Abstract
Tumor-derived exosomes (TEX) carry both immunosuppressive and immunostimulatory receptor/ligands that in part mimic the profiles of the parent tumor cells. Operating as an intercellular communication system, TEX deliver protumor or antitumor signals to immune and nonimmune cells reprogramming their functions. Mechanisms responsible for cellular reprogramming include cell surface signaling and/or uptake of TEX by recipient cells. Once internalized, TEX transfer mRNA, miRNA and proteins that promote transcriptional/translational activities. TEX-mediated signaling is contextual and, in the tumor microenvironment, TEX largely mediate suppression. TEX may interfere with immune therapies either by sequestration of therapeutic antibodies or elimination of vaccine-induced or adoptively-transferred immune effector cells. TEX are emerging as an ubiquitous subcellular system regulating immune responses in patients with cancer.
Keywords: : cancer, cellular reprogramming, immune escape, immune regulation, TEX, tumor-derived exosomes, tumor microenvironment
Recent studies of cancer development, progression and response to therapies have emphasized the critical role of the tumor microenvironment (TME) in achieving better outcome [1]. The TME of human solid tumors is characterized by immunosuppression which is orchestrated by the developing tumor to enable its escape from the host immune system. Immune escape of tumors has been considered a major barrier to successful immunotherapy of cancer [2]. Understanding of the intricate cellular, molecular and genetic interplay in the TME has been a major objective of multiple research studies aimed at reprogramming of the TME to drive antitumor responses and eliminate immune suppression [3]. Today, there is little doubt that the host immune system is intimately involved in the regulation of tumor progression and that tumor-induced or drug-induced impairments in antitumor immunity are associated with poor outcome of cancers [4]. Successful restoration of antitumor immune responses, as recently achieved through the advent of checkpoint inhibitor-based immune therapies to the clinic, has confirmed that elimination of immune suppression in the TME improves cancer outcome [5].
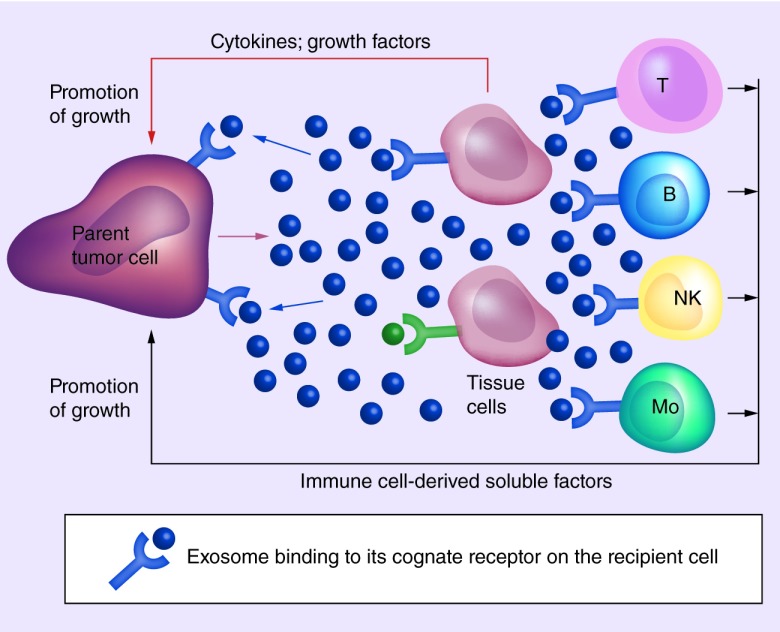
Among the numerous strategies human tumors have evolved to escape immune detection and immune elimination, the ability of tumors to produce populations of variously-sized extracellular vesicles has attracted recent attention [6]. Exosomes are a subset of extracellular vesicles with the smallest diameter (30–150 nm) produced by most, if not all cells, including tumor cells. They circulate freely in body fluids of patients with cancer serving as a major delivery system of signals that reprogram functions of various cells found in the TME, including immune cells [7]. Tumor-derived exosomes, dubbed as ‘TEX’, represent the communication network used by the tumor to drive autocrine, juxtacrine and paracrine signaling among various cells populating the TME (Figure 1). While, all cells produce exosomes, tumors are especially avid producers of exosomes. Levels of exosomes in plasma or other body fluids are elevated in patients with cancer relative to their levels in normal donors [8]. TEX are of special current interest because of their unique biogenesis, their potential to serve as noninvasive cancer biomarkers and their ability to modulate functions of immune cells and to suppress antitumor responses [7].
Figure 1. . Signaling of tumor-derived exosomes to cells present in the tumor microenvironment.
TEX recognizing cognate receptors/ligands on the surface of the parent cell mediate autocrine signals (blue arrows) that result in the promotion of tumor growth (proliferation, differentiation, migration). TEX can also signal to neighboring tissue cells (juxtacrine signaling) altering their functions and stimulating secretion of cytokines, chemokines or growth factors promoting tumor growth (red arrow). TEX also communicate with immune cells infiltrating tumors or present in tumor-draining lymph nodes (paracrine signaling) reprogram these cells and induce them to produce soluble factors supporting tumor growth (black arrow).
NK: Natural killer; TEX: Tumor-derived exosomes.
Adapted and reproduced with permission from [9] © John Wiley and Sons.
Exosome isolation & characterization
Exosomes can be isolated from supernatants of cultured cell lines and from body fluids of healthy donors or cancer patients by a variety of methods ranging from ultracentrifugation at high speed, precipitation, microfluidic separation, affinity capture to size-exclusion chromatography [10]. High-speed ultracentrifugation of precleared plasma, traditionally used for exosome isolation, is being slowly replaced by methods allowing for more efficient, rapid and clinically applicable isolation of exosomes that are partially depleted of plasma ‘contaminants’ and retain their vesicular morphology and integrity [11,12]. Exosome recovery from 1 ml of cancer plasma yields around 1012 particles [12]. Exosomes can be visualized by electron microscopy or atomic-force microscopy, counted in specially designed instruments, labeled with dyes, coincubated with cells, injected into experimental animals or loaded with drugs [13]. Since, TEX are a subset of total plasma exosomes, the ratios of TEX/normal cell-derived exosomes in cancer plasma varies, although in patients with advanced malignancies TEX represent a substantial fraction of total plasma exosomes [14]. TEX recovery from plasma of patients with melanoma has recently become possible in my laboratory, using an immunoaffinity-based selective capture of TEX by an antibody specific for the antigenic epitope expressed on melanoma but not on normal cells [15]. This offers an opportunity for ex vivo studies of the content and function of TEX present in cancer plasma as opposed to those produced by cultured tumor cells.
Exosomes, including TEX, originate from the endosomal cell compartment and thus carry a molecular cargo that partly mimics that of the parent tumor cell [16]. Exosomes acquire their molecular components through the well-defined series of coordinated inward membrane invaginations taking place in late endosomes and multivesicular bodies. Sorting and packaging of exosomes for release from the parent cell is executed by the endosomal sorting complex responsible for transport, which is parent-cell specific, and which might be responsible for directing exosomes to a predefined cellular address [17,18]. Multivesicular bodies fuse with a parent cell-surface membrane releasing exosomes into the extracellular space. This biogenesis process forms exosomes that contain elements derived from endosomes (e.g., TSG101, syntenin-1, ALIX) as well as from the cell-surface membrane and cytosol of the parent cell [16,19]. This biogenesis and the virus-like size of exosomes, distinguish them from larger (200–1000 nm) microvesicles which are a product of ‘blebbing’ or ‘budding’ of the parental cell-surface membrane [16]. Although the molecular and genetic contents as well as functions of exosomes and microvesicles might overlap, current evidence points to exosomes as the population of circulating vesicles that best approximates the antigenic content of parent cells and, in the case of TEX, might potentially be useful as ‘liquid tumor biopsies’.
Molecular signatures of tumor-derived exosomes
Much of what is known about TEX comes from studies of vesicles in supernatants of tumor cell lines, where all exosomes are products of tumor cells. The TEX molecular signature distinguishes them from exosomes derived from normal cells. Further, TEX released by different types of tumor cells have distinct molecular signatures [12,14]. TEX carry a cargo consisting of a broad variety of molecular species, including membrane-associated proteins, glycoproteins, lipids and glycolipids as well as a rich vesicular content (reviewed in [20]). The surface membrane of TEX is a lipid–protein bilayer that contains cholesterol, ceramides, sphingomyelins and phospholipids as well as numerous biologically active proteins such as the major histocompatibility complex molecules; tumor associated antigens (TAAs); inhibitory ligands such as FasL, TRAIL, PD-L1, TGF-β/LAP; adhesion molecules, notably ICAM, EPCAM, CD44, integrins; proteases such as mitochondrial membrane potential and CD26; ectonucleotidases engaged in adenosine production, CD39/CD73; transmembrane receptors such as CXCR4 and c-Met; heat shock proteins; and various tetraspanins (CD9, CD63, CD81) frequently used as ‘exosome markers’. In the TEX lumen are nucleic acids, including DNA, mRNA and miRNA; cytosolic proteins including various enzymes; soluble factors, such as PGE2; cytokines; histones; transport proteins such as ALIX, Rabs, dynamin, LAMPs; cytoskeletal proteins, including actin, tubulin, vimentin and others; oncoproteins; and a variety of signaling molecules, including MAPK, ERK1/2, Rho, catenin, Wnt and many others. The TEX molecular and genetic content recapitulates that of the parent cell. However, it is unclear how much of the parent cell content is passed on to TEX, and the estimates vary widely from 5 to 50% [21]. Nevertheless, TEX have been shown to be enriched in key molecules that are characteristic of the parent cell and therefore can, in part, serve as surrogates of the parent tumor cells [22].
Functional profiles of TEX
TEX are well equipped to serve as information transfer vehicles shuttling messages between parent tumor cells and other normal or malignant cells in the TME [23]. It is suspected that these messages might be addressed to reach specific recipient cells. Although, the mechanisms are not yet understood for TEX delivery and processing of their cargo in recipient cells, they may include initial ligand–receptor type of binding on the cell surface followed by endocytosis or phagocytosis [24]. Veladi et al. were first to report that mRNAs and miRNAs can be transferred via exosomes from one cell to another and induce changes in cellular functions of the recipient cells [25]. Whether TEX signal via cognate receptors on the vesicle surface or are internalized by the recipient cell, delivering their content of nucleic acids, TEX–recipient cell interactions culminate in a loss or gain of functions in recipient cells and in cellular reprogramming [20].
Immunosuppressive signaling by TEX
Initial experiments with TEX isolated from supernatants of cultured tumor cells, which contain only TEX and no other exosomes, indicated that TEX can effectively mediate suppression of immune cells in ex vivo assays and in vivo in experimental animals [26]. This was not an unexpected finding, as the TEX cargo is generally enriched in immunoinhibitory proteins and factors similar to those present in parental tumor cells and able to downregulate immune responses [6]. As tumor cells themselves inhibit functions of immune cells [27], TEX emerge as a novel mechanism able to effectively transmit suppressive signals to all types of immune cells. TEX-mediated suppression of immune-cell functions contributes to tumor growth and facilitates tumor escape from the host immune system [6].
The mechanistic aspects of TEX-mediated suppression of immune cells have been investigated using human or murine T cells coincubated with TEX. T cells express TcR and IL-2R, and these two receptors regulate T-cell responses and any interference with TcR-mediated or IL-2R-mediated signaling negatively impacts T-cell functions. We and others have observed that TEX negatively regulate functions of these receptors [27,28] and that T cells, especially CD8+ T cells, are very sensitive to TEX-mediated inhibition. When T cells are co-incubated with TEX, TEX-mediated down-regulation of the TcR ζ chain is consistently seen [29]. Additionally, TEX reduce phosphorylation in activated T cells and JAK expression, which is essential for IL-2, IL-7 and IL-15 function. These are cytokines that share the γ-chain of the IL-2R and are essential for T-cell expansion: downregulation of JAK expression/activity by TEX results in the inhibition of T-cell proliferation [30]. However, effects of TEX on T-cell subsets are complex. It was determined that TEX suppressed proliferation of CD8+ T cells but promoted expansion of CD4+ T cells, while exosomes released by normal cells promoted proliferation of all T cells [27]. We and others reported that TEX promoted expansion and activity of Treg, a functionally critical subset of CD4+ T cells [27,31,32]. TEX were also found to upregulate STAT5 phosphorylation in activated CD4+ T cells and to inhibit STAT5 phosphorylation in activated CD8+ T cells, suggesting that TEX modulate transcription-factor functions, such as those mediated by STATS, for example, in recipient T cells, although mechanisms behind these changes are not known [30].
We also asked whether TEX globally suppressed antitumor functions of all T cells or preferentially inhibited proliferation of human melanoma-specific CD8+ T cells. To this end, cocultures of human T cells with melanoma peptide-pulsed DC were set up. The data suggested that TEX inhibited melanoma antigen-specific T-cell responses as well as responses of other activated T cells [27]. It appears that T-cell activation rather than antigen specificity predisposes them to TEX-mediated suppression. There is strong evidence in support of the ability of TEX carrying the membrane form of FasL or PD-L1 to alter functions of Fas+ or PD-1+ T cells, respectively [12,27,33]. We showed that TEX-mediated signals leading to apoptosis of activated CD8+ T cells were associated with early membrane changes (i.e., Annexin V binding) in recipient cells, caspase-3 cleavage, cytochrome C release from mitochondria, loss of mitochondrial membrane potential and DNA fragmentation [33]. Thus, apoptosis was induced in activated CD8+ T cells by TEX via the engagement of extrinsic and intrinsic apoptotic cascades. Additional data indicated that the PI3K/AKT pathway was the key target for TEX-mediated demise of activated CD8+ T cells. We reported that time-dependent AKT dephosphorylation and concomitant decreases in expression levels of survival proteins, BCL-2, BCL-xL and MCL-1, accompanied by an increase in levels of proapoptotic BAX were observed in activated CD8+ T cells upon co-incubation with TEX [33].
Recent data suggest that nucleic acids, especially mRNA and miRNAs, transferred by TEX from the cancer to recipient cells are largely responsible for functional changes in the latter [34]. To evaluate contributions of mRNA transferred by TEX to the recipient T-cell reprogramming, we evaluated transcriptional activity and functional changes in T cells coincubated with TEX or DEX (exosomes produced by human monocyte-derived cultured DC and used as control for TEX). Subsets of human CD4+, CD8+ and CD4+CD39+ Treg cells isolated from peripheral blood of normal donors served as recipient cells [35]. Expression levels of 24 immunoregulatory genes were monitored by qRTPCR in these cells [35]. We observed massive changes in expression levels of multiple immunoinhibitory and immunostimulatory genes in recipient T cells following coincubation with TEX. Three factors had a significant impact on TEX-induced transcriptional activity in T cells: the presence/absence of TEX; the recipient cell type (CD4+, CD8+ or CD39+ Treg); and the activation status of the recipient T cells. Different immunoregulatory genes in TEX vs DEX induced changes in mRNA expression levels of recipient lymphocytes [31]. Some genes in Treg were also modulated differently in comparison with recipient CD4+ or CD8+ T cells. We also measured CD69 (an activation marker) expression levels in recipient CD4+ T effector cells by flow cytometry to demonstrate that the TEX-mediated mRNA changes translated into relevant functions. Consistent with TEX-induced immune suppression in CD4+ T cells, we saw significantly decreased expression levels of CD69 on the surface of these activated T cells [35]. Also, Treg coincubated with TEX, which carry CD39 and CD73 ectonucleotidases [36], significantly upregulated production of immunosuppressive adenosine in T cells in a concentration- and time-dependent manner [35]. The data suggest that mRNA transcripts delivered by TEX to recipient T cells are translated into immunosuppressive proteins, representing one of the mechanisms responsible for reprogramming. At the same time, in the context of our recently-reported results that activated T cells do not or only minimally internalize TEX [37], mRNA transfer may not be the major reprogramming mechanism in T cells.
Another potential mechanism used by TEX for reprogramming of recipient cells, especially those immune cells that readily up-take exosomes, such as B cells, monocytes or dendritic cells (DCs) [33] involves miRNAs [34]. These are small noncoding RNAs that bind within the 3′-untranslated region of mRNAs leading to destabilization of these RNAs and consequently to reduced expression of the encoded protein [34]. miRNAs are the major component of exosomes and can be transported to different cell types. As indicated in Table 1, cancer-derived exosomes carrying miRNAs shape the immune responses in the TME and largely account for the acquisition of protumorigenic functions in various recipient cells (Table 1). At the same time, the hypothesis that TEX negatively modulate T-cell responses by engaging surface receptors, such as PD-1, Fas or TRAILR, on recipient T cells and by activating molecular suppressive pathways in these cells remains a potentially attractive mechanism to consider and evaluate.
Table 1. . The miRNAs carried by tumor-derived exosomes and delivered to immune cells induce protumorigenic functions.
| miRNA carried by TEX | Tumor type | Recipient cell | Acquired functions | Consequences for recipient cells |
|---|---|---|---|---|
| miR-21 miR-29a | NSLC | TAMs |
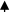 NF-κB pathway NF-κB pathway |
|
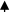 IL-6, IL-6,  TNF-α TNF-α |
 Proinflammatory phenotype Proinflammatory phenotype |
|||
| miR-203 miR-212p |
PAC | DCs |
 TLR-4 TLR-4 TNF-α, TNF-α,  IL-12 IL-12 |
 DC maturation DC maturation DC dysfunction DC dysfunction |
| miR-214 | Various human or mouse tumors | T cells |
 Treg Treg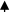 PTEN PTEN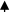 IL-10 IL-10 |
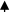 Immune
suppression Immune
suppression |
| miR-183 | Human tumor cell lines | NK cells |
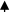 TGF-β TGF-β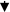 DAP12 DAP12 |
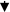 Lytic functions Lytic functions |
| miR-92a | Glioma | NKT cells |
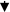 Perforin Perforin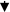 FasL FasL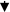 IFN-γ IFN-γ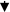 CD8+ T cells CD8+ T cells |
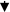 Tumor apoptosis Tumor apoptosis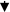 Antitumor activity Antitumor activity |
Selected examples of miRNA interactions with different types of immune cells are presented. For a detailed review of TEX-associated miRNAs and their biological effects, see [34].
DC: Dendritic cell; NK: Natural killer; NSLC: Non-small-cell lung cancer; PAC: Pancreatic adenocarcinoma; TAM: Tumor-associated macrophage; TEX: Tumor-derived exosome.
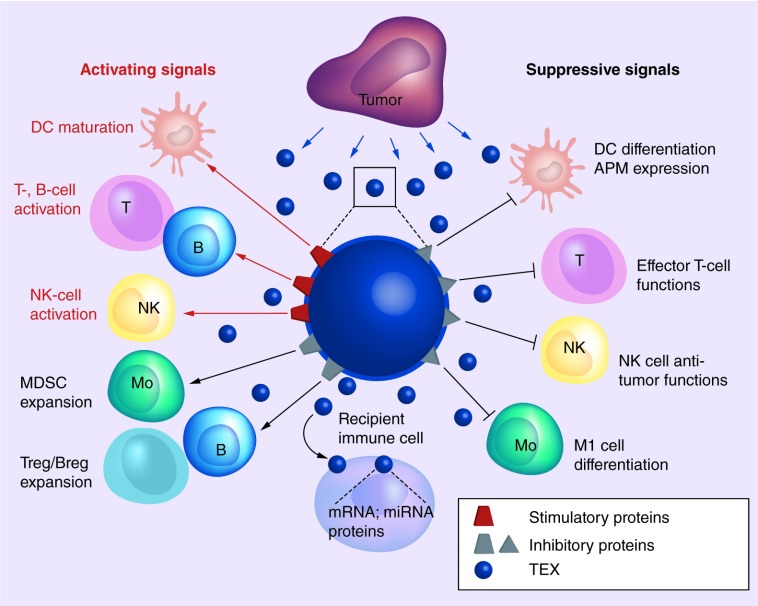
T-lymphocytes are not the only immune cells targeted by TEX, and functions of natural killer (NK) cells, B cells, monocytes/macrophages and DCs are impaired by co-incubation in the presence of TEX (Figure 2). TEX carrying MICA and MICB, ligands of the NKG2D, an activating receptor present on NK cells, downregulate its expression and suppress NK-cell functions [38]. TGF-β is displayed on TEX as TGF-LAP allowing TGF-β activation via binding to integrins, such as, a6βV. TGF-β inhibits NK-cell activation and cytotoxicity [38,39].
Figure 2. . Tumor-derived exosomes in the tumor microenvironment carry and deliver both immunoinhibitory (protumor) and immunostimulatory (antitumor) signals to immune cells.
Immune cells that recognize the inhibitory and stimulatory signals on the TEX surface engage TEX in a cross-talk that leads to the TEX uptake and transfer of mRNA, miRNAs and proteins from TEX to recipient cells. Molecular signals and nucleic acids delivered by TEX induce reprogramming of recipient cell functions via molecular/genetic mechanisms discussed in the text. Antitumor activities of most immune cells are blocked (black arrows), while the regulatory immune cells (Treg and Breg, MDSC) are stimulated by TEX to expand and acquire protumor suppressor functions. TEX also carry MHC molecules, tumor associated antigens and costimulatory molecules and thus can stimulate immune cells to upregulate antitumor activities (red arrows), including enhanced maturation of DCs and enhanced tumor antigen processing. Cross-talk of these DC with T and B cells leads to their activation. NK cells can be activated by soluble factors delivered by TEX. The TEX-immune cell cross-talk provides contextual reprogramming which in the TME is heavily biased toward immune suppression.
DC: Dendritic cell; MDSC: Myeloid-derived suppressor cell; MHC: Major histocompatibility complex; NK: Natural killer; TEX: Tumor-derived exosomes; TME: Tumor microenvironment.
TEX, which are able to make adenosine from ATP by virtue of carrying enzymatically active CD39 and CD73 [36] are implicated in inducing suppression of activated B cells [36]. Adenosine was shown to be able to convert activated B cells into regulatory B cells [40]. Additionally, TEX were reported to inhibit monocyte differentiation and to convert monocytes into TGF-β-expressing DCs. TGF-β-expressing DCs secrete prostaglandin E2 (PGE2) which interferes with the generation of cytolytic T cells [41,42]. Myeloid precursor cells were skewed toward developing into highly suppressive MDSCs by TEX, which was dependent on MyD88 signaling in monocytes and also the presence of TGF-β and PGE2 in the TEX cargo [43]. Overall, TEX appear to be biologically-active vesicles that can negatively impact functions of immune cells via mechanisms involving nucleic acids and/or proteins TEX carry.
TEX appear to be capable of simultaneously engaging one or several molecular and genetic pathways to induce protumorigenic functional changes in recipient cells.
Table 2. . Suppressive effects of tumor-derived exosomes on functions of immune cells in the tumor microenvironment.
| Direct TEX–immune cell interactions | Ref. |
|---|---|
| Apoptosis of activated antitumor effector T cells | [26,40] |
| Interference with normal differentiation | [41,42,44] |
| Inhibition of immune cell activation, proliferation and/or cytotoxicity | [14,26,46] |
| Polarization of the cytokine/chemokine profile to the tumor-promoting, proinflammatory profile | [23,29,38,41] |
| Regulation of immune cell migration to the tumor | [27,43,45,27] |
| Indirect interactions | |
| Promotion of CD4+CD25+FOXP3+ Treg proliferation | [31,47] |
| Enhancement of Treg suppressor functions via, e.g., increased adenosine production | [14,31,32] |
| Expansion of MDSC | [37] |
MDSC: Myeloid-derived suppressor cell; TEX: Tumor-derived exosomes.
Immunostimulatory signaling by TEX
The TEX cargo contains costimulatory molecules, major histocompatibility complex class I and class II molecules, TAA and intraluminal growth-promoting cytokines In addition to a plethora of immunoinhibitory molecules [7,20]. Clearly, TEX have dual functional capabilities suggesting that TEX are capable of stimulating as well as suppressing immune-cell responses (Figure 2). It appears that stimulatory and inhibitory signals TEX carry are delivered simultaneously. But it remains unclear how this mode of delivery of multiple signals able to stimulate or inhibit immune responses translates into specific functional alterations in recipient cells. Nevertheless, it could be that the content of TEX cargos, always containing a broad range of costimulatory as well as inhibitory molecules/genes may play a lesser role in the reprogramming than the nature of the recipient cell or the prevailing conditions in the TME. The perception that recipient cells might regulate the cross-talk between the tumor and the host immune system could explain some of the contradictory results in the literature. A large body of information supports the TEX potential to promote differentiation and antigen-processing capabilities of DC in the TME. The potential of TEX carrying TAA and costimulatory molecules to reprogram antigen-presenting cells (APC) may be especially useful in potentiating efficacy of the antitumor cancer vaccines [48]. Uptake of TEX by APC populating the tumor-draining lymph nodes (LN) might promote in situ production of proinflammatory cytokines, shifting the cytokine signature in the LNs to one rich in IL-6, IFN-γ and IL-12 but low in IL-10 and thus promoting the Th1 immune responses. Recent in vivo studies with TEX suggest that TEX exert stimulatory biological effects when incorporated into antitumor vaccines. Interestingly, immunostimulatory effects in the TME may not be mediated directly by TEX, but by M1 macrophages induced by TEX to produce exosomes that reprogram the LN cytokine milieu to one promoting the Th1 immune response [49]. This is an excellent example of TEX-mediated juxtacrine effects (Figure 1), where M1 macrophages or DCs in the TME are reprogrammed by TEX to promote antitumor immune responses.
Considerable efforts have been expanded to enlist TEX as a potential adjuvant component of future antitumor vaccines. Given the available data, it is clear that additional in vivo studies are needed to better define the role of TEX in potentiating the efficacy of antitumor vaccines. TEX have the ability to create a local inflammatory environment that shifts the balance from immune suppression to immune stimulation in the presence of a growing tumor. This finding recommends TEX as potentially more effective adjuvants for antitumor vaccines than those traditionally utilized to date. However, the selection of immunopotentiating exosomes for use in vaccines requires further studies. The molecular cargo of these exosomes should be enriched in costimulatory receptor/ligands on the surface of exosome membranes as well as abundant phosphatidyl serine to, respectively, ensure strong costimulatory signaling and efficient uptake by APCs, specifically by immature DCs. The lumen of the exosome is enriched in mRNA species that, when internalized, should redirect recipient cells to produce IL-12, TNF-α, IFN-γ and other cytokines promoting activation of APCs. To achieve this, it is necessary to rely either on the parent cell to provide exosomes with the necessary attributes or to ex vivo modify exosomes to fit the requirements defined above. The latter strategy is being currently pursued, and preliminary data suggest that it may be successful in the future.
The role of TEX in immunotherapy of cancer
The immunoinhibitory and immunostimulatory effects of TEX have led to a controversy regarding their biological role in cancer, with many investigators viewing TEX not as mediators of immune suppression but rather as vaccination-promoting vehicles capable of inducing effective antitumor immunity [50,51]. However, it is reasonable to expect that the vesicle-based communication system driven by the tumor is operating to benefit tumor progression and to impair antitumor immune responses. TEX carrying an excess of immunoinhibitory ligands not only directly or indirectly suppress antitumor functions of immune effector cells, but they also appear to interfere with immune therapies.
Evidence has been emerging that implicates TEX in reducing beneficial effects of immune therapies. For example, antibody-based cancer therapies are made less effective by TEX carrying TAAs which are targeted by therapeutic antibodies. Such TEX, ubiquitously present in all body fluids of cancer patients, can ‘soak’ therapeutic antibodies diminishing their antitumor effects or conceivably completely block the access of therapeutic Abs to the tumor [52]. Adoptively transferred activated T or NK cells may be especially vulnerable to TEX carrying multiple inhibitory ligands [46]. Furthermore, activated T cells could be highly sensitive to apoptosis by TEX carrying, for example, FasL among other inhibitory ligands, following the delivery of antitumor vaccines [47].
Our recent ex vivo experiments showed that pretherapy plasma of patients with relapsed refractory acute myelogenous leukemia enrolled in a Phase I clinical trial of adoptive cell therapy (ACT) with NK-92 cells containing high levels of exosomes enriched in the immunosuppressive cargo. These patients have not responded favorably to adoptive NK-92 therapy [53]. We speculated that the highly immunosuppressive microenvironment fueled by negatively-signaling exosomes in relapsed/refractory acute myelogenous leukemia patients could represent a major barrier to NK cell-based ACT. To test the hypothesis, we coincubated exosomes isolated from pretherapy plasma of these patients with NK-92 cells used for therapy. The isolated exosomes were shown to mediate potent inhibition of numerous NK-92 cell functions and to interfere with antileukemia activity of NK-92 cells [53]. Further, the ex vivo blockade of exosome-mediated suppression in part restored antileukemia functions of NK-92 cells. These results demonstrate that in malignancy, plasma-derived exosomes can interfere with immune cells used for ACT and may limit expected therapeutic benefits of ACT.
Future perspective
TEX are emerging as novel immunoregulatory elements in cancer. Their dual ability to carry and transfer to immune effector cells signals that are either inhibitory or stimulatory is intriguing and provocative. The key to this dual functional potential of TEX might be the cellular composition of the TME and the nature of cells targeted by exosomes. TEX-driven interactions can be direct or indirect, as illustrated above, and the presence/absence of immune recipient cells in the TME is likely to determine the outcome of TEX signaling. Tumors that are infiltrated by immune cells have a strong incentive to produce immunosuppressive TEX to disarm antitumor effector cells. These tumors may be responsive to immunotherapies blocking suppressive pathways induced in immune recipient cells by these TEX delivering the juxtacrine or paracrine signaling (Figure 1). Thus, in the TME, where tumor cells are actively engaged in suppression of antitumor immunity mediated by infiltrating activated T cells, TEX are primarily utilized as a highly effective mechanism that favors tumor escape. On the other hand, in tumors that are ‘sterile’ (i.e., are poorly infiltrated by immune cells), the TEX profiles might favor autocrine signaling to promote tumor growth. In this instance, with TEX committed to directly support tumor progression, immune therapies are likely to be ineffective, and conventional therapies designed to inhibit tumor progression warrant consideration. At the same time, it is necessary to remember that all cells in the TME produce exosomes and that immunostimulatory signals directly or indirectly induced by TEX can reprogram the milieu to one that supports immune activities and not the tumor. The balance of these multiple subcellular interactions in the TME will determine the fate of the tumor as well as activities of the local immune system. In the future, it might be possible to regulate TEX functions by molecular, genetic or immune interventions. Today, efforts are directed at the better understanding of molecular mechanisms driving TEX release, uptake by recipient cells and transcriptional or translational changes they induce.
Executive summary.
Tumor-derived exosomes (TEX) are novel immunoregulatory elements in cancer.
TEX carry molecular/genetic cargo that partially resembles that of a parent-tumor cell.
TEX maintain the cross-talk between tumor cells and all cells present in the tumor microenvironment.
TEX can mediate immuno-activating (antitumor) and immuno-inhibitory (protumor) activity.
TEX carry and deliver signals to immune recipient cells that are translated into functional alterations that modulate antitumor immune responses.
TEX use various mechanisms to deliver ‘messages’ to recipient cells, including transfer of nucleic acids, mRNA, miRNAs, DNA and proteins responsible for activation of inhibitory molecular pathways in immune recipient cells.
TEX exercising their protumor activity may interfere with immune therapies of cancer.
TEX responsible for immune suppression are being targeted by novel therapeutic strategies aimed at restoring effective antitumor immunity in patients with cancer.
Footnotes
Financial & competing interests disclosure
Supported in part by NIH grants RO-1 CA168628 and R21- CA205644 to TL Whiteside. The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Schoenhals JE, Seyedin SN, Anderson C, et al. Uncovering the immune tumor microenvironment in non-small cell lung cancer to understand response rates to checkpoint blockade and radiation. Transl. Lung Cancer Res. 2017;6(2):148–158. doi: 10.21037/tlcr.2017.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whiteside TL, Demaria S, Rodriguez-Ruiz ME, Zarour HM, Melero I. Emerging opportunities and challenges in cancer immunotherapy. Clin. Cancer Res. 2016;22(8):1845–1855. doi: 10.1158/1078-0432.CCR-16-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res. 2015;21(4):687–692. doi: 10.1158/1078-0432.CCR-14-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 2016;13(3):143–158. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 5.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whiteside TL. Tumor-derived exosomes and their role in tumor-induced immune suppression. Vaccines (Basel) 2016;4(4) doi: 10.3390/vaccines4040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv. Clin. Chem. 2016;74:103–141. doi: 10.1016/bs.acc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellwinkel JE, Redzic JS, Harland TA, Gunaydin D, Anchordoquy TJ, Graner MW. Glioma-derived extracellular vesicles selectively suppress immune responses. Neuro. Oncol. 2016;18(4):497–506. doi: 10.1093/neuonc/nov170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiteside TL. Exosomes carrying immunoinhibitory proteins and their role in cancer. Clin. Exp. Immunol. 2017;189(3):259–267. doi: 10.1111/cei.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Review and discussion of current methods for exosome isolation.
- 11.Welton JL, Webber JP, Botos LA, Jones M, Clayton A. Ready-made chromatography columns for extracellular vesicle isolation from plasma. J. Extracell. Vesicles. 2015;4:27269. doi: 10.3402/jev.v4.27269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong CS, Funk S, Muller L, Boyiadzis M, Whiteside TL. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J. Extracell. Vesicles. 2016;5:29289. doi: 10.3402/jev.v5.29289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nazarenko I, Rupp AK, Altevogt P. Exosomes as a potential tool for a specific delivery of functional molecules. Methods Mol. Biol. 2013;1049:495–511. doi: 10.1007/978-1-62703-547-7_37. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig S, Floros T, Theodoraki MN, et al. Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clin. Cancer Res. 2017;23(16):4843–4854. doi: 10.1158/1078-0432.CCR-16-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Links immunosuppressive content of the exosome cargo to disease activity in patients with cancer.
- 15.Sharma P, Ludwig S, Muller L, et al. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J. Extracell. Vesicles. 2017 doi: 10.1080/20013078.2018.1435138. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell. Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colombo M, Moita C, Van Niel G, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell. Sci. 2013;126(Pt 24):5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 18.Lo Cicero A, Stahl PD, Raposo G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr. Opin. Cell Biol. 2015;35:69–77. doi: 10.1016/j.ceb.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25(6):364–372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Whiteside TL. Exosomes and tumor-mediated immune suppression. J. Clin. Invest. 2016;126(4):1216–1223. doi: 10.1172/JCI81136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klinke DJ, 2nd, Kulkarni YM, Wu Y, Byrne-Hoffman C. Inferring alterations in cell-to-cell communication in HER2+ breast cancer using secretome profiling of three cell models. Biotechnol. Bioeng. 2014;111(9):1853–1863. doi: 10.1002/bit.25238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atay S, Godwin AK. Tumor-derived exosomes: a message delivery system for tumor progression. Commun. Integr. Biol. 2014;7(1):e28231. doi: 10.4161/cib.28231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyiadzis M, Whiteside TL. The emerging roles of tumor-derived exosomes in hematological malignancies. Leukemia. 2017;31(6):1259–1268. doi: 10.1038/leu.2017.91. [DOI] [PubMed] [Google Scholar]; • A comprehensive current text on the role of tumor-derived exosome (TEX) in leukemias.
- 24.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles. 2014:3. doi: 10.3402/jev.v3.24641. Online. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A good presentation of various uptake mechanisms for extracellular vesicles (EVs).
- 25.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]; • First report on ability of EVs to transport mRNA and miRNAs to recipient cells.
- 26.Wen SW, Sceneay J, Lima LG, et al. The biodistribution and immune suppressive effects of breast cancer-derived exosomes. Cancer Res. 2016;76(23):6816–6827. doi: 10.1158/0008-5472.CAN-16-0868. [DOI] [PubMed] [Google Scholar]
- 27.Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J. Immunol. 2009;183(6):3720–3730. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67(15):7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 29.Taylor DD, Gercel-Taylor C, Lyons KS, Stanson J, Whiteside TL. T-cell apoptosis and suppression of T-cell receptor/CD3-zeta by Fas ligand-containing membrane vesicles shed from ovarian tumors. Clin. Cancer Res. 2003;9(14):5113–5119. [PubMed] [Google Scholar]
- 30.Whiteside TL. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes) Biochem. Soc. Trans. 2013;41(1):245–251. doi: 10.1042/BST20120265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mrizak D, Martin N, Barjon C, et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J. Natl Cancer Inst. 2015;107(1):363. doi: 10.1093/jnci/dju363. [DOI] [PubMed] [Google Scholar]
- 32.Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg) PLoS ONE. 2010;5(7):e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Czystowska M, Han J, Szczepanski MJ, et al. IRX-2, a novel immunotherapeutic, protects human T cells from tumor-induced cell death. Cell Death Differ. 2009;16(5):708–718. doi: 10.1038/cdd.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fanini F, Fabbri M. Cancer-derived exosomic microRNAs shape the immune system within the tumor microenvironment: state of the art. Semin. Cell Dev. Biol. 2017;67:23–28. doi: 10.1016/j.semcdb.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; • An excellent summary of the role of miRNAs carried by TEX in reprogramming of immune cell functions.
- 35.Muller L, Mitsuhashi M, Simms P, Gooding WE, Whiteside TL. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci. Rep. 2016;6:20254. doi: 10.1038/srep20254. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Looks at TEX-induced changes in expression of multiple immunoregulatory genes (mRNA transcripts) in subsets of T cells coincubated with TEX.
- 36.Schuler PJ, Saze Z, Hong CS, et al. Human CD4(+) CD39(+) regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73(+) exosomes or CD73(+) cells. Clin. Exp. Immunol. 2014;177(2):531–543. doi: 10.1111/cei.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller L, Simms P, Hong CS, et al. Human tumor-derived exosomes (TEX) regulate Treg functions via cell surface signaling rather than uptake mechanisms. Oncoimmunology. 2017;6(8):e1261243. doi: 10.1080/2162402X.2016.1261243. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Shows that human T cells do not readily internalize exosomes.
- 38.Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica. 2011;96(9):1302–1309. doi: 10.3324/haematol.2010.039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong CS, Muller L, Whiteside TL, Boyiadzis M. Plasma exosomes as markers of therapeutic response in patients with acute myeloid leukemia. Front. Immunol. 2014;5:160. doi: 10.3389/fimmu.2014.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Levels in plasma and cargos of exosomes may serve as biomarkers of responses to therapy in acute myelogenous leukemia.
- 40.Figueiro F, Muller L, Funk S, Jackson EK, Battastini AM, Whiteside TL. Phenotypic and functional characteristics of CD39high human regulatory B cells (Breg) Oncoimmunology. 2016;5(2):e1082703. doi: 10.1080/2162402X.2015.1082703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiang X, Poliakov A, Liu C, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int. J. Cancer. 2009;124(11):2621–2633. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bretz NP, Ridinger J, Rupp AK, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J. Biol. Chem. 2013;288(51):36691–36702. doi: 10.1074/jbc.M113.512806. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Focus on interactions of exosomes with toll-like receptors on immune cells.
- 43.Liu Y, Xiang X, Zhuang X, et al. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am. J. Pathol. 2010;176(5):2490–2499. doi: 10.2353/ajpath.2010.090777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu S, Liu C, Su K, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J. Immunol. 2007;178(11):6867–6875. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- 45.Luga V, Zhang L, Viloria-Petit AM, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151(7):1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 46.Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007;67(7):2912–2915. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 47.Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin. Cancer Res. 2005;11(3):1010–1020. [PubMed] [Google Scholar]
- 48.Whiteside TL. Stimulatory role of exosomes in the context of therapeutic anti-cancer vaccines. Biotarget. 2017;1 doi: 10.21037/biotarget.2017.05.05. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng L, Wang Y, Huang L. Exosomes from M1-polarized macrophages potentiate the cancer vaccine by creating a pro-inflammatory microenvironment in the lymph node. Mol. Ther. 2017;25(7):1665–1675. doi: 10.1016/j.ymthe.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Features exosomes as potential adjuvants for anticancer vaccines.
- 50.Kunigelis KE, Graner MW. The deichotomy of tumor exosomes (TEX) in cancer immunity: is it all in the context? Vaccines (Basel) 2015;3(4):1019–1051. doi: 10.3390/vaccines3041019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014;14(3):195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Battke C, Ruiss R, Welsch U, et al. Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC. Cancer Immunol. Immunother. 2011;60(5):639–648. doi: 10.1007/s00262-011-0979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong CS, Sharma P, Yerneni SS, et al. Circulating exosomes carrying an immunosuppressive cargo interfere with cellular immunotherapy in acute myeloid leukemia. Sci. Rep. 2017;7:14684. doi: 10.1038/s41598-017-14661-w. [DOI] [PMC free article] [PubMed] [Google Scholar]