Abstract
Aim:
To characterize the clinical indications of females (<15 years old) undergoing ovarian tissue cryopreservation (OTC) through the Oncofertility Consortium's National Physicians Cooperative (OC-NPC).
Patients & methods:
The clinical indications of 114 females who underwent OTC were classified, and their incidence was compared with childhood cancer databases.
Results:
Leukemias/myeloproliferative diseases/myelodysplastic diseases and hemoglobinopathies were the most prevalent oncologic and nononcologic indications for OTC, respectively. The frequencies of malignant bone tumors and soft tissue and other extraosseous sarcomas were higher in the OC-NPC cohort relative to the general population, while CNS/intracranial/intraspinal neoplasms, retinoblastoma and hepatic tumors were lower.
Conclusion:
Those opting for OTC through the OC-NPC are at highest fertility risk, indicating that the appropriate patient populations are being identified.
Keywords: : childhood cancer, fertility preservation, oncofertility, ovarian tissue cryopreservation, pediatric, women's health
Graphical abstract
Cancer survivorship has steadily risen over the past four decades as treatments have become increasingly successful [1]. There are over 10,000 new cases of childhood cancer diagnosed per year, with a child <15 years of age in the USA having a 1 in 408 chance of receiving a cancer diagnosis [2]. Adult survival has improved by roughly 20% since the 1970s, with a current 5-year survival incidence of 69% [3]. Even more impressive are the changes in those with childhood cancer, in which the 5-year survival incidence in this population has improved from 58 to 83% since the 1970s [4]. As the population of cancer survivors increases, focus on long-term health and quality of post-treatment life is imperative. Unfortunately, the therapeutic regimens of chemotherapy and radiation have a deleterious effect on reproductive organs and increase a patient's risk for future infertility [5]. For example, women are born with a finite number of primordial follicles, which dictates their reproductive lifespan [6]. Radiation and chemotherapy can damage the follicular reserve resulting in compromised fertility and early menopause [7]. Alkylating drugs, total-body radiation and radiation below the diaphragm pose the highest risk for iatrogenic premature ovarian insufficiency (POI) [8].
The field of oncofertility was developed to help patients faced with potentially sterilizing treatments or conditions to identify appropriate fertility-preservation options [9,10]. The current accepted standards for fertility preservation in females are banking of either oocytes or embryos [11]. Both procedures involve hyperstimulation of the ovary to induce the growth of multiple follicles [12]. Following retrieval, mature oocytes or embryos obtained by in vitro fertilization (IVF), are cryopreserved and stored for the patient's future use [13]. These standard options, however, are not possible for those patients who cannot delay gonadotoxic treatment to undergo assisted reproduction technologies (ART) or prepubertal females. For these patient populations, ovarian tissue cryopreservation (OTC) with the potential for retransplantation is an emerging fertility-preservation method in which cortical ovarian tissue is removed, cryopreserved and transplanted to restore endocrine function and fertility [14]. In fact, there have been an estimated 86 live births and nine ongoing pregnancies worldwide following ovarian tissue transplantation [15]. The actual efficacy of OTC, however, is speculative as the total number of transplants attempted relative to the positive outcomes is typically not reported, and it cannot be proven that the restoration of reproductive function is due exclusively to the transplanted tissue [16]. In addition to OTC, in vitro growth of ovarian follicles is another promising fertility-preservation strategy, but it has not yet been used clinically [17].
OTC is the only fertility-preservation option for prepubertal girls because oocyte and embryo cryopreservation rely on a mature hypothalamic–pituitary–ovarian (HPO) axis, ovarian hyperstimulation and/or a sperm donor [11,18]. OTC followed by transplantation has also been used to restore endocrine function and fertility [19,20]. OTC can restore endocrine function that is impaired in POI. For example, normal endocrine function is possible after transplantation of cortical tissue for 5–7 years, and cortical strips have allowed patients to conceive naturally with normal endocrine function [21,22]. A live birth has been reported from tissue that was frozen prior to puberty but transplanted after [23]. There is also the potential of using ovarian tissue transplantation to induce puberty. However, concerns about this application exist due to the finite lifespan of the implanted tissue, limited and valuable nature of the tissue, and the abnormal variation of hormones in the HPO axis following transplant [24,25]. Nevertheless, this growing evidence provides tangible hope for young girls that endogenous hormone production through OTC will delay the deleterious health effects of menopause imposed by POI while also potentially restoring fertility.
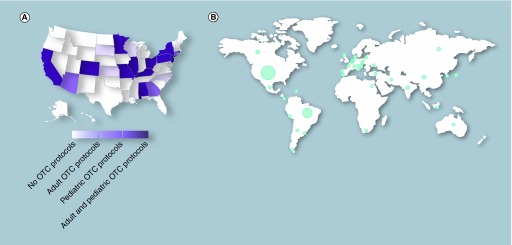
The Oncofertility Consortium's National Physicians Cooperative (OC-NPC) was established in 2007 to address fertility challenges faced by cancer treatment or other fertility-threatening conditions. The OC-NPC includes 38 member institutions in 20 states across the USA that offer, or have one time offered, OTC through Institutional Review Board-approved protocols (Figure 1A). Of the 20 states, 16 have member institutions that enroll pediatric ages ranging from 0 to 17 years into their OTC protocols (Figure 1A). The OC-NPC also has partner institutions in 35 countries across the globe, which together comprise the Oncofertility Consortium's Global Partners Network (Figure 1B). Use of OTC through the OC-NPC has been steadily increasing over the last decade, especially in young females [26]. The goal of this study was to gain further insight into the profile of participants (<15 years of age) who are accessing OTC through the OC-NPC by comparing the frequency of malignancies in this cohort to the general population. This age limit was selected so that we could directly compare OC-NPC data to existing databases for childhood cancers that report data in the same age cohort.
Figure 1. . The Oncofertility Consortium's National Physicians Cooperative presence in the USA and globally.
(A) The distribution of Oncofertility Consortium's National Physicians Cooperative sites within the USA according to which types of ovarian tissue cryopreservation protocols they currently have or have had approved. (B) The distribution of National Physicians Cooperative partner institutions across the globe. The circle width is proportional to the relative number of sites.
OTC: Ovarian tissue cryopreservation.
Materials & methods
Data acquisition & ethical conduct of research
At OC-NPC sites that offer OTC, ovarian tissue is harvested from individuals under Institutional Review Board-approved protocols and following informed consent or assent. Eighty percent of the tissue is cryopreserved for the participant's future clinical use to restore endocrine function and/or fertility and the remaining 20% can be donated for basic research [26]. Basic demographic and clinical information for each participant is collected and de-identified including age, clinical diagnosis and treatment history prior to OTC. We obtained de-identified clinical data from the OC-NPC database for females <15 years old who underwent OTC. From a total of 330 OTC cases that occurred between the inception of the OC-NPC through May 2016, 117 were from participants <15 years of age. Three of the 117 cases were excluded from further analysis due to incomplete data availability. The collected data from the remaining 114 OC-NPC cases are summarized in Tables 1 & 2. We then compared the OC-NPC data with that available for the same age cohorts in two existing databases, including the Surveillance, Epidemiology and End Results Program (SEER), a cancer surveillance program in the USA [27], and the Automated Childhood Cancer Information System (ACCIS), which reports cancer incidence in children and adolescents in Europe [28–30].
Table 1. . Age and clinical diagnoses of Oncofertility Consortium's National Physicians Cooperative participants who underwent ovarian tissue cryopreservation due to a nononcologic condition.
| OC-NPC ID# | Age (years) | Race | Ethnicity | Diagnosis | Treatment |
|---|---|---|---|---|---|
| Hemoglobinopathies | |||||
| NPC-2013-150 | 2 | White | Non-Hispanic | β-thalassemia intermedia | Chemotherapy |
| NPC-2015-238 | 2 | African–American/black | Non-Hispanic | Sickle cell anemia | None |
| NPC-2014-210 | 3 | Not reported | Non-Hispanic | β-thalassemia | None |
| NPC-2014-168 | 6 | African–American/black | Non-Hispanic | Sickle cell disease | Chemotherapy |
| NPC-2014-193 | 6 | Not reported | Non-Hispanic | β-thalassemia | None |
| NPC-2015-252 | 6 | Black | Non-Hispanic | Sickle cell disease | None |
| NPC-2014-182 | 7 | Not reported | Non-Hispanic | β-thalassemia | None |
| NPC-2013-144 | 8 | White | Non-Hispanic | Thalassemia | Chemotherapy |
| NPC-2015-253 | 8 | White | Non-Hispanic | Sickle cell disease | None |
| NPC-2014-200 | 10 | African–American/black | Non-Hispanic | Sickle cell anemia going to BMT | None |
| NPC-2011-80 | 10.5 | African–American/black | Non-Hispanic | Sickle cell anemia going to BMT | Chemotherapy |
| NPC-2013-147 | 11 | African–American/black | Non-Hispanic | Sickle cell anemia | None |
| NPC-2015-244 | 11 | Not reported | Non-Hispanic | β-thalassemia | BMT |
| NPC-2015-247 | 11 | African–American/black | Non-Hispanic | Sickle cell disease | None |
| NPC-2016-192 | 12 | Not reported | Non-Hispanic | β-thalassemia major | None |
| NPC-2016-278 | 12 | African–American/black | Non-Hispanic | Sickle cell | None |
| NPC-2015-259 | 12 | Not reported | Non-Hispanic | β-thalassemia major | None |
| NPC-2014-175 | 13 | African–American/black | Non-Hispanic | Sickle cell disease | None |
| DNA-repair disease | |||||
| NPC-2013-128 | 5 | Not reported | Not reported | Diamond–Blackfan anemia | None |
| NPC-2014-192 | 5 | African–American/black | Non-Hispanic | Fanconi's anemia | None |
| NPC-2013-151 | 7 | White | Non-Hispanic | Fanconi's anemia | None |
| NPC-2016-277 | 9 | White | Non-Hispanic | Fanconi's anemia | None |
| NPC-2013-145 | 11 | White | Non-Hispanic | Fanconi's anemia | None |
| NPC-2015-266 | 12 | White | Non-Hispanic | Fanconi's anemia | BMT |
| Bone marrow failure | |||||
| NPC-2015-241 | 5 | White | Non-Hispanic | Aplastic anemia | None |
| NPC-2014-180 | 7 | White | Non-Hispanic | Aplastic anemia | None |
| NPC-2015-214 | 8 | White | Non-Hispanic | Acquired aplastic anemia since 2008, developed clonal evolution consistent with hypocellular myelodysplastic syndrome, with megakaryocytic dysplasia | None |
| NPC-2015-254 | 11 | White | Non-Hispanic | Aplastic anemia | None |
| NPC-2013-153 | 13 | White | Non-Hispanic | Aplastic anemia | None |
| NPC-2014-207 | 14 | White | Hispanic | Aplastic anemia | None |
| Other | |||||
| NPC-2015-220 | 0.5 | Not reported | Non-Hispanic | Hemophagocytic lymphohistiocytosis | Chemotherapy |
| NPC-2015-256 | 0.6 | White | Non-Hispanic | Hemophagocytic lymphohistiocytosis | Chemotherapy |
| NPC-2016-276 | 0.9 | Not reported | Non-Hispanic | IFN-γ deficiency | None |
| NPC-2016-286 | 1 | White | Non-Hispanic | Hemophagocytic lymphohistiocytosis | Chemotherapy |
| NPC-2016-279 | 1.2 | White | Non-Hispanic | Hemophagocytic lymphohistiocytosis | Chemotherapy |
| NPC-2013-133 | 2 | White | Non-Hispanic | Hurler's syndrome | None |
| NPC-2012-116 | 5 | White | Non-Hispanic | Idiopathic hypereosinophilic syndrome | Other |
| NPC-2013-143 | 10 | White | Non-Hispanic | Turner's syndrome | None |
BMT: Bone marrow transplant; OC-NPC: Oncofertility Consortium's National Physicians Cooperative.
Table 2. . Age and clinical diagnoses of Oncofertility Consortium's National Physicians Cooperative participants who underwent ovarian tissue cryopreservation due to an oncologic condition.
| OC-NPC ID# | Age (years) | Race | Ethnicity | Diagnosis | Treatment |
|---|---|---|---|---|---|
| I: Leukemias, myeloproliferative diseases and myelodysplastic neoplasms | |||||
| NPC-2012-110 | 3.8 | White | Non-Hispanic | Chronic myeloid leukemia going to BMT | Chemotherapy |
| NPC-2015-219 | 4 | Not reported | Hispanic | Low-risk CNS-negative acute myeloid leukemia | Chemotherapy |
| NPC-2016-294 | 4 | White | Non-Hispanic | Chronic myeloid leukemia | None |
| NPC-2013-149 | 5 | White | Non-Hispanic | JJMML | Chemotherapy and HSCT |
| NPC-2013-136 | 6 | White | Non-Hispanic | B-cell precursor acute lymphoblastic leukemia | Chemotherapy and radiation |
| NPC-2015-225 | 6 | African–American/black | Non-Hispanic | AML with WBC 39,000 and 55% blasts on smears and large paraspinal and intraspinal chloroma with spinal cord compression | Chemotherapy |
| NPC-2015-273 | 6 | White | Non-Hispanic | Pre B acute lymphoblastic leukemia | Chemotherapy |
| NPC-2011-82 | 8 | White | Non-Hispanic | Acute myeloid leukemia | Chemotherapy |
| NPC-2013-130 | 8 | White | Non-hispanic | T-cell acute lymphoblastic leukemia | Chemotherapy and HSCT |
| NPC-2016-300 | 8 | White | Non-Hispanic | Acute lymphoblastic leukemia (Ph + ALL) | Chemotherapy |
| NPC-2012-123 | 8 | White | Non-Hispanic | Very high-risk acute lymphoblastic leukemia due to cytogenetics | Chemotherapy |
| NPC-2012-125 | 9 | White | Non-Hispanic | Fanconi anemia; myelodysplastic syndrome | None |
| NPC-2015-255 | 9 | White | Non-Hispanic | Trisomy 8 myelodysplastic syndrome | None |
| NPC-2014-204 | 10 | White | Non-Hispanic | Acute myeloid leukemia after acute lymphoblastic leukemia going to BMT | Chemotherapy |
| NPC-2012-119 | 12 | African–American/black | Non-Hispanic | Acute lymphoblastic leukemia in March 2012 (hypoploidy; which puts her at very high risk for relapse during chemotherapy); will go to BMT with thiotepa, cytoxan, TBI | Chemotherapy |
| NPC-2014-178 | 12 | White | Non-Hispanic | Acute myeloid leukemia | Chemotherapy |
| NPC-2011-73 | 14 | Other | Non-Hispanic | Acute myeloid leukemia going to BMT | Chemotherapy |
| NPC-2013-139 | 14 | White | Non-Hispanic | Very high-risk acute lymphoblastic leukemia (hypodiploid) | Chemotherapy |
| NPC-2009-24 | 14.5 | White | Non-Hispanic | Myelodysplastic syndrome + trisomy 8 | None |
| NPC-2011-85 | 14.6 | White | Non-Hispanic | Myelodysplastic syndrome, go for transplantation | None |
| II: Lymphomas and reticuloendothelial neoplasms | |||||
| NPC-2014-167 | 12 | White | Non-Hispanic | Primary mediastinal B-cell lymphoma, localized to her chest | Chemotherapy |
| NPC-2012-124 | 13 | White | Non-Hispanic | Hodgkin disease, stage II | None |
| NPC-2014-166 | 13 | White | Non-Hispanic | Primary mediastinal B-cell lymphoma, localized to her chest | Chemotherapy |
| NPC-2016-284 | 13 | White | Non-Hispanic | Hodgkin's lymphoma | Chemotherapy |
| NPC-2016-288 | 13 | White | Non-Hispanic | Hodgkin's lymphoma | Chemotherapy |
| NPC-2009-29 | 13.9 | White | Non-Hispanic | Lymphoma | None |
| NPC-2014-197 | 14 | White | Hispanic | Natural killer cell lymphoma | Chemotherapy |
| III: CNS and miscellaneous intracranial and intraspinal neoplasms | |||||
| NPC-2016-280 | 2 | White | Hispanic | Primitive neuroectodermal tumor-metastatic | Chemotherapy |
| NPC-2016-282 | 8 | White | Non-Hispanic | Medulloblastoma | None |
| NPC-2011-66 | 12.5 | White | Non-Hispanic | Pineoblastoma | None |
| NPC-2015-217 | 14 | White | Non-Hispanic | Optic pathway pilocytic astrocytoma | Chemotherapy |
| IV: Neuroblastoma and other peripheral nervous cell tumors | |||||
| NPC-2011-65 | 2 | White | Non-Hispanic | Neuroblastoma | Chemotherapy and surgery |
| NPC-2014-205 | 4 | White | Hispanic | Neuroblastoma | Chemotherapy and radiation |
| NPC-2014-185 | 5 | African–American/black | Non-Hispanic | Neuroblastoma | None |
| NPC-2016-281 | 5 | Not reported | Non-Hispanic | High-risk neuroblastoma | Chemotherapy and HSCT |
| NPC-2015-262 | 7 | White | Non-Hispanic | Neuroblastoma | Chemotherapy |
| NPC-2015-272 | 9 | White | Non-Hispanic | High-risk neuroblastoma, stage IV | Chemotherapy |
| NPC-2014-165 | 10 | White | Non-Hispanic | High-risk neuroblastoma | Chemotherapy and surgery |
| NPC-2010-43 | 11.4 | Not reported | Not reported | High-risk neuroblastoma | Chemotherapy |
| VI: Renal tumors | |||||
| NPC-2015-240 | 1.9 | White | Non-Hispanic | Stage III Wilms tumor, CNS relapse | Chemotherapy and radiation |
| NPC-2014-203 | 3 | White | Non-Hispanic | Relapsed Wilms tumor | Chemotherapy |
| NPC-2015-222 | 4 | White | Non-Hispanic | Stage IV favorable histology Wilms tumor | Chemotherapy |
| NPC-2015-224 | 4 | White | Non-Hispanic | Wilms tumor, bilateral multifocal | Chemotherapy |
| NPC-2015-239 | 4 | White | Non-Hispanic | Clear cell sarcoma of the left kidney | None |
| NPC-2008-17 | 8.5 | Not reported | Not reported | Wilms tumor | Chemotherapy and radiation |
| NPC-2011-78 | 10 | White | Non-Hispanic | Wilms tumor | Chemotherapy and radiation |
| NPC-2015-216 | 10 | White | Non-Hispanic | History of Wilms tumor stage III now secondary pancreatic mass consistent with Ewing sarcoma | Chemotherapy and radiation |
| VIII: Malignant bone tumors | |||||
| NPC-2015-250 | 7 | White | Non-Hispanic | Ewing sarcoma, right clavicle | None |
| NPC-2015-257 | 8 | White | Non-Hispanic | Osteosarcoma | None |
| NPC-2014-194 | 9 | White | Non-Hispanic | Ewing sarcoma | None |
| NPC-2015-246 | 10 | Not reported | Non-Hispanic | Ewing sarcoma | None |
| NPC-2012-112 | 11.7 | White | Non-Hispanic | Ewing sarcoma, localized superficial disease right thigh | None |
| NPC-2014-162 | 13 | N/A | Non-Hispanic | Ewings; sarcoma | None |
| NPC-2014-187 | 13 | White | Non-Hispanic | Ewing sarcoma left foot | None |
| IX: Soft tissue and other extraosseous sarcomas | |||||
| NPC-2010-40 | 1.4 | White | Non-Hispanic | Rhabdomyosarcoma | Chemotherapy |
| NPC-2015-275 | 1.5 | White | Non-Hispanic | Vaginal rhabdomyosarcoma | None |
| NPC-2015-231 | 1.8 | White | Non-Hispanic | Rhabdomyosarcoma (right chest wall: liver) | None |
| NPC-2013-155 | 3 | Not reported | Not reported | Rhabdomyosarcoma | None |
| NPC-2014-183 | 3 | African–American/black | Non-Hispanic | Vaginal embryonal rhabdomyosarcoma | Chemotherapy |
| NPC-2016-304 | 3 | White | Non-Hispanic | Rhabdomyosarcoma | None |
| NPC-2014-161 | 4 | >1 | Non-Hispanic | Alveolar rhabdomyosarcoma | Chemotherapy and radiation |
| NPC-2016-295 | 4 | Asian | Non-Hispanic | Stage IV embryonal rhabdomyosarcoma, primary abdominal tumor | Chemotherapy |
| NPC-2015-232 | 5 | African–American/black | Non-Hispanic | Pelvic rhabdomyosarcoma | None |
| NPC-2015-263 | 7 | White | Non-Hispanic | Alveolar rhabdomyosarcoma | Chemotherapy |
| NPC-2016-291 | 7 | White | Non-Hispanic | Embryonal rhabdomyosarcoma | Chemotherapy |
| NPC-2015-249 | 9 | Not reported | Not reported | Embryonal sarcoma | None |
| NPC-2011-72 | 9.6 | White | Non-Hispanic | Vaginal rhabdomyosarcoma | Chemotherapy |
| NPC-2014-189 | 10 | White | Non-Hispanic | Synovial sarcoma abdomen | Chemotherapy |
| NPC-2008-10 | 12 | Not reported | Not reported | Rhabdomyosarcoma | None |
| NPC-2015-268 | 12 | White | Non-Hispanic | Parameningeal embryonal rhabdomyosarcoma | None |
| NPC-2016-283 | 12 | White | Non-Hispanic | Synovial cell sarcoma | Chemotherapy |
| NPC-2014-202 | 14 | White | Non-Hispanic | Embryonal rhabdomyosarcoma | Chemotherapy |
| NPC-2015-223 | 14 | White | Non-Hispanic | Nasal rhabdomyosarcoma (alveolar subtype) | None |
| X: Germ cell tumors, trophoblastic tumors and neoplasms of gonads | |||||
| NPC-2015-218 | 6 | White | Non-Hispanic | Sacrococcygeal germ cell tumor | Chemotherapy and HSCT |
| NPC-2016-293 | 14 | White | Non-Hispanic | Bilateral ovarian tumors, elevated AFP | None |
| XI: Other malignant epithelial neoplasms and malignant melanomas | |||||
| NPC-2014-169 | 13 | White | Non-Hispanic | Small cell carcinoma of ovary | None |
AML: Acute myeloid leukemia; BMT: Bone marrow transplant; HSCT: Hematopoietic stem-cell transplantation; JJMML: Juvenile myelomonocytic leukemia; OC-NPC: Oncofertility Consortium's National Physicians Cooperative; TBI: Total body irradiation; WBC: White blood cell count.
Data analysis
The OC-NPC cases were classified into two broad categories: oncologic and nononcologic. Oncologic conditions were further divided into the 12 categories specified by the International Classification of Childhood Cancer (ICCC; ICD-O-3; Tables 2 & 3) [31]. Nononcologic conditions were grouped into broad disease categories including hemoglobinopathies, DNA-repair diseases and bone marrow failure (Table 1). The frequency of malignancies between the OC-NPC cohort was compared with that in the general population obtained through the ACCIS and SEER databases. These two databases were selected due to their use of ICCC categories and reporting malignancy incidence for each category in individuals <15 years of age.
Table 3. . Categories of pediatric malignancies per the WHO's International Classification of Childhood Cancer system.
| ICCC category | Malignancies |
|---|---|
| I | Leukemias, myeloproliferative diseases and myelodysplastic diseases |
| II | Lymphomas and reticuloendothelial neoplasms |
| III | CNS and miscellaneous intracranial and intraspinal neoplasms |
| IV | Neuroblastoma and other peripheral nervous cell tissues |
| V | Retinoblastoma |
| VI | Renal tumors |
| VII | Hepatic tumors |
| VIII | Malignant bone tumors |
| IX | Soft tissue and other extraosseous sarcomas |
| X | Germ cell tumors, trophoblastic tumors and neoplasms of gonads |
| XI | Other malignant epithelial neoplasms and malignant melanomas |
| XII | Other and unspecified malignant neoplasms |
ICCC: International Classification of Childhood Cancer.
Results
Profile of OC-NPC participants (<15 years old) who underwent ovarian tissue cryopreservation
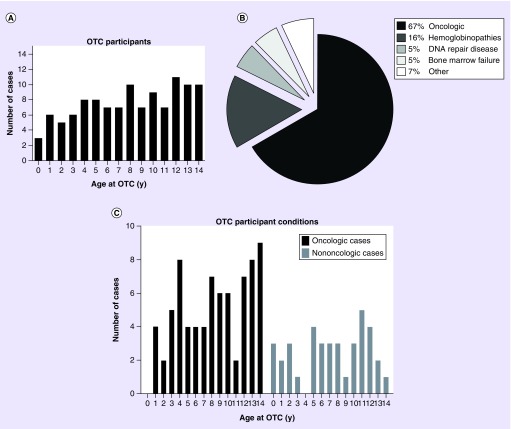
In the cohort of OC-NPC participants we examined, the age ranged from 0.5 to 14.6 years, with a mean age of 8.1 years and a median age of 8 years (Figure 2A). The average age of menarche in the USA is 12.5 years, and only 18% of participants in this cohort were older than this [32]. Thus, we believe that the majority of the females in our study were likely to be premenarchal, which is significant because OTC would be the only fertility-preservation option available in this cohort. For patients where race was reported 81% were white, 16% were African–American/black, 1% Asian, 1% were more than one race and 1% were other. Ninety percent of these patients were non-Hispanic. Reported previous treatment history for patients utilizing OTC varied, with 49% having had no prior treatment, 37% having undergone chemotherapy, 6% having been treated with chemotherapy and radiation, 3% with chemotherapy and hematopoietic stem-cell transplantation (HSCT), 2% treated with chemotherapy and surgery, 2% with bone marrow transplant and 1% other (Tables 1 & 2).
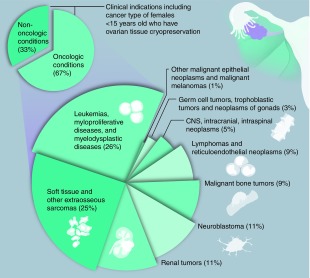
Figure 2. . Characteristics of participants <15 years old who have undergone ovarian tissue cryopreservation through the Oncofertility Consortium's National Physicians Cooperative.
(A). The age distribution in years of OC-NPC participants who have undergone OTC. The number of cases at each age is shown. (B) Distribution of clinical indications for OTC in the OC-NPC cohort. Oncologic conditions are clustered together. Nononcologic conditions are subdivided, and the relative percentages of each condition are noted. Additional details are in Tables 1 & 2. (C) The age distribution in years of participants who have undergone OTC for oncologic and nononcologic conditions. The number of cases at each age is shown.
OC-NPC: Oncofertility Consortium's National Physicians Cooperative; OTC: Ovarian tissue cryopreservation; y: Years.
The largest indication for OTC was oncologic conditions, comprising 67% of cases (Figure 2B). Nononcologic conditions comprised the remainder of the cases and included hemoglobinopathies (16%; sickle cell anemia and β-thalassemia); DNA-repair diseases (5%; Diamond–Blackfan anemia and Fanconi anemia); bone marrow failure (5%; aplastic anemia); and other (7%) (Figure 2B & Table 1). The majority of African–American/black participants underwent OTC for nononcologic versus oncologic conditions (67 vs 33%; Tables 1 & 2). This is not surprising given the higher prevalence of sickle cell anemia in this population [33]. Both oncologic and nononcologic conditions were distributed across ages (Figure 2C).
Characterization & evaluation of the OC-NPC cohort according to the ICCC malignancy categories
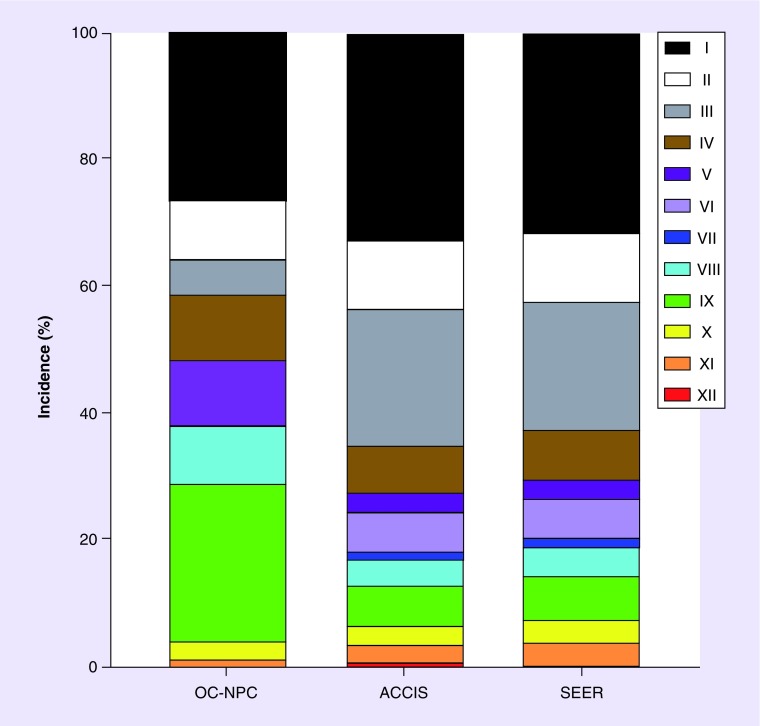
The oncologic conditions in the OC-NPC cohort were classified according to the 12 established categories of the ICCC system, which categorizes malignancies according to histological characteristics as opposed to primary site (Tables 2 & 3). The ICCC was utilized due to its acceptance within the international community for epidemiological studies and cancer registries, such as the SEER and ACCIS databases [34]. The breakdown of the OC-NPC cohort according to ICCC categories is shown in Figure 3. Leukemias, myeloproliferative diseases and myelodysplastic diseases (category I) were the largest oncologic indication (26%) in the OC-NPC cohort and included myeloid leukemia, lymphoid leukemia and myelodysplastic syndrome. Soft tissue and other extraosseous sarcomas (category IX), such as rhabdomyosarcoma and synovial sarcoma, were the second largest indication for OTC in the OC-NPC cohort (25%). Neuroblastoma (category IV) and renal tumors (category VI), including Wilms tumor and clear cell sarcoma, both accounted for 11% of oncologic cases. Lymphomas and reticuloendothelial neoplasms (category II), including Hodgkin lymphoma, non-Hodgkin lymphoma and B-cell lymphoma, and malignant bone tumors (category VIII), including Ewing sarcoma and osteosarcoma, each accounted for 9% of the OC-NPC oncologic cases. Five percent of the OC-NPC cases were CNS, intracranial and intraspinal neoplasms (category III) consisting of medulloblastoma, pineoblastoma, astrocytoma and primitive neuroectodermal tumors. Three percent of cases were germ cell tumors, trophoblastic tumors and neoplasms of gonads (category X). The one case in the other malignant epithelial neoplasms and malignant melanomas (category XI) case was small cell carcinoma of the ovary. There were no cases of retinoblastoma (category V), hepatic tumors (category VII) or other and unspecified malignant neoplasms (category XII; Table 2 & Figure 3).
Figure 3. . The incidence of International Classification of Childhood Cancer malignancy categories in the Oncofertility Consortium's National Physicians Cooperative cohort compared with the general population.
The percent incidence of malignancies in individuals <15 years old in each category was plotted from the three databases (OC-NPC cohort, ACCIS, and SEER Program). More detail on each International Classification of Childhood Cancer category is in Table 3.
ACCIS: Automated Childhood Cancer Information System; OC-NPC: Oncofertility Consortium's National Physicians Cooperative; SEER: Surveillance, Epidemiology and End Results Program.
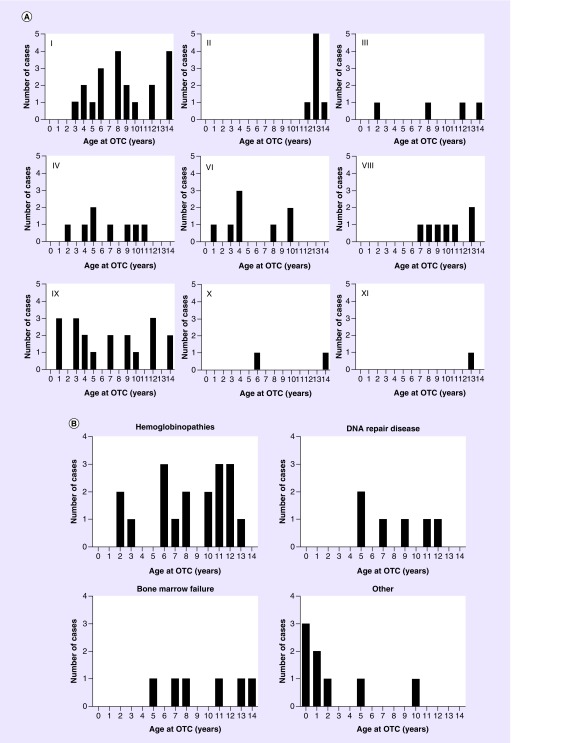
Within each ICCC category, the ages of the OC-NPC cohort were displayed in histograms (Figure 4A). Participants with leukemias, myeloproliferative diseases and myelodysplastic diseases (category I); CNS, intracranial and intraspinal neoplasms (category III); renal tumors (category VI); and soft tissue and other extraosseous sarcomas (category IX), were distributed across the age spectrum. However, lymphomas and reticuloendothelial neoplasms (category II) and malignant bone tumors (category VIII) were more prevalent in older aged participants (Figure 4A). The OC-NPC participants with malignancies in category II were 12 years or older, and those in category VIII were 7 years or older. These findings are consistent with epidemiologic data, as lymphomas and reticuloendothelial neoplasms occur at a higher incidence in children >10 years, especially those with Hodgkin's disease [27]. Epidemiologic data for malignant bone tumors (category VIII) show rising incidence in individuals age 5–10 years, with an increased incidence associated with growth peaking at age 13 [27]. There were no OC-NPC participants that had retinoblastoma (category V), hepatic tumors (category VII) or unspecified malignant neoplasms (category XII). Limited numbers of OC-NPC cases had malignancies in category X (germ cell tumors, trophoblastic tumors and neoplasm of gonads) or category XI (malignant epithelial neoplasms and malignant melanomas). For nononcologic conditions, those with hemoglobinopathies were distributed across ages, whereas those with DNA-repair diseases and bone marrow failure were noted to be in females above 5 years of age (Figure 4B). However, this dataset is limited.
Figure 4. . Age histogram of the Oncofertility Consortium's National Physicians Cooperative cohort according to clinical condition.
(A) The number of OTC cases at each age is shown for the oncologic conditions specified by the relevant International Classification of Childhood Cancer malignancy categories and (B) the nononcologic conditions.
OTC: Ovarian tissue cryopreservation.
We next compared the incidence of ICCC malignancy categories between the OC-NPC cohort and the general population. To do this, we examined the frequency of each ICCC malignancy category for data obtained from three separate databases: the OC-NPC; ACCIS; and SEER (Figure 3). ACCIS and SEER are databases for childhood cancer, and as expected, there was concordance in the frequency distribution of malignancy categories between these two databases (Figure 3). However, when comparing the malignancy incidence within the OC-NPC cohort to the general population (ACCIS and SEER), differences in five main categories were observed (Figure 3). Categories III, V and VII (CNS/intracranial/intraspinal malignancies, retinoblastoma and hepatic tumors) were under-represented in the OC-NPC cohort relative to the general population (Table 4). In contrast, categories VIII and IX (malignant bone tumors and soft tissue/extraosseous sarcomas) were over-represented in the OC-NPC cohort (Table 4).
Table 4. . Categories with lower and higher frequency in the Oncofertility Consortium's National Physicians Cooperative cohort than the general population.
| ICCC category | OC-NPC (%) | ACCIS (%) | SEER (%) |
|---|---|---|---|
| Lower frequency categories | |||
| III | 5 | 21.5 | 20.2 |
| V | No cases | 2.9 | 3.1 |
| VII | No cases | 1.1 | 1.3 |
| Higher frequency categories | |||
| VIII | 9 | 4.1 | 4.5 |
| IX | 25 | 6.3 | 7.0 |
ACCIS: Automated Childhood Cancer Information System; ICCC: International Classification of Childhood Cancer; OC-NPC: Oncofertility Consortium's National Physicians Cooperative; SEER: Surveillance, Epidemiology and End Results.
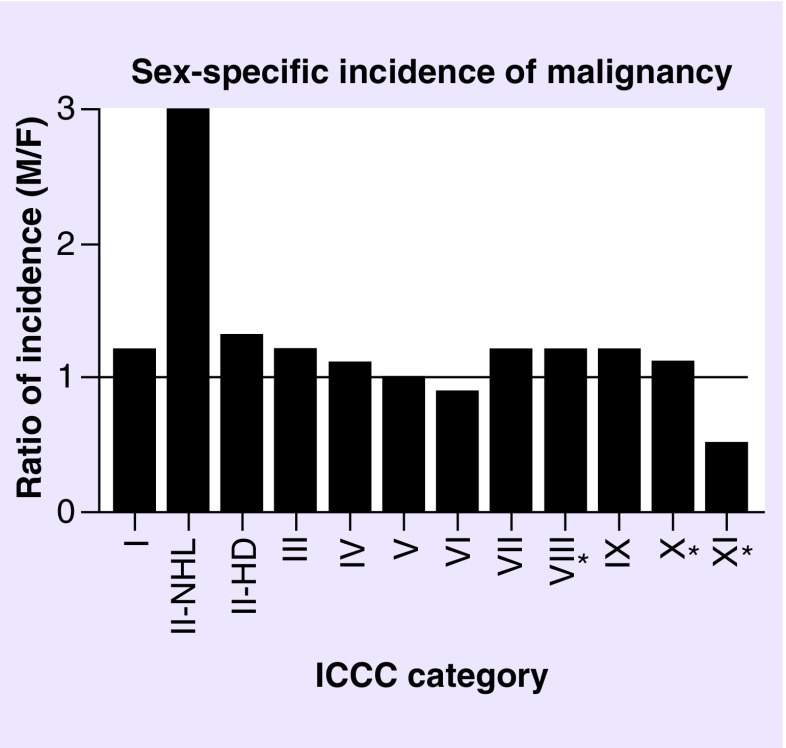
A limitation of comparing the OC-NPC data to that in the ACCIS and SEER databases is that the male and female malignancy incidences are combined. We therefore examined whether there were sex-specific differences in malignancy incidences in the general population. In the SEER database, malignancy incidences are separated by sex and reported for individuals <15 and <20 years old. For the majority of malignancy categories, the male-to-female ratio was close to one (Figure 5). The sex-specific incidence for category XII was not reported due to a low number of cases but was not highlighted as having a sex bias. Thus overall, these malignancies appear to have similar incidences between males and females (Figure 5). However, category II (non-Hodgkin lymphoma) had a higher incidence in males (M:F ratio: 3.0), and category XI (malignant epithelial neoplasms) had a higher incidence in females (M:F ratio: 0.5; Figure 5). Importantly, the malignancy categories that appeared different between the OC-NPC cohort and the ACCIS and SEER populations (categories III, V, VII, VIII and IX) did not exhibit sex-specific differences in incidence (Figure 5 & Table 4).
Figure 5. . The sex-specific incidence of malignancies according to International Classification of Childhood Cancer categories.
Malignancy incidences were separated by sex and reported for individuals <15 or <20 (marked with asterisk) years old in the Surveillance, Epidemiology and End Results Program database. For each International Classification of Childhood Cancer category, the data are shown as a ratio of male-to-female incidence. Specific numerical data were not available for category XII. The horizontal line highlights equal incidence or a ratio of one.
F: Female; ICCC: International Classification of Childhood Cancer; M: Male.
Discussion
The OC-NPC represents the largest formal fertility-preservation network in the USA, and we previously documented that usage of OTC has steadily increased among young females (<18 years old) relative to the adult female population in this network [26]. The purpose of this study was to further investigate the characteristics of this young cohort that has utilized OTC. However, we narrowed our focus to females <15 years old so that we could directly compare our data to existing databases of cancer incidences. Understanding how OTC is used among patients has broad implications because the usage of this technique is widespread and increasing globally. A recent study of OTC in Danish girls <18 years of age reported a similar increased frequency in indications in both malignant (malignant bone tumors and soft tissue/other extraosseous sarcomas) and nonmalignant conditions (hemoglobinopathies, bone marrow failure, DNA-repair diseases), with a peak incidence in hematologic malignancies [35]. This is reassuring that our data regarding indications for OTC coincides with this study, though the Danish study investigated a wider age group in a single country. The experimental label and restrictions placed on OTC have been challenged in Israel, indicating that this procedure may become an even more prevalent fertility-preservation option [36].
In our cohort, the largest indication for OTC was oncologic conditions, with the top two malignancy classifications being leukemias, myeloproliferative diseases and myelodysplastic diseases (category I) and soft tissue and other extraosseous sarcomas (category IX). This is expected due to the fertility risk of the therapy regimens involved in treating these malignancies, including alkylating chemotherapy, radiation of tumors and radioablation in preparation for HSCT [37,38]. Importantly, studies suggest that over 60% of patients undergoing HSCT experience POI [39]. It is also clear from our analysis that OTC in young females is performed for indications beyond the setting of oncofertility. A third of patients had nononcologic conditions that required fertility preservation due to either the sterilizing nature of the disease treatment or the disease process itself. For example, patients with hemoglobinopathies, DNA-repair diseases, bone marrow failure and inborn errors of metabolism can all be treated with HSCT [40–44]. Thus, fertility-preservation options are offered in these scenarios. In the case of sickle cell anemia, there has been a successful birth from a patient utilizing her cryopreserved cortical grafts that were preserved a decade prior, before she had entered puberty [23]. These results are encouraging and underscore the importance of OTC as a fertility-preservation option for both oncologic as well as nononcologic conditions. Leukemias comprised the largest indication for OTC in our cohort; autotransplantation of ovarian tissue from patients with hematologic malignancies imparts a risk of re-seeding malignancy [45]. A recent study describes autotransplantation of ovarian tissue from a small number of patients with leukemia with no detection of malignant cells within the sample tissue or reoccurrence of malignancy, though this tissue was harvested after adjuvant chemotherapy prior to HSCT and conditioning regimens [36]. Careful counseling of patients with hematologic malignancies before OTC is needed because of the risk of re-seeding malignancy and investigation of tissue for malignant cells is still required. Other possible methods to decrease risk of re-seeding malignancies include the isolation of follicles from cryopreserved ovarian tissue and utilizing an artificial ovary or in vitro follicle growth [17,46,47]. These methods, however, are still experimental and have not been translated clinically.
When we compared the types and incidence of malignancies in the OC-NPC cohort who underwent the OTC procedure relative to the age-matched general population, we observed that several disease categories were either relatively over- or under-represented. These discrepancies appear to correlate with the fertility risk of these specific conditions or their treatment. For example, conditions with higher incidence within the OC-NPC cohort were malignant bone tumors and soft tissue and other extraosseous sarcomas. Standard treatment for bone tumors and rhabdomyosarcoma utilizes alkylating agent chemotherapy (cisplatin, cyclophosphamide, and ifosfamide) and radiation in excess of 30 Gy, which may be targeted to the pelvis for vaginal rhabdomyosarcoma [38,48,49]. Because these categories use therapies that impose the highest risk to fertility, it is expected and reassuring that these patients are being educated about fertility preservation and electing to undergo OTC. In contrast, conditions that were under-represented in the OC-NPC cohort relative to the general population included CNS/intracranial/intraspinal malignancies, retinoblastoma and hepatic tumors. Of note, there were no cases of retinoblastoma or hepatic tumors reported in the OC-NPC cohort. CNS/intracranial/intraspinal malignancies are typically treated with a combination of surgical resection and radiation, which could damage the HPO axis [50]. However, this exposure would not directly damage the ovaries and, therefore, OTC would not necessarily be indicated. Some cases involve spinal irradiation or alkylating agent chemotherapy, both of which would warrant discussion of the fertility preservation [51]. Patients with CNS/intracranial/intraspinal malignancies may require fertility preservation depending on their treatment modality, though it is likely that these fertility-threatening regimens were not utilized in the OC-NPC patients within this category due to a low observed incidence. Retinoblastoma affects the retina and is typically treated via enucleation, though 20% of retinoblastoma cases are metastatic and involve alkylating agents and radiation [52]. Retinoblastoma has a 5-year recurrence rate of 62% [53]. In most cases, OTC is not relevant for this condition, and this may explain why we did not observe any individuals with this diagnosis in the OC-NPC cohort. On the other hand, hepatic tumors are treated with alkylating agent chemotherapy, which represents a high risk to fertility [54]. Although patients with hepatic tumors may be candidates for OTC, the incidence of this malignancy is low during childhood, accounting for 1.1% of all malignancies in children <20 years of age [27]. This low frequency could account why this population was not represented in the OC-NPC cohort.
Ovarian tissue should only be removed from individuals that are at the highest risk of sterilization. This study provides important insight into the profile of young girls who have undergone OTC for fertility preservation for oncologic and nononcologic conditions. Although our sample size is small, it appears that the fertility risk imparted by disease treatments corresponds to the disease categories of individuals that have undergone OTC. Our findings indicate that the physicians in the OC-NPC are correctly identifying and extending OTC to young patients with the highest risk of POI imposed by their treatment. These findings provide evidence that providers and patients are aware of fertility-preservation options. One limitation of our study is that we do not know, of the patients who were counseled, how many elected to undergo fertility preservation. Although not electing to pursue OTC is a valid decision, these patients may have also been correctly identified as the highest need for fertility preservation but would not have been included in our analysis. Our analysis is also confined to the OC-NPC, which is comprised of large interdisciplinary teams primarily at large academic centers that are trained and committed to providing fertility preservation to men, women and children in the USA [9,55]. Whether these findings can be extrapolated from this biased population across larger populations warrants additional investigation. In addition, it is simply a matter of years before those who banked their ovarian tissue during childhood return to use it for transplantation. Global efforts are needed to monitor transplantation efficiency and outcomes in these individuals and to develop novel methods to restore fertility and endocrine function with these tissues [56].
Conclusion
The population of those surviving and experiencing long-term health effects of cancer treatment is increasing. Fertility preservation is an important option for cancer patients, which allows restoration of endocrine function and/or fertility in those whose disease or treatment may have the unintended consequence of compromised reproductive function. OTC followed by transplantation is an experimental procedure that has successfully restored fertility (86 live births) and hormone production (up to 7 years). Importantly, OTC is the only fertility-preservation method suitable for prepubertal girls. Nearly 25% of OTC cases that have been done through the OC-NPC network were in girls <15 years of age. Removal of ovarian tissue, especially in young individuals, is experimental, must be done under appropriate ethical approvals, and represents a drastic fertility-preservation measure. Thus, it is imperative that physicians are educated to appropriately identify only those patients at highest risk of reproductive failure as candidates for OTC. By examining the malignancy categories that are predominant in the OC-NPC cohort relative to the general population, we observed congruence between the diagnoses of participants who underwent OTC and the relative fertility risk associated with the treatment for their condition. This finding validates that the physicians in the OC-NPC are correctly identifying and extending OTC to young patients at the highest risk and in most need of fertility preservation.
Summary points.
Between the inception of the Oncofertility Consortium's National Physicians Cooperative (OC-NPC) in 2007 and May 2016, 330 individuals have undergone ovarian tissue cryopreservation (OTC), and 117 of these individuals were <15 years old.
A third of the girls (<15 years old) that have undergone OTC through the OC-NPC have done so for nononcologic conditions that have a high risk of infertility due to the conditions themselves or their treatment.
The top two oncologic indications for OTC through the OC-NPC in girls (<15 years old) include the International Classification of Childhood Cancer categories of leukemias, myeloproliferative diseases, and myelodysplastic diseases (I) and soft-tissue and other extraosseous sarcomas (IX).
Differences exist in malignancy distribution between the general population and girls (<15 years old) who have undergone OTC through the OC-NPC. These differences can be traced to the relative fertility risk imparted by the specific conditions and/or their treatment.
OC-NPC physicians are correctly identifying and extending fertility preservation to patients at highest risk and in most need of fertility preservation.
Acknowledgements
The authors would like to thank the Oncofertility Consortium's National Physicians Cooperative participants and sites, the Oncofertility Consortium, and the University of Kansas Medical Center.
Footnotes
Financial & competing interests disclosure
This work was supported by the Center for Reproductive Health After Disease (P50 HD076188–02) from the National Centers for Translational Research in Reproduction and Infertility (NCTRI). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.American Society of Clinical Oncology. The state of cancer care in America, 2014: a report by the American Society of Clinical Oncology. J. Oncol. Pract. 2014;10(2):119–142. doi: 10.1200/JOP.2014.001386. [DOI] [PubMed] [Google Scholar]
- 2.Ward E, Desantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 2014;64(2):83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 5.Tonorezos ES, Hudson MM, Edgar AB, et al. Screening and management of adverse endocrine outcomes in adult survivors of childhood and adolescent cancer. Lancet Diabetes Endocrinol. 2015;3(7):545–555. doi: 10.1016/S2213-8587(15)00038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376(9744):911–921. doi: 10.1016/S0140-6736(10)60355-8. [DOI] [PubMed] [Google Scholar]
- 7.Brougham MF, Wallace WH. Subfertility in children and young people treated for solid and haematological malignancies. Br. J. Haematol. 2005;131(2):143–155. doi: 10.1111/j.1365-2141.2005.05740.x. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt KT, Larsen EC, Andersen CY, Andersen AN. Risk of ovarian failure and fertility preserving methods in girls and adolescents with a malignant disease. BJOG. 2010;117(2):163–174. doi: 10.1111/j.1471-0528.2009.02408.x. [DOI] [PubMed] [Google Scholar]
- 9.Woodruff TK. Oncofertility: a grand collaboration between reproductive medicine and oncology. Reproduction. 2015;150(3):S1–S10. doi: 10.1530/REP-15-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384(9950):1302–1310. doi: 10.1016/S0140-6736(14)60834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnez J, Dolmans MM. Ovarian tissue freezing: current status. Curr. Opin. Obstet. Gynecol. 2015;27(3):222–230. doi: 10.1097/GCO.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 12.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013;31(19):2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopeika J, Thornhill A, Khalaf Y. The effect of cryopreservation on the genome of gametes and embryos: principles of cryobiology and critical appraisal of the evidence. Hum. Reprod. Update. 2015;21(2):209–227. doi: 10.1093/humupd/dmu063. [DOI] [PubMed] [Google Scholar]
- 14.Salama M, Woodruff TK. New advances in ovarian autotransplantation to restore fertility in cancer patients. Cancer Metastasis Rev. 2015;34(4):807–822. doi: 10.1007/s10555-015-9600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J. Assist. Reprod. Genet. 2017;34(3):325–336. doi: 10.1007/s10815-016-0843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filatov MA, Khramova YV, Kiseleva MV, Malinova IV, Komarova EV, Semenova ML. Female fertility preservation strategies: cryopreservation and ovarian tissue in vitro culture, current state of the art and future perspectives. Zygote. 2016;24(5):635–653. doi: 10.1017/S096719941600006X. [DOI] [PubMed] [Google Scholar]
- 17.Xiao S, Zhang J, Romero MM, Smith KN, Shea LD, Woodruff TK. In vitro follicle growth supports human oocyte meiotic maturation. Sci. Rep. 2015;5:17323. doi: 10.1038/srep17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salama M, Isachenko V, Isachenko E, Rahimi G, Mallmann P. Updates in preserving reproductive potential of prepubertal girls with cancer: systematic review. Crit. Rev. Oncol. Hematol. 2016;103:10–21. doi: 10.1016/j.critrevonc.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Ernst E, Kjaersgaard M, Birkebaek NH, Clausen N, Andersen CY. Case report: stimulation of puberty in a girl with chemo- and radiation therapy induced ovarian failure by transplantation of a small part of her frozen/thawed ovarian tissue. Eur. J. Cancer. 2013;49(4):911–914. doi: 10.1016/j.ejca.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 20.Poirot C, Abirached F, Prades M, Coussieu C, Bernaudin F, Piver P. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet. 2012;379(9815):588. doi: 10.1016/S0140-6736(11)61781-9. [DOI] [PubMed] [Google Scholar]
- 21.Andersen CY, Silber SJ, Bergholdt SH, Jorgensen JS, Ernst E. Long-term duration of function of ovarian tissue transplants: case reports. Reprod. Biomed. Online. 2012;25(2):128–132. doi: 10.1016/j.rbmo.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Donnez J, Dolmans MM, Pellicer A, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil. Steril. 2013;99(6):1503–1513. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 23.Demeestere I, Simon P, Dedeken L, et al. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum. Reprod. 2015;30(9):2107–2109. doi: 10.1093/humrep/dev128. [DOI] [PubMed] [Google Scholar]
- 24.Anderson RA, Hindmarsh PC, Wallace WH. Induction of puberty by autograft of cryopreserved ovarian tissue in a patient previously treated for Ewing sarcoma. Eur. J. Cancer. 2013;49(13):2960–2961. doi: 10.1016/j.ejca.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 25.Von Wolff M, Stute P, Fluck C. Autologous transplantation of cryopreserved ovarian tissue to induce puberty – the endocrinologists’ view. Eur. J. Pediatr. 2016;175(12):2007–2010. doi: 10.1007/s00431-016-2771-1. [DOI] [PubMed] [Google Scholar]
- 26.Duncan FE, Pavone ME, Gunn AH, et al. Pediatric and teen ovarian tissue removed for cryopreservation contains follicles irrespective of age, disease diagnosis, treatment history, and specimen processing methods. J. Adolesc. Young Adult Oncol. 2015;4(4):174–183. doi: 10.1089/jayao.2015.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ries LAG Seer Program (National Cancer Institute [US]) In: Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program 1975–1995. Ries LAG, Smith MA, Gurney JG, et al., editors. National Cancer Institute, SEER Program; Bethesda, USA: 1999. [Google Scholar]
- 28.ACCIS. National estimates of incidence rates standardized to world standard population age 0–14. 2003. http://accis.iarc.fr/
- 29.SEER. Cancer incidence and survival among children and adolescents: United States SEER program 1975–1995. https://seer.cancer.gov/archive/publications/childhood/childhood-monograph.pdf
- 30.ACCIS. National estimates of incidence rates standardized to world standard population age 0–14. http://accis.iarc.fr/results/2003/pdfs/summaryincidencetables.pdf
- 31.Institute NC. ICCC Recode ICD-O-3/WHO 2008. 2008. https://seer.cancer.gov/iccc/iccc-who2008.html
- 32.Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of US women: data from the 2002 National Survey of Family Growth. Vital Health Stat. 2005;23(25):1–160. [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention (CDC) Incidence of sickle cell trait – United States, 2010. MMWR Morb. Mortal. Wkly Rep. 2014;63(49):1155–1158. [PMC free article] [PubMed] [Google Scholar]
- 34.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer. 2005;103(7):1457–1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 35.Jensen AK, Rechnitzer C, Macklon KT, et al. Cryopreservation of ovarian tissue for fertility preservation in a large cohort of young girls: focus on pubertal development. Hum. Reprod. 2017;32(1):154–164. doi: 10.1093/humrep/dew273. [DOI] [PubMed] [Google Scholar]
- 36.Meirow D, Ra'Anani H, Shapira M, et al. Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil. Steril. 2016;106(2):467–474. doi: 10.1016/j.fertnstert.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 37.Committee IP, Kim SS, Donnez J, et al. Recommendations for fertility preservation in patients with lymphoma, leukemia, and breast cancer. J. Assist. Reprod. Genet. 2012;29(6):465–468. doi: 10.1007/s10815-012-9786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment – where do we stand? A state of the art review. Cancer Treat Rev. 2014;40(4):523–532. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Joshi S, Savani BN, Chow EJ, et al. Clinical guide to fertility preservation in hematopoietic cell transplant recipients. Bone Marrow Transplant. 2014;49(4):477–484. doi: 10.1038/bmt.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angelucci E, Matthes-Martin S, Baronciani D, et al. Hematopoietic stem cell transplantation in thalassemia major and sickle cell disease: indications and management recommendations from an international expert panel. Haematologica. 2014;99(5):811–820. doi: 10.3324/haematol.2013.099747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fumani HK, Zokaasadi M, Kasaeian A, et al. Allogeneic hematopoietic stem cell transplantation for adult patients with fanconi anemia. Mediterr. J. Hematol. Infect. Dis. 2016;8(1):e2016054. doi: 10.4084/MJHID.2016.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dietz AC, Lucchini G, Samarasinghe S, Pulsipher MA. Evolving hematopoietic stem cell transplantation strategies in severe aplastic anemia. Curr. Opin. Pediatr. 2016;28(1):3–11. doi: 10.1097/MOP.0000000000000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parini R, Deodato F, Di Rocco M, et al. Open issues in Mucopolysaccharidosis type I-Hurler. Orphanet J. Rare Dis. 2017;12(1):112. doi: 10.1186/s13023-017-0662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shenoy S, Boelens JJ. Advances in unrelated and alternative donor hematopoietic cell transplantation for nonmalignant disorders. Curr. Opin. Pediatr. 2015;27(1):9–17. doi: 10.1097/MOP.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dolmans MM, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood. 2010;116(16):2908–2914. doi: 10.1182/blood-2010-01-265751. [DOI] [PubMed] [Google Scholar]
- 46.Laronda MM, Rutz AL, Xiao S, et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat. Commun. 2017;8:15261. doi: 10.1038/ncomms15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abir R, Ben-Aharon I, Garor R, et al. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy. Hum. Reprod. 2016;31(4):750–762. doi: 10.1093/humrep/dew007. [DOI] [PubMed] [Google Scholar]
- 48.Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group. J. Clin. Oncol. 2012;30(33):4148–4154. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hawkins DS, Gupta AA, Rudzinski ER. What is new in the biology and treatment of pediatric rhabdomyosarcoma? Curr. Opin. Pediatr. 2014;26(1):50–56. doi: 10.1097/MOP.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vern-Gross TZ, Bradley JA, Rotondo RL, Indelicato DJ. Fertility in childhood cancer survivors following cranial irradiation for primary central nervous system and skull base tumors. Radiother. Oncol. 2015;117(2):195–205. doi: 10.1016/j.radonc.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Tosoni A, Balestrini D, Brandes AA. Fertility preservation in women with CNS tumors. Expert Rev. Anticancer Ther. 2017;17(5):439–445. doi: 10.1080/14737140.2017.1316195. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez-Galindo C, Chantada GL, Haik BG, Wilson MW. Treatment of retinoblastoma: current status and future perspectives. Curr. Treat. Options Neurol. 2007;9(4):294–307. doi: 10.1007/s11940-007-0015-4. [DOI] [PubMed] [Google Scholar]
- 53.Kim JW, Abramson DH, Dunkel IJ. Current management strategies for intraocular retinoblastoma. Drugs. 2007;67(15):2173–2185. doi: 10.2165/00003495-200767150-00005. [DOI] [PubMed] [Google Scholar]
- 54.Aronson DC, Czauderna P, Maibach R, Perilongo G, Morland B. The treatment of hepatoblastoma: its evolution and the current status as per the SIOPEL trials. J. Indian Assoc. Pediatr. Surg. 2014;19(4):201–207. doi: 10.4103/0971-9261.142001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woodruff TK. From the bench to bedside to babies: translational medicine made possible by funding multidisciplinary team science. J. Assist. Reprod. Genet. 2013;30(10):1249–1253. doi: 10.1007/s10815-013-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ataman LM, Rodrigues JK, Marinho RM, et al. Creating a global community of practice for oncofertility. J. Glob. Oncol. 2016;2(2):83–96. doi: 10.1200/JGO.2015.000307. [DOI] [PMC free article] [PubMed] [Google Scholar]