavirus large T antigen in Merkel cell carcinoma. Virology. 2010;398(2):273–279. doi: 10.1016/j.virol.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 42.Moshiri AS, Nghiem P. Milestones in the staging, classification, and biology of Merkel cell carcinoma. J. Natl Compr. Canc. Netw. 2014;12(9):1255–1262. doi: 10.6004/jnccn.2014.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iyer JG, Afanasiev OK, Mcclurkan C, et al. Merkel cell polyomavirus-specific CD8(+) and CD4(+) T-cell responses identified in Merkel cell carcinomas and blood. Clin. Cancer. Res. 2011;17(21):6671–6680. doi: 10.1158/1078-0432.CCR-11-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng Q, Gomez BP, Viscidi RP, et al. Development of a DNA vaccine targeting Merkel cell polyomavirus. Vaccine. 2012;30(7):1322–1329. doi: 10.1016/j.vaccine.2011.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ibrani D, Iyer JG, Vandeven N, et al. Society For Investigative Dermatology Annual Meeting. Atlanta, GA, USA: 6–9 May 2015. Identifying Merkel cell polyomavirus-specific CD4+ and CD8+ T-cells in Merkel cell carcinoma patients’ tumor-infiltrating lymphcytes. Presented at. Abstract 015. [Google Scholar]
- 46.Paulson KG, Iyer JG, Tegeder AR, et al. Transcriptome-wide studies of merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J. Clin. Oncol. 2011;29(12):1539–1546. doi: 10.1200/JCO.2010.30.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Indicates that MCPyV-specific T cells express targetable markers of exhaustion.
- 47.Andea AA, Coit DG, Amin B, Busam KJ. Merkel cell carcinoma: histologic features and prognosis. Cancer. 2008;113(9):2549–2558. doi: 10.1002/cncr.23874. [DOI] [PubMed] [Google Scholar]
- 48.Sihto H, Bohling T, Kavola H, et al. Tumor infiltrating immune cells and outcome of Merkel cell carcinoma: a population-based study. Clin. Cancer. Res. 2012;18(10):2872–2881. doi: 10.1158/1078-0432.CCR-11-3020. [DOI] [PubMed] [Google Scholar]
- 49.Afanasiev OK, Yelistratova L, Miller N, et al. Merkel polyomavirus-specific T cells fluctuate with merkel cell carcinoma burden and express therapeutically targetable PD-1 and Tim-3 exhaustion markers. Clin. Cancer. Res. 2013;19(19):5351–5360. doi: 10.1158/1078-0432.CCR-13-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lyngaa R, Pedersen NW, Schrama D, et al. T-cell responses to oncogenic merkel cell polyomavirus proteins distinguish patients with merkel cell carcinoma from healthy donors. Clin. Cancer. Res. 2014;20(7):1768–1778. doi: 10.1158/1078-0432.CCR-13-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paulson KG, Iyer JG, Simonson WT, et al. CD8+ lymphocyte intratumoral infiltration as a stage-independent predictor of Merkel cell carcinoma survival: a population-based study. Am. J. Clin. Pathol. 2014;142(4):452–458. doi: 10.1309/AJCPIKDZM39CRPNC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paulson KG, Tegeder A, Willmes C, et al. Downregulation of MHC-I expression is prevalent but reversible in Merkel cell carcinoma. Cancer Immunol. Res. 2014;2(11):1071–1079. doi: 10.1158/2326-6066.CIR-14-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willmes C, Adam C, Alb M, et al. Type I and II IFNs inhibit Merkel cell carcinoma via modulation of the Merkel cell polyomavirus T antigens. Cancer Res. 2012;72(8):2120–2128. doi: 10.1158/0008-5472.CAN-11-2651. [DOI] [PubMed] [Google Scholar]
- 54.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999;5(12):1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 55.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin. Immunol. 2014;153(1):145–152. doi: 10.1016/j.clim.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 57.Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 2015;39(1):98–106. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lipson EJ, Vincent JG, Loyo M, et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol. Res. 2013;1(1):54–63. doi: 10.1158/2326-6066.CIR-13-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108(1):19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 61.Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin. Cancer. Res. 2005;11(8):2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 62.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat. Rev. Immunol. 2004;4(3):211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gehad AE, Lichtman MK, Schmults CD, et al. Nitric oxide-producing myeloid-derived suppressor cells inhibit vascular E-selectin expression in human squamous cell carcinomas. J. Invest. Dermatol. 2012;132(11):2642–2651. doi: 10.1038/jid.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Afanasiev OK, Nagase K, Simonson W, et al. Vascular E-selectin expression correlates with CD8 lymphocyte infiltration and improved outcome in Merkel cell carcinoma. J. Invest. Dermatol. 2013;133(8):2065–2073. doi: 10.1038/jid.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huen AO, Rook AH. Toll receptor agonist therapy of skin cancer and cutaneous T-cell lymphoma. Curr. Opin. Oncol. 2014;26(2):237–244. doi: 10.1097/CCO.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 66.Shahzad N, Shuda M, Gheit T, et al. The T antigen locus of Merkel cell polyomavirus downregulates human Toll-like receptor 9 expression. J. Virol. 2013;87(23):13009–13019. doi: 10.1128/JVI.01786-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pacini L, Savini C, Ghittoni R, et al. Downregulation of toll-like receptor 9 expression by beta human papillomavirus 38 and implications for cell cycle control. J. Virol. 2015;89(22):11396–11405. doi: 10.1128/JVI.02151-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chapuis AG, Afanasiev OK, Iyer JG, et al. Regression of metastatic Merkel cell carcinoma following transfer of polyomavirus-specific T cells and therapies capable of re-inducing HLA class-I. Cancer Immunol. Res. 2014;2(1):27–36. doi: 10.1158/2326-6066.CIR-13-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dowlatshahi M, Huang V, Gehad AE, et al. Tumor-specific T cells in human Merkel cell carcinomas: a possible role for Tregs and T-cell exhaustion in reducing T-cell responses. J. Invest. Dermatol. 2013;133(7):1879–1889. doi: 10.1038/jid.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 72.Kim HJ, Cantor H. CD4 T-cell subsets and tumor immunity: the helpful and the not-so-helpful. Cancer Immunol. Res. 2014;2(2):91–98. doi: 10.1158/2326-6066.CIR-13-0216. [DOI] [PubMed] [Google Scholar]
- 73.Oleinika K, Nibbs RJ, Graham GJ, Fraser AR. Suppression, subversion and escape: the role of regulatory T cells in cancer progression. Clin. Exp. Immunol. 2013;171(1):36–45. doi: 10.1111/j.1365-2249.2012.04657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burkholder B, Huang RY, Burgess R, et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim. Biophys. Acta. 2014;1845(2):182–201. doi: 10.1016/j.bbcan.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 75.Smith TR, Kumar V. Revival of CD8+ Treg-mediated suppression. Trends Immunol. 2008;29(7):337–342. doi: 10.1016/j.it.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 76.Schmieder A, Michel J, Schonhaar K, Goerdt S, Schledzewski K. Differentiation and gene expression profile of tumor-associated macrophages. Semin. Cancer Biol. 2012;22(4):289–297. doi: 10.1016/j.semcancer.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 77.Brunner M, Thurnher D, Pammer J, et al. Expression of VEGF-A/C, VEGF-R2, PDGF-alpha/beta, c-kit, EGFR, Her-2/Neu, Mcl-1 and Bmi-1 in Merkel cell carcinoma. Mod. Pathol. 2008;21(7):876–884. doi: 10.1038/modpathol.2008.63. [DOI] [PubMed] [Google Scholar]
- 78.Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS ONE. 2013;8(11):e80908. doi: 10.1371/journal.pone.0080908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell. Mol. Immunol. 2013;10(3):230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pahl J, Cerwenka A. Tricking the balance: NK cells in anti-cancer immunity. Immunobiology. 2015 doi: 10.1016/j.imbio.2015.07.012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 81.Chao MP, Majeti R, Weissman IL. Programmed cell removal: a new obstacle in the road to developing cancer. Nat. Rev. Cancer. 2012;12(1):58–67. doi: 10.1038/nrc3171. [DOI] [PubMed] [Google Scholar]
- 82.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19(11):1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sihto H, Kukko H, Koljonen V, Sankila R, Bohling T, Joensuu H. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J. Natl Cancer Inst. 2009;101(13):938–945. doi: 10.1093/jnci/djp139. [DOI] [PubMed] [Google Scholar]
- 84.Bhatia K, Goedert JJ, Modali R, Preiss L, Ayers LW. Merkel cell carcinoma subgroups by Merkel cell polyomavirus DNA relative abundance and oncogene expression. Int. J. Cancer. 2010;126(9):2240–2246. doi: 10.1002/ijc.24676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schrama D, Peitsch WK, Zapatka M, et al. Merkel cell polyomavirus status is not associated with clinical course of Merkel cell carcinoma. J. Invest. Dermatol. 2011;131(8):1631–1638. doi: 10.1038/jid.2011.115. [DOI] [PubMed] [Google Scholar]
- 86.Handschel J, Muller D, Depprich RA, et al. The new polyomavirus (MCPyV) does not affect the clinical course in MCCs. Int. J. Oral Maxillofac. Surg. 2010;39(11):1086–1090. doi: 10.1016/j.ijom.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 87.Asioli S, Righi A, De Biase D, et al. Expression of p63 is the sole independent marker of aggressiveness in localised (stage I-II) Merkel cell carcinomas. Mod. Pathol. 2011;24(11):1451–1461. doi: 10.1038/modpathol.2011.100. [DOI] [PubMed] [Google Scholar]
- 88.Goh G, Walradt T, Markarov V, et al. Mutational landscape of MCPyV-positive and MCPyV-negative merkel cell carcinomas with implications for immunotherapy. Oncotarget. 2015;7(3):3403–3415. doi: 10.18632/oncotarget.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wong SQ, Waldeck K, Vergara IA, et al. UV-associated mutations underlie the etiology of MCV-negative Merkel cell carcinomas. Cancer Res. 2015;75(24):5228–5234. doi: 10.1158/0008-5472.CAN-15-1877. [DOI] [PubMed] [Google Scholar]; •• Indicates the MCPyV-negative tumors have high mutation burden with a UV-signature that is associated with increased immune response.
- 90.Harms PW, Vats P, Verhaegen ME, et al. The distinctive mutational spectra of polyomavirus-negative Merkel cell carcinoma. Cancer Res. 2015;75(18):3720–3727. doi: 10.1158/0008-5472.CAN-15-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 92.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prieto I, De La Fuente TP, Medina S, et al. Merkel cell carcinoma: an algorithm for multidisciplinary management and decision-making. Crit. Rev. Oncol. Hematol. 2015;98:170–179. doi: 10.1016/j.critrevonc.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 95.Allen PJ, Bowne WB, Jaques DP, Brennan MF, Busam K, Coit DG. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J. Clin. Oncol. 2005;23(10):2300–2309. doi: 10.1200/JCO.2005.02.329. [DOI] [PubMed] [Google Scholar]
- 96.Santamaria-Barria JA, Boland GM, Yeap BY, Nardi V, Dias-Santagata D, Cusack JC., Jr Merkel cell carcinoma: 30-year experience from a single institution. Ann. Surg. Oncol. 2013;20(4):1365–1373. doi: 10.1245/s10434-012-2779-3. [DOI] [PubMed] [Google Scholar]
- 97.Iyer JG, Blom A, Doumani R, et al. The 2014 American Society for Clinical Oncology Annual Meeting. Chicago, Illinois, USA: 29 May–2 June 2014. Response rates and durability of chemotherapy for metastatic Merkel cell carcinoma among 62 patients. Presented at. Abstract 9091. [Google Scholar]
- 98.Van Der Jeught K, Bialkowski L, Daszkiewicz L, et al. Targeting the tumor microenvironment to enhance antitumor immune responses. Oncotarget. 2015;6(3):1359–1381. doi: 10.18632/oncotarget.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Highlights distinct mechanistic activity of three therapeutic approaches strongly implicating that the use of combination therapy is required for maximal antitumor efficacy.
- 100.Tang C, Wang X, Soh H, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol. Res. 2014;2(9):831–838. doi: 10.1158/2326-6066.CIR-14-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Iyer JG, Parvathaneni U, Gooley T, et al. Single-fraction radiation therapy in patients with metastatic Merkel cell carcinoma. Cancer Med. 2015;4(8):1161–1170. doi: 10.1002/cam4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cha E, Daud A. Plasmid IL-12 electroporation in melanoma. Hum. Vaccin. Immunother. 2012;8(11):1734–1738. doi: 10.4161/hv.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yue FY, Geertsen R, Hemmi S, et al. IL-12 directly up-regulates the expression of HLA class I, HLA class II and ICAM-1 on human melanoma cells: a mechanism for its antitumor activity? Eur. J. Immunol. 1999;29(6):1762–1773. doi: 10.1002/(SICI)1521-4141(199906)29:06<1762::AID-IMMU1762>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 105.Bhatia S, Iyer J, Ibrani D, et al. The European Cancer Congress 2015. Vienna, Austria: 25–29 September 2015. Intratumoral delivery of Interleukin-12 DNA via in vivo electroporation leads to regression of injected and non-injected tumors in Merkel cell carcinoma: final results of a phase 2 study. Presented at. Abstract 504. [Google Scholar]
- 106.Matzner P, Sorski L, Shaashua L, et al. Perioperative treatment with the new synthetic TLR-4 agonist GLA-SE reduces cancer metastasis without adverse effects. Int. J. Cancer. 2015;138(7):1754–1764. doi: 10.1002/ijc.29885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bhatia S, Ibrani D, Vandeven N, et al. The 2015 American Society for Clinical Oncology Annual Meeting. Chicago, Illinois, USA: 29 May–2 June 2015. Pilot study of intratumoral G100, toll-like receptor-4 (TLR4) agonist, therapy in patients with Merkel cell carcinoma (MCC) Presented at. Abstract 3083. [Google Scholar]
- 108.Nakajima H, Takaishi M, Yamamoto M, et al. Screening of the specific polyoma virus as diagnostic and prognostic tools for Merkel cell carcinoma. J. Dermatol. Sci. 2009;56(3):211–213. doi: 10.1016/j.jdermsci.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 109.Pickard C, Smith AM, Cooper H, et al. Investigation of mechanisms underlying the T-cell response to the hapten 2,4-dinitrochlorobenzene. J. Invest. Dermatol. 2007;127(3):630–637. doi: 10.1038/sj.jid.5700581. [DOI] [PubMed] [Google Scholar]
- 110.Herrmann G, Groth W, Krieg T, Mauch C. Complete remission of Merkel cell carcinoma of the scalp with local and regional metastases after topical treatment with dinitrochlorbenzol. J. Am. Acad. Dermatol. 2004;50(6):965–969. doi: 10.1016/j.jaad.2003.11.049. [DOI] [PubMed] [Google Scholar]
- 111.Fisher TS, Kamperschroer C, Oliphant T, et al. Targeting of 4–1BB by monoclonal antibody PF-05082566 enhances T-cell function and promotes anti-tumor activity. Cancer Immunol. Immunother. 2012;61(10):1721–1733. doi: 10.1007/s00262-012-1237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shelved 4–1BB Antibodies Make Comeback. Cancer Discov. 2015;5(11):1118. doi: 10.1158/2159-8290.CD-ND2015-003. [DOI] [PubMed] [Google Scholar]
- 113.Uherek C, Tonn T, Uherek B, et al. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood. 2002;100(4):1265–1273. [PubMed] [Google Scholar]
- 114.Brack SS, Silacci M, Birchler M, Neri D. Tumor-targeting properties of novel antibodies specific to the large isoform of tenascin-C. Clin. Cancer. Res. 2006;12(10):3200–3208. doi: 10.1158/1078-0432.CCR-05-2804. [DOI] [PubMed] [Google Scholar]
- 115.Tothill R, Estall V, Rischin D. Merkel cell carcinoma: emerging biology, current approaches, and future directions. Am. Soc. Clin. Oncol. Educ. Book. 2015 doi: 10.14694/EdBook_AM.2015.35.e519. [DOI] [PubMed] [Google Scholar]
- 116.Ascierto PA, Marincola FM. 2015: The year of anti-PD-1/PD-L1s against melanoma and beyond. EBioMedicine. 2015;2(2):92–93. doi: 10.1016/j.ebiom.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Topalian SL, Sznol M, Mcdermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hodi FS, O'day SJ, Mcdermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N. Engl. J. Med. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 121.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 122.Robert L, Tsoi J, Wang X, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin. Cancer. Res. 2014;20(9):2424–2432. doi: 10.1158/1078-0432.CCR-13-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Callahan MK, Wolchok JD. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J. Leukoc. Biol. 2013;94(1):41–53. doi: 10.1189/jlb.1212631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin. Cancer. Res. 2015;21(19):4286–4293. doi: 10.1158/1078-0432.CCR-14-2607. [DOI] [PubMed] [Google Scholar]
- 126.Nghiem P, Bhatia S, Lipson EJ, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N. Engl. J. Med. 2016 doi: 10.1056/NEJMoa1603702. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reports remarkably high response rates in MCC patients treated with anti-PD-1 pembrolizumab.
- 127.Boyerinas B, Jochems C, Fantini M, et al. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol. Res. 2015;3(10):1148–1157. doi: 10.1158/2326-6066.CIR-15-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sundar R, Cho BC, Brahmer JR, Soo RA. Nivolumab in NSCLC: latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2015;7(2):85–96. doi: 10.1177/1758834014567470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chapuis AG, Thompson JA, Margolin KA, et al. Transferred melanoma-specific CD8+ T cells persist, mediate tumor regression, and acquire central memory phenotype. Proc. Natl Acad. Sci. USA. 2012;109(12):4592–4597. doi: 10.1073/pnas.1113748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hunder NN, Wallen H, Cao J, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N. Engl. J. Med. 2008;358(25):2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Johnson DB, Peng C, Sosman JA. Nivolumab in melanoma: latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2015;7(2):97–106. doi: 10.1177/1758834014567469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Shows that CD8 T cell intratumoral infiltration correlates with PD-L1 expression and predicts response to pembrolizumab.
- 134.Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Indicates that higher mutational burden, increased neoantigen load and elevated expression of cytolytic markers predict response to iplimimumab treatment for melanoma patients.
- 135.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]